Abstract
Inflammatory bowel disease (IBD) belongs to the group of diseases characterized by idiopathic inflammation of the gastrointestinal organs. Two basic IBD types are distinguished: ulcerative colitis and Crohn’s disease. The IBD symptoms including vomiting and diarrhea, abdominal pain, rectal hemorrhage, and anemia have a significant negative impact on the general patient’s state of health. More than four million people in the USA and Europe suffer from IBD, while the general incidence of this disease in the developed countries exceeds 0.5% of the population. Besides, IBD is associated with a significant risk of colitis-associated malignancy. In the last decades, considerable progress has been achieved in the IBD therapy due to application of drugs suppressing the local gastrointestinal tract inflammation, such as antibodies to TNF-α (infliximab and adalimumab), corticosteroids, salicylates, etc. At the same time, this strategy, unfortunately, does not result in the repair of the damaged tissues, primarily ulcers of the colon, in many IBD patients. To achieve the mucosa healing and stable remission in IBD patients, novel approaches are required, cell therapy, actively used since the beginning of 2000s, being one of them. In our book chapter, we discuss the advantages and problems of application of mesenchymal stem cells (MSCs) which are most actively used in the cell therapy of IBD. The results of the most important preclinical and clinical studies are covered.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Clinical trials
- Inflammatory bowel disease
- Ulcerative colitis
- Crohn’s disease
- Cell therapy
- Mesenchymal stem cells
- Regenerative medicine
Introduction
Inflammatory bowel disease (IBD) is a group of chronic inflammatory conditions of the gastrointestinal (GI) tract characterized by the augmented immune response of the mucosa. Crohn’s disease (CD) and ulcerative colitis (UC) are the two basic types of IBD. Long-lasting IBD results in GI tract damage. CD may affect any part of the GI tract from the mouth to the anus. The terminal part of the small intestine (ileum) is most frequently affected near the place where it joins the large intestine. CD may manifest itself in the form of “patches,” involving some parts of the GI tract and leaving the other parts intact. The inflammation in CD may spread through the whole colon wall thickness (Sairenji et al. 2017). In UC, only the colon and rectum are affected. The inflammation appears only in the innermost layer of the colon mucosa. It usually starts in the rectum and lower parts of the colon, but may also spread continuously and affect the entire large intestine. IBD shares some symptoms such as persistent diarrhea, abdominal pain, rectal bleeding/bloody stool, weight loss, and fatigue. In some cases, it is difficult to determine whether a patient has CD or UC. Such cases are classified as indeterminate colitis (Guindi and Riddell 2004).
The exact cause of IBD is unknown, but there is an assumption that it results from a defective immune system. The immune system of an IBD patient wrongly reacts to the environmental triggers that cause the GI tract inflammation. Such a wrong reaction of the immune system arises, supposedly, in people with a corresponding family history who inherited genes determining the susceptibility to IBD (Khor et al. 2011).
More than four million people in the USA and Europe suffer from IBD, while the general incidence of this disease in the developed countries exceeds 0.5% of the population. Seventy thousand new IBD cases are diagnosed yearly in the USA only, and in general, the yearly financial burden of IBD in the USA exceeds 31 billion dollars (CCFA 2014; GBD 2020). The majority of patients receive the diagnosis of IBD at the age of less than 35 years. In particular, 80,000 children suffer from IBD in the USA. These lifelong chronic conditions essentially affect the quality of life and medical expenses of patients. Besides, IBD patients are susceptible to the risk of developing of other serious diseases such as colon cancer, thrombosis, and primary sclerosing cholangitis (PSC).
In some cases, surgical removal of the damaged GI parts is required for the therapy of severe IBD forms. However, due to the achieved success of the drug therapy of IBD, it has been generally used in the last decades, with five basic types of drugs (CCFA 2014).
Aminosalicylates such as sulfasalazine, balsalazide, mesalamine, and olsalazine administered per os or rectally reduce the colon wall inflammation and are applied primarily for the UC treatment. At the same time, they are less efficient in the CD treatment.
Corticosteroids, such as prednisone, prednisolone, and budesonide, keep the immune system under control. Therefore, they are efficient in the short-term management of exacerbations. But unfortunately, their side effects include infections, weight gain, sleep disorders, etc.
Immunomodulators, such as azathioprine, 6-mercaptopurine, and methotrexate, influence the immune system activity; they are toxic and usually used to sustain the remission in those patients who do not respond to other drugs, or respond to steroids only.
