Abstract
The treeline ecotone, which lies between subalpine forests and the alpine grasslands, offers special habitats and niches for several unique, representative, and sensitive biodiversity elements. Present study, with a focus on distribution patterns of macrolichens, attempts to describe such elements in high-altitude landscape associated with Tungnath, west Himalayan treeline ecotone. Two representative elevation transects [Kalsir–Chandrashila (KCT), 2080–3677 m asl and Pothibhasha–Chandrashila (PCT), 2120–3677 m asl] were investigated. Together these transects harbor 108 macrolichen species (41 genera, 15 families). PCT with 104 species (40 genera, 15 families) was species rich compared to KCT (73 species, 34 genera, 15 families). While family Parmeliaceae (46), Physciaceae (18), and Cladoniaceae (14) were species rich, genera like Parmotrema (12), Heterodermia (11), and Cladonia (8 species) had maximum species. Among families Candelariaceae, Coccocarpaceae, Nephromataceae, and Teloschistaceae had one species only. The species richness peaked at 2500 and 2600 m altitude bands (62 spp.). The presence of two foliicolous and caliciale lichen communities was interesting in view of their indicator value. Existence of the foliicolous lichens is indicative of human disturbance and increasing subtropical elements in high-elevation forests. Occurrence of caliciale lichens is associated with old growth forests. The distribution of macrolichens has implications for management of high−altitude forests including treelines.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Treeline ecotone, a transition between alpine grasslands and subalpine forests, provides habitats and niches for unique and distinct biodiversity from both these ecosystems with many species sensitive to change in the climate (Rawal and Dhar 1997). Being highest in the world, treeline in the Himalaya is considered a special indicator of climate change (Singh 2018). Some studies in the region have established its uniqueness, and floristic sensitivity (Rawal and Dhar 1997). Few other studies have described responses of treeline ecotone vegetation to anthropogenic disturbances (Gairola et al. 2009, 2015; Rai et al. 2012), and its conservation in Western Himalaya (Dhar 2000; Singh 2018; Rawal et al. 2018). More recently, a comprehensive compilation of diverse studies on timberline of Indian Himalayan Region (IHR) has been a major outcome (Singh 2018). The study has pointed out that the Himalaya is warming with a rapid rate which has made timberlines more vulnerable. Despite being identified as conservation entities, the studies conducted on these regions are meager.
Among others, owing to their high-altitude location and relatively less anthropogenic pressure, the Himalayan treeline provides a suitable habitat with congenial environment for growth of different lichen species. As the lichens are well recognized for their sensitivity toward pollution (Vokou et al. 1999), they thrive very well in higher altitude areas where the pollution level remains low as compared to low-land areas. Lichens are the most successful symbiotic organisms in nature which dominate 8% or more of the Earth’s terrestrial area (Ahmadjian 1995). They are better indicators of diverse environmental conditions (Will-Wolf et al. 2002; Shukla et al. 2014). High sensitivity of lichens to changing climate events is well recognized (Gauslaa 2014). Their slow growth rate allows the lichens to integrate the climatic conditions and modify their habitat as per the requirements (Aptroot 2009).
While lichens contribute to richness of forest biodiversity (Pharo et al. 1999), they form an important forage for several animals (Rosentreter et al. 1997), nesting material for birds (Starkey and Hagar 1999), special habitats for many invertebrates (Pettersson et al. 1995), and are also involved in nutrient cycling (Essen et al. 1996). However, being habitat specific, presence of some particular lichen communities in a forest indicates its status, and their diversity on a given site also reflects on habitat heterogeneity (McCune 2000). During recent years some lichenological investigations were conducted in high-altitude zones of Kumaun, west Himalaya, including glaciers (Bisht 2018; Bisht et al. 2018a, b; 2019a), enumeration of medicinally important lichens of high-altitude regions (Bisht et al. 2019b), discoveries of new species (Joshi et al. 2016, 2018a), new records of lichens (Joshi et al. 2018b), lichen growth rates (Bisht et al. 2020). However, in spite of all these studies, lichens in Himalaya, in general, and high-altitude regions, in particular (especially the timberline ecotone of IHR), have remained poorly investigated. Keeping this lacuna in view and considering the overall importance of lichens as components of biodiversity, the present study was conducted to: (i) enumerate the lichen diversity along the representative elevation transects [i.e., Kalsir–Chandrashila transect (KCT), and Pothibhasha–Chandrashila transect (PCT)], (ii) identify the indicator lichen communities, and (iii) understand the status of lichen diversity across the elevation bands.
