Abstract
Despite advancements in drug discovery techniques, developing medications for diverse ailments still remains challenging. As a result, novel techniques such as drug repurposing are required to develop therapeutics to treat both common and rare disorders. Drug repurposing is a potential approach in drug discovery for identifying new therapeutic applications for existing medications that are distinct from the original medical indication. Identification of new indications for existing drugs by drug repurposing has the potential to supplement traditional drug development by reducing the substantial monetary and time costs and hazards associated with the latter. To date, most of the repurposed drugs are a result of serendipitous discovery through careful observations by physicians, medical staffs and basic researchers. Repurposing approaches involving experimental screening and computational approaches are already developed to increase the speed and ease of the repurposing process. With the advancement of technologies such as proteomics, genomics, transcriptomics, metabolomics and the availability of massive databases resources such as drug omics data, disease omics data and so on, there are a plethora of opportunities to discover drugs by combining all of the above methods/approaches.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Translation of fundamental research findings into meaningful medicinal breakthroughs is an essential objective of biomedical research. Despite advancements in drug discovery techniques, developing medications for diverse ailments still remains challenging (Gribkoff and Kaczmarek 2017). As a result, novel techniques such as drug repurposing are required to develop therapeutics to treat both common and rare disorders. Attempts to create novel remedies for diseases are typically expensive and unsatisfactory, necessitating both extensive timeframes and large expenditures. The repurposing of safe existing medications to new indications, on the other hand, offers a cost-effective and time-saving alternative (Morofuji and Nakagawa 2020). Drug repurposing is a revolutionary method of discovering new applications for existing medications that are not covered by the original medical indication (Pushpakom 2022). Drug repurposing makes advantage of the adaptability of approved medications to reassign them to a new function (Nosengo 2016). Other phrases commonly employed in this context include drug repositioning, drug reprofiling and drug re-tasking, all of which have somewhat different meanings but are used interchangeably with drug repurposing. This alternate method of drug discovery fast-tracking is gaining popularity (Morofuji and Nakagawa 2020). Some of the early examples of repurposing depended on serendipity and retrospective clinical experience, resulting in the effective repurposing of previously unsuccessful medications such as thalidomide and sildenafil in a variety of illness situations. Modern repurposing methodologies, on the other hand, make use of an ever-expanding pool of drug- and disease-related data, computationally driven hypothesis development and high throughput screening methods to identify fresh applications for existing medications (Pushpakom 2022). Furthermore, systematic drug repurposing involving network analysis, data mining and machine learning is also expected to play an important role in future treatment developments.
1.2 Rationale of Drug Repurposing
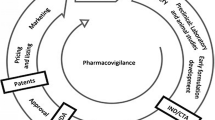
Drug development is a complicated, time-consuming and expensive process with high failure rates. An average of 12–15 years is required for a drug to be approved (Wouters et al. 2020). The investment necessary to get it rises exponentially as the drug progresses through the regulatory development phase, which precedes clinical stages, until it is eventually approved for marketing by the respective regulatory bodies. Furthermore, clinical translation of results from sophisticated animal and cellular models is limited (Leenaars et al. 2019). In this context, drug repurposing has gained prominence in recent years as a means of expediting the drug development process (Pushpakom et al. 2019) (Fig. 1.1). Drug repurposing is a potential approach in drug discovery for identifying new therapeutic applications for existing medications that are distinct from the original medical indication (Cantrell et al. 2021). Drug repurposing, using known drugs and compounds for new indications, offers a number of advantages over traditional approaches to de novo drug discovery and development, as these ‘old drugs’ have already been proven safe in humans. The advantages include (a) Faster drug development timeline compared to the conventional method; (b) reduced healthcare cost; (c) faster regulatory approval; (d) risk reduction; (e) higher odds of success and faster investment return and (f) further understanding of disease mechanisms which may lead to development of novel entities structurally similar but more potent to the repurposed drug (Cantrell et al. 2021; Pushpakom 2022). In recent years, drug repurposing has emerged as a viable strategy to increase the overall productivity of drug discovery. According to estimates, drug repurposing can possibly make a treatment ready for usage in patients within 3–12 years at a total cost of $40–80 million, as opposed to at least 13–15 years and a cost of $2–3 billion for creating a new drug (Cha et al. 2018; Pushpakom 2022). Drug repurposing is also seen to be an acceptable strategy for discovering treatments for orphan and rare diseases, and it is predicted to play a significant role in this area in the future. Indeed, despite the fact that rare diseases impact over 350 million people globally, creating de novo therapies for their limited individual markets is not profitable enough to attract economic interest (Gelosa et al. 2020a, b).
