Abstract
Adaptation of plant species to cold environment and short growing season in the Himalayan high altitudes could render them highly sensitive to future climate change. In this study, we analyzed the effect of elevated CO2 concentration on the growth, productivity, physiology, and various biochemical parameters of four alpine treeline herbaceous species, viz. Acomastylis elata, Anaphalis nepalensis, Bistorta macrophylla, and Trillium govanianum. We planted seedlings of the selected plant species in open top chambers and CO2 concentration was raised from ambient (400 ppm) to elevated (650 ppm) levels. Elevated CO2 stimulated net assimilation rate (34–38%), growth and productivity of A. elata and A. nepalensis, whereas B. macrophylla and T. govanianum showed decrease (18%) in photosynthesis. The sugar content in all the species increased (36–78%); however, foliar N decreased (17–37%), possibly due to dilution effect of high carbohydrate content. Reduced tissue N can probably affect the activity of key photosynthetic enzyme Rubisco and therefore, decreased carbon assimilation. From this study, it can be deduced that long-term effects of elevated CO2 can be species specific and might be affected by availability of nutrients. More such studies in Himalayan regions involving different plant communities are needed to develop a better understanding of plant responses to climate change.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
18.1 Introduction
The carbon dioxide (CO2) concentration in the earth’s atmosphere has varied over the geological time scale. CO2 concentrations as low as 180–200 ppm had been known to exist during the last two ice ages around 13,000–30,000 and 140,000–160,000 years ago. Direct atmospheric sampling data have shown that CO2 increased from 315 ppm in the late 1950s to 355 ppm in the early 1990s (Keeling et al. 1989) and has been rising continuously since then. The rise in atmospheric CO2 has become more profound since the beginning of the industrial revolution and is expected to continue in the coming years with drastic effects on the earth’s climate. The rise in atmospheric carbon dioxide will not only affect climatic events but is likely to have significant implications on the global carbon cycle and plants life too. Plants of the alpine regions are specifically more sensitive toward changes in the climatic factors chiefly temperature.
The upper limit of a closed forest is called as timberline, whereas a treeline is the upper limit of the “tree” life form showing an upright growth. The transitional zone between the timberline and treeline is called as the alpine treeline ecotone (Körner 2012). The trees that generally form treeline are Abies spectabilis, Sorbus foliolosa, Betula utilis, etc. Many herbaceous species are also found growing below canopies, along, and above treelines. Herbs are one of the major flowering plant life-forms in these regions. It has been estimated that approximately 2.6% land area besides Antarctica, covering the alpine belt, inhabits about 4% of all the flowering plant species on Earth. In other words, the alpine regions are quite rich in plant diversity than the lower elevation ecosystems (Körner 2018).
A number of herbaceous species can be restricted to the treeline or below it due to a more hospitable habitat than the open alpine meadows which are directly exposed to abiotic extremities, viz heavy snowfall, frost, and solar radiation. This higher distribution of herbaceous species is mainly based on their morphological adaptations, like small stature and dense structure, which help them restrict aerodynamic exchange with the atmosphere. This causes heat accumulation from solar radiation providing warm temperatures to operate unlike trees, which are upright and more ventilated. Alpine herbaceous plants produce short lived leaves and the sunlight heated soil provides a thermally buffered environment to their meristems which are positioned close to the ground in comparison to trees which form longer living and slowly maturing leaves along with meristems positioned above from the ground where they are fully exposed to cold temperatures (Körner 2018).
Increasing CO2 concentration can cause positive effect on plant growth with an increase in leaf area (Allen Jr 1991). Plants that are exposed to higher levels of CO2 usually have amplified growth, water use efficiency, and photosynthesis rate (Amthor 1995; Wittwer 1995). Being a very important raw material for the process of photosynthesis, an increase in atmospheric CO2 concentration can have direct effect on plant photosynthesis. However, plants growing in alpine region and glacier fore-fields in the Swiss Alps were found to be carbon saturated at ambient CO2 concentrations of 390 ppm (Körner 2018). It was further reported that doubling the concentration of atmospheric CO2 for over four successive seasons had no effect on net primary productivity of alpine plants with exception of some species. Hence, in long run, a possibility of slow but certain shifts in species composition is expected with some species getting suppressed and some gaining ground (Körner 2018).
