Abstract
Kawasaki disease (KD) is the most common acute coronary vasculitis syndrome that mainly affects genetically susceptible kids under 5 years of age. Aside from the standard diagnostic five criteria, patients with KD may also experience a variety of nonspecific clinical symptoms and signs. Anemia is the most common clinical feature in KD patients. In 2001, the scientists have the discovery of a liver-derived peptide hormone named as hepcidin began revolutionizing the understanding of anemia’s relation to a number of inflammatory diseases, including KD. This chapter focuses on hepcidin-induced iron deficiency’s relation to transient hyposideremia, anemia, and disease outcomes in KD patients, and goes on to suggest possible routes of KD study.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Kawasaki Disease Is the Most Common Vasculitis of the Coronary Arteries in Children
Kawasaki disease (KD) is an acute multisystem vasculitis syndrome that mainly affects genetically susceptible infants and kids under 5 years of age. Although the cause of KD is not clearly known, it is temporarily defined as an infection–immune–genetic pathogenesis for this mysterious disease [1]. Tomisaku Kawasaki is the first doctor to publish 50 cases of KD in English in 1974 [2]. KD shows signs and symptoms such as high fever for several days, conjunctivitis, diffuse mucosal inflammation, polymorphous skin rashes, peeling of the skin on the hands and feet (especially on their tips), and nonsuppurative lymphadenopathy [1]. Inflammation in small- and medium-sized blood vessels, particularly the coronary arteries, may be also manifested in KD patients. The most serious complication in KD is coronary artery lesions (CAL), including myocardial infarction and coronary artery aneurysm. If left untreated, 20% of the affected kids may suffer from a sequelae of vasculitis with coronary aneurysms [3]. Currently, a single dose of 2 mg/Kg intravenous immunoglobulin (IVIG) is the main treatment for coronary artery lesions (CAL) in KD patients [4]. The global prevalence of KD among children is the highest in Japan (218/105) and the lowest (4.7/105) in kids of European descent, while the incidence in Taiwan is 66/105 [1]. During our recent study period, the incidence of KD in Taiwan more than doubled from 28.58 to 60.08 per 100,000 [5]. Therefore, the prevalence of KD in Asia is nearly 10 times higher than in Europe and the USA. However, for clinicians in the pediatric emergency department, the biggest challenge is to identify KD itself in the early stage, because KD shares many of the same clinical signs with other childhood diseases with high fever [6]. Even worse, 20–30% of KD patients do not fully meet the diagnostic criteria and are considered to suffer an incomplete KD, making the diagnosis even more challenging for inexperienced pediatricians [7, 8].
Anemia in Patients with Kawasaki Disease
Aside from the standard diagnostic criteria, patients with KD may also experience a variety of nonspecific clinical symptoms, such as uveitis, aseptic meningitis, abdominal pain, hydrocystic gallbladder, skin rash at the site of BCG-Carinella inoculation, impaired liver function, hypoalbuminemia, and anemia [9,10,11,12]. Among them, anemia is the most common clinical feature in KD patients and is considered to have a longer duration of active inflammation [13,14,15,16]. A study involving 783 people including 441 KD patients and 342 febrile controls showed that hemoglobin level is one of the seven variables with the largest absolute value of the diagnostic coefficient [17]. In addition, Lin et al. observed that hemoglobin is a useful marker for telling KD shock syndrome from toxic shock syndrome in pediatric intensive care units [18]. Although severe hemolytic anemia that requires blood transfusion is rare, it may be related to IVIG infusion [14, 15, 19]. The main cause of hemolysis is usually related to anti-A and anti-B IgM antibodies and anti-Rh IgG antibodies [20]. In fact, the IVIG products used today are generally safe and effective. They consist of at least 98% IgG and very low anti-A (1:8) and anti-B (1:4) IgM antibodies, but no anti-D IgG antibodies [22, 31]. In addition, Rh-negative blood types are much less common in the Asian populations (0.3%) than in the Caucasian populations (15%). The phenomena and literature concerning hemolysis after IVIG in KD patients may thus be more commonly reported in European ancestry than Asian ancestry. We also found that there were no significant differences in total bilirubin and haptoglobin levels between KD patients before and after IVIG treatment [11]. Therefore, we believe that the key etiological link can explain the relationship between KD and anemia.
Anemia associated with inflammation represents a serious, highly common clinical problem [21]. Anemia of chronic disease is usually observed in various inflammatory states, such as infections, inflammatory diseases, and certain cancers [22,23,24,25]. In 2000, Krause et al. described a peptide that was first called liver-expressed antimicrobial peptide-1 or LEAP-1 but later named “hepcidin” due to its liver expression and antibacterial activity [26]. It is understood that hepcidin plays a vital role in preventing the subsequent iron influx into the plasma: duodenal absorption, macrophage release, mobilization of iron stored in liver cells, and inflammatory anemia [21, 27]. In anemia associated with inflammatory diseases such as infections [39, 40], autoimmune diseases [41, 42], severe diseases [43, 44], obesity [28], and acute myocardial infarction [29], abnormally elevated levels of hepcidin have also been observed.