1.3 Role of Drug Repurposing in Conventional Pharmaceutical Market
Over the last 30 years, it has been abundantly evident that the pharmaceutical business is experiencing an ever-increasing productivity gap (Scannell et al. 2012). Despite greater expenditures in cutting-edge technology and a better understanding of numerous human diseases, in conjunction with advancements in fields such as genomics and proteomics, the pharmaceutical industry has struggled to translate these into viable therapeutic outcomes. The global pharmaceutical industry is confronting a high medication attrition rate, rising drug development costs ($2–3 billion per medicine), and increased delay to bring novel chemical entities to market (average of 12 years) (Pushpakom 2022). Many lead compounds that demonstrate success in pre-clinical studies fail in later clinical trials. Rising research costs, high failure rates and an ever-increasing time to bring a molecule from bench to approval have made the pharmaceutical sector a less appealing investment. The pharmaceutical sector returns less than a $1 for every dollar invested on research and development (R&D). Drug repurposing is largely seen as a viable solution to this ‘problem’ (Pushpakom 2022). It is frequently appropriate to repurpose medications or failed drug candidates for new use (Naylor 2015). Most successful drug repositioning cases are aimed to repurposing drugs for a new indication (Pantziarka et al. 2018). However, most drug repositioning cases occur more by chance than a systematical design (Huang 2020). Identification of new indications for existing drugs by drug repurposing has the potential to supplement traditional drug development by reducing the substantial monetary and time costs and hazards associated with the latter (Cha et al. 2018). A prospective repurposing medicine will have a well-established safety and toxicity profile, with data previously amassed in preparation for regulatory approval. Repurposing a medicine with an existing favourable safety profile onto the market for a different indication not only saves time but also increases possible returns on investment (Ashburn and Thor 2004; Pushpakom 2022).
1.4 Roadmap to Modern Drug Repurposing
Historically, drug repurposing has largely been an unintended, fortuitous process that occurs when a medicine is discovered to have an off-target impact or a previously undetected on-target effect that might be put to another purpose. The discovery of two of the most successful medication repurposing instances, thalidomide and sildenafil citrate, was entirely inadvertent and serendipitous, and was based on retrospective clinical experience. Sildenafil, which was originally developed for angina pectoris and failed as a cardiovascular drug, has been repurposed for the treatment of erectile dysfunction and, subsequently, pulmonary arterial hypertension (Ghofrani et al. 2006). In March 1998, the FDA approved Viagra (sildenafil) for the treatment of men with erectile dysfunction. It was approved for the treatment of patients suffering from pulmonary arterial hypertension by the FDA in 2005 (Pushpakom 2022). Thalidomide, which was originally developed as a sleep-inducing drug but discontinued due to foetal teratogenicity, is now repurposed for use in Erythema Nodosum Leprosum and also used against multiple myeloma (Kim and Scialli 2011). In 1998, the FDA approved thalidomide for the treatment of ENL. Thalidomide in combination with Dexamethasone was officially approved by the FDA in 2006 for the treatment of multiple myeloma (Pushpakom 2022). The need for novel approaches to medication research and development, along with the emergence of large data repositories and accompanying analytical technologies, has fuelled interest in creating systematic ways to drug repurposing in recent years (Cha et al. 2018). A systematic strategy based on drug- and disease-related data, utilizing the power of high-performance computational tools, and employing high-throughput screening procedures (termed as ‘systematic repurposing’), has emerged as the way ahead in drug repurposing (Fig. 1.2). The majority of repurposing endeavours presently rely on systematic repurposing approaches, which may be broadly classified as experimental screening approaches and in silico approaches that use existing data to uncover possible novel drug–disease connections (Pushpakom 2022).