12.2 Material and Methods
12.2.1 Study Area
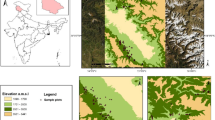
The study was conducted in two representative elevation transects [Kalsir–Chandrashila (KCT), and Pothibhasha–Chandrashila (PCT)] in Tungnath area of Indian Himalayan region of Uttarakhand. Considering its association with treeline ecotone, we have termed the study landscape as Treeline Landscape (TL). Major parts of these transects fall in Rudraprayag district and a small part in Chamoli district. Elevation range of study transects varied from 2082 to 3677 m asl for KCT and 2120 to 3677 m asl for PCT. The Tungnath area is of great religious importance due to the presence of famous Lord Shiva shrine, which is recognized among famous Five Kedars of Garhwal region. The vegetation of the region broadly represents temperate mixed broad leaf and coniferous forests.
12.2.2 Sample Collection, Identification, and Herbarium Documentation
Several field expeditions were conducted in both the transects between the years 2016 and 2018 and an exhaustive search for lichen species was undertaken. Each transect was divided into 18 elevation bands with an interval of 100 m between each band. In each elevation band, 3 plots (50 m × 50 m) were laid and the representative lichen specimens were collected from the major available substrates (i.e., wood, soil, and rock). The collected specimens were packed in poly bags and then air dried. The air-dried specimens were then brought to the laboratory and the morphological characteristics of the species were examined under a stereo-zoom dissecting microscope (Olympus SZ2-ILST). The anatomical studies of handmade sections were done using a research microscope (CX21iLEDFS1). Spot test reactions on thalli, medulla, and fruiting bodies were tested with the standard reagents: 10% potassium hydroxide (K), sodium hypochlorite (C), and para-phenylenediamine (Pd). The specimens were identified using the available literature (Awasthi 2007; Divakar and Upreti 2005; Singh and Sinha 2010). The identified specimens have been deposited in the herbarium of G. B. Pant Institute (GBP).
Species richness in each elevation band was determined by pooling all species identified in different plots in that band. Whittaker’s beta diversity across the elevation bands was analyzed by the following method mentioned in Rawal et al. (2018). Similarity coefficient between different selected elevations was calculated as per Sorenson’s Similarity Index (Sorenson 1948).
12.3 Results
12.3.1 Macrolichen Species Diversity and Composition
Collectively in both the transects, the study revealed occurrence of 108 macrolichen species (41 genera and 15 families). The detailed inventory across transects and elevation bands of TL is presented in Table 12.1. Distribution of macrolichen species rich families is presented in Fig. 12.1. While the KCT harbored 73 species (belonging to 15 families and 34 genera), PCT was comparatively species rich with 104 species (belonging to 15 families and 40 genera). Family Parmeliaceae (46 species; 18 genera) was the dominant family followed by Physciaceae (18 species; 6 genera) and Cladoniaceae (14 species; 2 genera). In case of genera, Parmotrema (12 spp.) followed by Heterodermia (11 spp.) and Cladonia (8 spp.) were species rich. Among the families, Candelariaceae, Coccocarpaceae, Nephromataceae, and Teloschistaceae were species poor, represented by only one species. Likewise, a total of 18 (43.9%) genera, viz. Candelaria, Canoparmelia, Cetraria, Coccocarpia, Collema, Dolichousnea, Flavoparmelia, Leucodermia, Melanelia, Nephroma, Parmelinella, Physconia, Protoparmeliopsis, Rhizoplaca, Sulcaria, Sticta, Xanthoparmelia, and Xanthoria were represented by only one species. The species rich PCT had seven genera (viz Collema, Melanelia, Myelochroa, Parmelinella, Physconia, Remototrachyna, and Sticta) restricted to this transect whereas, transect Kalsir–Chandrashila had only one genus, that is, Sulcaria restricted to this transect. Considering the diverse substrates, maximum macrolichen species (41; 37.9%) were recorded exclusively on woody (corticolous) substrate followed by 15 (13.9%) species on rock (saxicolous) and 15 (13.9%) on soil (terricolous) substrate. While 10 spp. (9.2%) were common to all the substrates, wood and rock shared 19 (17.5%), wood and soil shared 5 spp. (4.6%), and soil and rock substrate share 3 spp. (2.7%). The distribution of species by substratum is presented (Fig. 12.2).