At higher altitudes, the impact of elevated CO2 on plants can vary due to low ambient partial pressure of CO2, short growing season, and a number of abiotic stresses mainly extreme low-temperature regimes. CO2 exposure duration can also cause varying responses in plants as initial exposure can enhance the net assimilation rate and biomass (Ainsworth et al. 2008; Chaturvedi et al. 2009; Chaturvedi et al. 2013). With increasing duration of CO2 exposure plant can show photosynthetic acclimation, lowering of leaf chlorophyll and nitrogen followed by declined (Zelikova et al. 2014) or no significant variation in biomass (Schäppi and Körner 1997; Ward et al. 1999).
Initial studies on the effect of warming have shown that the Himalayas have a higher warming, more prominent at higher altitudes, as compared to the global average rate (Shrestha et al. 1999; Liu and Chen 2000) with higher increases in the winter and autumn temperatures than the summer. There have been only a few studies regarding the impact of CO2 enrichment on the Himalayan alpine species (Joshi et al. 2007; Chaturvedi et al. 2009; Chaturvedi et al. 2013) and one long-term study on alpine grassland biomass and community structure (Zhu et al. 2020). However, a considerate amount of work and long-term studies on warming and CO2 enrichment effect on the European Alps treeline, community structure, and species have been carried out (Schäppi and Körner 1996; Schäppi and Körner 1997; Inauen et al. 2012; Dawes et al. 2013). The treeline in the Himalayan highlands ranges from 3200 m to 4900 mamsl (Singh et al. 2019) bearing the highest peaks of the world, whereas the treeline in the Alps ranges from 1750 m to slightly above 2350 mamsl from north to south (Paulsen and Körner 2001). Therefore, the responses of treeline species and community structure to climate change particularly relating to species migration and distribution patterns at the Himalayas might differentiate from the other alpine regions.
Since the alpine regions are dominated by herbaceous species which are also a key component of biodiversity, this study aims to estimate the effect of elevated CO2 on growth-productivity, physiology, and biochemistry of alpine–treeline herbs. Due to low temperature and partial pressure of gases in the alpine regions, the effect of CO2 on plant speciesof these regions can vary from species of lower elevations, however, the photosynthesis rate, pigment content, and biomass of the herbs were expected to increase as a result of CO2 fertilization effect as found in some earlier studies. Since in the earlier studies these responses have been found varying in different species; therefore, four species were subjected to CO2 treatment in this study.
In order to properly understand the treeline dynamics with reference to anthropogenic warming of the planet, it is essential to carry out more reliable assessments of forest treelines across the globe to preserve the ecological state of alpine ecotone biodiversity and to forge forest conservation policies (Holtmeier and Broll 2010; Mishra and Mainali 2017.
18.2 Materials and Methods
18.2.1 Study Site
This study was conducted at alpine field station of High Altitude Plant Physiology Research Center at Tungnath, (3400 m amsl, 30°14′N and 79°13E) in the Western-Central Himalaya, India. The study site is a part of Kedarnath Wild Life Sanctuary and exhibits a number of endemic species. The area usually remains snow covered from December to May and the growing period for herbaceous species is between May to October. The study area generally receives an annual rainfall ranging from of 300 to 500 cm with the month of July and August being the wettest. Daily mean temperatures from June to September fluctuate between 8 and 18 °C.
18.2.2 Growth Conditions
Two open top chambers (OTCs) were established at Tungnath. The OTCs were round in structure having 4 × 4 m (height × diameter) dimension. Due to high wind velocity at the study site, OTCs were preferred over free air CO2 enrichment (FACE) experiment. The frame of the OTCs was made of galvanized iron pipes. The OTCs were covered with polycarbonate sheet which provided 90% transmittance of light. The OTCs were equipped with programmable logic controller coupled with supervisory control and data acquisition (SCADA) system which automatically recorded air temperature, relative humidity, and CO2 concentration. The CO2 concentration of one OTC was raised and maintained to 650 ppm during the growing period (June to mid-October). This performed automatically via a solenoid valve controlled by SCADA system.