1.5 Drug Repurposing Strategies and Approaches
Through the drug repurposing approach, commercial compounds can be identified for new therapeutic uses (Tables 1.1 and 1.2) that complements the traditional drug research method by reducing time and cost. Before considering the candidate medicine for repurposing, drug repurposing procedures include three steps.
-
Identify the candidate medicine for a specific ailment by generating hypotheses.
-
Evaluation of efficacy in phase II clinical trials (assuming that adequate safety data are available from phase I trials of original indication).
-
Mechanistic evaluation of the drug’s therapeutic potential in preclinical studies. Furthermore, after selecting the appropriate medicine for an indication of choice, several methodologies, such as systematic approaches, can be employed for drug repurposing. The systemic method is further separated into computational and experimental approaches, both of which are used synergistically.
1.5.1 Computational Approaches
This technique is mostly data-driven, involving the systematic review of data such as chemical structure, gene expression, electronic health records, genotyping and proteomic data to generate repurposing hypotheses (Hurle et al. 2013). Below are the most often utilized computational methodologies for medication repurposing (Chen 2021).
Signature matching: It is based on comparing a drug’s ‘signature’, or unique marker, which can be generated from a database of medications such as proteome, transcriptome (RNA) and metabolomic data, as well as adverse event profiles and chemical structures, to another disease, drug or clinical characteristic (Keiser et al. 2009). The method of matching transcriptome signatures can be used to compare drug–disease and drug–drug similarity (Iorio et al. 2013). In the first case, a drug’s transcriptomic signature is determined by comparing the genetic expression of cells or tissues before and after administration of the drug; the resulting molecular signature of the drug is then compared to a disease-linked expression profile determined similarly by comparing disease versus healthy conditions. The computational technique is based on the signature reversion principle (SRP), which states that if a medicine has the ability to reverse the genetic expression that is associated with a given disorder phenotype, then that drug may also be able to reverse the disease phenotype. Furthermore, because this premise is so basic, it has been effectively used to identify innovative drug repurposing prospects across a wide range of therapeutic domains. Based on anticancer drug-resistance profiles, the SRP has also been successful in identifying medications that could be repositioned as chemo-sensitizers (Wagner et al. 2015; Hsieh et al. 2016; Mirza et al. 2017). Another method of medication repositioning signature matching is based on chemical structures and their therapeutical relevance. Furthermore, comparing the chemical signatures of two drugs to see if they have any molecular similarities could imply that they have similar therapeutical action. The method entails choosing a set of chemical traits for each drug and then building networks based on the shared chemical features. Chemical similarity approaches have their own set of limitations, such as errors in chemical structures and physiological activity that exists outside of the structural relationship (e.g. a metabolite of an initial drug with a modified chemical structure could be an active molecule), which may limit their use in drug repurposing (Dudley et al. 2011a, b).
Genome-wide association studies (GWAS): Following breakthroughs in genotyping methodology and the completion of the Human Genome Project, which reduced genotyping costs, GWAS research have been done primarily in the previous 10 years. GWAS aims to identify genetic variants associated with diseases’ common shared mechanisms and provide deep insights into disease pathophysiology. The data generated may also be useful in identifying new drug targets, as some of the targets may be shared across diseases, resulting in therapeutic repositioning. Sanseau et al enhanced the USNHGRI (National Human Genome Research Institute) database of published GWAS traits and found that genes associated with illness characteristics are likely coded for proteins that are ‘druggable’ in comparison to the rest of the genome (Sanseau et al. 2012). Furthermore, Grover et al discovered that a bioinformatics approach can be used to match gene targets identified for coronary artery disease with drug information obtained from various drug–target databases such as DrugBank, PharmGKB and Therapeutic Target Database, which could be useful for identifying potential drug repositioning opportunities (Grover et al. 2015). Although there are certain limitations to using information from GWAS for drug repurposing, its utility is currently unclear.
