Abstract
Ultraviolet (UV) is a part of solar spectrum and has 3 subtypes: UV-A, UV-B, and UV-C. Out of them, UV-B has major impact on biological systems. It acts as an abiotic stress and also informational signal for plant growth and development. The photoreceptor responsible for UV signaling is UVR8, which changes its conformation on UV exposure leading to signaling pathways involving factors, like COP-1, FHY3, HY5, HYH, RUP1, RUP2, BIM1, WRK36, MYB73/MYB77, CRY, and many more. UVR8 signaling has holistic impact on plant, including various physiological effects, such as DNA alteration, defense, morphogenesis, phototropism, circadian clock, and induction of flowering. COP1 with UVR8 affects photomorphogenesis, gene expression, propanoid accumulation, and hypocotyl growth. The later involves HYH and HY5 for action. Cryptochromes and UVR8 lead to change in flavonoid levels aiding UV tolerance. RUP1 and RUP2 along with UVR8 lead to changes in flowering pattern as well as morphogenesis and UV acclimation. On the other hand, MYB, under the effect of UVR8, regulates root growth and development. It also alters expression of auxin-responsive genes, which further leads to multiple effects. BIM1 with UVR8 affect brassinosteroid-responsive genes affecting plant growth. As UVR8 helps multiple factors to link with each other, henceforth, the mode of action, signaling, and impact on plant growth is detailed in the sections ahead.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
10.1.1 UV Radiations
Various environmental stresses affect the development of plants under natural conditions. This triggers them to adapt and evolve copious strategies to exist under such hostile conditions. Ultraviolet (UV) radiations are part of the solar spectrum and have three types: UV-A, UV-B, and UV-C. Less than 0.5% of solar energy reaching the surface of the earth is contributed by UV-B (Blumthaler 1993). Factors, like altitude, latitude, stratospheric ozone, solar angle, and troposphere pollution, play a major role in this (Paul and Gwynn-Jones 2003; McKenzie et al. 2007). Every stress triggers a definite set of signaling factors for the perception of the stimulus, regulators of transcription, and downstream-responsive genes for stress acclimation and its tolerance.
UV-B light of wavelength 280–315 nm is latent stress and signal, which proves to control plant growth and its development (Tilbrook et al. 2013a, b). Its low fluence rate acts as a stimulus that mediates many chief plant physiological responses, such as circadian rhythm, photomorphogenesis, and photoprotection. Plant growth, development, and biochemical composition are well regulated by a low fluence of ambient UV-B (Boccalandro 2001; Suesslin and Frohnmeyer 2003) and mark the gene expression (Casati and Walbot 2003, 2004; Jenkins 2009). In comparison, UV-B at high fluence may trigger both specific as well as non-specific pathways instigating the formation of reactive oxygen species (ROS), inhibition of photosynthetic processes, protein synthesis, and damage to DNA and proteins (Jenkins 2009; Frohnmeyer and Staiger 2003). Subsequently, this leads to a varied range of alterations in morphology as well as at molecular levels (Brown et al. 2005; Kataria et al. 2014; Meegahakumbura et al. 2018). Also, high doses of UV-B mutilate cell membranes, lipids, as well as photosynthetic machinery, leading to changes at the cellular level and viability, posing stunted growth and a decline in crop yield as well as quality (Frohnmeyer and Staiger 2003). Subsequently, various ways had been evolved by plants to dodge UV-B damage (Tilbrook et al. 2013a, b). Primitive organisms, viz., cyanobacteria, and many eukaryotic algae form UV protective substances, like mycosporine-like amino acids (MAAs) (Llewellyn and Airs 2010; Rastogi and Incharoensakdi 2013; Rozema et al. 2002). As the complexity increases from algae to higher plants, there occur changes in the UV-B absorbing with reference to environmental UV-B levels (Rozema et al. 2002; Kataria et al. 2013). There exist an array of very sensitive and intricate photoreceptors for combating changes in light duration, quality, quantity, as well as direction.
10.1.2 UVR8
During the course of evolution, plants have developed different photoreceptors for the light of specific wavelengths: phototropins (phot1 and phot2) for blue light, phytochromes (phyA–E) to detect red/far-red light; cryptochromes (CRY1 and CRY2), Zeitlupe family proteins (ztl, fkf1, and lkp1), and the UV Resistance Locus 8 (UVR8) for ultraviolet (UV)-B (Rizzini et al. 2011; Tilbrook et al. 2013a, b; Jenkins 2014; Galvao and Fankhauser 2015). In higher plants, phytochromes are also involved in the UV-B protection mechanism and it makes UV-B response more complex (Kreslavski et al. 2020). Unlike photosynthetic complexes, a majority of photoreceptors have merely a single chromophore. Therefore, a single site is responsible for photon absorption as well as subsequent photoreactions leading to protein conformational changes with no transfer of excitation energy.
Previous researches proved the role of UVR8 photoreceptor in UV-B-induced responses. It has been observed by the action spectrum of UVR8 that although the major UV absorption occurs around 280 nm, the most important physiological responses occur by absorbing a wavelength of ≈300 nm with a minor absorption peak. UVR8 is a β-propeller protein, containing integral Trp residues that act as the base for the reception of UV-B (Christie et al. 2012; Liu et al. 2014). UVR8 from Arabidopsis thaliana is made up of total 440 amino acids containing two discrete domains: a core domain (seven-bladed β-propeller section) and a C-terminal area of 27 amino acids (Cloix et al. 2012; Kliebenstein et al. 2002). Unlike various infrared and visible photoreceptors (Möglich et al. 2010; Rockwell et al. 2006; Spudich et al. 2000), UVR8 has no external chromophore, and rather it utilizes its tryptophan (W or Trp) residues for the perception of light (Christie et al. 2012; O’Hara and Jenkins 2012; Ulm and Jenkins 2015; Wu et al. 2012). Precisely, intrinsic Trp285 and Trp337 play a major role in the absorption of UV-B light directly (Christie et al. 2012; Rizzini et al. 2011; Wu et al. 2012). Intriguingly, UVR8 has highly organized light-harvesting networks for photoreception (Rizzini et al. 2011). As these Trp networks are highly conserved among different species (Tilbrook et al. 2016; Fernández et al. 2016; Soriano et al. 2018), the light-harvesting mechanism possibly could have arisen early during the evolution of UVR8.
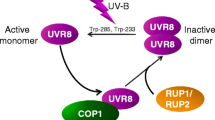
It is noteworthy that there exist the homodimer and the monomer states of UVR8, which are transitional, reversible, and dynamic. In the ground or “dark” state, UVR8 exists as a homodimer with its interface being well decorated with numerous inter-subunit salt bridges and hydrogen bonds (Rizzini et al. 2011; Christie et al. 2012; Wu et al. 2012). However, in the presence of UV-B, the tryptophan residues of UVR8 undergo structural changes after UV absorption and lead to the immediate formation of active monomer units (Rizzini et al. 2011; Christie et al. 2012; Wu et al. 2012; Zeng et al. 2015). Every monomer of UVR8 consists of 14 tryptophan residues. Apart from the unstructured C-terminal one, the remaining 13 structural Trp residues are generally classified into three different groups, viz., a distal ring (6Wd), a peripheral outlier (3Wp, viz., W198, W250, and W302), and a pyramidal center (4Wc) (Christie et al. 2012; O’Hara and Jenkins 2012; Liu et al. 2014; Wu et al. 2012, 2014, 2015; Voityuk et al. 2014; Zeng et al. 2015). The dimer form of UVR8 consists of two symmetry-related pyramid centers (4Wc); each is shaped by van der Waals clustering of 3Wc (W285, W233, and W337) from one monomer and a fourth Wc (W94) from the opposing monomer. Moreover, six residues of Trp, 6Wd, are concealed at the center of the beta-propellers and lead to the formation of a highly symmetrical ring. Out of all the tryptophan residues, only two pyramid center Trp (W285 and W233) of the Wc pyramid play the chief role during light-induced monomerization (Christie et al. 2012; Liu et al. 2014; O’Hara and Jenkins 2012; Wu et al. 2012, 2014, 2015; Voityuk et al. 2014; Zeng et al. 2015).
Li et al. used a site-directed mutagenesis approach and found that there exists a significant difference in absorption spectra longer than 300 nm among the Trp residues. The distal group of 6Wd residues has negligible absorbance beyond 310 nm; the absorption spectrum of the 3Wp extends to 314 nm, while that of 4Wc reaches beyond 320 nm. As a result of differential absorption, there exist differences in selective excitation of Wd, Wp, or Wc, leading to diverse emission spectra with peaks at 320 nm, 340 nm, and 350 nm, respectively. Despite UV-B absorption by all the Trp residues, only the interfacial pyramid remains the chief site for the critical reaction leading to dimer dissociation (Christie et al. 2012; O’Hara and Jenkins 2012; Rizzini et al. 2011; Wu et al. 2012; Wu et al. 2014, 2015; Liu et al. 2014; Voityuk et al. 2014; Zeng et al. 2015; Mathes et al. 2015). Conclusively, it can be stated that Trp residues not just play a structural role but also the distal and peripheral tryptophan networks have a chief functional role in harvesting and funneling UV-B energy to 4Wc (the pyramid perception centers), which leads to induction of the reaction and unfold the dimer interface resulting in a further signaling cascade.
Moreover, current structural studies propose the importance of the C-terminal tail of UVR8. This includes C27 and C17 domains, making diverse conformational changes for action. For instance, compact and the extended states of their structure have a key role in regulating the activity of UVR8 (Camacho et al. 2019).
UVR8 protein is reported to be present throughout the plant body (Rizzini et al. 2011). Even in the absence of UV-B, it is frequently located in the cytoplasm, whereas a small amount is found in the nucleus. Within minutes of UV-B exposure, UVR8 hoards in the nucleus, whereas its major amount remains in the cytoplasm (Kaiserli and Jenkins 2007; Yin et al. 2016; Qian et al. 2016). This nuclear localization of UVR8, as well as its monomerization, is critical for its role in controlling signal transduction and photomorphogenesis, resulting from changes in expression of genes, followed by acclimation responses (Kaiserli and Jenkins 2007; Rizzini et al. 2011).
10.2 UVR8-Mediated Signaling
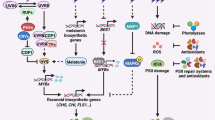
UV-B, with the help of UVR8, induces many physiological responses, which lead to growth regulation and developmental changes. It is also a signal which helps modulate photomorphogenesis, which involves accretion of flavonoids and anthocyanins, inhibition of hypocotyl elongation, and increased expression of UV-B-responsive genes (Jenkins 2009; Ballaré et al. 2012; Heijde and Ulm 2012; Wargent and Jordan 2013). On the perception of UV-B, the UVR8 triggers the signaling pathway and further leads to alteration of gene expression via molecular signaling. UVR8 helps acclimation for low fluence, while promotion of tolerance to high fluence of UV-B light (Kliebenstein et al. 2002; Brown et al. 2005; Favory et al. 2009).