12.3.2 Diversity of Macrolichen in Different Elevation Bands
While considering elevation patterns of species richness, number of macrolichen species peaked at 2500 and 2600 m elevation bands (62 spp.) and thereafter a gradual decline was noticed with minimum diversity at 3700 m elevation (16 spp.). In general, the elevation pattern of lichens showed a hump shaped distribution pattern (Fig. 12.3). One-fourth of the total species were restricted to 2500 m altitude. Whereas, 8 species, viz. Cetraria islandica, Cladonia pyxidata, Capitites ramulosa, Cladonia rangiferina, Hypotrachyna pindarensis, Parmelinella wallichiana, Stereocaulon alpinum, and Stereocaulon massartianum were recorded only above 3000 m elevation. Fifteen species were confined to mid elevation zone, that is, 2400–3200 m altitude. Three species Heterodermia diademata, Leucodermia boryi, and Phaeophyscia hispidula, were, however, present in all the elevation bands. Other 8 species, that is, Heterodermia dissecta (2500 m), Heterodermia indica (2600 m), Heterodermia leucomela (2800 m), Heterodermia obscurata (2000 m), Leptogium trichophorum (2400 m), Remototrachyna adducta (2100 m), Rinorea crenata (2300 m) and Usnea stigmatoides (2900 m) were recorded from a single elevation band only. Fifteen species were frequently distributed (found in every elevation band) between 2000 and 2800–3400 m altitude. The species to family ratio (SR/F) across the elevation ranged from 2.4 at 3300 m to 6.2 at 2600 m. The species richness and genera ratio (SR/G) was maximum at 2200 m (2.5) and was minimum at 3300 m (1.41).
12.3.3 Lichen Diversity in Closed Canopy Forests
The moisture and shade loving species of lichen families like Collemataceae (Collema spp. and Leptogium spp.), Lobariaceae (Lobaria spp.), and Peltigeraceae (Peltigera spp.) were found growing under closed canopy forests.
12.3.4 Lichen Diversity at Treeline Ecotone
Members of lichen families such as Caliciaceae (Pyxine spp.), Parmeliaceae (Hypotrachyna spp., Nephroma spp., Parmelia spp., Parmotrema spp., and Usnea spp.), Physciaceae (Heterodermia spp.), and Ramalinaceae (Ramalina spp.) were dominant at the treeline ecotone of the studied landscape. The species of these families grow well in open places with direct sunlight.
12.3.5 Lichen Diversity in Alpine Tundra
The alpine meadows above treeline were dominated by the members of families Cladoniaceae (Cladonia spp. and Stereocaulon spp.) on soil, while the rocks above treeline were colonized by the species of families Lecanoraceae (Protoparmeliopsis spp. and Rhizoplaca spp.), Umbilicariaceae (Umbilicaria spp.), and Teloschistaceae (Xanthoria spp.).
12.3.6 Beta (β) Diversity across Elevation Bands
Whittaker’s β diversity values, calculated for the studied transects and selected 18 altitude bands, are presented in Table 12.2. Perusal of the data revealed variations in macrolichen species across the elevations and transects. The maximum β diversity (5.75) was recorded at the 3700 m elevation. Across the elevation bands β diversity mostly increased with increase in elevation.