Hepcidin Expression Is Associated with the Prognosis of Kawasaki Disease
We have previously reported that before receiving IVIG treatment, the plasma hepcidin and IL-6 levels of KD patients were higher than those of febrile controls [30]. After IVIG treatment, the levels of hepcidin and IL-6 decreased significantly. Interestingly, changes in hepcidin levels are related to the resistance to IVIG treatment and the formation of CAL, which supports the theory that inflammation markers and increased IVIG anergy may be related to the development of CAL in KD patients [30].
It is proven that IVIG can effectively reduce the incidence of CAL [4], but it is still unclear what role aspirin plays and how much aspirin should be administered on KD patients. In the past few decades, even before IVIG was used, aspirin-related practices have been administered in KD treatment [3]. Moreover, anemia and significant bleeding are associated with the use of aspirin [31]. In a study of 851 patients with KD, we have reported that high-dose aspirin in acute-phase KD does not benefit the disease outcomes but may be harmful in reducing disease inflammation [32]. In addition, this is the first study to show that high-dose aspirin can lower hemoglobin levels and hinder the ability to lower hepcidin levels after IVIG treatment. Therefore, high-dose aspirin may not be an essential part of acute-phase KD treatment. Nevertheless, more randomized placebo-controlled studies are needed to clarify the function of high-dose aspirin in KD.
Iron Deficiency Caused by Hepcidin Is Related to Transient Hypoferremia and Anemia in KD Patients
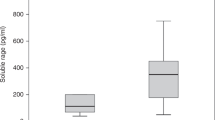
Hepcidin is essential in coordinating iron metabolism and the pathogenesis of inflammatory anemia [33, 34]. After hepcidin interacts with ferroportin, the ferroportin is internalized and degraded, which ultimately leads to a decrease in iron chelation and iron absorption in the cells [35]. Currently, ferroportin is the only known exporter of mammalian iron and is essential for transporting iron from one cell type to another [35]. Hepcidin not only controls the absorption of iron, but also has a direct inhibitory effect on erythropoiesis [36]. Moreover, it is also proved that hepcidin can directly affect the proliferation and survival of erythroid precursors, because the formation of erythrocyte colony [37] is consistent with the observation of transient erythrocytopenia in bone marrow aspiration in patients with KD [38]. In our previous study, hemoglobin levels kept on decreasing significantly after IVIG treatment, showing that myelosuppression in KD patients does not quickly reverse after IVIG treatment. In our age-matched 27 health controls and 117 KD patients, the hemoglobin level increased 3 weeks after IVIG treatment, and the hemoglobin level was completely restored during the 6-month follow-up (Fig. 1). Thus, we recommend that patients with KD do not need to supplement iron.
Comparison of hemoglobin levels in age-matched healthy controls (N = 27) with Kawasaki disease (KD) (N = 117) patients before and after receiving intravenous immunoglobulin therapy. The data are expressed as mean ± standard error. * indicates p < 0.05 between groups (reproduced with permission from https://encyclopedia.pub/3623)
HAMP Promoter Hypomethylation and Elevated Hepcidin Levels Are Biomarkers of Kawasaki Disease
The genome-wide DNA methylation method shows that DNA methylation occurs in the epigenetic regulation mechanism, which is used for the transcriptional inhibition of HAMP in alcohol-related hepatocellular carcinoma [39]. In our previous study, univariate analysis showed that the methylation status of the HAMP promoter was significantly and negatively correlated with plasma hepcidin levels in KD patients and controls [40]. Since DNA methylation is the most famous control mechanism of gene expression, our in vitro research results also show that promoter DNA methylation under epigenetic regulation can be used as a regulatory mechanism for the transcription regulation of HAMP genes. In addition, these findings highlight the epigenetic hypomethylation of the HAMP promoter and the upregulation of hepcidin expression. These results establish a new significance for the epigenetic hypomethylation of HAMP in KD patients and indicate that a significant increase in the level of hepcidin can be used as a biomarker for KD. In 2021, we further demonstrated a novel scoring system, which has good ability to distinguish children with Kawasaki disease from other kids with high fever and highlights the importance of eosinophils in Kawasaki disease. Using this novel scoring system to evaluate factors, including hemoglobin, average red blood cell hemoglobin, and average red blood cell hemoglobin concentration level, can help first-line doctors diagnose and treat Kawasaki disease as soon as possible [41].