White light can travel through the root tissues through an effect known as light piping and leads to photomorphogenic changes in the roots as that is not directly exposed to light (Lee et al. 2016). It is proven that CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1), Far-Red Elongated Hypocotyl 3 (FHY3), and ELONGATED HYPOCOTYL 5 (HY5) are three common factors playing a chief role in promoting light signaling and inducing photomorphogenesis. Transcription factors viz FHY3, COP1, and HY5 affect positively (Stracke et al. 2010; Huang et al. 2012; Binkert et al. 2014a, b) and the negative regulators RUP1 and RUP2 (Gruber et al. 2010; Heijde and Ulm 2013) ally the signaling pathway mediated by UVR8. Also, UVR8 precisely controls UV-B photomorphogenesis, which includes seedling de-etiolation, leaf development, phototropism, lateral root development, stomatal movements, floral transition, and stress tolerance (Wargent et al. 2009; Demkura and Ballaré 2012; Li et al. 2013; Tossi et al. 2014; Vandenbussche et al. 2014; Jenkins 2017; Yin and Ulm 2017; Arongaus et al. 2018; Liu et al. 2019; Liang et al. 2019; Vanhaelewyn et al. 2019).
Moreover, BES1-INTERACTING MYC-LIKE 1 (BIM1), WRKY36, and MYB DOMAIN PROTEIN 73 and 77 (MYB73/MYB77) also play a role in regulating gene expression (Brown and Jenkins 2008; Favory et al. 2009; Huang et al. 2012; Liang et al. 2018, 2019; Yang et al. 2018, 2020). So, it is established that certain genes are known to be reporters for UVR8 signaling: HY5, CHS, HYH, ELIP1, CRYD, GPX7, SIG5, PHR1, and WAKL8 with the first two as most implemented examples (Ulm et al. 2004a, b; Cloix and Jenkins 2008; Favory et al. 2009; Stracke et al. 2010; Binkert et al. 2014a, b; Yin et al. 2015; Moriconi et al. 2018).
Recently, it has been found that there occur multiple physiological responses that are regulated by UVR8: phototropism, auxin signaling, thermomorphogenesis, entrainment of the circadian clock, defense, salt stress tolerance, shade avoidance, chloroplast development, stomatal density and closure, leaf epinasty, photoprotective flavonoid biosynthesis, inhibition of hypocotyl extension, leaf expansion, endoreduplication, tolerance to Botrytis, and response to osmotic stress (Kliebenstein et al. 2002; Favory et al. 2009; Rizzini et al. 2011; Tilbrook et al. 2013a, b; Hayes et al. 2014; Jenkins 2017; Yin and Ulm 2017). A major hallmark for UV-B sensitivity can be the accumulation of photoprotective pigment. Interestingly, UV-B augments H2O2 production, thereby increasing the levels of nitric oxide and subsequently magnifying the expression of UVR8 (Wu et al. 2016).
It has been understood that by microarray and reverse transcription PCR analyses that UVR8 can regulate the expression of genes related to photooxidative damages (ELIP1, SIG5), protection against oxidative stress (PDX1), UV protection (flavonoid, PHR1), a number of genes encoding signaling components, transcription factors, transporters, proteases, and several proteins with unknown functions (Brown et al. 2005) (Fig. 10.1).
10.2.1 CONSTITUTIVELY PHOTOMORPHOGENIC 1 and Photomorphogenesis
CONSTITUTIVELY PHOTOMORPHOGENIC 1 is a chief negative regulator of plant photomorphogenesis in the dark and visible light, therefore involved in many light signaling pathways (Lau and Deng 2012; Podolec and Ulm 2018). Intriguingly, being a repressor of photomorphogenesis, COP1 plays a role in protein selection for ubiquitination and degradation (Lau and Deng 2012).
Being an E3 ubiquitin ligase, COP1 interacts directly with the photoactivated UVR8 monomer, proving this association crucial for the UVR8 signal transduction (Tilbrook et al. 2016). As UV-B is needed for UVR8–COP1 association (Rizzini et al. 2011; Cloix et al. 2012), followed by COP1 accumulation in the nucleus, implies that COP1 associates with the UVR8 monomer and UV-B signaling occur inside the nucleus (Oravecz et al. 2006; Favory et al. 2009). The truncated UVR8 has only the C27 domain, which is very critical to interact constitutively with COP1. For the interaction, COP1 utilizes its C-terminal WD40-repeat domain to interact with the two domains (beta-propeller) of UVR8 (Cloix et al. 2012; Yin et al. 2015). The UVR8 C-terminal tail alters to compact and extended conformations (Camacho et al. 2019). Numerous proteins join the C27 domain due to the extended conformation, while the compact version prospectively constrains UV-B signaling. Lin et al. (2020) suggested that C17 of COP1 hinders both the types of associations: the UVR8–COP1 as well as the C27–COP1. Although the interaction of C17 with UVR8 leads to C17 folding back onto full-length UVR8 which further forms more packed C-terminal conformation inhibiting the C27–COP1 association. In the dearth of UV-B treatment, the C27 domain of full-length UVR8, being protected inside the homodimer interface, is barred from COP1 association (Cloix et al. 2012; Yin et al. 2015).
There are two suggested models for UVR8 and COP1 interaction: (1) The nuclear co-import model, where UV-B induces the formation of UVR8 monomer leading to cytoplasmic COP1 association. The COP1-containing nuclear localization sequence aids in the co-import of UVR8 to the nucleus. So, the UVR8–COP1 association is important for UVR8 transport to the nucleus (Qian et al. 2016; Yin et al. 2016). (2) The nuclear retention model: UV-B-dependent nuclear localization of UVR8 monomer and its transportation into the nucleus by an unidentified mechanism, whereas nuclear COP1 prevents instant nuclear export of UVR8 (Yin and Ulm 2017). Although COP1 is an E3 ubiquitin ligase, the association of UVR8 and COP1 checks the degradation of UVR8 (Favory et al. 2009). Moreover, UV-B radiation upsurges COP1 protein post-transcriptionally in a UVR8-dependent manner, and the probable reason being relegated is autoubiquitination of COP1 (Favory et al. 2009). Additionally, the COP1 transcript gets enhanced due to transcription factors FHY3 and HY5, which work with UVR8 signaling on exposure of UV-B (Huang et al. 2012), and the binding sites for both the transcription factors on the COP1 promoter are adjacent to each other (Huang et al. 2012). It has also been studied that far-red light represses, while UV-B induces the expression of FHY3 (Lin et al. 2007; Huang et al. 2012).
ELONGATED HYPOCOTYL 5 acts as a photomorphogenesis-promoting bZIP transcription factor. COP1 associates with HY5 as well as many other positive regulators of photomorphogenesis for 26S proteasomal degradation (Osterlund et al. 2000). Contrastingly, under UV-B exposure, COP1 guards HY5 from 26S proteasome-mediated degradation (Huang et al. 2013; Ren et al. 2019). The prevention of HY5 ubiquitination can be due to the competition between UVR8 and HY5 for association with COP1 interaction (Yin et al. 2015; Lau et al. 2019). Hence, there occurs the maintenance of HY5 and the upregulation of multiple target genes, which encode proteins for photomorphogenesis (Huang et al. 2012; Binkert et al. 2014a, b). Most likely, this leads to related overlapping photomorphogenic responses, for instance, changes in gene expression, hypocotyl growth inhibition, phenylpropanoid accretion, along with induction of flowering (Galvao and Fankhauser 2015; Jenkins 2017; Yin and Ulm 2017; Podolec and Ulm 2018; Arongaus et al. 2018; Wang et al. 2018).
Moreover, it has also been suggested that COP1 associates with SPA1 (SUPPRESSOR OF PHYA) as well as other components of E3 ubiquitin ligase complexes which stimulate ubiquitination and degradation of transcription factors HY5 and HYH (HY5 HOMOLOG) (Lau and Deng 2012). The interaction of UVR8 and COP1 controls COP1 functioning and likely promotes functional dissociation of the COP1–SPA core complexes. This helps in forming an exceptional association, including UVR8–COP1–SPA1, and thereby controls the ubiquitination of HY5 (Huang et al. 2013). Conclusively, UV-B exposure initiates the association of UVR8 and COP1 and helps HY5 expression as well as protein stabilization, which further induces expression of UV-B-responsive gene leading to photomorphogenesis.
10.2.2 HY5 and HYH
Transcription factor HY5 is one of those genes which are activated upon UV exposure and has a chief role in signaling (Stracke et al. 2010; Huang et al. 2012). It is a bZIP transcription factor that responds to multiple wavelengths of light for directing gene expression involved in UV-B photomorphogenesis along with flavonoid biosynthesis (Lee et al. 2007; Brown and Jenkins 2008; Stracke et al. 2010). The HY5 induction and photomorphogenesis are critically dependent on the photoactivated nuclear UVR8 because cytoplasmic UVR8 is incapable of the same (Qian et al. 2016; Yin et al. 2016).
It has been studied that the expression of HY5 is dependent on UVR8 and COP1 with the exposure of UV-B (Oravecz et al. 2006; Favory et al. 2009). Intriguingly, the chromatin binding site of UVR8 and HY5 genomic locus is adjacent to each other (Brown et al. 2005; Cloix and Jenkins 2008). HY5, along with HYH, is commonly known to regulate many UV-regulated transcription (Brown and Jenkins 2008). It is also true that HY5 associates with HY5 Homolog (HYH), which regulates photomorphogenesis positively. This implies that HYH participates in UVR8-mediated signaling. As a mark of early photomorphogenic UV-B response, HY5 and HYH transcripts upsurge (Vanhaelewyn et al. 2019). HY5 plays an important role for UVR8-mediated UV-B signaling because it is deployed in COP1 and proteasome-mediated degradation in the absence of light (Osterlund et al. 2000). Three cis-regulatory elements (a T/G-box, an ACG-box (ACG), and an E-box) transcriptionally activate HY5, where the ACG-box is a light-induced HY5 repression factor (Binkert et al. 2014a, b). Furthermore, after induction of HY5 expression, one of the three ACGT-containing elements (ACEs) binds to the COP1 promoter leading to its increased expression (Huang et al. 2012). A supplementary characteristic attribute of HY5 is its re-engagement, which helps maintain a functional implication in older seedlings as well as mature plants (Oravecz et al. 2006). Upon UV-B exposure, HY5 and its homolog HYH attach to the T/G-box in the HY5 promoter to alter the expression of HY5 (Binkert et al. 2014a, b). HY5 further controls the expression of several UV-B-responsive genes, like RUPs, COP1, and flavonoid biosynthesis genes (Ulm et al. 2004a, b). WRKY36 is a transcription factor and uses its C-terminal DNA-binding domain for interaction with the C27 domain of UVR8. Under UV absence, UVR8 chiefly limits in the cytosol, whereas WRKY36 confines in the nucleus to negatively impact HY5 transcription and enhance hypocotyl elongation (Yang et al. 2018; Liang et al. 2018). While, on UV-B exposure, UVR8 monomerization leads to its accumulation in the nuclear compartment and associates with the WRKY36 to impede its DNA-binding feature. Additionally, HY5 affects auxin signaling by promoting the expression of its negative regulators, and thereby HY5 acting as a signaling links light and hormone signaling (Cluis et al. 2004).