Antibiotics, such as ciprofloxacin and metronidazole, are of moderate use in treating CD patients with the affected colon or perianal region. In particular, antibiotics are administered in the case of infections, e.g., abscesses.
TNF inhibitors include adalimumab, certolizumab pegol, golimumab, and infliximab. These drugs have a pronounced anti-inflammatory effect and are used in the therapy of patients suffering from severe forms of IBD in the absence of a satisfactory and sound effect from the standard treatment. However, the application of these drugs, regretfully, is not always efficient, as well. In particular, the long-term infliximab administration has shown that up to one-third of patients do not respond to the anti-cytokine therapy, either due to primary resistance to the drug or the development of secondary resistance (Magro and Portela 2010). Besides, severe complications may occasionally emerge, including bacterial, viral, and fungal infections, increased risk of lymphoma, colorectal cancer, and other oncological diseases.
IBD Therapy with MSCs
According to estimates, application of contemporary methods of IBD therapy leads to a 20–30% rate of remission, with a maximum of 50% when using a combinatorial therapy approach (Ocansey et al. 2020). Furthermore, cell therapy has shown to be very effective and extremely promising in treating IBD (Cassinotti et al. 2012; Irhimeh and Cooney 2016; Lopez-Santalla et al. 2020). Therefore, the use of mesenchymal stem cells (MSCs) is of particular interest regarding this approach.
MSCs Properties
MSCs are multipotent stromal cells which may be derived from the bone marrow, adipose tissue, dental pulp, skeletal muscle, etc. (Lei et al. 2006; Tolar et al. 2010; Williams et al. 1999; Zuk et al. 2001; Gronthos et al. 2011). MSCs express molecules of the major histocompatibility complex (MHC) class I at a low level and do not express molecules of MHC class II, hence they may be used in allogeneic transplantation (Prockop 2009; Haider et al. 2011). They constitute a heterogeneous population of cells and are characterized by the expression of specific surface markers including CD73, CD90, and CD105 markers, while lacking the expression of CD14, CD11b, CD79 and Cd19, CD34 and CD45 hemopoietic stem cell–specific markers, as well as CD31 endothelial markers (Lv et al. 2014; Haider 2018). Besides surface marker expression, they show specific adherence to the plastic surface and possess trilineage differentiation potential to adopt adipocyte, osteoblast, and chondrocyte phenotypes (Caplan and Correa 2011; Wang et al. 2018). This criterion of characterization has been set forth by the International Society of Cell Therapy (ISCT) which has significantly helped in harmonizing the nomenclature and biological characterization of the cell preparations being used in the experimental and clinical studies. Their autologous availability and robust nature, therefore, can be genetically manipulated to delivery genes of interest to the target organ for angiomyogenic repair as well as to enhance their therapeutic potential (Jiang et al. 2006; Haider et al. 2008) and reprogramming in to pluripotency (Buccini et al. 2012). They have also been combined with other stem cell types for combinatorial cell therapy approach (Hosseini et al. 2018).
Mechanisms of MSCs’ Action
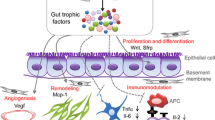
The first and the primary mechanism of MSCs’ action is their transdifferentiation into morphofunctionally competent cell types and achieve the phenotype of interest, which allows the replacement of damaged cells and contribute to the repair and restoration of damaged tissues (cartilage, bones, etc.). The second mechanism of MSCs’ action is associated with the ability of MSCs to move to the sites of damage and inflammation, and secrete cytokines and growth factors, and lipid vesicles rich in bioactive cargo of proteins, lipids, and RNA as part of their paracrine activity to reduce inflammation and restore the damaged tissues (Caplan and Correa 2011; Caplan 2016; Bernardo and Fibbe 2013) (Fig. 1). Besides the abovementioned two primary mechanisms, MSCs also have immunosuppressive and anti-inflammatory effects via the suppression of proliferation and differentiation of T cells (CD4+ and CD8+ lymphocytes), reducing the activity of NK cells and activating Т regulatory cells. In addition, MSCs reduce the secretion of pro-inflammatory cytokines (IL-1β, IL-6, TNFα, and IFN-γ) and boost the secretion of anti-inflammatory IL-4 and IL-10 (Spaggiari et al. 2008; Ghannam et al. 2010). More recently, MSCs surface markers including PDL1, Gal-9, CXCR4 etc., have been implicated as part of the immunosuppressive activity of MSCs (Siyu et al. 2020). Concomitantly, proangiogenic activity of MSCs induces neovascularization regionally at the site of engraftment to restore regional blood flow (Maacha et al. 2020), while apoptosis and oxidative stress are inhibited (Terai and Tsuchiya 2017). Put together, the mechanism of MSCs’ therapeutic benefits is multifactorial as summarized in Fig. 1.