12.3.7 Similarity Coefficient between the Elevation Bands
Considerable variation in similarity coefficient was apparent across elevation bands (Table 12.3). Among all the bands, highest similarity was recorded between 3500 and 3600 m (100%) followed by 2000 and 2100 m, 3500 and 3700 m, and 3600 and 3700 m (98%, 97%, and 97%) respectively. The distantly located elevation bands (i.e., 2200 and 3500 m, 2200 and 3600 m, 2200 and 3700 m, 2300 and 3500 m, 2300 and 3600 m, and 2300 and 3700 m) exhibited low similarity (8% each).
12.3.8 Indicator Lichen Communities
Besides the composition and diversity of macorlichens across the elevation bands, two special indicator lichen groups, viz. foliicolous (leaf colonizing lichens) and caliciales (pin head lichens), were encountered during the field survey. Leaves of Persea duthiei from Pothibhasha (2100 m) to Dugalbhitta (2300 m) were heavily colonized by a foliicolous lichen, that is, Strigula complanata (Fig. 12.4a). Similarly, most of the trees of Abies spectabilis were found colonized by the caliciale lichens (Fig. 12.4b).
12.4 Discussion
The elevation transects of present study represented about 4% and 10% of the total lichen diversity reported from India and Uttarakhand, respectively. In a previous study, a total of 85 species of macrolichens covering an elevation range of 1500–3700 m in a similar study region were reported (Negi 2000). While comparing these species with the species observed along the elevation transects of present study, a total of 69 species (81%) were common in both the studies. This suggests that most of the macrolichens have wide distribution in the study area. However, 16 species (viz Cetreliopsis rhytidocarpa (Cetraria rhytidocarpa), Cladonia chlorophaea, Cladonia gymnopoda, Cladonia parasitica, Cladonia pleurota, Cladonia scabriuscula, Heterodermia angustiloba, Heterodermia punctifera, Hypotrachyna awasthii (Parmelia awasthi), Hypotrachyna crenata (Parmelia crenata), Parmelinella simplicior (Parmelia simplicior), Peltigera rufescens, Ramalina roesleri, Usnea baileyi, Usnea eumitrioides, and Usnea perplexans) were reported in previous report only. Likewise 39 species have been reported in the present study only. However, putting both studies together it is reasonable to suggest the existence of 124 species of macrolichens in Tungnath area. Despite the maximum number of species were growing only on woody substrates, these species are at risk of disappearance from the region. It was observed that most of the trees were colonized by mosses and bryophytes and were devoid of lichens. In due course of time, with expected colonization of bryophyte species on whole trunk would lead to exclusion of lichens from them. In this case host specific (only corticolous) species are likely to suffer and struggle for their substrate requirements.
Elevation in Himalayan mountains remains an important factor for determining the diversity and distribution of biodiversity elements (Upadhya 2017; Rawal et al. 2018; Upadhyay et al. 2018). Along the elevation range, largely three types of distribution patterns of organisms exist: (1) monotonic decline, (2) increase with altitude, and (3) hump-shaped or intermediate relationship. Our study on macrolichens suggests, by and large, prevalence of monotonic decline. Although, there is an indication of increase in species number around 2600–2700 m altitude, thereby forming a small hump in species distribution. The study, however, established that the number of macrolichen species decline considerably with increasing elevation, specifically above 2600 m asl. In this context, species richness pattern of Tungnath differs from hump-shaped species relationship, with maximum richness at approximately 4000 m asl, reported in neighboring Nepalese Himalaya (Baniya et al. 2010). Considerable difference in richness of species in the two study transects (i.e., 73 species in KCT; 104 species in PCT) indicates that macrolichen diversity varied due to diversity in microclimatic conditions. Presence of maximum macrolichen species at an elevation of 2600 m asl may be due to the high atmospheric moisture and cooler summer temperature which are favorable for the growth and development of the macrolichens. Occurrence of 8 species of macrolichens (Cetraria islandica, Cladonia pyxidata, Cladonia ramulosa, Cladonia rangiferina, Hypotrachyna pindarensis, Parmelinella wallichiana, Stereocaulon alpinum, and Stereocaulon massartianum) only above 3000 m asl suggests that they are the ecotonal species. Three species, viz. Heterodermia diademata, Leucodermia boryi, and Phaeophyscia hispidula, with existence in all altitude bands, exhibited their wide ecological amplitude and resistance for growing in any environmental condition.