Network/Pathway mapping: Reconstruct disease-specific pathways that give significant targets for repositioning medications using disease omics data, accessible signalling or metabolic pathways and protein interaction networks. These approaches have the advantage of being able to narrow down huge signalling networks to a specialized network with only a few proteins or target molecules. It has mostly been used to identify therapeutic targets and medicines with repurposing potential. As previously discussed, some potential targets identified using GWAS or other methods may become immediately acquiescent as drug targets; nonetheless, these genes may not always be excellent druggable targets. In such circumstances, a network/pathway-based method may provide a pool of data on genes that are either downstream or upstream of the GWAS-associated target, which can be investigated for drug repositioning potential (Greene and Voight 2016). Network analysis entails building drug or illness networks based on gene expression sequences, disease pathophysiology, drug–protein interactions and GWAS data to identify the best repurposing molecule (Pushpakom et al. 2019). A recent study found that employing a network-wide association study (NetWAS) to explore disease–gene interaction by combining genetic variant data from GWAS with tissue-specific interaction networks is more efficient than using GWAS alone. In addition, a study found that pathway analysis of a gene expression database that covers a wide range of respiratory viruses in human host infection models identified 67 widely shared biological processes that play a crucial role in respiratory viral infections (Pushpakom et al. 2019). Furthermore, these pathways are compared to the Drug Bank, allowing numerous medicines with potential effects on host-viral targets to be tested. For instance, pranlukast is an LT-I (leukotriene 1) antagonist used to treat asthma, and amrinone is a phosphodiesterase (PDE) inhibitor used to treat congestive heart failure (CHF). Despite their propensity to modify immune cell responses, both of these medications have been shown to be effective in treating viral infections.
Computational molecular docking: Molecular docking is a structure-based computational method for predicting the ligand (drug) and target binding sites (receptor). If information about receptors and enzymes as targets that are pathologically involved in a disease already exists, various medicines could be tested against the specific target. Furthermore, drug libraries can be tested against a variety of target receptors using inverse docking, which involves numerous targets and one ligand, to identify novel interactions that can be used for repurposing. Dakshanamurthy et al used high-throughput screening to perform molecular fit computations on 3671 FDA-approved medicines versus 2335 human protein structures. According to these findings, the anti-parasitic medication mebendazole has the structural capacity to block vascular endothelial growth factor 2 (VEGFR2) is an angiogenesis mediator (Dakshanamurthy et al. 2012). However, there are a few drawbacks to using computational docking for medication repurposing. Because drug targets are typically membrane proteins, such as G protein-coupled receptors (GPCRs), 3D structures for some target proteins may not be available (Cooke et al. 2015). Furthermore, well-established macromolecular target databases that could provide correct structural information have flaws. Finally, the effectiveness of docking algorithms in predicting binding affinities has been called into question, as has variance in software predictability, such as mode of binding and entropic effects (Huang et al. 2018).
Retrospective clinical analysis: This method employed retrospective clinical data analysis to investigate the medication having repositioning potential. Furthermore, repurposing breakthrough from the retrospective clinical analyses approach is the use of aspirin in colorectal cancer, which is also helpful in preventing cardiovascular disease. Another drug, raloxifene, has been approved by the FDA for reducing the risk of breast cancer in postmenopausal females who are at a higher risk of the disease. Electronic health records (EHR), clinical trial data and post-marketing surveillance data are all good places to look for retrospective clinical data. EHRs contain a wealth of information about patient outcomes, both structured and unstructured, such as diagnostic and pathophysiological data and clinical descriptions of patient symptoms and signs, respectively (Pushpakom et al. 2019). Additionally, the data in EHRs could be used to recognize signals for drug repositioning and this enormous EHR data also provides high statistical power. Despite the fact that there are numerous obstacles, including ethical and legal obstacles, retrieving the unstructured results available in this databank is tough. Other significant sources include post-marketing surveillance (PMS) and clinical trial data; however, access to this data is restricted for commercial and confidentiality reasons. As a result, there is a requirement to have access to this data in order to benefit future medication development research.