18.2.3 Plant Materials
Morphologically similar seedlings of Acomastylis elata (Wall. ex G.Don) F.Bolle (Syn: Geum elatum, family: Rosaceae), Anaphalis nepalensis (Spreng.) Hand.-Mazz (Asteraceae), Bistrota macrophylla (D. Don) Soják (Polygonaceae), and Trillium govanianum Wall. ex. D. Don., (Melanthiaceae) were collected from surrounding areas and planted in triplicates (each having 10 seedlings) in each OTC. These species are naturally distributed from treeline to upper alpine regions, while T. govanianum grows in subalpine regions. Seedlings of all the 4 species were transplanted to OTCs and left for a month to acclimatize, after which the treatment was given. The OTCs were established at native site of these species, the soil inside the OTCs was not modified, and no fertilizers were added. Transplantation was done in the month of July in the first year (2019) and therefore, the treatment in the first season was given only for 30 ± 2 days. In the second year (2020), the treatment lasted from June to September. The seedlings growing under elevated CO2 chamber were compared with seedlings growing at ambient CO2 concentration in an OTC.
18.2.4 Gas Exchange Measurement
Leaf gas exchange parameters were recorded on leaves of nine randomly selected individuals (3 from each replicate) of each species from each chamber using Licor, Li-6400, a portable infrared gas analyzer (IRGA). The instrument was equipped with red blue LED light source and CO2 mixer (for maintaining desired CO2 concentration). The photosynthetic rate (Pn, μmol CO2/m2/s), stomatal conductance (gs, mol H2O/m2/s), transpiration rate (E, mmol H2O/m2/s), and intracellular CO2 (Ci, μmol CO2/mol air) were recorded between 9:00 and 11:00 h. The study was conducted under saturated light conditions (photosynthetic photon flux density—1250 μmol/m2-s). The water use efficiency (WUE = Pn/E) and intrinsic water use efficiency (iWUE = Pn/gs) were calculated.
18.2.5 Pigment, Carbohydrate, and Nitrogen Estimation
Leaf samples of all the fourspecies were collected (5 samples for each species) from both ambient (aCO2) and elevated (eCO2) chambers. Samples were further analyzed through UV-VIS spectrophotometer for chlorophyll a, b and total carotenoids following Holm et al. (1965) and total soluble sugars following (McCready et al., 1950). Total N was estimated from oven-dried leaves using Kjeldhal Nitrogen analyzer (Pelican Instruments).
18.2.6 Growth and Biomass
Plant height, leaf area, aboveground, and belowground biomass were recorded on five randomly selected individuals from each species grown under aCO2 and eCO2 chambers.
18.2.7 Data Analysis
All the growth parameters of plants from aCO2 and eCO2 were recorded after 120 days of CO2 treatment. One way analysis of variance was performed to assess the effect of elevated CO2 and further significant difference between ambient and elevated CO2 was tested using paired sample t-test for each parameter.
18.3 Results
The daytime air temperature under eCO2 increased by 0.63 °C and the temperature difference between aCO2 and eCO2 was greater in May and June. The total precipitation (Measured by tipping bucket rain gauge) during our study was 2130 mm and 60 to 80% of total precipitation took place between July and August (Fig. 18.1).
CO2 enrichment experiment showed significant changes in physiology, growth, and biochemistry of herbaceous species; however, the effects are species specific.
18.3.1 Effect on Gaseous Exchange
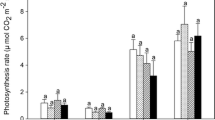
CO2 exposure strongly affected the physiological process of photosynthesis (Pn). Pn under eCO2 significantly (p = 0.05) increased by 38 and 34% in A. nepalensis and A. elata, respectively; whereas it decreased (18%, p < 0.05) in T. govanianum and remained unaffected in B. macrophylla. The stomatal conductance (gs) under eCO2 increased significantly in A. elata (+59%, p < 0.01); other species showed non-significant reduction in gs. CO2 is considered as an ideal antitranspirant and in our observation we found reduced transpiration rate (E) under eCO2 for A. nepalensis (−54%, p < 0.05), B. macrophylla (−54%, 0.08), and T. govanianum (−59%, <0.01); however, A. elata showed an increased E (+36%, <0.01). Elevated CO2 improved the water use efficiency (WUE) in A. nepalensis (+2-folds) and T. govanianum (+35%), whereas WUE of A. elata and B. macrophylla was unaffected. The intrinsic WUE (iWUE) significantly improved by twofolds only in A. nepalensis (Table 18.1).