Other Studies on Hepcidin in Kawasaki Disease
Macrophages play a vital role in regulating iron homeostasis, which is closely related to polarization during innate immunity. The iron homeostasis of macrophages is related to the functional polarization and plasticity of these cells and plays an extreme role in the process of inflammation, immune regulation, and inflammation regression [42]. According to the Mosser and Edwards model, macrophages are grouped by their functional characteristics into three populations, including host defense (M1), wound healing (M2a), and immune regulation (M2b/c). Under a conceptual framework these three basic macrophage populations can be blended into a large number of different macrophage subpopulations [43]. Polarization characteristics usually refer to the cytokine profile that has been extensively studied in KD patients. However, no studies have yet been made to resolve the exact macrophage polarization in KD. Since iron is an essential growth factor for most bacteria and parasites, they have developed various mechanisms to separate iron from the host. Doing so makes M1-macrophages a major iron storage site under inflammatory conditions [42]. In contrast, M2-macrophages increase ferroportin to promote iron release [44]. However, little is known about whether iron homeostasis affects the ability of the macrophage polarization program and molecular mechanisms involved in the KD disease process.
Conclusion
Inflammation-induced hepcidin can bring about transient hypoironemia, anemia, and disease outcomes in acute-phase KD (Fig. 2, published Int J Mol Sci. 2017 Apr 12; 18 (4): 820. doi: 10.3390/ijms18040820, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5412404/). However, further research is needed to better clarify the role of hepcidin in the pathogenesis of KD.
Proposed mechanism of transient anemia and coronary artery lesions caused by hepcidin in patients with Kawasaki disease. Although the exact cause of Kawasaki disease (KD) is still uncertain, we have reported that KD stimulates the abnormal upregulation of most TLRs, and these TLR arteries upregulate the expression of Hepcidin. After hepcidin interacts with ferroportin, the ferroportin is internalized and degraded, which ultimately leads to the reduction of intracellular iron chelation and duodenal iron absorption. Hepcidin not only controls the absorption of iron, but also has a direct inhibitory effect on erythropoiesis, which can cause transient hypoironemia and anemia in patients with KD. The iron homeostasis of macrophages is related to the functional polarization and plasticity of these cells and makes M1-macrophages a major iron storage site under inflammatory conditions. However, little is known about whether iron homeostasis affects the macrophage polarization program and the ability of the molecular mechanisms involved in the KD disease process. (Reproduced with permission from Int J Mol Sci. 2017 Apr 12; 18 (4): 820. doi: 10.3390/ijms18040820)
References
Wang CL, Wu YT, Liu CA, Kuo HC, Yang KD. Kawasaki disease: infection, immunity and genetics. Pediatr Infect Dis J. 2005;24:998–1004.
Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–6.
Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–7.
Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–9.
Huang YH, Lin KM, Ho SC, Yan JH, Lo MH, Kuo HC. Increased incidence of Kawasaki disease in Taiwan in recent years: a 15 years nationwide population-based cohort study. Front Pediatr. 2019;7:121.
Hao S, Jin B, Tan Z, et al. A classification tool for differentiation of Kawasaki disease from other febrile illnesses. J Pediatr. 2016;176:114–20. e8
McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–99.
Liu YC, Lin MT, Wang JK, Wu MH. State-of-the-art acute phase management of Kawasaki disease after 2017 scientific statement from the American Heart Association. Pediatr Neonatol. 2018;59:543–52.
Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis and Kawasaki disease, council on cardiovascular disease in the young, American Heart Association. Circulation. 2004;110:2747–71.
Tseng HC, Ho JC, Guo MM, et al. Bull’s eye dermatoscopy pattern at bacillus Calmette-Guerin inoculation site correlates with systemic involvements in patients with Kawasaki disease. J Dermatol. 2016;43:1044–50.
Huang YH, Kuo HC, Huang FC, et al. Hepcidin-induced iron deficiency is related to transient anemia and hypoferremia in Kawasaki disease patients. Int J Mol Sci. 2016;17:715.
Kuo HC, Hsu YW, Wu MS, Chien SC, Liu SF, Chang WC. Intravenous immunoglobulin, pharmacogenomics, and Kawasaki disease. J Microbiol Immunol Infect. 2016;49:1–7.
Alves NR, Magalhaes CM, Almeida Rde F, Santos RC, Gandolfi L, Pratesi R. Prospective study of Kawasaki disease complications: review of 115 cases. Rev Assoc Med Bras. 2011;57:295–300.
Fukushige J, Takahashi N, Ueda Y, Ueda K. Incidence and clinical features of incomplete Kawasaki disease. Acta Paediatr. 1994;83:1057–60.