1.5.2 Experimental Approaches
Phenotypic screening: Compounds that have potential disease-specific effects in model systems without prior knowledge of the target are tested using this method. If the chemicals under examination are licenced or investigational, this could imply repurposing opportunities that can be pursued promptly in the context of medicine repurposing. In vitro phenotypic screens typically use a 96-well size and a range of cell-based testing. Iljin et al used four prostate cancer cell lines and two non-malignant prostate epithelial cell lines to conduct high-throughput cell-based screening of a library of 4910 drug-like small-molecule compounds, with proliferation as the primary endpoint (Iljin et al. 2009). They discovered that disulfiram, a drug used to treat alcoholism, is a selective anticancer agent, which they proved by genome-wide gene expression studies. Whole-organism phenotypic assays are also used in drug repurposing. Moreover, Cousin et al tested 39 FDA-approved medicines for tobacco addiction in a zebrafish model and discovered that apomorphine and topiramate changed nicotine- and ethanol-induced behaviour in this species (Pushpakom et al. 2019; Cousin et al. 2014).
Binding assays: Proteomic techniques like mass spectrometry and affinity chromatography have been used to investigate drug binding targets for various drugs. Analyses of drug targets and off-targets, as well as drug repurposing, have become natural bedfellows in the chemical biology era for target validation. The Cellular ThermoStability Assay (CETSA) assay, for example, has been developed to map target engagement in cells using biophysical concepts that anticipate thermal stabilization of target proteins by drug-like ligands with the proper cellular affinity. The confirmation of cellular targets for the tyrosine kinase inhibitor (TKI) crizotinib and the finding of quinone reductase 2 (NQO2) as a cellular off-target of acetaminophen (paracetamol) are two early triumphs with this technique (Pushpakom et al. 2019; Alshareef et al. 2016; Miettinen and Björklund 2014).
1.6 Opportunity and Challenges in Drug Repurposing
Drug repositioning, in contrast to traditional medication discovery which is a difficult and time-consuming procedure with high development costs and risk of failure, decreases the time and expense of drug development. Repositioning drugs is another low-risk method. The performance of drug repositioning has been greatly enhanced using a computational or machine learning technique. Experimental procedures that provide a direct evidence-based understanding of linkages between medications and diseases are more reliable and believable than computational approaches. In recent years, however, computational tools have been frequently paired with experimental procedures to uncover novel indications for existing medications, a process known as mixed approaches. Biological experiments and clinical studies are used to validate computational approaches in this approach. The mixed approach to repositioning allows for a systematic and thorough examination of all repositioning options, taking into account enhanced access to databases and technology advancements. Furthermore, compared to traditional drug research, the R&D expenditure needed for drug repositioning is cheaper. As a result, drug repositioning allows numerous pharmaceutical companies to manufacture treatments at cheaper costs (Matthews et al. 2013). The mixed approach of DR allows for more successful and rapid development of repositioned medications. From a market standpoint, a vast number of diseases necessitate the development of novel medications, with possible market needs and economic implications. As a result, repositioning medications for the treatment of uncommon, neglected, orphan diseases or difficult-to-treat diseases is a possibility. There are about 6000 uncommon diseases for which there is no effective treatment. Approximately 5% of them are being studied. Due to the high attrition rates, high expenditure and slow process of drug discovery, repurposing already marketed drugs to treat both common and rare diseases is becoming a more appealing area of research (Talevi and Bellera 2020; de Oliveira and Lang 2018). It involves the use of drug molecules with a lower risk of failure and development time and cost. With the advancement of technologies such as proteomics, genomics, transcriptomics, metabolomics and the availability of massive databases resources such as drug omics data, disease omics data and so on, there are a plethora of opportunities to discover drugs by combining all of the above methods/approaches. Researchers now have the most up-to-date tools and data to investigate novel unknown mechanisms of action/pathways based on illness-specific target proteins, genes and specific biomarkers linked to disease development. Public databases and software for genomes, proteomics, metabolomics and pathway analysis are accessible. Researchers are currently equipped with the latest reliable tools and data to explore the novel unknown mechanism of actions/pathways based upon disease-specific target proteins/genes and specific biomarkers associated with the progression of the disease. Several computational strategies are already developed to increase the speed and ease of the repurposing process. However, opportunities come often with many challenges in drug repositioning. The identification of a new therapeutic indication for an existing drug poses a major challenge in repositioning. Drug repositioning, on the other hand, is a complicated process including a variety of aspects such as technology, patents, commercial models, and investment and market demands. Choosing the appropriate therapeutic area for the drug under investigation, as well as concerns with clinical studies, such as the necessity to start new trials from the beginning if data from preclinical or clinical trials for the existing drug or drug product is obsolete or unsatisfactory (Rudrapal et al. 2020; Pushpakom et al. 2019).