10.2.3 Cryptochromes (CRYs)
Cryptochromes are a diverse array of flavoproteins, which are sensitive to blue light and have roles in maintaining circadian rhythms and magnetic field sensing. There exist two different genes for coding distinct proteins, viz., CRY1 and CRY2. In the presence of blue light, the monomer (inactive form) of cryptochrome modifies to homodimer (active) (Wang et al. 2016). Blue-light inhibitors of cryptochromes (BIC1) and BIC2 directly bind to cryptochromes and inhibit their dimerization, showing negative feedback regulation (Wang et al. 2016, 2017). Interestingly, there exists an overlapping in the absorption spectra of CRYs and UVR8. The absorption spectra of CRY range from UV-B to green wavelength (Ahmad et al. 2002; Zeugner et al. 2005; Banerjee et al. 2007), whereas those of UVR8 range from UV-C to the violet wavelength. This implied interaction between CRYs and UVR8. Cryptochrome-mediated blue-light signaling exhibit certain fascinating similarities to UVR8 UV-B signaling. Tissot and Ulm (2020) showed that the UVR8 photoequilibrium is responsive to blue-light signaling mediated by cryptochrome, thus identifying a novel photoreceptor co-action mechanism that balances UV-B sensitivity of plants under the polychromatic spectrum of sunlight. Fascinatingly, both non-functional CRYs and UVR8 impede the survival of plants under UV-B (Morales et al. 2013; Rai et al. 2019). In the presence of UV-B, flavonoid levels upsurge with the help of UVR8. When CRYs and UVR8 interact with COP1, it leads to stabilization of HY5 and HYH, further regulating the expression of many blue- and UV-responsive genes. Such genes, like CHALCONE SYNTHASE (CHS), DIHYDROFLAVONOL 4-REDUCTASE (DFR), EARLY LIGHT-INDUCED PROTEIN 2 (ELIP2), CHALCONE ISOMERASE (CHI), and SOLANESYL DIPHOSPHATE SYNTHASE 1 (SPS1), are induced by UV and blue light with the help of UVR8 and CRY (Favory et al. 2009; Yu et al. 2010; Nawkar et al. 2017). Also, active cryptochromes inhibit the COP1 to stabilize HY5 and help its accumulation (Lian et al. 2011; Liu et al. 2011; Zuo et al. 2011; Holtkotte et al. 2017; Lau et al. 2019).
Both photoreceptors CRY and UVR8 require to bind with COP1 for signaling cascade (Mao et al. 2005). Morales et al. (2013) proposed that the survival of the plant in the presence of UV is due to other pathways as well, which are independent of UVR8. Besides Fuglevand et al. (1996), Liu et al. (2018) also studied that CRY1 helps induce CHS in the presence of blue light, while Gruber et al. (2010) studied the RUP2 induction under blue light. Moreover, Rai et al. (2019) showed that together CRYs and UVR8 are essential for transcript accumulation of CHI in the presence of UV-A.
It is noteworthy that CRY1, CRY2, and UVR8 are crucial for survival under natural sunlight (Rai et al. 2019). So, the interactions between phytochrome, cryptochrome, and UVR8 signaling pathways help in UV-B tolerance, which aids plant survival in sunlight in natural conditions (Tissot and Ulm 2020).
10.2.4 Repressor of UV-B Photomorphogenesis 1 (RUP1) and RUP2
RUP1 and RUP2 (REPRESSOR OF UV-B PHOTOMORPHOGENESIS) are two important regulators that affect the UVR8 pathway negatively and are induced by UV-B (Gruber et al. 2010). Under UV-B exposure, UVR8, COP1, and HY5 upsurge the transcription of RUP1 and RUP2. RUPs directly interact with UVR8 and are important for UV responses as well as plant growth. COP1 promotes, while RUPs repress UVR8 accumulation (Qian et al. 2016; Yin et al. 2016). RUPs are vastly homologous to WD40-repeat proteins and both promote UVR8 redimerization (Gruber et al. 2010; Heijde and Ulm 2013). They are also phylogenetically related to COP1 and the SPA proteins and their overexpression leads to early flowering and impedes the inhibition of hypocotyl growth in UV-B absence, irrespective of photoperiods (Wang et al. 2011).
Podolec et al. (2021) suggested that UVR8 redimerization can occur through two stages: First is RUPs outcompete COP1 and other VP-domain interactors, separating them from UVR8, while second is RUPs facilitate UVR8 redimerization. uvr8-17D has been studied to be a UV-B-hypersensitive UVR8 allele allied with a single glycine-101 to serine amino acid alteration. This hypersensitivity is linked with its monomeric conformation in vivo, indicating that redimerization facilitated by RUPs is compromised.
Expression RUPs increase due to the interaction of UVR8-RUP1/RUP2 (Gruber et al. 2010). Heijde and Ulm (2013) indicated a significant role of RUPs in UVR8 redimerization, and this is independent of COP1. Under UV-B irradiated conditions RUPs negatively regulate UV-B signaling by mediating UVR8 redimerization, which further interposes the association of UVR8 with COP1. Additionally, RUP proteins hinder UV-B signaling by degradation of HY5 (Ren et al. 2019). C27 domain mediates binding between RUP1 and RUP2 with UVR8 (Cloix et al. 2012; Yin et al. 2015). It is proposed that C17 inhibits the association of C27 to RUP proteins because the binding between C44 and RUP is not as strong as C27 and RUP. Here, the Val-Pro residues within C27 play a major role in the reformation of UVR8 homodimers (Yin et al. 2015). RUP1 and RUP2 are likely to be a part of a CUL4-DDB1-based E3 ubiquitin ligase that aims HY5 for degradation (Ren et al. 2019). It is also likely that many signaling pathways triggered by environmental signals beyond light perception can affect RUP1 and RUP2, thus involved in further cross-regulation of UVR8-mediated photomorphogenesis and UV-B acclimation (Tissot and Ulm 2020).
10.2.5 MYB (Myeloblastosis) Transcription Factors and Regulation of Root Growth and Development
It is known that UVR8 leads to plant growth reduction under drought conditions (Kliebenstein et al. 2002; Brown et al. 2005). Fasano et al. (2014) made a phenotypic analysis of UVR8-overexpressing plants and found a negative effect on root growth on light exposure. This inhibitory action was due to a decrease in cell enlargement than in cell numbers.
MYB is a group of different transcription factors which interact with UVR8 to modulate lateral root growth as well as cotyledon development (Qian et al. 2020). Yang et al. (2020) studied that UVR8 limits lateral root growth with UV-B irradiation. This is due to the downregulation of the diverse auxin-responsive genes. There exists a binding between UVR8 and the two MYB types (MYB73 and MYB77) under UV-B presence. Both the MYB types are positive regulators that aid in the upregulation of auxin-responsive genes. The interaction between UVR8 and MYB73/77 revokes the DNA-binding ability of the two MYBs and consequently controls the expression of auxin-responsive genes (Yang et al. 2020). Qian et al. (2020) has put light onto the global UV-B-responsive transcriptome and found that MYB13 expression level promptly increased by nucleus-localized UVR8. MYB13 is explicitly expressed in cotyledons and is required for UV-B-responsive cotyledon expansion as well as flavonoid biosynthesis. Here, MYB13 associates with UVR8 under the presence of UV-B and further controls MYB13 DNA binding to its target promoters (Qian et al. 2020). Collectively, UV-activated UVR8 binds with either MYB73/77 in roots or MYB13 in cotyledons. This association of UVR8 and MYB transcription factors leads to transcriptional alterations regulating lateral root growth, cotyledon expansion, as well as stress acclimation. MYB13 directly interacts with the promoter sequences of auxin-responsive genes to regulate gene expression. UVR8–MYB interactions indicate the complexity of the UVR8 interactome. MYB73 and MYB77 have a key role in lateral root growth as well as they also partly regulate hypocotyl elongation.
It is noteworthy that the growth of the lateral roots is governed by auxin. Fasano et al. (2014) studied elevated levels of flavonoids and a reduction in IAA conjugates content in UVR8-overexpressing plants. (Casimiro et al. 2001; Bhalerao et al. 2002; De Smet and Jürgens 2007). Conclusively, more flavonoids are related to a decrease in cell expansion, that later alters polar auxin transport. UVR8 can control the flavonoid concentration as well as auxin movements within roots, which is significant for root growth, and hence connect light and hormone signaling pathways together. MYB12 being an explicit transcription factor for FLAVONOL SYNTHASE (FLS) leads to the increase in the concentration of flavonoids with UV-B irradiation. Moreover, its expression is regulated by HY5 (Stracke et al. 2010). MYB4 is another transcription factor, which suppresses C4H, 4CL, LAR, CHS, and ANR2 to arbitrate UV-B-dependent anthocyanin and phenylpropanoid formation (Schenke et al. 2011; Li et al. 2017). Also, bHLH is an alternative group of the transcription factor playing multiple roles extending from regulation of floral development to flavonoids biosynthesis (Sorensen et al. 2003; Ohno et al. 2011). Moreover, MYB4a is a negative regulator of C4H, 4CL, CHS, LAR, and ANR2 which works by binding to target gene promoters (Li et al. 2017).
10.2.6 The UVR8–BES1 (BRI1-EMS Suppressor1)/BIM1 Pathway
Brassinosteroids are phytohormones, which play role in controlling plant growth and development, like photomorphogenesis and skotomorphogenesis, and in combatting abiotic and biotic stresses (Clouse 2011). The surface receptor kinase BRASSINOSTEROID INSENSITIVE 1 (BRI1) responds to Brassinosteroids and leads to activation of BES1 and BZR1 (BRASSINAZOLE RESISTANT 1) (He et al. 2002; Nam and Li 2002). There exist several mechanisms through which the GSK3-like kinase BIN2 (BR-INSENSITIVE 2) leads to inhibition of BES1 and BZR1 by phosphorylation, in the absence of BR (Li and Jin 2007). BIN2 activity gets inhibited in the presence of BR, and as a result, dephosphorylated BES1 and BZR1 get accumulated inside the nucleus (Belkhadir and Jaillais 2015). This promotes the upregulation of BR target genes (Wang et al. 2002; Yin et al. 2002). BIM1 interacts with BES1 for regulation of BR-induced gene as well as hypocotyl elongation (Yin et al. 2005). Also, UVR8 interacts with BIM1 to modulate transcriptional events (Liang et al. 2018). Additionally, UVR8 binds with the dephosphorylated BES1 (active state) to control BR signaling. Dephosphorylated BES1 can bind with DNA under the influence of BR (Vert and Chory 2006). UVR8 can interact with BES1 homolog BZR1 as well as the long isoform of BES1 (BES1-L), that comprises a supplementary N-terminal nuclear localization signal (Jiang et al. 2015). Also, the interaction of UVR8 and BIM1 leads to inhibition of the BR-responsive genes and decreases the hypocotyl elongation (Sun and Zhu 2018). UVR8 interacts with the C-terminus of BIM1 as well as the BIN2 phosphorylation domain of BES1. UVR8 interacts weakly with the basic helix–loop–helix (bHLH) domain of BIM1, whereas the same interacts strongly with the bHLH domain combined with either the N-terminus or C-terminus of BIM1. BIM1 and dephosphorylated BES1, as they are transcriptional factors, are sited mainly in the nuclear compartment, whereas UVR8 is found in the nucleus due to UV stimulus. Conclusively, the UVR8–BES1/BIM1 complex, in the nucleus stimulated by UV exposure, regulates hypocotyl elongation and photomorphogenesis. This is mediated by inhibition of BR signaling and altogether it helps fine-tune plant growth (Liang et al. 2018).