Kuo HC, Wang CL, Liang CD, et al. Persistent monocytosis after intravenous immunoglobulin therapy correlated with the development of coronary artery lesions in patients with Kawasaki disease. J Microbiol Immunol Infect. 2007;40:395–400.
Kuo HC, Yang KD, Liang CD, et al. The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatr Allergy Immunol. 2007;18:354–9.
Ling XB, Lau K, Kanegaye JT, et al. A diagnostic algorithm combining clinical and molecular data distinguishes Kawasaki disease from other febrile illnesses. BMC Med. 2011;9:130.
Lin YJ, Cheng MC, Lo MH, Chien SJ. Early differentiation of Kawasaki disease shock syndrome and toxic shock syndrome in a pediatric intensive care unit. Pediatr Infect Dis J. 2015;34:1163–7.
Nakagawa M, Watanabe N, Okuno M, Kondo M, Okagawa H, Taga T. Severe hemolytic anemia following high-dose intravenous immunoglobulin administration in a patient with Kawasaki disease. Am J Hematol. 2000;63:160–1.
Thorpe SJ. Specifications for anti-a and anti-B in intravenous immunoglobulin: history and rationale. Transfusion. 2015;55(Suppl 2):S80–5.
Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28:671–81. vi
Keel SB, Abkowitz JL. The microcytic red cell and the anemia of inflammation. N Engl J Med. 2009;361:1904–6.
Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23.
Hohaus S, Massini G, Giachelia M, et al. Anemia in Hodgkin's lymphoma: the role of interleukin-6 and hepcidin. J Clin Oncol. 2010;28:2538–43.
Lee SH, Jeong SH, Park YS, et al. Serum prohepcidin levels in chronic hepatitis C, alcoholic liver disease, and nonalcoholic fatty liver disease. Korean J Hepatol. 2010;16:288–94.
Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–50.
Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. 2016;127:2809–13.
del Giudice EM, Santoro N, Amato A, et al. Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J Clin Endocrinol Metab. 2009;94:5102–7.
Sasai M, Iso Y, Mizukami T, et al. Potential contribution of the hepcidin-macrophage axis to plaque vulnerability in acute myocardial infarction in human. Int J Cardiol. 2017;227:114–21.
Kuo HC, Yang YL, Chuang JH, et al. Inflammation-induced hepcidin is associated with the development of anemia and coronary artery lesions in Kawasaki disease. J Clin Immunol. 2012;32:746–52.
Gaskell H, Derry S, Moore RA. Is there an association between low dose aspirin and anemia (without overt bleeding)? Narrative review. BMC Geriatr. 2010;10:71.
Kuo HC, Lo MH, Hsieh KS, Guo MM, Huang YH. High-dose aspirin is associated with anemia and does not confer benefit to disease outcomes in Kawasaki disease. PLoS One. 2015;10:e0144603.
Le NT, Richardson DR. Ferroportin1: a new iron export molecule? Int J Biochem Cell Biol. 2002;34:103–8.
Ward DM, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta. 1823;2012:1426–33.
Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3.
Kim A, Nemeth E. New insights into iron regulation and erythropoiesis. Curr Opin Hematol. 2015;22:199–205.
Dallalio G, Law E, Means RT Jr. Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrations. Blood. 2006;107:2702–4.
Frank GR, Cherrick I, Karayalcin G, Valderrama E, Lanzkowsky P. Transient erythroblastopenia in a child with Kawasaki syndrome: a case report. Am J Pediatr Hematol Oncol. 1994;16:271–4.
Udali S, Guarini P, Ruzzenente A, et al. DNA methylation and gene expression profiles show novel regulatory pathways in hepatocellular carcinoma. Clin Epigenetics. 2015;7:43.
Huang YH, Kuo HC, Li SC, Cai XY, Liu SF, Kuo HC. HAMP promoter hypomethylation and increased hepcidin levels as biomarkers for Kawasaki disease. J Mol Cell Cardiol. 2018;117:82–7.
Tsai CM, Chu CH, Liu X, et al. A novel score system of blood tests for differentiating Kawasaki disease from febrile children. PLoS One. 2021;16:e0244721.
Jung M, Mertens C, Brune B. Macrophage iron homeostasis and polarization in the context of cancer. Immunobiology. 2015;220:295–304.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69.
Corna G, Campana L, Pignatti E, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95:1814–22.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Huang, YH., Kuo, HC. (2022). Anemia in Kawasaki Disease. In: Kuo, HC. (eds) Kawasaki Disease. Springer, Singapore. https://doi.org/10.1007/978-981-19-2944-1_15
Download citation
DOI: https://doi.org/10.1007/978-981-19-2944-1_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2943-4
Online ISBN: 978-981-19-2944-1
eBook Packages: MedicineMedicine (R0)