1.7 Conclusion
Despite a large rise in pharmaceutical company investment, the rate of new medication approvals stays consistent, owing mostly to high attrition rates. This, along with a significant unmet need in treating a wide range of disorders, generates an urgent need for innovation in bringing viable therapeutics to market. In recent years, drug repurposing has emerged as a feasible technique for increasing the overall productivity of drug discovery and development. Drug repurposing (also known as drug repositioning, reprofiling or re-tasking) is a promising area in drug discovery for uncovering new therapeutic applications for previously investigated medications. Drug repositioning may simply be summarized as expanding effective medications and renewing unsuccessful ones. Drug repurposing is a direct application of polypharmacology, which characterizes the drugs’ potential to act on multiple targets and disease pathways. The identification of new molecular targets and pharmacological effects opens up new treatment avenues for clinically utilized medications. Drug repurposing holds the potential to bring medications with known safety profiles to new patient populations. This strategy provides for speedier drug development with less expenditure and risk than the development of a de novo drug. The majority of repurposing endeavours presently rely on systematic repurposing approaches that make use of an ever-expanding pool of drug- and disease-related data, computationally driven hypothesis development, and high-throughput screening methods to identify fresh applications for existing medications to increase the speed and ease of the repurposing process.
References
Alshareef A, Zhang HF, Huang YH, Wu C, Zhang JD, Wang P, El-Sehemy A, Fares M, Lai R (2016) The use of cellular thermal shift assay (CETSA) to study crizotinib resistance in ALK-expressing human cancers. Sci Rep 6(1):1–2. https://doi.org/10.1038/srep33710
Ashburn TT, Thor KB (2004) Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3(8):673–683. https://doi.org/10.1038/nrd1468
Cantrell MS, Soto-Avellaneda A, Wall JD, Ajeti AD, Morrison BE, Warner LR, McDougal OM (2021) Repurposing drugs to treat heart and brain illness. Pharmaceuticals 14(6):573. https://doi.org/10.3390/ph14060573
Cha Y, Erez T, Reynolds IJ, Kumar D, Ross J, Koytiger G, Kusko R, Zeskind B, Risso S, Kagan E, Papapetropoulos S (2018) Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol 175(2):168–180. https://doi.org/10.1111/bph.13798
Chen J (2021) Computational drug repurposing: a review in modern application. In: AIP conference proceedings, vol. 2350, no. 1, pp 020012. AIP Publishing LLC. https://doi.org/10.1063/5.0049200
Cooke RM, Brown AJ, Marshall FH, Mason JS (2015) Structures of G protein-coupled receptors reveal new opportunities for drug discovery. Drug Discov Today 20(11):1355–1364. https://doi.org/10.1016/j.drudis.2015.08.003
Cousin MA, Ebbert JO, Wiinamaki AR, Urban MD, Argue DP, Ekker SC, Klee EW (2014) Larval zebrafish model for FDA-approved drug repositioning for tobacco dependence treatment. PLoS One 9(3):e90467. https://doi.org/10.1371/journal.pone.0090467
Dakshanamurthy S, Issa NT, Assefnia S, Seshasayee A, Peters OJ, Madhavan S, Uren A, Brown ML, Byers SW (2012) Predicting new indications for approved drugs using a proteochemometric method. J Med Chem 55(15):6832–6348. https://doi.org/10.1021/jm300576q