10.3 Molecular Mechanism of Photoreceptor-Mediated Signaling
Christie et al. (2012) suggested that β-propeller subunits constituting a group of tryptophan residues form a dimer interface which gets stabilized with the help of a complex salt-bridge network. UV-B sensing occurs with the help of a UV-B antenna, which is formed by a Trp pyramid, having Trp 233, Trp 285, and Trp 337, along with Trp 94 (integral tryptophans in UVR8). Post UV-B perception, an excited electron gets transferred from the excitonically coupled Trp pyramid that lies adjacent to arginine(s), leading to charge neutralization, subsequent breaking of cross-dimer salt bridges, and hence dimer destabilization and dissociation. The Trp pyramid becomes critical for UVR8 photoperception, where W285 plays a chief role. Correspondingly, W233 helps maintain excitation coupling for photoreception, while W337 and W94 have secondary roles. For UVR8 photoreception, there exists a conserved sequence of Gly-Trp-Arg-His-Thr repeat forms a “triad” of tryptophan residues: W233, W285, and W337 having a tight packing. The piling of W285 with adjacent R286 is critical for dimerization and connecting UV-B photoreception with salt-bridge formation. To sum up, the extensive packing congregation of the conserved aromatic cluster neighboring the Trp pyramid and the salt bridges zipping the dimer interface imply that UVR8 has evolved a strenuous mechanism for the perception of UV-B and signaling.
Wu et al. (2012) studied and unleashed the mechanism of UVR8-dependent UV-B sensing. The study successfully explained that UV-B perception by UVR8 requires its chromophore with its two tryptophan residues, Trp 285 and Trp 233. These experimental studies and the information on tryptophan fluorescence explain a mechanistic model for UVR8-dependent UV-B perception. The exposure of UV-B leads to excitation of the indole rings of Trp 285 and Trp 233. This excitation is believed to unsettle the P bond over the indole rings and further result to destabilize the intramolecular cation–π interactions. These changes cause distinct conformational alterations to the side chain comprising Arg 286 and Arg 338 that fails to uphold intermolecular hydrogen bonding with Asp or Glu residues from the adjacent UVR8 molecule and leading to UVR8 monomerization. There exists an excited-state proton transfer to the indole rings undergo that makes the indole ring positively charged, leading to destabilization of the cation–π interactions. This results in quenching of intrinsic tryptophan fluorescence and hence results in a slight decrease of fluorescence signal. As proton donors for the same, Trp 233 and Trp 285 lie adjacent to Asp 129, Glu 182, and Arg 234. The reformation of homodimers is possible because there exists no covalent modification of UVR8 on the perception of UV-B.
Voityuk et al. (2014) proposed three steps for the photodissociation mechanism of UVR8 through high-level quantum chemical calculations:
-
1.
On conversion of dimeric UVR8 to monomers, the intrinsic tryptophan residues establish a broad light-harvesting system, where the excited form of Trp 233 undergoes strong electrostatic stabilization by the protein environment.
-
2.
Charge separation leads to fast decay of the locally excited state, which creates the radical ion pair Trp 285(+)-Trp 233(−), with a dipole moment of ≈18 D.
-
3.
The resultant dipole moment destabilizes the salt bridges between the two monomer subunits.
Yin et al. (2015) studied the two distinct domains of UVR8 and suggested that they interact with COP1: the β-propeller domain of UVR8 facilitates association with the WD40-repeats-based predicted β-propeller domain of COP1 on exposure to UV, while the C-terminal C27 domain of UVR8 networks with COP1. UV-B exposure leads to its absorption by Trp residues next to Arg residues and forms salt bridges across the dimer interface. These changes dissociate the UVR8 homodimers by disordering the salt bridges instantly. The resultant UVR8 monomer, along with its seven-bladed β-propeller domain (C27), joins the WD40 domain (a structurally related seven-bladed β-propeller) of COP1. This UVR8–COP1 complex stabilizes HY5 and upsurges the expression of the two RUP genes, leading to a negative feedback loop. HY5, being a basic leucine zipper transcription factor, has a critical role in the process of de-etiolation (Osterlund et al. 2000; Saijo et al. 2003; Yi and Deng 2005). RUP1 and RUP2 are WD40-repeat proteins and hold phylogenetic and structural similarities with COP1. Their association with the C27 domain disrupts the UVR8–COP1 complex promoting the formation of UVR8 dimers again.
RUP1/RUP2-UVR8–COP1 complex forms briefly when RUPs remain associated with UVR8 by its C27 domain, whereas UVR8 and COP1 still remain associated. Also, the differences in the UVR8–COP1 and UVR8-RUP1/RUP2 associations are because of variances in their modes of interaction. The exposed β-propeller surface of UVR8 monomers has the tendency to bind with COP1 but not with RUP proteins. Also, COP1 and RUPs have a discrete capability to associate with the C-terminal. Moreover, COP1 binding needs UV-B activation and UVR8 monomer formation but RUPs can associate with inactivated homodimers as well.
Zeng et al. (2015) crystallized UVR8 (12–381 residues) and studied light-induced structural alterations. They concluded that the two clusters of strong positive and negative difference densities occur at the dimer interface explicitly linked with Trp 285/Trp 233 and a water molecule. The water molecule establishes hydrogen bond at the dimer interface, which includes Trp 285/Arg 286 in one subunit and Asp 96/Trp 94/Asp 107 in another one. Due to strong attraction, Trp 285 and Trp 233 strike and result in a change in conformations around them. This leads to the breakage of inter-subunit interactions at the dimer interface leading to monomerization.
Heilmann and Jenkins (2013) studied and suggested that the UVR8 monomerization is never de novo. Also, redimerization reversal is a complex process and is assisted by numerous factors: occurrence of intact cells, translation due to UV irradiation, and association of UVR8 C-terminal with multiple factors.
10.3.1 DNA Alterations and Damage Repair
UV-B causes harm to DNA by producing two photoproducts, pyrimidine photoproducts (6–4 PP), and, mainly, cyclobutane pyrimidine dimer (CPD). In prokaryotes as well as eukaryotes, the chief repair pathway for CPD and 6–4 PP includes photolyases (Britt 2004). One of the important photolyases being type II photolyase PHR1 can regulate several genes for UV defense as well as damage repair (Brown et al. 2005). On exposure to the wavelength of 350–450 nm, these light-dependent photolyases join with dimers to restore the native DNA by reversal of damage without any error (Jansen et al. 1998).
DNA methylation for gene regulation is known to be a conserved mechanism that can have quintessential roles in transposon silencing, imprinting, development, and environmental responses (Pikaard and Scheid 2014; Schübeler 2015). Jiang et al. (2021) studied that UV-B suppresses DNA methylation through DRM2 and derepresses the dependent reporter genes in UVR8-dependent fashion. UVR8 can interact with DNA methyltransferase DRM2 within the nucleus, mediated by the ubiquitin-associated (UBA) domains of DRM2. UVR8 impedes DRM2 chromatin association and catalytic activity. Taken together, UVR8 is a negative regulator of DRM2 to begin a mechanistic assembly between light signaling and DNA methylation in plants.
10.3.2 Plant Defense
Sinapate is a precursor for syringyl-type lignin synthesis and helps in cell wall synthesis as well as is capable of preventing fungal hyphae penetration within the plant cell (Kishimoto et al. 2006; Quentin et al. 2009; Lloyd et al. 2011). It is suggested that UV-B radiations provide defense against fungal infections in Arabidopsis, and it is most likely that this can be due to increased sinapate levels involving UVR-8 in the process (Demkura and Ballaré 2012).
Jasmonic acid (JA) synthesis, as well as signaling genes transcript buildup, takes place, and that is mediated by UVR8 on UV exposure. The responsible JA biosynthesis genes (Allene Oxide Synthase [AOS], Allene Oxide Cyclase 1 [AOC1], AOC3, and Oxophytodienoate Reductase 3) and JA signaling transcription factors (WRKY70, Jasmonate Zim Domain 1 [JAZ1], Syntaxin-Related Protein 1) play a major role (Morales et al. 2013). This indicates that the UVR8 and JA signaling pathways offer pathogen resistance and defense against herbivores (Izaguirre et al. 2003; Demkura et al. 2010; Demkura and Ballaré 2012).
Volatile terpenoids are biosynthesized through the mevalonate and methylerythritol phosphate (MEP) pathways (Schwab et al. 2008). AACT1 HMGR, HMGS, and DXS, PMK, and MVK from either the MVA or MEP pathway are all boosted by light, jasmonic acid, and ethylene (Hemmerlin et al. 2012). Shamala et al. (2020) studied that all these genes except HGMR-2, PMK-1 DXR, and DXS are upregulated by UV exposure indicating the participation of UVR8. Many volatile terpenoids were found to be greater due to UV exposure (Gil et al. 2012; Liu et al. 2017).
10.4 Inhibition of Plant Shade Avoidance and Thermomorphogenesis
Under scarce sunlight due to neighboring vegetation, plants have evolved shade avoidance responses to compete, enhance growth, and acquire light (Fraser et al. 2016). UVR8 is involved in shade avoidance responses with the help of auxin and gibberellins (GAs). UVR8–COP1 interaction upsurge the levels of HY5 and HYH, leading to augmented GA2ox1 (Gibberellin 2 oxidase) transcript. This decreases GA levels while stabilizing DELLA protein (a negative regulator of GA), and suppresses PIFs (Phytochrome Interacting Factor 4 and Phytochrome Interacting Factor 5) (de Lucas et al. 2008; Feng et al. 2008). DELLAs are repressor proteins of growth and inactivate PIF function (Feng et al. 2008). In a parallel HY5/HYH-independent pathway, photoactivated UVR8 prevents low R:FR-mediated induction of Indole-3-pyruvate monooxygenase genes YUCCA2, YUCCA5, YUCCA8, and YUCCA9 (genes which are involved in auxin biosynthesis and convert indole-3-pyruvic acid (IPA) into indole-3-acetic acid (IAA)) and the auxin-responsive genes IAA29 and GH3.3, so hindering auxin formation. UV-B is responsible for PIF degradation and stabilizes DELLAs to impede the function of PIF (de Lucas et al. 2008). This inhibits PIF from triggering the expression of auxin biosynthesis genes, therefore leading to inhibition of shade avoidance responses (Hayes et al. 2014). UVR8 regulates PIF4 and controls shade avoidance responses as well as thermomorphogenesis (Hayes et al. 2014, 2017). PIF4 is known to be a key positive regulator for thermomorphogenesis response (Koini et al. 2009; Hayes et al. 2017). The UVR8–COP1 complex inhibits PIF4 transcription and also prevents shade-promoting hypocotyl elongation by regulating the protein stability and function of PIF4 and PIF5, which helps in shade avoidance responses (Lorrain et al. 2008). Hayes et al. (2014) suggested that UVR8 activation is linked to PIF degradation. This decreases auxin activity, and further inhibits elongation and leads to suppression of shade avoidance.
UV-B-stabilized HFR1 (LONG HYPOCOTYL IN FAR RED) forms a competitive complex with PIFs to hinder their DNA-binding ability (Hayes et al. 2017; Yin and Ulm 2017). Tavridou et al. (2020) studied that UVR8-mediated inhibition of the COP1 leads to balance HFR1. This suggests that HFR1 is a molecular effector of UVR8 photoreceptor signaling to regulate plant shade avoidance. The stabilized HFR1 inhibits non-degraded PIF4 and PIF5 under UV-B through heterodimer formation and prevents their binding to DNA (Hornitschek et al. 2009), therefore antagonizing the effect of UVR8 on both thermomorphogenesis and shade responses (Hayes et al. 2017; Tavridou et al. 2020).
Moreover, SAS is also inhibited through phytochrome-, cryptochrome-, and UVR8-mediated induction of the bZIP transcription factors HY5 and HYH (Moriconi et al. 2018).