Whittaker’s β diversity revealed that the high-altitude bands exhibit higher heterogeneity with respect to macrolichens. The higher beta diversity in high-altitude bands presumably reflects greater spatial variation in substrate and microclimatic conditions. This is obvious as <3200 m asl altitude bands are trees that are dominating elements, whereas >3200 m altitude bands are mostly small shrubs and herbaceous elements that dominate the landscape.
Overall heterogeneity in macrolichen species distribution across the elevation range is further reflected with lower similarity among low- and high-altitude bands. For instance, if the low-altitude bands (2000–2500 m) are compared with high-altitude bands (3100–3700 m), similarity in most cases remains less than 25% and even goes down to 8–9% in certain cases.
The lichens are considered bioindicators of forest health and ecological continuity, as well as atmospheric pollution in different regions of the world (McCune 2000; Kricke and Loppi 2002; Brunialti and Giordani 2003; Wolseley et al. 2006; Upadhya 2017). In this context, two interesting indicator communities (i.e., foliicolous and caliciales) were present in the study area. Existence of foliicolous lichens (lichens colonizing leaves of vascular plants), which are among the most abundant epiphytes in tropical rain forests (Lücking 2001; Anthony et al. 2002), indicates existence of higher human disturbance and microclimatic conditions of the studied forests (Lücking 1997). In India the distribution of foliicolous lichens is mostly confined to tropical regions of Eastern Himalaya and Western Ghats and over 135 species have been documented (Singh and Sinha 2010). Recently, Upadhyay et al. (2015) reported 6 species of foliicolous lichens from Nandhaur wildlife sanctuary of Uttarakhand. Presence of foliicolous lichens in some subtropical areas (Vězda 1983; Sérusiaux 1996; Puntillo and Ottonello 1997) and temperate regions (Malcolm and Galloway 1997; Lücking et al. 2003) has also been reported. Therefore, frequent occurrence of these lichens in studied transects that represent temperate climatic conditions is of special interest.
The lower parts of PCT, Pothibhasha (2100 m) to Dugalbhitta (2300 m), are broad leaved mixed forests. The leaves of Persea duthiei were heavily colonized by foliicolous lichens (Fig. 12.4a). This suggests that these lichens of tropical or subtropical climates are finding suitable habitats even at these elevations. In other words, it can be concluded that these lichens are extending their distributional range to temperate areas. These evidences correspond well with the reports of greater warming rate in higher elevations, in general, and the present study area, in particular (Singh 2018). However, more evidences from other transects would be required for a better generalization. Also, this will establish bio-indicator potential of these lichens to monitor the climatic conditions of various forest types across the Himalayan landscape.
The caliciales (Pin lichens) are the tiny unremarkable lichens which resemble with small pins that arise from a bed of green algae. Their association with late-successional and old-growth forests is, however, well documented (Rose 1992). They are substrate specific. Some of these lichen assemblages tend to be found on gymnosperms generally and on angiosperms rarely and vice versa (Selva 1994). Most of the Abies spectabilis trees in the areas of studied transects were colonized by caliciale lichens (Fig. 12.4b). The presence of these lichens on the bark of Abies trees is indicative of old growth of these forests that have reached the climax stage of succession. Such old growth forests that have taken a longer period of time to acquire the characteristics of microhabitats suitable for establishment of the rare caliciales have been described as “ancient forests” by Selva (1994).