de Oliveira EA, Lang KL (2018) Drug repositioning: concept, classification, methodology, and importance in rare/orphans and neglected diseases. J Appl Pharma Sci 8(8):157–165. https://doi.org/10.7324/JAPS.2018.8822
Dudley JT, Deshpande T, Butte AJ (2011a) Exploiting drug–disease relationships for computational drug repositioning. Brief Bioinform 12(4):303–311. https://doi.org/10.1093/bib/bbr013
Dudley JT, Sirota M, Shenoy M, Pai RK, Roedder S, Chiang AP, Morgan AA, Sarwal MM, Pasricha PJ, Butte AJ (2011b) Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci Transl Med 3(96):96ra76. https://doi.org/10.1126/scitranslmed.3002648
Gelosa P, Castiglioni L, Camera M, Sironi L (2020a) Drug repurposing in cardiovascular diseases: opportunity or hopeless dream? Biochem Pharmacol 177:113894. https://doi.org/10.1016/j.bcp.2020.113894
Gelosa P, Castiglioni L, Camera M, Sironi L (2020b) Repurposing of drugs approved for cardiovascular diseases: opportunity or mirage? Biochem Pharmacol 177:113895. https://doi.org/10.1016/j.bcp.2020.113895
Ghofrani HA, Osterloh IH, Grimminger F (2006) Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov 5(8):689–702. https://doi.org/10.1038/nrd2030
Greene CS, Voight BF (2016) Pathway and network-based strategies to translate genetic discoveries into effective therapies. Hum Mol Genet 25(R2):R94–R98. https://doi.org/10.1093/hmg/ddw160
Gribkoff VK, Kaczmarek LK (2017) The need for new approaches in CNS drug discovery: why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 120:11–19. https://doi.org/10.1016/j.neuropharm.2016.03.021
Grover MP, Ballouz S, Mohanasundaram KA, George RA, Goscinski A, Crowley TM, Sherman CD, Wouters MA (2015) Novel therapeutics for coronary artery disease from genome- wide association study data. BMC Med Genet 8(2):1. https://doi.org/10.1186/1755-8794-8-S2-S1
Hsieh YY, Chou CJ, Lo HL, Yang PM (2016) Repositioning of a cyclin-dependent kinase inhibitor GW8510 as a ribonucleotide reductase M2 inhibitor to treat human colorectal cancer. Cell Death Discov 2(1):1–8. https://doi.org/10.1038/cddiscovery.2016.27
Huang J (2020) Can drug repositioning work as a systematical business model? ACS Med Chem Lett 11(6):1074–1075. https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00122
Huang H, Zhang G, Zhou Y, Lin C, Chen S, Lin Y, Mai S, Huang Z (2018) Reverse screening methods to search for the protein targets of chemopreventive compounds. Front Chem 6:138. https://doi.org/10.3389/fchem.2018.00138
Hurle MR, Yang L, Xie Q, Rajpal DK, Sanseau P, Agarwal P (2013) Computational drug repositioning: from data to therapeutics. Clin Pharmacol Therap 93(4):335–341. https://doi.org/10.1038/clpt.2013.1
Iljin K, Ketola K, Vainio P, Halonen P, Kohonen P, Fey V, Grafström RC, Perälä M, Kallioniemi O (2009) High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clin Cancer Res 15(19):6070–6078. https://doi.org/10.1158/1078-0432.CCR-09-1035
Iorio F, Rittman T, Ge H, Menden M, Saez-Rodriguez J (2013) Transcriptional data: a new gateway to drug repositioning? Drug Discov Today 18(7–8):350–357. https://doi.org/10.1016/j.drudis.2012.07.014
Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R (2009) Predicting new molecular targets for known drugs. Nature 462(7270):175–181. https://doi.org/10.1038/nature08506
Kim JH, Scialli AR (2011) Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci 122(1, 1):–6. https://doi.org/10.1093/toxsci/kfr088
Leenaars CH, Kouwenaar C, Stafleu FR, Bleich A, Ritskes-Hoitinga M, De Vries RB, Meijboom FL (2019) Animal to human translation: a systematic scoping review of reported concordance rates. J Transl Med 17(1):1–22. https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-019-1976-2