18.3.2 Effect on Pigments, Carbohydrates, and Nitrogen Content
CO2 enrichment affected the pigment contents and the effect varied from species to species. The chl a and b (chlorophyll a and b) significantly (p < 0.05) increased in A. elata (+19%) and A. nepalensis (+21%) under eCO2. Other species, i.e., T. govanianum and B. macrophylla, showed reduced chl a content under eCO2; however, the chl b was unaffected. The total carotenoids showed most prominent response toward eCO2. All species growing at eCO2 chamber showed increased carotenoids (+23 to 46%). The TSS under eCO2 increased significantly (p < 0.05) for A. elata (+46%), A. nepalensis (+36%), T. govanianum (+78%), and non-significantly for B. macrophylla (15%). The plant N in eCO2 significantly reduced for A. elata (−17%), A. nepalensis (−23%), and T. govanianum (−37%), although the effect of eCO2 on N reduction of B. macrophylla was non-significant (Table 18.2).
18.3.3 Effect on Growth and Biomass
Under eCO2, the plant height and leaf area of A. nepalensis and A. elata significantly increased; however, the species B. macrophylla and T. govanianum did not show any significant change in morphometrics. Height of A. nepalensis and A. elata increased by 55 and 24%, respectively under eCO2 (Fig. 18.2). Similarly, leaf area in these two species also increased by 97 and 32%, respectively (Fig. 18.3). The accumulation of dry matter in shoot and root system was measured and in comparison to aCO2, the aboveground biomass in eCO2 increased significantly by 50 and 116% in A. elata and A. nepalensis, respectively. The below ground biomass in these two species also increased, although the increment was significant only in A. nepalensis (+55%), while the other two species did not show any significant change in above and belowground biomass (Fig. 18.4).
18.4 Discussion
This study brings out the short-term exposure effects of CO2 enrichment on four alpine treeline ecotone herbaceous species having different leaf structures and morphology.
Elevated CO2 led to an increase in the daytime air temperature inside the chamber as compared to ambient CO2 levels. This can be attributed to the heat absorbing and radiating nature of CO2 and its activity as a greenhouse gas. Elevated CO2 led to an increase in the photosynthesis rate in two species namely A. elata and A. nepalensis. Similar results were obtained in a number of other studies (Ainsworth and Long 2005; van der Kooi et al. 2016) where elevated CO2 caused a rise in the photosynthetic rate with subsequent rise in growth and biomass. A higher CO2 concentration has a fertilizing effect especially on C3 plants because Rubisco in C3 plants is not CO2 saturated at its current atmospheric levels (Yu et al. 2012; Singh and Reddy 2016). Elevated CO2 also reduces the instance of photorespiration, thereby having positive effects on the carboxylase activity of Rubisco (Zheng et al. 2019). However, the other two species, viz. T. govanianum and B. macrophylla either had negative or no significant effect on their photosynthetic activity as a result of elevated CO2. This could be possibly because of differences in their carbon saturation threshold and the ability to adapt to a carbon richer environment. The down-regulation photosynthetic activity could also be due to lower concentration and activity of Rubisco. Kanemoto et al. (2009) also reported a decline in the photosynthetic activity of soybean plants under elevated CO2. In this study, a decline in the plant nitrogen (N) content was also seen. N is an important element of tissue protein and amino acid, and under elevated CO2 a decline in tissue N may affect the concentration of Rubisco.