The study suggests that there is an opportunity to harness the full bioindicator potential of different lichen communities to assess the ecosystem health in the study area. The identification of “indicator” lichens can provide a basis for appropriate management prescriptions and can effectively be used to assess climatic changes and potential forest recovery in areas where deforestation has caused changes in microclimate and thereby in the phanerogamic communities as well (Wolseley and Aguirre-Hudson 2007). The study also provides baseline information about the macrolichen community structure which can be used for long-term monitoring, temporal changes taking place in community structure, and migration of taxa from one habitat to another, which may be the consequence of change in climatic conditions. Any alteration in the lichen species and community structure may be further used for predicting future climate scenarios.
References
Ahmadjian V (1995) Lichens are more important than you think. Bio Sci 45:124
Anthony PA, Holtum JAM, Jackes BR (2002) Shade acclimation of rainforest leaves to colonization by lichens. Funct Ecol 16:808–816
Aptroot A (2009) Lichens as an indicator of climate and global change. In: Letcher TM (ed) Climate change: observed impacts on planet earth. Elsevier, Oxford, p 444
Awasthi DD (2007) A compendium of the macrolichens from India, Nepal and Sri Lanka. Bishen Singh Mahendra Pal Singh Publication, Dehra Dun; pp. 580
Baniya CB, Solhøy T, Gauslaa Y, Palmer MW (2010) The elevation gradient of lichen species richness in Nepal. Lichenologist 42(1):83–96
Bisht K (2018) Impact of climate change on glaciers of Kumaun Himalaya: a lichenometric approach. Ph.D. thesis, Kumaun University, Nainital, Uttarakhand, India. http://hdl.handle.net/10603/257037
Bisht K, Joshi Y, Upadhyay S, Mehta P (2018a) Recession of Milam Glacier, Kumaun Himalaya, observed via lichenometric dating of moraines. J Geol Soc India 92(2):173–176
Bisht K, Joshi Y, Upadhyay S, Chandra K (2018b) Assessment of climate change impact on recession of Adi Kailash glacier, Kumaun Himalaya: a lichenometric observation. ENVIS Bull Himal Ecol 25:24–27
Bisht K, Upadhyay S, Joshi Y (2019a) Cairns as hurdles for lichenometric studies on Himalayan glaciers. J Geol Soc India 94(5):545–546
Bisht K, Upadhyay S, Joshi Y (2019b) Timberline forests: potential habitats for conserving Himalayan medicinal lichen diversity in Kailash Sacred Landscape part of India. ENVIS Bull Himal Ecol 26:24–27
Bisht K, Upadhyay S, Joshi Y (2020) Higher growth rates of saxicolous lichens in alpine regions of western Himalaya, a consequence of warming climate? ENVIS Bull Himal Ecol 27:69–72
Brunialti G, Giordani P (2003) Variability of lichen diversity in a climatically heterogeneous area (Liguria, NW Italy). Lichenologist 35:55–69
Dhar U (2000) Prioritization of conservation sites in the timberline zone of west Himalaya. In: Singh S, Sastry ARK, Mehta R, Uppal V (eds.) Setting biodiversity conservation priorities for India; pp. 193 ̶ 211
Divakar PK, Upreti DK (2005) Parmelioid lichens in India. A revisionary study. Bishen Singh Mahendra Pal Singh Publicaton, Dehradun, p 488
Essen PA, Renhorn KE, Pettersson RB (1996) Epiphytic lichen biomass in managed and old-growth boreal forests: effects of branch quality. Ecol Appl 6:228–238
Gairola S, Rawal RS, Dhar U (2009) Patterns of litter fall and return of nutrients across anthropogenic disturbance gradients in three subalpine forests of West Himalaya, India. J For Res 14:73–80