General MSCs’ effects grouped by the two fundamental mechanisms: (1) direct transdifferentiation of the transplanted MSCs (into cells of adipose, bone, cartilage, and muscle tissues) to replace damaged cells and (2) induction of cytokines secreted by MSCs as a part of their paracrine activity into the inflammatory medium, affecting the recipient’s immune system. Abbreviations: (IL-6: Interleukin-6; PGE2: Prostaglandin E2; TGF-β: β-transforming growth factor; IDO: Indoleamine-2,3-dioxygenase; CCL-2: С-С-chemokine ligand 2; IL-10: Interleukin −10; HGF: Hepatocyte growth factor; MMP: Matrix metalloproteinases; HLA-G: Human leukocyte antigen-G)
Preclinical Studies
Yabana et al. demonstrated that in rats with dextran sodium sulfate (DSS)-induced colitis, MSCs, administered intravenously to the animals, migrated to the lamina propria of the damaged colon, where they activated the expression of alpha-smooth muscle actin (α-SMA), which facilitated the restoration of the epithelium (Yabana et al. 2009). It was also shown that MSCs participated in sustaining the epithelial barrier function by the repeated assembly of claudins, apical proteins of tight junctions.
The most critical role in the IBD pathogenesis is evidently played by enhanced proliferation and defective apoptosis of immune cells, which is likely related to the imbalance of Bcl-2 and Bax, essential proteins controlling apoptosis (Dias et al. 2014).
Akiyama et al. showed that systemic infusion of bone marrow–derived MSCs (BM-MSCs) induced apoptosis of T cells via the Fas-ligand (FasL)-dependent pathway and could improve the disease course in experimental murine DSS-induced colitis (Akiyama et al. 2012). However, FasL/MSCs did not induce apoptosis of recipients’ T -cells and could not positively influence the colitis course. It was shown that Fas-regulated secretion of MCP-1 protein by BM-MSCs recruited T cells for FasL-mediated apoptosis. Apoptosis of T cells, in turn, leads to the induction of macrophages producing a high level of TGFβ. This results in an increased number of T-regulatory cells and, finally, in the immune tolerance of the organism.
IBD is also associated with the imbalance in subpopulations of T cells. As a result, the pro-inflammatory cytokines level grows: in CD – due to differentiation of Th1 and Th17 cells, and in UC – due to differentiation of Th2 cells. In contrast, the level of T-regulatory (Treg) cells is depressed in the peripheral blood of IBD patients (Sisakhtnezhad et al. 2017). Among Treg cells, the crucial role in the immune system suppression and sustaining the tolerance belongs to CD4+CD25+FoxP3+ cells (Akiyama et al. 2012).
Chen et al. demonstrated that intravenous MSCs administration significantly reduced the clinical severity of murine UC (weight loss, diarrhea, and inflammation) induced by trinitrobenzene sulfonic acid (TNBS) and enhanced the survival of animals (Chen et al. 2013). It was shown that MSCs reached the damaged colon and facilitated the proliferation of intestinal epithelial cells and differentiation of intestinal stem cells (determined by detecting Lgr5+-cells). Furthermore, it was mediated by suppressing both Th1 and Th17 cell–induced autoimmune and inflammatory reactions (IL-2, TNF-α, IFN-γ, T-bet; IL-6, IL-17, RORγt), as well as by enhanced activity of Th2 cells (IL-4, IL-10, and GATA-3). Besides, it was shown that MSCs induced activated CD4+CD25+Foxp3+ Т-regulatory cells (TGF-β, IL-10, Foxp3).
Macrophages, dendritic cells, and B cells, known as antigen-presenting cells (APC), are also involved in the IBD pathogenesis due to their specialization in presenting an antigen to T cells and the subsequent generation of the T cell response. Macrophages play a critical role in sustaining normal intestinal homeostasis, but they may also participate in the IBD pathogenesis (Han et al. 2021). In a healthy colon, resident macrophages exhibit an M2 phenotype, while inflammatory M1 macrophages dominate in the inflamed intestinal mucosa. In this regard, changing the balance of macrophage population to the M2 phenotype is being adopted as a novel approach in IBD therapy (Ahluwalia et al. 2018). Numerous preclinical studies have shown that MSCs can induce immunomodulating macrophages and macrophages mediate their therapeutic efficiency in experimental UC with an M2-like phenotype (Hidalgo-Garcia et al. 2018).