10.4.1 Regulation of Leaf Morphogenesis
UV-B leads to inhibition of leaf growth and shape (Searles et al. 2001). UVR8 is responsible for mediating photomorphogenesis in response to UV-B (Jenkins 2017). Photomorphogenesis is the result of altered gene expression, which is due to signaling events triggered by the perception of UV-B and blue by UVR8 and CRYs, respectively (Jenkins 2017). The epidermis holds a key role in directing leaf growth and shape (Savaldi-Goldstein et al. 2007). Yet, on UV-B exposure, epidermal cell division has been studied to be mostly independent of UVR8.
The regulation of endopolyploidy, which is correlated with increased cell size, entails UVR8 on exposure to UV-B (Wargent et al. 2009). It has been found that UVR8 has a regulatory role in other developmental events. Hence, UVR8 is a key signaling component in regulating important morphogenetic activity in the leaves.
10.4.2 Phototropism
Positive phototropism is a directional growth of plants toward the light, allowing plants to orient the photosynthetic tissues toward the incoming light (Whippo and Hangarter 2006; Preuten et al. 2013; Vandenbussche et al. 2014). Phototropism is important to optimize photosynthesis, increase pollination efficiency, and reproductive success (Serrano et al. 2018). The epidermis plays an important role in UV-B sensing, signaling, and driving a considerable bending response mediated by UVR8. UVR8 is well related to different signaling pathways, including hormonal cascades (Vanhaelewyn et al. 2016). Auxin is a known plant growth promoter that not just causes cell division and cell elongation but also regulates development. It is also noteworthy that auxin works under the control of UVR8 in seedlings (Hayes et al. 2014; Vandenbussche et al. 2014; Fierro et al. 2015).
Phototropism also decides flower position and hence impacts pollination (Serrano et al. 2018). The action spectrum of phototropism was found to be 280–500 nm, indicating the role of UV-A and blue light for the process (Christie and Murphy 2013). Phototropins can perceive not just blue light but also UV-A and UV-B (Briggs and Christie 2002; Guo et al. 2005; Vandenbussche et al. 2014). The UVR8 requires auxin efflux and functional PINOID (PID; Vandenbussche et al. 2014). UVR8 has a leading role in the UV-B-mediated phototropism and controls hormonal pathways, which results in the bending of the stem toward the UV-B. HY5 plays a central role for UV-B-mediated phototropism in seedlings (Vandenbussche et al. 2014), while it was found outmoded with HYH in inflorescence stems. There occurs disparity in the distribution pattern of HY5 and HYH, with high levels found at the irradiated side of stems and extremely low levels at the shaded side of stems (Vanhaelewyn et al. 2019).
10.4.3 Circadian Clock
There exists an inter-relationship between the circadian clock and photomorphogenic UV-B light. Feher et al. (2011) highlighted the involvement of low-intensity, non-damaging UV-B for the light-mediated entrainment of the circadian clock. This involves UVR8 and COP1 in the process, although HY5 and HYH do not contribute. Responsive clock genes that undergo transcriptional activation are needed for UV-B-mediated photomorphogenic circadian rhythm. It is suggested that temporal restriction of low-intensity UV-B responses by the circadian clock is likely to be utilized for saving resources during acclimation and not for increasing stress tolerance. It is noteworthy that, within the roots, red light travels better than blue light to entrain the circadian clock in unexposed tissues (Nimmo 2018).
10.4.4 Flavonoid and Alkaloid Pathways
UVR8 is required for the induction of genes involved in flavonoid and alkaloid pathways (Brown et al. 2005; González Besteiro et al. 2011; Demkura and Ballaré 2012). These genes help in UV protection, the best studied being the UV-B induction of CHS, while other genes help in flavonoid biosynthesis, which have free radical scavenging action, and also work as a sunscreen by absorbing UV radiation (Jenkins et al. 1997). CHS is the first enzyme in the flavonoid biosynthesis pathway. There are distinct phototransduction pathways for UV-B and UV-A/blue light (CRY1) for CHS regulation (Jenkins et al. 1997). Calmodulin antagonist W-7 is responsible for inhibition of UV-B-mediated induction of CHS, but this does not hold true for UV-A/blue light (CRY1) mediated CHS induction (Christie and Jenkins 1996). CHS, FLS, and several other genes belonging to distinct pathways are target genes for HY5 that undergo upregulation by UV-B. Moreover, UV-B leads to stabilization and transcriptional induction of HY5 (Oravecz et al. 2006; Huang et al. 2013). Radiation-mediated responses, including gene expression and phenolics biosynthesis, can get triggered within a few minutes to a few hours (Morales et al. 2013). However, accumulation is dependent on turnover rate, which is slower for phenolics than for gene transcripts.
On UV-B exposure, UVR8 leads to alterations in the concentrations of phenolic compounds in the leaf epidermis and increases the content of epidermal flavonoids (Demkura and Ballaré 2012; Morales et al. 2013). However, the induction of phenolic compounds was mainly done by the blue component of sunlight (Siipola et al. 2015). UV exposure leads to an increased concentration of flavonoid glycosides and hydroxycinnamic acids (HCAs), which are the two chief groups of phenolic compounds with UV-B absorbing features (Burchard et al. 2000). Moreover, UVR8 is a positive regulator of the UV-B induction of kaempferol-3-glucoside, quercetin, and quercetin-3-glucoside (Morales et al. 2013).
Vanhaelewyn et al. (2019) suggested that UV-B irradiation can penetrate the endodermis and pith of the stem, hence reaching radial cell layers where the UVR8 signal induces flavonoid accumulation. Additionally, the UVR8- dependent flavonoid accumulation is a tissue-independent process, indicating that flavonoid synthesis occurs locally (Buer et al. 2007). Intriguingly, genes for flavonoid biosynthesis overlap with the genes responsible for light signaling as well as abiotic stresses (Vandenbussche et al. 2018; Georgii et al. 2017). Also, UVR8–COP1 can regulate some transcriptional factors, like R2R3-MYB, bHLH, and WD40 (MBW ternary complexes), which further regulate multiple enzymatic processes involved in flavonoid biosynthesis (Mano et al. 2007; Zhao et al. 2013; Shamala et al. 2020).
10.4.5 Protection from Photoinhibition and Photooxidative Stress
Oxidative stress triggers the synthesis of antioxidants, like vitamins C and E, carotenoids, and glutathione (Chen and Xiong 2005). Pyridoxine (vitamin B6) is an essential antioxidant which helps in UV-B protection (Brosché et al. 2002; Ulm et al. 2004a, b; Kalbina et al. 2008; Ristilä et al. 2011) and makes use of two proteins for its biosynthesis––Pyridoxine Biosynthesis 1 (PDX1) and PDX2. Ristilä et al. (2011) studied that UV-B exposure to Arabidopsis thaliana leaves leads to the accumulation of PDX1 and vitamin B6. However, at a low fluence, UV-B can regulate PDX1.3 (homolog of PDX1) transcripts.
ELIPs (Early Light Inducible Proteins), the thylakoid proteins, are encoded by light-responsive nuclear genes and lead to tolerance to photoinhibition and photooxidative stress (Adamska et al. 2001). Brown and Jenkins (2008) proposed that the expression of ELIP1 can be controlled by the HY5 and, therefore, it is regulated by the UVR8-dependent UV-B signaling pathway.
10.4.6 Other Pathways
Photosynthetic competence is modulated by UVR8 by regulating the expression of genes, like chloroplastic proteins (SIG5 and ELIP1), in UV-B-dependent manner (Davey et al. 2012). Among them, SIG5 encoding the plastid RNA polymerase sigma factor regulates PsbD (a transcript of PsbD-BLR-P encoding the PSII D2 proteins) (Kanamaru and Tanaka 2004; Brown and Jenkins 2008).
Gibberellins play an important role in enhancing germination and flowering. Also, they promote growth by deactivating growth inhibitor DELLA proteins (Hauvermale et al. 2012). GA2 oxidases regulate the levels of bioactive GA in Arabidopsis by inactivating GA by hydroxylation. GA2 oxidases are target genes for HY5, and hence, UV-B reduces GA levels as well as growth (Ulm et al. 2004a, b; Weller et al. 2009; Hayes et al. 2014). The UVR8-mediated upregulation of the GA signal inhibits DELLA proteins at the irradiated side of the endodermis and cortex. This overlaps with this zone of activity and hence shows a direct control of growth by light.
Increased exposure is not just taken in the context of light capture and photosynthesis but also taken as maximized flower visibility and increased inflorescence temperature, which is considered to be significant factors for plant–pollinator interactions (Serrano et al. 2018). Exposure to UV-B can induce the synthesis of floral volatiles for attracting pollinators (Falara et al. 2013; Amarasinghe et al. 2015) and flavonoid-derived pigments for determining flower color (Khoo et al. 2017; Serrano et al. 2018).
Davey et al. (2012) studied substantial photoinhibition in UVR8, demonstrating that PSII is comparatively more sensitive to UV-B-induced damage. Even low doses of UV-B can also be deleterious for photosynthetic machinery. The defense is offered through UVR8-regulated gene expression. It can be done directly via induction of chloroplastic proteins and indirectly via regulating the phenylpropanoid and other secondary metabolites pathway, photomorphogenesis, and DNA repair. Therefore, UVR8 has a chief role in plant acclimation and distinct responses to UV-B.
Moreover, Raipuria et al. (2021) studied Static Magnetic Field-stimulated tolerance toward UV-B stress during early seedling growth and indicated that nitric oxide could be an important signaling molecule. Also, NOS initiates SMF-triggered NO production in soybean seedlings on exposure to UV-B.
10.5 Conclusion
UV exposure to plants poses several effects on plant growth, development, reproduction, defense, flowering, senescence, and overall metabolism. These effects involve the role of an important photoreceptor called as UVR8. This photoreceptor is a β- propeller protein, which resides in the cytoplasm in its homodimer state. On UV exposure, it monomerizes and moves to the nucleus for various signaling cascades. UVR8 allies with different factors, like COP1, RUP1, RUP2, CRYs, MYB, HY5, HYH, and BES1/BIM1 to give several responses, like photomorphogenesis, circadian rhythms, defense mechanism, flavonoid synthesis, regulation of root growth, flowering induction, and many more. To give the effects, there occur distinct signaling pathways which have been studied very extensively so far, but there are still several areas to explore the detailed mechanism of action.