The stomatal conductance in three out of the four studied species was found to be declined. Increased levels of atmospheric CO2 cause smaller stomatal apertures, thereby decreasing leaf conductance for water vapor (Morison 1987). A decrease in stomatal conductance of these species also led to a decline in the transpiration rate. Stomatal conductance of A. elata increased along with an increase in its transpiration rate. Changes in stomatal conductance and transpiration rate are also greatly influenced by the species type (Ward et al. 2013; Haworth et al. 2013). Stomatal conductance and transpiration rates are also influenced by changes in stomatal density, which have been found to be either decreased or increased in different species under elevated CO2 (Xu et al. 2016). The WUE was significantly increased in T. govanianum and A. nepalensis as a result of decline in the transpiration rate. In the other two species, viz. A. elata and B. macrophylla WUE remained unaffected. A significant increase in the pigment content, i.e., chlorophyll in A. nepalensis and A. elata, can be correlated with significantly higher Pn in both the species. Pigments trap light energy which is utilized to convert trapped carbon to carbohydrates by the process of photosynthesis. An increase in the carotenoid content of all the species has been found under elevated CO2. Carotenoids moderate the effect of increased temperature by protecting the plants from photo-oxidative stress (Strzalka et al. 2003). Carotenoid content has shown variable responses under elevated CO2 in a number of studies. Some studies have reported increase, whereas some had reported a decline in the carotenoid content with respect to elevated CO2 (Loladze et al. 2019).
TSS increased under elevated CO2 in all the studied species. Higher substrate availability can be the most probable reason for increase in TSS in comparison to ambient conditions. Higher Pn and TSS content leads to accumulation of carbohydrates stored as starch causing an increase in the biomass of the plants. The biomass accumulation was very significant in A. nepalensis and A. elata and so were the Pn rates and TSS content indicating the competence of these two species to utilize increased carbon more efficiently than the other two species. However, initial responses showed enhanced photosynthetic capacity and increase in biomass, but this might tend to saturate after a certain time period; since, with the continuously increasing or higher CO2 levels, some or most plants initially grow rigorously, and later soil microbes enhance the immobilization of limiting nutrients thus hampering further growth (Shaw et al. 2002). A decline in the tissue nitrogen was observed in all the species under elevated CO2. Previous studies have found that long-term elevated CO2 leads to down-regulation of photosynthetic activity, termed as photosynthetic acclimation. This acclimation causes less uptake of nitrogen and thus a reduction in tissue nitrogen (Temperton et al. 2003; Leakey et al. 2006; Zheng et al. 2019), which then limits photosynthetic capacity (Ewa Jach and Ceulemans 1999).
An increase in the leaf area under CO2 enrichment has been reported in a number of studies (Centritto et al. 1999; Pritchard et al. 1999; Masle 2000; Usuda 2006). Leaves are the point of interaction for carbon transfer and capture. Elevated CO2 can bring changes in the internal structure of leaves (Pritchard et al. 1999). Stomatal density has been found to either decrease or increase with elevated CO2 depending on the species. An additional variation is alteration in epicuticular wax on elevated CO2 grown leaves (Thomas and Harvey 1983; Prior et al. 1997). Plant height increased in A. nepalensis and A. elata, whereas there was no significant change in the height of T. govanianum and B. macrophylla. The stems of A. nepalensis and A. elata were slightly branched having internodes, whereas T. govanianum and B. macrophylla had erect unbranched stems. The leaves of T. govanianum and B. macrophylla were also thinner in comparison to the thick and hairy leaves of A. nepalensis and A. elata. Elevated CO2 can cause the stimulation of cell division at the shoot apical meristem (Pritchard et al. 1999) by reducing the time period between consecutive cell divisions as demonstrated by Masle (2000). A number of studies have shown an increase in leaf area and stem length or branch elongation in plants exposed to CO2 enrichment without any change in the number of nodes (Downton et al. 1990; Pritchard et al. 1999). But these results have also varied among species as can be seen in this study.