Gairola S, Rawal RS, Todaria NP (2015) Effect of anthropogenic disturbance on vegetation characteristics of sub-alpine forests in and around valley of flowers National Park, a world heritage site of India. Trop Ecol 56(3):357–365
Gauslaa Y (2014) Rain, dew and humid air as drivers of morphology, function and spatial distribution in epiphytic lichens. Lichenologist 46:1–16
Joshi Y, Upadhyay S, Chandra K, Bisht K, Falswal A (2016) A new species of Plectocarpon (Roccellaceae, Lichenised Ascomycetes) from India. Act Bot Hung 58(3–4):257–264
Joshi Y, Tripathi M, Bisht K, Upadhyay S, Kumar V, Pal N, Gaira A, Pant S, Rawat KS, Bisht S, Bajpai R, Halda JP (2018a) Further contributions to the documentation of lichenicolous fungi from India. Kavaka 50:26–33
Joshi Y, Bisht K, Upadhyay S, Chandra K (2018b) Three new records of lichens from India. Nelumbo 60(1):90–94
Kricke R, Loppi S (2002) Bioindication: the I.a.P. approach. In: Nimis PL, Scheidegger C, Wolseley PA (eds) Monitoring with lichens – monitoring lichens. Kluwer Academic Press, Dordrecht, pp 21–37
Lücking R (1997) The use of foliicolous lichens as bioindicators in the tropics, with special reference to the microclimate. Abst Bot 21:99–116
Lücking R (2001) Lichens on leaves in tropical rainforests: life in a permanently ephemerous environment. In: Gottsberger G, Liede S (eds.), Life forms and dynamics in tropical forests; pp. 41–77
Lücking R, Wirth V, Ferrarom CMES (2003) Foliicolous lichens from Valdivian temperate rain forest of Chile and Argentina: evidence of an austral element, with the description of seven new taxa. Glob Ecol Biogeogr 12:21–36
Malcolm WM, Galloway DJ (1997) New Zealand Lichens. In: Checklists, key and glossary. Wellington, Museum of New Zealand Te Papa Tongarewa, p 192
McCune B (2000) Lichen communities as indicators of forest health. Bryologist 103(2):353–356
Negi HR (2000) On the patterns of abundance and diversity of macrolichens of Chopta-Tungnath in the Garhwal Himalaya. J Biosci 25(4):367–378
Pettersson RB, Ball JP, Renhorn KE, Essen PA, Sjberg K (1995) Invertebrate communities in boreal forest canopies as influenced by forestry and lichens with implications for passerine birds. Biol Conserv 74:57–63
Pharo EJ, Beattie AJ, Binns D (1999) Vascular plant diversity as a surrogate for bryophyte and lichen diversity. Conserv Biol 3:242–262
Puntillo D, Ottonello D (1997) A new foliicolous lichen station in Italy. Lichenologist 29:388–390
Rai ID, Adhikari BS, Rawat GS, Bargali K (2012) Community structure along timberline ecotones in relation to micro-topography and disturbance in West Himalaya. Not Sci Biol 4:41–52
Rawal RS, Dhar U (1997) Sensitivity of timberline flora in Kumaun Himalaya, India: conservation implications. Arct Alp Res 29:112–121
Rawal RS, Rawal R, Rawat B, Negi VS, Pathak R (2018) Plant species diversity and rarity patterns along altitude range covering treeline ecotones in Uttarakhand: conservation implications. Trop Ecol 59(2):225–239
Rose F (1992) Temperate forest management: its effects on bryophyte and lichen floras and habitats. In: Bates JW, Farmer AM (eds) Bryophytes and lichens in a changing environment. Clarendon, Oxford, pp 211 ̶–21233
Rosentreter R, Hayward GH, Wicklow-Howard M (1997) Northern flying squirrel seasonal food habits in the interior conifer forests of Central Idaho, USA. Northwest Sci 71:97–101
Selva SB (1994) Lichen diversity and stand continuity in the Northern Hardwoods and Spruce-Fir forests of Northern New England and Western New Brunswick. Bryologist 97:424–429