References
Adamska I, Kruse E, Kloppstech K (2001) Stable insertion of the early light induced proteins in to etioplast membranes requires chlorophyll a. J Biol Chem 276:8582–8587
Ahmad M, Grancher N, Heil M, Black RC, Giovani B, Galland P, Lardemer D (2002) Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiol 129:774–785
Amarasinghe R, Poldy J, Matsuba Y, Barrow RA, Hemmi JM, Pichersky E, Peakall R (2015) UV-B light contributes directly to the synthesis of chiloglottone floral volatiles. Ann Bot 115:693–703
Arongaus AB, Chen S, Pireyre M, Glockner N, Galvao VC, Albert A, Winkler JB, Fankhauser C, Harter K, Ulm R (2018) Arabidopsis RUP2 represses UVR8-mediated flowering in non-inductive photoperiods. Genes Dev 32:1332–1343
Ballaré CL, Mazza CA, Austin AT, Pierik R (2012) Canopy light and plant health. Plant Physiol 160:145–155
Banerjee R, Schleicher E, Meier S, Viana RM, Pokorny R, Ahmad M, Bittl R, Batschauer A (2007) The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J Biol Chem 282:14916–14922
Belkhadir Y, Jaillais Y (2015) The molecular circuitry of brassinosteroid signaling. New Phytol 206:522–540
Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29:325–332
Binkert M, Kozma-Bognar L, Terecskei K, De Veylder L, Nagy F, Ulm R (2014a) UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell 26:4200–4213
Binkert M, Kozma-Bognár L, Terecskei K, De Veylder L, Nagy F, Ulm R (2014b) UV-B- responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell 26:4200–4213
Blumthaler M (1993) Solar UV measurements. Environmental effects of UV (ultraviolet) radiation. Lewis Publishers, Boca Raton
Boccalandro HE (2001) Ultraviolet B radiation enhances a phytochrome B-mediated photomorphogenic response in Arabidopsis. Plant Physiol 126:780–788
Briggs WR, Christie JM (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci 7:204–210
Britt AB (2004) Repair of DNA damage induced by solar UV. Photosynth Res 81:105–112
Brosché M, Schuler MA, Kalbina I, Connor L, Strid Å (2002) Gene regulation by low level UV-B radiation: identification by DNA array analysis. Photochem Photobiol Sci 1:656–664
Brown BA, Jenkins GI (2008) UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol 146:576–588
Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI (2005) A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci U S A 102:18225–18230
Buer CS, Muday GK, Djordjevic MA (2007) Flavonoids are differentially taken up and transported long distances in Arabidopsis. Plant Physiol 145:478–490
Burchard P, Bilger W, Weissenbock G (2000) Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV-A and UV-B radiation in developing rye primary leaves as assessed by ultraviolet-induced chlorophyll fluorescence measurements. Plant Cell Environ 23:1373–1380
Camacho IS, Theisen A, Johannissen LO, Diaz-Ramos LA, Christie JM, Jenkins GI, Bellina B, Barran P, Jones AR (2019) Native mass spectrometry reveals the conformational diversity of the UVR8 photoreceptor. Proc Natl Acad Sci U S A 116:1116–1125
Casati P, Walbot V (2003) Gene expression profiling in response to ultraviolet radiation in maize genotypes with varying flavonoid content. Plant Physiol 132:1739–1754
Casati P, Walbot V (2004) Rapid transcriptome responses of maize (Zea mays) to UV-B in irradiated and shielded tissues. Genome Biol 5:1–19
Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13:843–852
Chen H, Xiong L (2005) Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stresses. Plant J 44:396–408
Christie JM, Jenkins GI (1996) Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 8:1555–1567
Christie JM, Murphy AS (2013) Shoot phototropism in higher plants: new light through old concepts. Am J Bot 100:35–46
Christie JM, Arvai AS, Baxter KJ, Heilmann M, Pratt AJ, O’Hara A, Kelly SM, Hothorn M, Smith BO, Hitomi K, Jenkins GI, Getzoff ED (2012) Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges. Science 335:1492–1496
Cloix C, Jenkins GI (2008) Interaction of the Arabidopsis UV-B specific signaling component UVR8 with chromatin. Mol Plant 1:118–128
Cloix C, Kaiserli E, Heilmann M, Baxter KJ, Brown BA, O’Hara A, Smith BO, Christie JM, Jenkins GI (2012) C-terminal region of the UV-B photoreceptor UVR8 initiates signaling through interaction with the COP1 protein. Proc Natl Acad Sci U S A 109:16366–16370
Clouse SD (2011) Brassinosteroids. In: The Arabidopsis. American Society of Plant Biologists, pp 9
Cluis CP, Mouchel CF, Hardtke CS (2004) The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J 38:332–347
Davey MP, Susanti NI, Wargent JJ, Findlay JE, Quick WP, Paul ND, Jenkins GI (2012) The UV-B photoreceptor UVR8 promotes photosynthetic efficiency in Arabidopsis thaliana exposed to elevated levels of UV-B. Photosynth Res 114:121–131
de Lucas M, Davière JM, Rodríguez-Falcón M, Mariela P, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blázquez MA, Titarenko E, Prat S (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451:480–484
De Smet I, Jürgens G (2007) Patterning the axis in plants-auxin in control. Curr Opin Genet Dev 17:337–343
Demkura PV, Ballaré CL (2012) UVR8 mediates UV-B-induced Arabidopsis defence responses against Botrytis cinerea by controlling sinapate accumulation. Mol Plant 5:642–652
Demkura PV, Abdala G, Baldwin IT, Ballaré CL (2010) Jasmonate-dependent and -independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defence. Plant Physiol 152:1084–1095
Falara V, Amarasinghe R, Poldy J, Pichersky E, Barrow RA, Peakall R (2013) The production of a key floral volatile is dependent on UV light in a sexually deceptive orchid. Ann Bot 111:21–30
Fasano R, Gonzalez N, Tosco A, Piaz FD, Docimo T, Serrano R, Grillo S, Leone A, Inzé D (2014) Role of Arabidopsis UV RESISTANCE LOCUS 8 in plant growth reduction under osmotic stress and low levels of UV-B. Mol Plant 7:773–791
Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, Seidlitz HK, Nagy F, Ulm R (2009) Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J 28:591–601
Feher B, Kozma-BognaR L, Kevei E, Hajdu A, Binkert M, Davis SJ, Schafer E, Ulm R, Nagy F (2011) Functional interaction of the circadian clock and UV RESISTANCE LOCUS 8-controlled UV-B signaling pathways in Arabidopsis thaliana. Plant J 67:37–48
Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S, Schäfer E, Fu X, Fan LM, Deng XW (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451:475–479
Fernández MB, Tossi V, Lamattina L, Cassia RA (2016) Comprehensive phylogeny reveals functional conservation of the UV-B photoreceptor UVR8 from green algae to higher plants. Front Plant Sci 7:1698
Fierro AC, Leroux O, De Coninck B, Cammue BP, Marchal K, Prinsen E, Van Der Straeten D, Vandenbussche F (2015) Ultraviolet-B radiation stimulates downward leaf curling in Arabidopsis thaliana. Plant Physiol Biochem 93:9–17
Fraser DP, Hayes S, Franklin KA (2016) Photoreceptor crosstalk in shade avoidance. Curr Opin Plant Biol 33:1–7
Frohnmeyer H, Staiger D (2003) Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol 133:1420–1428
Fuglevand G, Jackson JA, Jenkins GI (1996) UV-B, UV-A, and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell 8:2347–2357
Galvao VC, Fankhauser C (2015) Sensing the light environment in plants: photoreceptors and early signaling steps. Curr Opin Neurobiol 34:46–53
Georgii E, Jin M, Zhao J, Kanawati B, Schmitt-Kopplin P, Albert A, Winkler JB, Schaffner AR (2017) Relationships between drought, heat and air humidity responses revealed by transcriptome-metabolome co-analysis. BMC Plant Biol 17:1–23
Gil M, Pontin M, Berli F, Bottini R, Piccoli P (2012) Metabolism of terpenes in the response of grape (Vitis vinifera L.) leaf tissues to UV-B radiation. Phytochemistry 77:89–98
González Besteiro MA, Bartels S, Albert A, Ulm R (2011) Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J 68:727–737
Gruber H, Heijde M, Heller W, Albert A, Seidlitz HK, Ulm R (2010) Negative feedback regulation of UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Proc Natl Acad Sci U S A 107:20132–20137
Guo H, Kottke T, Hegemann P, Dick B (2005) The phot LOV2 domain and its interaction with LOV1. Biophys J 89:402–412
Hauvermale AL, Ariizumi T, Steber CM (2012) Gibberellin signaling: a theme and variations on DELLA repression. Plant Physiol 160:83–92
Hayes S, Velanis CN, Jenkins GI, Franklin KA (2014) UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proc Natl Acad Sci U S A 111:11894–11899
Hayes S, Sharma A, Fraser DP, Trevisan M, Cragg-Barber CK, Tavridou E, Fankhauser C, Jenkins GI, Franklin KA (2017) UV-B perceived by the UVR8 photoreceptor inhibits plant thermomorphogenesis. Curr Biol 27:120–127
He JX, Gendron JM, Yang Y, Li J, Wang ZY (2002) The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci U S A 99:10185–10190
Heijde M, Ulm R (2012) UV-B photoreceptor-mediated signaling in plants. Trends Plant Sci 17:230–237
Heijde M, Ulm R (2013) Reversion of the Arabidopsis UV-B photoreceptor UVR8 to the homodimeric ground state. Proc Natl Acad Sci U S A 110:1113–1118
Heilmann M, Jenkins GI (2013) Rapid reversion from monomer to dimer regenerates the ultraviolet-B photoreceptor UV Resistance Locus 8 in intact Arabidopsis plants. Plant Physiol 161(1):547–555
Hemmerlin A, Harwood JL, Bach TJ (2012) A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Progress Lipid Res 51:95–148
Holtkotte X, Ponnu J, Ahmad M, Hoecker U (2017) The blue light-induced interaction of cryptochrome 1 with COP1 requires SPA proteins during Arabidopsis light signaling. PLoS Genet 13:e1007044
Hornitschek P, Lorrain S, Zoete V, Michielin O, Fankhauser C (2009) Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J 28:3893–3902
Huang X, Ouyang X, Yang P, Lau OS, Li G, Li J, Chen H, Deng XW (2012) Arabidopsis FHY3 and HY5 positively mediate induction of COP1 transcription in response to photomorphogenic UV-B light. Plant Cell 24:4590–4606
Huang X, Ouyang X, Yang P, Lau OS, Chen L, Wei N, Deng XW (2013) Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1- SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proc Natl Acad Sci U S A 110:16669–16674
Izaguirre MM, Scopel AL, Baldwin IT, Ballaré CL (2003) Convergent responses to stress: solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol 132:1755–1767
Jansen MAK, Gaba V, Greenberg BM (1998) Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci 3:131–135
Jenkins GI (2009) Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol 60:407–431
Jenkins GI (2014) The UV-B photoreceptor UVR8: from structure to physiology. Plant Cell 26:21–37