18.5 Conclusion
From the results of the present and previous studies, a general assumption that can be drawn is that the response of any plant species of any ecosystem or form depends on a number of factors, viz its morphology, habitat and community structure, microbial and other associations, nutrient and water availability, carbon saturation threshold, limit to withstand environmental extremes, etc. Under elevated CO2 the photosynthetic yield increases due to availability of carbon substrate but later due to impaired phloem loading capacity, source—sink imbalance can be observed in the long term. Changes in microbial activity under elevated CO2 might also affect soil nutrients consequently affecting their availability and uptake. Overall the effect of CO2 enrichment in high altitude are majorly species specific and an increased growth and productivity might be beneficial for establishment of an individual species but can adversely affect community structure and distribution of other species. Therefore, studies especially in the Himalayan alpine regions on effect of CO2 enrichment and warming on community structure, species response, and distribution are required on a large scale to make precise predictions.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–372. https://doi.org/10.1111/j.1469-8137.2004.01224.x
Ainsworth EA, Leakey ADB, Ort DR, Long SP (2008) FACE-ing the facts: inconsistencies and interdependence among field, chamber and modeling studies of elevated [CO2] impacts on crop yield and food supply. New Phytol 179(1):5–9. https://doi.org/10.1111/j.1469-8137.2008.02500.x
Allen Jr LH (1991) Effects of increasing carbon dioxide levels and climate change on plant growth, evapotranspiration, and water resources. In: Managing water resources in the west under conditions of climate uncertainty: a proceedings. The National Academies Press, Washington, DC; pp 101–147
Amthor JS (1995) Terrestrial higher-plant response to increasing atmospheric [CO2] in relation to the global carbon cycle. Glob Chang Biol 1:243–274
Centritto M, Lee H, Jarvis PG (1999) Increased growth in elevated [CO2]: an early, short-term response? Glob Chang Biol 5(6):623–633. https://doi.org/10.1046/j.1365-2486.1999.00263.x
Chaturvedi AK, Vashistha RK, Rawat N, Prasad P, Nautiyal MC (2009) Effect of CO2 enrichment on photosynthetic behavior of Podophyllum hexandruman endangered medicinal herb. J Am Sci 5(5):113–118
Chaturvedi AK, Prasad P, Nautiyal MC (2013) Impact of elevated CO2 on growth, morphology and dry matter partitioning in alpine growth forms of north western Himalayas. Indian J Plant Physiol 18(2):118–124
Dawes MA, Hagedorn F, Handa IT, Streit K, Ekblad A, Rixen C, Körner C, Hättenschwiler S (2013) An alpine treeline in a carbondioxide-rich world; synthesis of a nine-year free-air carbon dioxide enrichment study. Oecologia 171(3):623–637. https://doi.org/10.1007/s00442-012-2576-5
Downton WJS, Grant WJR, Chacko EK (1990) Effects of elevated carbon dioxide on the photosynthesis and early growth of mangosteen (Garcinia mangostana L.). Sci Hortic 44:215–225. https://doi.org/10.1016/0304-4238(90)90121-T
Ewa Jach M, Ceulemans R (1999) Effects of elevated atmospheric CO2 on phenology, growth and crown structure of scots pine (Pinus sylvestris) seedlings after two years of exposure in the field. Tree Physiol 19(4–5):289–300. https://doi.org/10.1093/treephys/19.4-5.289
Haworth M, Elliott-Kingston C, McElwain JC (2013) Co-ordination of physiological and morphological responses of stomata to elevated [CO2] in vascular plants. Oecologia 171:71–82. https://doi.org/10.1007/s00442-012-2406-9
Holm-Hansen O, Lorenzen CJ, Holmes RW, Strickland JD (1965) Fluorometric determination of chlorophyll. ICES J Mar Sci 30(1):3–15
Holtmeier KF, Broll G (2010) Altitudinal and polar treelines in the northern hemisphere causes and response to climate change (Obere und polareBaumgrenze auf der nördlichenHemisphäreUrsachen und Antwort auf den Klimawandel). Polarforschung 79:139–153