Matthews H, Usman-Idris M, Khan F, Read M, Nirmalan N (2013) Drug repositioning as a route to anti-malarial drug discovery: preliminary investigation of the in vitro anti- malarial efficacy of emetine dihydrochloride hydrate. Malar J 12(1):1. https://doi.org/10.1186/1475-2875-12-359
Miettinen TP, Björklund M (2014) NQO2 is a reactive oxygen species generating off-target for acetaminophen. Mol Pharm 11(12):4395–4404. https://doi.org/10.1021/mp5004866
Mirza N, Sills GJ, Pirmohamed M, Marson AG (2017) Identifying new antiepileptic drugs through genomics-based drug repurposing. Hum Mol Genet 26(3):527–537. https://doi.org/10.1093/hmg/ddw410
Morofuji Y, Nakagawa S (2020) Drug development for central nervous system diseases using in vitro blood-brain barrier models and drug repositioning. Curr Pharm Des 26(13):1466–1485. https://doi.org/10.2174/1381612826666200224112534
Naylor DM (2015) Therapeutic drug repurposing, repositioning and rescue. Drug Discov. 57. https://www.ddw-online.com/therapeutic-drug-repurposing-repositioning-and-rescue-part-ii-business-review-888-201504/
Nosengo N (2016) Can you teach old drugs new tricks? Nature 534(7607):314–316. https://ideas.repec.org/a/nat/nature/v534y2016i7607d10.1038_534314a.html
Pantziarka P, Pirmohamed M, Mirza N (2018) New uses for old drugs. BMJ 361:k2701. https://doi.org/10.1136/bmj.k2701
Pushpakom S (2022) Introduction and historical overview of drug repurposing opportunities. pp 1–13. https://doi.org/10.1039/9781839163401-00001
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A (2019) Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18(1):41–58. https://www.nature.com/articles/nrd.2018.168
Rudrapal M, Khairnar SJ, Jadhav AG (2020) Drug repurposing (DR): an emerging approach in drug discovery. Drug repurposing hypothesis. Mol Aspects Ther Appl. https://www.intechopen.com/chapters/72744
Sanseau P, Agarwal P, Barnes MR, Pastinen T, Richards JB, Cardon LR, Mooser V (2012) Use of genome-wide association studies for drug repositioning. Nat Biotechnol 30(4):317–320. https://doi.org/10.1038/nbt.2151
Scannell JW, Blanckley A, Boldon H, Warrington B (2012) Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov 11(3):191–200. https://doi.org/10.1038/nrd3681
Talevi A, Bellera CL (2020) Challenges and opportunities with drug repurposing: finding strategies to find alternative uses of therapeutics. Expert Opin Drug Discov 15(4):397–401. https://doi.org/10.1080/17460441.2020.1704729
Wagner A, Cohen N, Kelder T, Amit U, Liebman E, Steinberg DM, Radonjic M, Ruppin E (2015) Drugs that reverse disease transcriptomic signatures are more effective in a mouse model of dyslipidemia. Mol Syst Biol 11(3):791. https://doi.org/10.15252/msb.20145486
Wouters OJ, McKee M, Luyten J (2020) Estimated research and development investment needed to bring a new medicine to market, 2009-2018. JAMA 323(9):844–853. https://jamanetwork.com/journals/jama/article-abstract/2762311
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Joon, P. et al. (2023). Drug Repurposing: An Advance Way to Traditional Drug Discovery. In: Sobti, R.C., Lal, S.K., Goyal, R.K. (eds) Drug Repurposing for Emerging Infectious Diseases and Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-19-5399-6_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-5399-6_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5398-9
Online ISBN: 978-981-19-5399-6
eBook Packages: MedicineMedicine (R0)