Jo et al. cocultured immature dendritic cells and lipopolysaccharide (LPS)-treated mature dendritic cells with MSCs for 48 h, and then analyzed the profiles of surface markers and cytokines and the regulatory role of those DC for primary splenocytes (Jo et al. 2018). Besides, the therapeutic effects of MSCs and DC cocultured with MSCs were compared for UC-affected mice. The authors demonstrated that following the coculture of MSCs with immature dendritic cells (MSCs-DC) or LPS-treated mature dendritic cells (LPS + MSCs-DC), the expression of CD11c, CD80, CD86, IL-6, TNF-α, and IFN-γ was significantly decreased. In contrast, the expression of CD11b, IL-10, and TGF-β was elevated. Besides, MSCs-DC and LPS + MSCs-DC induced CD4, CD25, and Foxp3 in primary mice-derived splenocytes. In mice with DSS-induced UC, MSCs and MSCs-DC increased the length of the colon, body weight, and survival, and caused a histological improvement. Moreover, in the MSCs and MSCs-DC groups, the expression of IL-6, TNF-α, and IFN-γ in the colon tissues was also inhibited, while the expression of IL-10, TGF-β, and Foxp3 was elevated. These data assumed that MSCs stimulate differentiation of dendritic cells into regulatory dendritic cells leading to improved chronic colitis therapy.
It was also shown that administration of MSCs could suppress activation and proliferation of B cells secreting IgG and, oppositely, stimulate the formation of CD5+ regulatory B cells (Bregs) producing IL-10. Besides, it was shown that MSCs could depress the proliferation of NK cells secreting pro-inflammatory cytokines (Liu et al. 2020).
MSCs-Derived Exosomes for Experimental IBD Therapy
MSCs-derived exosomes – extracellular vesicles obtained from MSCs – contain a large number of essential factors (Haider and Aramini 2020). In intercellular communication, exosomes are identified as efficient carriers for nucleic acids, functional proteins, lipids, mRNA, and microRNA (Samoylova et al. 2017). Thus, MSCs-derived exosomes, similar to MSCs themselves, have a potent physiological action affecting the damaged tissue repair (Zhao et al. 2019; Haider and Aramini 2020). At the same time, exosomes are more stable than MSCs and in principle are nonimmunogenic.
It was demonstrated earlier by several research groups that exosomes secreted by MSCs had a pronounced regenerative effect in the therapy of many diseases causing tissue damage, including IBD (Mianehsaz et al. 2019; Mendt et al. 2019; Mao et al. 2017). For instance, Mao et al. showed that exosomes released from human umbilical cord–derived MSCs (hUC-MSCs) positively influenced the treatment of DSS-induced colitis and studied the primary mechanism of their effect (Mao et al. 2017). Similarly, exosomes labeled with indocyanine green (ICG) reached the colon tissue of IBD-affected mice 12 h after the injection. The IL-10 gene expression was increased, while the expression of TNF-α, IL-1β, IL-6, iNOS, and IL-7 genes was decreased in the colon and spleen tissues of mice treated with MSCs-derived exosomes. Besides, macrophages infiltration in the colon tissues was significantly reduced. It was also shown that coculturing in vitro with exosomes suppressed the expression of iNOS and IL-7 in macrophages isolated from the peritoneal cavity of normal mice. In addition, the researchers found that IL-7 expression in the colon tissue was higher for colitis patients than healthy participants of the control group. In general, the data obtained have demonstrated a potent effect of hUC-MSCs-derived exosomes on the relief of DSS-induced experimental IBD. The observed effects may be mechanistically mediated via the modulation of IL-7 expression in macrophages at molecular levels.