Inauen N, Körner C, Hiltbrunner E (2012) No growth stimulation by CO2 enrichment in alpine glacier forefield plants. Glob Chang Biol 18(3):985–999. https://doi.org/10.1111/j.1365-2486.2011.02584.x
Joshi SC, Chandra S, Palni LMS (2007) Differences in photosynthetic characteristics and accumulation of osmoprotectants in saplings of evergreen plants grown inside and outside a glasshouse during the winter season. Photosynthetica 45:594–600. https://doi.org/10.1007/s11099-007-0102-5
Kanemoto K, Yamashita Y, Ozawa T et al (2009) Photosynthetic acclimation to elevated CO2 is dependent on N partitioning and transpiration in soybean. Plant Sci 177:398–403
Keeling CD, Bacastow RB, Carter AF, Piper SC, Whorf TP, Heimann M, Mook WG, Roeloffzen H (1989) A three-dimensional model of atmospheric CO2 transport based on observed winds: 1. Analysis of observational data, in aspects of climate variability in the Pacific and the Western Americas, geophysical monograph 55, AGU, Washington, DC; pp 165–236
Körner C (2012) Alpine treelines functional ecology of the global high elevation tree limits. Springer, Basel
Körner C (2018) Alpine ecosystems and the high-elevation treeline. In: Sven Erik J, Brian DF (eds) Encyclopedia of ecology. Academic Press, Oxford, pp 407–413
Leakey ADB, Uribelarrea M, Ainsworth EA, Naidu SL, Rogers A, Ort DR, Long SP (2006) Photosynthesis, productivity and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol 140(2):779–790. https://doi.org/10.1104/pp.105.073957
Liu XD, Chen BD (2000) Climatic warming in the Tibetan plateau during recent decades. Int J Climatol 20(14):1729–1742
Loladze I, Nolan JM, Ziska LH, Knobbe AR (2019) Rising atmospheric CO2 lowers concentrations of plant carotenoids essential to human health: a meta-analysis. Mol Nutr Food Res 63:1801047
Masle J (2000) The effects of elevated CO2 concentrations on cell division rates, growth patterns, and blade anatomy in young wheat plants are modulated by factors related to leaf position, vernalization, and genotype. Plant Physiol 122(4):1399–1416. https://doi.org/10.1104/pp.122.4.1399
Mccready RM, Guggolz J, Silviera V, Owen HS (1950) Determination of starch and amylase in vegetables. Anal Chem 22:1156–1158
Mishra NB, Mainali KP (2017) Greening and browning of the Himalaya: spatial patterns and the role of climatic change and human drivers. Sci Total Environ 587:326–339. https://doi.org/10.1016/j.scitotenv.2017.02.156
Morison JIL (1987) Intercellular CO2 concentration and stomatal response to CO2. In: Zeiger E, Farquhar GD, Cowan IR (eds) Stomatal function. Stanford University Press, Stanford, pp 229–252
Paulsen J, Körner C (2001) GIS-analysis of tree-line elevation in the Swiss Alps suggests no exposure effect. J Veg Sci 12:817–824. https://doi.org/10.2307/3236869
Prior SA, Pritchard SG, Runion GB, Rogers HH, Mitchell RE (1997) Influence of atmospheric CO2 enrichment, soil N, and water stress on needle surface wax formation in Pinus palustris (Pinaceae). Am J Bot 84(8):1070–1077
Pritchard SG, Rogers HH, Prior SA, Peterson CM (1999) Elevated CO2 and plant structure: a review. Glob Chang Biol 5(7):807–837. https://doi.org/10.1046/j.1365-2486.1999.00268.x
Schäppi B, Körner C (1996) Growth responses of an alpine grassland to elevated CO2. Oecologia 105(1):43–52. https://doi.org/10.1007/BF00328790
Schäppi B, Körner C (1997) In situ effects of elevated CO2 on the carbon and nitrogen status of alpine plants. Funct Ecol 11(3):290–299. https://doi.org/10.1046/j.1365-2435.1997.00084.x
Shaw MR et al (2002) Grassland responses to global environmental changes suppressed by elevated CO2. Science 298:1987–1990
Shrestha AB, Wake CP, Mayewski PA, Dibb JE (1999) Maximum temperature trends in the Himalaya and its vicinity: an analysis based on temperature records from Nepal for the period 1971–94. J Clim 12(9):2775–2786
Singh SK, Reddy VR (2016) Methods of mesophyll conductance estimation: its impact on key biochemical parameters and photosynthetic limitations in phosphorus stressed soybean across CO2. Physiol Plant 157:234–254