Sérusiaux E (1996) Foliicolous lichens from Madeira, with the description of a new genus and two new species and a world-wide key of foliicolous Fellhanera. Lichenologist 28:197–227
Shukla V, Upreti DK, Bajpai R (2014) Lichen diversity in different lichenogeographical regions of India. In: Shukla V, Upreti DK, Bajpai R (eds) Lichen to biomonitor the environment. Springer, India, pp 61–96
Singh SP (2018) Research on Indian Himalayan treeline ecotone: an overview. Trop Ecol 59(2):163–176
Singh KP, Sinha GP (2010) Indian lichens: an annotated checklist. Botanical Survey of India, Kolkata, p 571
Sorenson T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analysis of the vegetation on Danish commons. Biol Skrifter Kongelige Danske videnskabernes Selskab 5:1–34
Starkey E, Hagar JC (1999) Biodiversity of young Forest-role of birds. Final Report to US Department of Interior, Blue River District
Upadhya S (2017) Distribution and diversity pattern of macrolichens along an altitudinal gradient in Kumaun Himalaya with an emphasis to their indicator value. Ph.D. thesis. Kumaun University, Nainital
Upadhyay S, Shukla S, Joshi Y, Pinokiyo A (2015) Range extension of foliicolous lichens in India: a case study from Nandhaur Forest range, Lakhan Mandi, Haldwani, Uttarakhand, India. G - J Env Sci Tech 3:101–104
Upadhyay S, Jugran AK, Joshi Y, Suyal R, Rawal RS (2018) Ecological variables influencing the diversity and distribution of epiphytic macrolichens colonizing Quercus leucotrichophora in Thal Ke Dhar forest, Pithoragarh district, Uttarakhand. J Mt Sci 15:307. https://doi.org/10.1007/s11629-017-4397-9
Vězda A (1983) Foliicole Flechtenaus der Kolchis (West-Transkaukasien, UdSSR). Fol Geobot Phyto Praha 18:45–70
Vokou D, Pirintsos SA, Lopppi S (1999) Lichens as bioindicators of temporal variation in air quality around Thessaloniki, northern Greece. Ecol Res 14:89–96
Will-Wolf S, Neitlich P, Essen PA (2002) Monitoring biodiversity and ecosystem functions: forest. In: Nimis PL, Scheidegger C, Wolseley P (eds) Monitoring with lichens monitoring lichens, NATO science series. Kluwer Academic Publishers, The Hague, pp 203–222
Wolseley PA, Aguirre-Hudson B (2007) Lichens as indicators of environmental changes in the tropical forests of Thailand. Glob Ecol Biogeogr Lett 1(6):170–175
Wolseley PA, Stofer S, Mitchell R, Truscott AM, Vanbergen A, Chimonides J, Scheidegger C (2006) Variation of the lichen communities with landuse in Aberdeenshire, UK. Lichenologist 38(4):307–322
Acknowledgements
Financial support from Indian Himalayan Timberline Project (IHTP) under the National Mission on Himalayan Studies (NMHS) of Govt. of India is gratefully acknowledged. Coordinator IHTP, Prof SP Singh is thanked for his encouragement. Thanks are due to Head, Department of Botany, S.S.J. Campus, Kumaun University, Almora and Director NIHE for laboratory facilities. We extend our thanks to Mr. Sunil Joshi, Ms. Renu Rawal, Ms. Poonam Mehta, Ms. Kamini Durgapal, and Ms. Medha Durgapal, NIHE, for supporting the authors (KB and SU) in the field.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bisht, K., Upadhyay, S., Rawal, R.S., Joshi, Y. (2023). Lichen Diversity in High Elevations of Western Himalaya with Special Reference to Treeline Ecotone: Conservation and Indicator Value. In: Singh, S.P., Reshi, Z.A., Joshi, R. (eds) Ecology of Himalayan Treeline Ecotone. Springer, Singapore. https://doi.org/10.1007/978-981-19-4476-5_12
Download citation
DOI: https://doi.org/10.1007/978-981-19-4476-5_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-4475-8
Online ISBN: 978-981-19-4476-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)