In a study by Yang et al., exosomes derived from MSCs preconditioned with IFN-γ were transplanted in an experimental mice model of DSS-induced colitis that essentially improved the index of activity and histological assessment of colitis, as well as reduced the fraction of Th17 cells and augmented the fraction of Treg cells (Yang et al. 2020). Molecular studies revealed that the administration of exosomes markedly inhibited the expression of Stat3 and p-Stat3, suppressing differentiation of Th17 cells. Interestingly, treatment with exosomes derived from MSCs preconditioned with IFN-γ showed the highest inhibition. Furthermore, the preliminary treatment with IFN-γ increased the level of miR-125a and miR-125b in MSCs-derived exosomes, which directly targeted Stat3, suppressing differentiation of Th17 cells. Moreover, concomitant infusion of miR-125a and miR-125b also demonstrated a therapeutic effect in colitis, accompanied by a simultaneous decrease in the Th17 cell fraction. In general, this study demonstrated that the IFN-γ treatment enhanced the efficiency of MSCs-derived exosomes in the relief of colitis, owing to increasing the level of miR-125a and miR-125b, which are bound to 3′-UTR of Stat3, to suppress differentiation of Th17 cells.
Clinical Studies
Completed Clinical Studies
Due to their therapeutic properties, MSCs (obtained mainly from the bone marrow and adipose tissue) have been actively used in numerous clinical trials on IBD therapy, with both local injections of cells and intravenous (systemic) infusions (Table 1).
Local MSCs Injections
Local administration of MSCs is used primarily for the therapy of fistulizing (extra-luminal) form of CD (Ko et al. 2021). For example, Panes et al. have conducted a double-blind, randomized, placebo-controlled study (ADMIRE CD Study) to establish the safety and study the long-term efficiency of a single local administration of allogeneic adipose tissue–derived MSCs (Сх601), for the treatment of CD patients with hard-to-treat draining complicated perianal fistulas (Panés et al. 2018). The study was conducted in 49 clinical centers of Europe and Israel. The trials enrolled a total of 212 patients (ClinicalTrials.gov: NCT01541579). The patients were randomly distributed (1:1) into groups, which received either a single local injection of Cx601 (120 × 106 cells) or placebo (control group) in addition to the standard care. The final indices of efficiency, estimated in the modified population of intended to treat (randomly assigned, receiving the treatment, with one or several efficiency estimations after the basic level) at week 52, including a combined remission (closure of all the treated external fistula tracts, draining at the initial level in the absence of accumulations of >2 cm, confirmed by magnetic resonance imaging; MRI) and clinical remission (absence of draining fistulas). Earlier, the same researchers reported a primary endpoint of a study at week 24 (combined remission in 51.5% patients receiving Сх601, compared to 35.6% in the control group, the difference being 15.8%; 97.5% confidence interval 0.5–31.2; P = 0.021) (Panés et al. 2016). At week 52, a significantly larger section of the patients receiving Сх601 reached the combined remission (56.3%, as compared to the control group with 38.6%, 17.7%difference; 95% confidence interval 4.2–31.2; P = 0.010) and the clinical remission (59.2% vs. 41.6% of the control group with the 17.6% difference; 95% confidence interval 4.1–31.1; P = 0.013). The safety was sustained for 52 weeks; side effects were observed in 76.7% of group Сх601 patients and 72.5% of the control group patients.
The researchers concluded that according to the results of the phase 3 study of CD patients with treatment-resistant perianal fistulas, the researchers have concluded that Cx601 is safe and efficient for closures of external fistulas, compared to placebo, in one year of the study. Based on the ADMIRE CD Study results, Darvadstrocel (Alofisel), a medication based on MSCs derived from the adipose tissue, has been developed (Scott 2018). This is the first MSCs-based cell preparation approved in the EU to treat complicated perianal fistulas in adult patients with nonactive/moderately active luminal CD when fistulas do not respond to one or more standard therapies.
Herreros et al. have published the data of a clinical study that assessed 45 patients with 52 surgically resistant anal fistulas of various etiology (of them 18 patients with perianal fistulas caused by CD) (Herreros et al. 2019). The patients’ response to MSCs therapy of different types was monitored, with cells, including allogeneic MSCs from the adipose tissue (ASCs), autologous ASCs, and a stromal vascular fraction (SVF), which were believed to contain ASCs with a minimal addition of adipocytes and erythrocytes.
In 40 out of 42 cases of perianal fistulas (95.2%), either healing or improvement was shown in 6.6 weeks on average (in the observation time of 2–36 weeks). The cure occurred in 22 out of 42 cases (52.4%). Most of the patients were cured in 5.8 months on average (in the observation time of 0.5–24 months). The disease course in the 42 patients was assessed depending upon the applied cell preparations. The degree of cure reached 13/23 (56.5%) for SVF, 3/9 (33.3%) for autologous ASCs, and 6/10 (60%) for allogeneic ASCs. The administered cell dose was also analyzed, with the average value of 43.9 million (in the range of 3–210 million cells) for the cases of cure.