Singh SP, Sharma S, Dhyani PP (2019) Himalayan arc and treeline: distribution, climate change responses and ecosystem properties. Biodivers Conserv 28:1997–2016. https://doi.org/10.1007/s10531-019-01777-w
Strzalka K, Gugala AK, Latowski D (2003) Carotenoids and environmental stress in plants: significance of carotenoid-mediated modulation of membrane physical properties. Russ J Plant Physiol 50(2):168–173
Temperton VM, Grayston SJ, Jackson G, Barton CVM, Millard P, Jarvis PG (2003) Effects of elevated carbon dioxide concentration on growth and nitrogenfixation in Alnusglutinosain a long-term field experiment. Tree Physiol 23:1051–1059. https://doi.org/10.1093/treephys/23.15.1051
Thomas JF, Harvey CN (1983) Leaf anatomy of four species grown under continuous CO2 enrichment. Bot Gaz 144(3):303–309
Usuda H (2006) Effects of elevated CO2 on the capacity for photosynthesis of a single leaf and a whole plant, and on growth in a radish. Plant Cell Physiol 47(2):262–269. https://doi.org/10.1093/pcp/pci244
van der Kooi CJ, Reich M, Löw M, De Kok LJ, Tausz M (2016) Growth and yield stimulation under elevated CO2 and drought: a meta-analysis on crops. Environ Exp Bot 122:150–157. https://doi.org/10.1016/j.envexpbot.2015.10.004
Ward JK, Tissue DT, Thomas RB, Strain BR (1999) Comparative responses of model C3 and C4 plants to drought in low and elevated CO2. Glob Chang Biol 5(8):857–867
Ward EJ, Oren R, Bell DM et al (2013) The effects of elevated CO2 and nitrogen fertilization on stomatal conductance estimated from 11 years of scaled sap flux measurements at Duke FACE. Tree Physiol 33:135–151. https://doi.org/10.1093/treephys/tps118
Wittwer SH (1995) Food, climate and carbon dioxide – the global environment and world food production. CRC Press, Boca Raton, FL
Xu Z, Jiang Y, Jia B, Zhou G (2016) Elevated-CO2 response of stomata and its dependence on environmental factors. Front Plant Sci 7:657. https://doi.org/10.3389/fpls.2016.00657
Yu J, Chen L, Xu M (2012) Effects of elevated CO2 on physiological responses of tall fescue to elevated temperature, drought stress, and the combined stress. Crop Science 52:1848–1858
Zelikova TJ, Blumenthal DM, Williams DG, Souza L, LeCain DR, Morgan J, Pendall E (2014) Long-term exposure to elevated CO2 enhances plant community stability by suppressing dominant plant species in a mixed-grass prairie. Proc Natl Acad Sci U S A 111(43):15456–15461
Zheng Y, Li F, Hao L et al (2019) Elevated CO2 concentration induces photosynthetic down-regulation with changes in leaf structure, non-structural carbohydrates and nitrogen content of soybean. BMC Plant Biol 19:255. https://doi.org/10.1186/s12870-019-1788-9
Zhu J, Zhang Y, Yang X, Chen N, Jiang L (2020) Synergistic effects of nitrogen and CO2 enrichment on alpine grassland biomass and community structure. New Phytol 228:1283–1294. https://doi.org/10.1111/nph.16767
Acknowledgements
We gratefully acknowledge the financial support from G.B. Pant National Institute of Himalayan Environment (GBPNIHE) under the National Mission on Himalayan Studies (NMHS) program and Space Applications Centre, Indian Space Research Organization, Ahmedabad, under SHRESTI - HIMADRI program. We would also like to thank Director HAPPRC, HNB Garhwal University for his support during the study and Prof. B. P. Nautiyal, VCSG Uttarakhand University of Horticulture and Forestry for reviewing the manuscript and providing valuable suggestions.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chandra, S., Chandola, V., Singh, A., Singh, C.P., Nautiyal, M.C. (2023). Responses of Herbaceous Species of Alpine Treeline to Elevated CO2. In: Singh, S.P., Reshi, Z.A., Joshi, R. (eds) Ecology of Himalayan Treeline Ecotone. Springer, Singapore. https://doi.org/10.1007/978-981-19-4476-5_18
Download citation
DOI: https://doi.org/10.1007/978-981-19-4476-5_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4475-8
Online ISBN: 978-981-19-4476-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)