Jenkins GI (2017) Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ 40:2544–2557
Jenkins GI, Fuglevand G, Christie JM (1997) Plants and UV-B: responses to environmental change. Cambridge University Press, Cambridge, pp 135–156
Jiang J, Zhang C, Wang X (2015) A recently evolved isoform of the transcription factor BES1 promotes brassinosteroid signaling and development in Arabidopsis thaliana. Plant Cell 27:361–374
Jiang J, Liu J, Sanders D, Qian S, Ren W, Song J, Liu F, Zhong X (2021) UVR8 interacts with de novo DNA methyltransferase and suppresses DNA methylation in Arabidopsis. Nat Plants 7(2):184–197
Kaiserli E, Jenkins GI (2007) UV-B promotes rapid nuclear translocation of the Arabidopsis UV-B-specific signaling component UVR8 and activates its function in the nucleus. Plant Cell 19:2662–2673
Kalbina I, Li S, Kalbin G, Björn LO, Strid Å (2008) Two separate UV-B radiation wavelength regions control expression of different molecular markers in Arabidopsis thaliana. Funct Plant Biol 35:222–227
Kanamaru K, Tanaka K (2004) Roles of chloroplast RNA polymerase sigma factors in chloroplast development and stress response in higher plants. Biosci Biotechnol Biochem 68:2215–2223
Kataria S, Guruprasad KN, Ahuja S, Singh B (2013) Enhancement of growth, photosynthetic performance and yield by exclusion of ambient UV components in C3 and C4 plants. J Photochem Photobiol B 127:140–152
Kataria S, Jajoo A, Guruprasad KN (2014) Impact of increasing ultraviolet-B (UV-B) radiation on photosynthetic processes. J Photochem Photobiol B 137:55–66
Khoo HE, Azlan A, Tang ST, Lim SM (2017) Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res 61:1361779
Kishimoto K, Matsui K, Ozawa R, Takabayashi J (2006) Components of C6-aldehyde-induced resistance in Arabidopsis thaliana against a necrotrophic fungal pathogen, Botrytis cinerea. Plant Sci 170(4):715–723
Kliebenstein DJ, Lim JE, Landry LG, Last RL (2002) Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol 130:234–243
Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA (2009) High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol 19:408–413
Kreslavski VD, Huang X, Semenova G, Khudyakova A, Shirshikova G, Hummatov N, Zharmukhamedov SK, Li X, Allakhverdiev SI, Nie C, Shabala S (2020) Linking sensitivity of photosystem II to UV-B with chloroplast ultrastructure and UV-B absorbing pigments contents in A. thaliana L. phyAphyB double mutants. Plant Growth Regul 91(1):13–21
Lau OS, Deng XW (2012) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17:584–593
Lau K, Podolec R, Chappuis R, Ulm R, Hothorn M (2019) Plant photoreceptors and their signaling components compete for COP1 binding via VP peptide motifs. EMBO J 38:e102140
Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19:731–749
Lee HJ, Ha JH, Kim SG, Choi HK, Kim ZH, Han YJ, Kim JI, Oh Y, Fragoso V, Shin K, Hyeon T (2016) Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots. Sci Signal 9(452):ra106
Li J, Jin H (2007) Regulation of brassinosteroid signaling. Trends Plant Sci 12:37–41
Li J, Yang L, Jin D, Nezames CD, Terzaghi W, Deng XW (2013) UV-B-induced photomorphogenesis in Arabidopsis. Protein Cell 4:485–492
Li M, Li Y, Guo L, Gong N, Pang Y, Jiang W, Liu Y, Jiang X, Zhao L, Wang Y, Xie DY (2017) Functional characterization of tea (Camellia sinensis) MYB4a transcription factor using an integrative approach. Front Plant Sci 8:943
Lian HL, He SB, Zhang YC, Zhu DM, Zhang JY, Jia KP, Sun SX, Li L, Yang HQ (2011) Blue-light-dependent interaction of cryptochrome 1 with SPA1 defines a dynamic signaling mechanism. Genes Dev 25:1023–1028
Liang T, Mei S, Shi C, Yang Y, Peng Y, Ma L, Wang F, Li X, Huang X, Yin Y, Liu H (2018) UVR8 interacts with BES1 and BIM1 to regulate transcription and photomorphogenesis in Arabidopsis. Dev Cell 44:512–523
Liang T, Yang Y, Liu H (2019) Signal transduction mediated by the plant UV-B photoreceptor UVR8. New Phytol 221:1247–1252
Lin R, Ding L, Casola C, Ripoll DR, Feschotte C, Wang H (2007) Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318:1302–1305
Lin L, Dong H, Yang G, Yin R (2020) The C-terminal 17 amino acids of the photoreceptor UVR8 is involved in the fine-tuning of UV-B signaling. J Integr Plant Biol 62(9):1327–1340
Liu B, Zuo Z, Liu H, Liu X, Lin C (2011) Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light. Genes Dev 25:1029–1034
Liu Z, Li X, Zhong FW, Li J, Wang L, Shi Y, Zhong D (2014) Quenching dynamics of ultraviolet-light perception by UVR8 photoreceptor. J Phys Chem Lett 5:69–72
Liu H, Cao X, Liu X, Xin R, Wang J, Gao J, Wu B, Gao L, Xu C, Zhang B, Grierson D (2017) UV-B irradiation differentially regulates terpene synthases and terpene content of peach. Plant Cell Environ 40:2261–2275
Liu CC, Chi C, Jin LJ, Zhu J, Yu JQ, Zhou YH (2018) The bZip transcription factor HY5 mediates CRY1a-induced anthocyanin biosynthesis in tomato. Plant Cell Environ 41:1762–1775
Liu X, Zhang Q, Yang G, Zhang C, Dong H, Liu Y, Yin R, Lin L (2019) Pivotal roles of tomato photoreceptor SlUVR8 in seedling development and UV-B stress tolerance. Biochem Biophys Res Commun 1:177–183
Llewellyn CA, Airs RL (2010) Distribution and abundance of MAAs in 33 species of microalgae across 13 classes. Mar Drugs 8:1273–1291
Lloyd AJ, Allwood JW, Winder CL, Dunn WB, Heald JK, Cristescu SM, Sivakumaran A, Harren FJM, Mulema J, Denby K, Goodacre R, Smith AR, Mur Luis AJ (2011) Metabolomic approaches reveal that cell wall modifications play a major role in ethylene mediated resistance against Botrytis cinerea. Plant J 67:852–868
Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth promoting bHLH transcription factors. Plant J 53:312–323
Mano H, Ogasawara F, Sato K, Higo H, Minobe Y (2007) Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple fleshed sweet potato. Plant Physiol 143:1252–1268
Mao J, Zhang YC, Sang Y, Li QH, Yang HQ (2005) A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc Natl Acad Sci U S A 102:12270–12275
Mathes T, Heilmann M, Pandit A, Zhu J, Ravensbergen J, Kloz M, Fu Y, Smith BO, Christie JM, Jenkins GI, Kennis JT (2015) Proton-coupled electron transfer constitutes the photoactivation mechanism of the plant photoreceptor UVR8. J Am Chem Soc 137:8113–8120
McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M (2007) Changes in biologically- active ultraviolet radiation reaching the Earth’s surface. Photochem Photobiol Sci 6:218–231
Meegahakumbura MK, Wambulwa MC, Li MM, Thapa KK, Sun YS, Möller M, Xu JC, Yang JB, Liu J, Liu BY, Li DZ (2018) Domestication origin and breeding history of the tea plant (Camellia sinensis) in China and India based on nuclear microsatellites and cpDNA sequence data. Front Plant Sci 8:2270
Möglich A, Yang X, Ayers RA, Moffat K (2010) Structure and function of plant photoreceptors. Annu Rev Plant Biol 61:21–47
Morales LO, Brosche M, Vainonen J, Jenkins GI, Wargent JJ, Sipari N, Strid A, Lindfors AV, Tegelberg R, Aphalo PJ (2013) Multiple roles for UV RESISTANCE LOCUS8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar ultraviolet radiation. Plant Physiol 161:744–759
Moriconi V, Binkert M, Costigliolo C, Sellaro R, Ulm R, Casal JJ (2018) Plant Physiol 177:75–81
Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110:203–212
Nawkar GM, Kang CH, Maibam P, Park JH, Jung YJ, Chae HB, Chi YH, Jung IJ, Kim WY, Yun DJ, Lee SY (2017) HY5, a positive regulator of light signaling, negatively controls the unfolded protein response in Arabidopsis. Proc Natl Acad Sci U S A 114:2084–2089
Nimmo HG (2018) Entrainment of Arabidopsis roots to the light: dark cycle by light piping. Plant Cell Environ 41:1742–1748
O’Hara A, Jenkins GI (2012) In vivo function of tryptophans in the Arabidopsis UV-B photoreceptor UVR8. Plant Cell 24:3755–3766
Ohno S, Hosokawa M, Hoshino A, Kitamura Y, Morita Y, Park KI, Nakashima A, Deguchi A, Tatsuzawa F, Doi M, Lida S (2011) A bHLH transcription factor, DvIVS, is involved in regulation of anthocyanin synthesis in dahlia (Dahlia variabilis). J Exp Bot 62:5105–5116
Oravecz A, Baumann A, Máté Z, Brzezinska A, Molinier J, Oakeley EJ, Adám E, Schäfer E, Nagy F, Ulm R (2006) Constitutively photomorphogenic 1 is required for the UV-B response in Arabidopsis. Plant Cell 18:975–1990
Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405:462–466
Paul ND, Gwynn-Jones D (2003) Ecological roles of solar UV radiation: towards an integrated approach. Trends Ecol Evol 18:48–55
Pikaard CS, Scheid OM (2014) Epigenetic regulation in plants. Cold Spring Harbor Persp Biol 6(12):a019315
Podolec R, Ulm R (2018) Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr Opin Plant Biol 45:18–25
Podolec R, Lau K, Wagnon TB, Hothorn M, Ulm R (2021) A constitutively monomeric UVR8 photoreceptor confers enhanced UV-B photomorphogenesis. Proc Natl Acad Sci U S A 118(6)
Preuten T, Hohm T, Bergmann S, Fankhauser C (2013) Defining the site of light perception and initiation of phototropism in Arabidopsis. Curr Biol 23:1934–1938
Qian C, Mao W, Liu Y, Ren H, Lau OS, Ouyang X, Huang X (2016) Dual-source nuclear monomers of UV-B light receptor direct photomorphogenesis in Arabidopsis. Mol Plant 9:1671–1674
Qian C, Chen Z, Liu Q, Mao W, Chen Y, Tian W, Liu Y, Han J, Ouyang X, Huang X (2020) Coordinated transcriptional regulation by the UV-B photoreceptor and multiple transcription factors for plant UV-B responses. Mol Plant 13(5):777–792
Quentin M, Allasia V, Pegard A, Allais F, Ducrot PH, Favery B, Levis C, Martinet S, Masur C, Ponchet M, Roby D, Schlaich NL, Jouanin L, Keller H (2009) Imbalanced lignin biosynthesis promotes the sexual reproduction of homothallic oomycete pathogens. PLoS Pathog 5:e1000264
Rai N, Neugart S, Yan Y, Wang F, Siipola SM, Lindfors AV, Winkler JB, Albert A, Brosché M, Lehto T, Morales LO (2019) How do cryptochromes and UVR8 interact in natural and simulated sunlight? J Exp Bot 70(18):4975–4990
Raipuria RK, Kataria S, Watts A, Jain M (2021) Magneto-priming promotes nitric oxide via nitric oxide synthase to ameliorate the UV-B stress during germination of soybean seedlings. J Photochem Photobiol B 220:112211
Rastogi RP, Incharoensakdi A (2013) UV radiation-induced accumulation of photoprotective compounds in the green alga Tetraspora sp. CU2551. Plant Physiol Biochem 70:7–13
Ren H, Han J, Yang P, Mao W, Liu X, Qiu L, Qian C, Liu Y, Chen Z, Ouyang X, Chen X, Deng XW, Huang X (2019) Two E3 ligases antagonistically regulate the UV-B response in Arabidopsis. Proc Natl Acad Sci U S A 116:4722–4731
Ristilä M, Strid H, Eriksson LA, Strid A, Sävenstrand H (2011) The role of the pyridoxine (vitamin B6) biosynthesis enzyme PDX1 in ultraviolet-B radiation responses in plants. Plant Physiol Biochem 49:284–292
Rizzini L, Favory JJ, Cloix C, Faggionato D, O'Hara A, Kaiserli E, Baumeister R, Schafer E, Nagy F, Jenkins GI, Ulm R (2011) Perception of UV-B by the Arabidopsis UVR8 protein. Science 332:103–106
Rockwell NC, Su Y, Lagarias JC (2006) Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol 57:837–858
Rozema J, Björn LO, Bornman JF, Gaberščik A, Häder DP, Trošt T, Germ M, Klisch M, Gröniger A, Sinha RP, Lebert M (2002) The role of UV-B radiation in aquatic and terrestrial ecosystems—an experimental and functional analysis of the evolution of UV-absorbing compounds. J Photochem Photobiol B 66:2–12
Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, Ma L, Hoecker U, Deng XW (2003) The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev 17:2642–2647
Savaldi-Goldstein S, Peto C, Chory J (2007) The epidermis both drives and restricts plant shoot growth. Nature 446:199–202
Schenke D, Böttcher C, Scheel D (2011) Crosstalk between abiotic ultraviolet-B stress and biotic (flg22) stress signalling in Arabidopsis prevents flavonol accumulation in favor of pathogen defence compound production. Plant Cell Environ 34:1849–1864
Schübeler D (2015) Function and information content of DNA methylation. Nature 517(7534):321–326
Schwab W, Davidovich-Rikanati R, Lewinsohn E, Yishay R (2008) Biosynthesis of plant-derived flavor compounds. Plant J 54:712–732
Searles PS, Flint SD, Caldwell MM (2001) A metaanalysis of plant field studies simulating stratospheric ozone depletion. Oecologia 127:1–10
Serrano AM, Arana MV, Vanhaelewyn L, Ballare CL, Van Der Straeten D, Vandenbussche F (2018) Following the star: inflorescence heliotropism. Environ Exp Bot 147:75–85
Shamala LF, Zhou HC, Han ZX, Wei S (2020) UV-B induces distinct transcriptional re-programing in UVR8-signal transduction, flavonoid, and terpenoids pathways in Camellia sinensis. Front Plant Sci 11:234
Siipola SM, Kotilainen T, Sipari N, Morales LO, Lindfors AV, Robson TM, Aphalo PJ (2015) Epidermal UV-A absorbance and whole leaf flavonoid composition in pea respond more to solar blue light than to solar UV radiation. Plant Cell Environ 38:941–952
Sorensen A, Kro S, Unte US, Huijser P, Dekker K, Saedler H (2003) The Arabidopsis ABORTED MICROSPORES (AMS) gene encodes a MYC class transcription factor. Plant J 33:413–423
Soriano G, Cloix C, Heilmann M, Núñez-Olivera E, Martínez-Abaigar J, Jenkins GI (2018) Evolutionary conservation of structure and function of the UVR8 photoreceptor from the liverwort Marchantia polymorpha and the moss Physcomitrella patens. New Phytol 217:151–162
Spudich JL, Yang C, Jung K, Spudich EN (2000) Retinylidene proteins: structures and functions from archaea to humans. Annu Rev Cell Dev Biol 16:365–392
Stracke R, Favory JJ, Gruber H, Bartelniewoehner L, Bartels S, Binkert M, Funk M, Weisshaar B, Ulm R (2010) The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ 33:88–103
Suesslin C, Frohnmeyer H (2003) An Arabidopsis mutant defective in UV-B light-mediated responses. Plant J 33:591–601
Sun K, Zhu Z (2018) Illuminating the nucleus: UVR8 interacts with more. Trends Plant Sci 23:279–281
Tavridou E, Schmid-Siegert E, Fankhauser C, Ulm R (2020) UVR8-mediated inhibition of shade avoidance involves HFR1 stabilization in Arabidopsis. PLoS Genet 16(5):e1008797
Tilbrook K, Arongaus A B, Binkert M, Heijde M, Yin R, Ulm R (2013a) The UVR8 UV-B photoreceptor: perception, signaling, and response. In: The Arabidopsis. American Society of Plant Biologists, pp 11
Tilbrook K, Arongaus AB, Binkert M, Heijde M, Yin R, Ulm R (2013b) The UVR8 UV-B photoreceptor: perception, signaling and response. Arabidopsis Book 11:e0164
Tilbrook K, Dubois M, Crocco CD, Yin R, Chappuis R, Allorent G, Schmid-Siegert E, Goldschmidt-Clermont M, Ulm R (2016) UV-B perception and acclimation in Chlamydomonas reinhardtii. Plant Cell 28:966–983
Tissot N, Ulm R (2020) Cryptochrome-mediated blue-light signalling modulates UVR8 photoreceptor activity and contributes to UV-B tolerance in Arabidopsis. Nat Commun 11(1):1–10
Tossi V, Lamattina L, Jenkins GI, Cassia RO (2014) Ultraviolet-B- induced stomatal closure in Arabidopsis is regulated by the UV RESISTANCE LOCUS8 photoreceptor in a nitric oxide- dependent mechanism. Plant Physiol 164:2220–2230
Ulm R, Jenkins GI (2015) Q & A: how do plants sense and respond to UV-B radiation? BMC Biol 13:45
Ulm R, Baumann A, Oravecz A, Mate Z, Adam E, Oakeley EJ, Schafer E, Nagy F (2004a) Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc Natl Acad Sci U S A 101:1397–1402
Ulm R, Baumann A, Oravecz A, Máté Z, Ádám É, Oakeley EJ, Schäfer E, Nagy F (2004b) Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc Natl Acad Sci U S A 101:1397–1402
Vandenbussche F, Tilbrook K, Fierro AC, Marchal K, Poelman D, Van Der Straeten D, Ulm R (2014) Photoreceptor- mediated bending towards UV-B in Arabidopsis. Mol Plant 7:1041–1052
Vandenbussche F, Yu N, Li W, Vanhaelewyn L, Hamshou M, Van Der Straeten D, Smagghe G (2018) Plant Sci 268:54–63
Vanhaelewyn L, Prinsen E, Van Der Straeten D, Vandenbussche F (2016) Hormone-controlled UV-B responses in plants. J Exp Bot 67:4469–4482
Vanhaelewyn L, Viczian A, Prinsen E, Bernula P, Serrano AM, Arana MV, Ballare CL, Nagy F, Van Der Straeten D, Vandenbussche FJ (2019) Differential UVR8 signal across the stem controls UV-B-induced inflorescence phototropism. Plant Cell 31:2070–2088
Vert G, Chory J (2006) Downstream nuclear events in brassinosteroid signalling. Nature 441:96–100
Voityuk AA, Marcus RA, Michel-Beyerle ME (2014) On the mechanism of photoinduced dimer dissociation in the plant UVR8 photoreceptor. Proc Natl Acad Sci U S A 111:5219–5224
Wang ZY, Nakano T, Gendron J, He J, Chen M, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T, Chory J (2002) Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2:505–513
Wang W, Yang D, Feldmann KA (2011) EFO1 and EFO2, encoding putative WD-domain proteins, have overlapping and distinct roles in the regulation of vegetative development and flowering of Arabidopsis. J Exp Bot 62:1077–1088
Wang Q, Zuo Z, Wang X, Gu L, Yoshizumi T, Yang Z, Yang L, Liu Q, Liu W, Han YJ, Kim JI (2016) Photoactivation and inactivation of Arabidopsis cryptochrome 2. Science 354:343–347
Wang X, Wang Q, Han YJ, Liu Q, Gu L, Yang Z, Su J, Liu B, Zuo Z, He W, Wang J (2017) A CRY-BIC negative-feedback circuitry regulating blue light sensitivity of Arabidopsis. Plant J 92:426–436
Wang Q, Zuo Z, Wang X, Liu Q, Gu L, Oka Y, Lin C (2018) Beyond the photocycle-how cryptochromes regulate photoresponses in plants? Curr Opin Plant Biol 45:120–126
Wargent JJ, Jordan BR (2013) From ozone depletion to agriculture: understanding the role of UV radiation in sustainable crop production. New Phytol 197:1058–1076
Wargent JJ, Gegas VC, Jenkins GI, Doonan JH, Paul ND (2009) UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation. New Phytol 183:315–326
Weller JL, Hecht V, Vander Schoor JK, Davidson SE, Ross JJ (2009) Light regulation of gibberellin biosynthesis in pea is mediated through the COP1/HY5 pathway. Plant Cell 21:800–813
Whippo CW, Hangarter RP (2006) Phototropism: bending towards enlightenment. Plant Cell 18:1110–1119
Wu D, Hu Q, Yan Z, Chen W, Yan C, Huang X, Zhang J, Yang P, Deng H, Wang J, Deng X (2012) Structural basis of ultraviolet-B perception by UVR8. Nature 484:214–219
Wu M, Strid A, Eriksson LA (2014) Photochemical reaction mechanism of UV-B-induced monomerization of UVR8 dimers as the first signaling event in UV-B-regulated gene expression in plants. J Phys Chem B 118:951–965
Wu Q, Huang B, Niehaus TA, Yang X, Fan J, Zhang RQ (2015) The role of tryptophans in the UV-B absorption of a UVR8 photoreceptor—a computational study. Phys Chem Chem Phys 17:10786–10794
Wu Q, Su N, Zhang X, Liu Y, Cui J, Liang Y (2016) Hydrogen peroxide, nitric oxide and UV RESISTANCE LOCUS8 interact to mediate UV-B-induced anthocyanin biosynthesis in radish sprouts. Sci Rep 6:1–12
Yang Y, Liang T, Zhang L, Shao K, Gu X, Shang R, Shi N, Li X, Zhang P, Liu H (2018) UVR8 interacts with WRKY36 to regulate HY5 transcription and hypocotyl elongation in Arabidopsis. Nat Plants 4:98–107
Yang Y, Zhang L, Chen P, Liang T, Li X, Liu H (2020) UV-B photoreceptor UVR8 interacts with MYB73/MYB77 to regulate auxin responses and lateral root development. EMBO J 39(2):e101928
Yi C, Deng XW (2005) COP1—from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol 15:618–625
Yin R, Ulm R (2017) How plants cope with UV-B: from perception to response. Curr Opin Plant Biol 37:42–48
Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J (2002) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109:181–191
Yin Y, Vafeados D, Tao Y, Yoshida S, Asami T, Chory J (2005) A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 120:249–259
Yin R, Arongaus AB, Binkert M, Ulm R (2015) Two distinct domains of the UVR8 photoreceptor interact with COP1 to initiate UV-B signaling in Arabidopsis. Plant Cell 27:202–213
Yin R, Skvortsova MY, Loubery S, Ulm R (2016) COP1 is required for UV-B induced nuclear accumulation of the UVR8 photoreceptor. Proc Natl Acad Sci U S A 113:4415–4422
Yu X, Liu H, Klejnot J, Lin C (2010) The cryptochrome blue light receptors. Arabidopsis Book 8:e0135
Zeng X, Ren Z, Wu Q, Fan J, Peng PP, Tang K, Zhang R, Zhao KH, Yang X (2015) Dynamic crystallography reveals early signalling events in ultraviolet photoreceptor UVR8. Nat Plants 1:1–6
Zeugner A, Byrdin M, Bouly JP, Bakrim N, Giovani B, Brettel K, Ahmad M (2005) Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function. J Biol Chem 280:19437–19440
Zhao L, Gao L, Wang H (2013) The R2R3-MYB, bHLH, WD40, and related transcription factors in flavonoid biosynthesis. Funct Integr Genomics 13:75–98
Zuo Z, Liu H, Liu B, Liu X, Lin C (2011) Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr Biol 21:841–847
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Laad, P., Patel, P., Guruprasad, K.N. (2022). UVR8 Signaling, Mechanism, and Integration with Other Pathways. In: Kataria, S., Singh, V.P. (eds) UV-B Radiation and Crop Growth. Plant Life and Environment Dynamics. Springer, Singapore. https://doi.org/10.1007/978-981-19-3620-3_10
Download citation
DOI: https://doi.org/10.1007/978-981-19-3620-3_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-3619-7
Online ISBN: 978-981-19-3620-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)