If to focus on perianal fistulas caused by CD, 18/18 patients (100%) demonstrated healing or improvement/partial response, beginning from 5.3 weeks on average (in the observation time of 2–12 weeks). The cure occurred in 10/18 (55.5%) cases. Most of the patients were cured in 6.5 months (in the observation time of 0.5–24 months).
The disease course in those 18 patients was also assessed depending on the cell preparations used. The degree of cure reached 40% for SVF, 66.6% for autologous ASCs, and 55.5% for allogeneic ASCs. The mean administered dose in the cure cases was 43.9 million (in the range of 3–210 million cells). In all the cases of CD-associated perianal fistulas, the surgical technique was applied: the curettage, closing of the fistula tract, and injection of cells (Herreros et al. 2019).
The phase 2 clinical trial on the application of autologous ASCs for CD-associated perianal fistulas with a high rate of recurrence has shown their safety and therapeutic potential with a stable response for 2 years (Cho et al. 2015). In this phase 2 study, 41 patients initially participated. In 24 months, the complete cure was observed in 27 out of 36 patients (75.0%) (the data from 5 patients were absent in 24 months). No ASCs-based treatment-related adverse effects were observed. Moreover, the complete closure of the fistula was stable after the initial treatment. These results also testified that autologous ASCs were efficient in the treatment of CD-associated fistulas.
De la Portilla et al. have conducted an open-label, single-arm clinical trial which included 24 CD patients with perianal fistulas from six hospitals in Spain (de la Portilla et al. 2013). Twenty million ASCs were administered locally in one draining fistula tract. At week 12, if the fistula had not completely closed, 40 million more ASCs were administered. The patients were monitored up to week 24 after the first treatment.
During 6 months of follow-up, no serious adverse events were observed, attesting the treatment as sufficiently safe. At week 24, the number of fistulas was reduced in 69.2% of patients, the complete closure of treated fistulas was observed in 56.3% of the patients, and in 30% of the cases all the fistulas were completely closed. The criteria used to grade the extent of closure were the following: absence of draining and complete re-epithelization, and the MRI-confirmed absence of accumulations. The MRI Score of Severity showed a noticeable reduction at week 24. Thus, the applied ASCs-based therapy appeared safe and fairly efficient for CD-associated perianal fistulas.
A double-blind dose-finding study on the allogeneic BM-MSCs treatment of refractory perianal fistulizing CD was conducted at Leiden University Medical Center in 2012–2014 (Barnhoorn et al. 2020). The study involved 21 patients; three regimes of local MSCs administrations were applied: cohort 1 – five patients, 1 × 107 cells, cohort 2 – five patients, 3 × 107 cells, and cohort 3 – five patients, 9 × 107 cells. The patients were assessed for 4 years, with the registration of clinical events, monitoring the fistula closure, and measuring the level of anti-HLA antibodies, pelvic MRI, and rectoscopy.
The long-term follow-up was performed in 13 patients (four from cohort 1, four from cohort 2, and five from cohort 3). No serious side effects of the therapy were observed. In two patients, malignancies were observed; however, these were reported as unrelated to the cell-based therapy. During 4 years of follow-up, the closure of fistulas was observed in all the cohort 2 patients, in 63% of cohort 1 patients, and in 43% of cohort 3 patients. No anti-HLA antibodies were detected in 24 weeks and 4 years of posttreatment follow-up. The fistula tracts became notably smaller, according to the MRI data. This study demonstrated that local application of BM-MSCs was safe and efficient for fistula closures.
A promising variation of the MSCs treatment for perianal fistulas is the use of a bioabsorbable matrix as a carrier for the cells. A Gore BioA Fistula Plug based on a bioabsorbable material was earlier tried in a multicenter study of high anal fistulas, including those in CD patients (Ommer et al. 2012). The study showed a rather high efficiency of such plugs in the treatment of fistulas; in particular, two out of four study participants with CD had complete healing in 6 months. Another development of the plug technique was its combined use with MSCs. A six-months-long study at Mayo Clinic (ClinicalTrials.gov Identifier: NCT01915927), including 12 patients, was dedicated to the treatment of fistulas with autologous ASCs deposited onto a Gore BioA Fistula Plug (Dietz et al. 2017). ASCs were harvested from the patients for autologous transplantation, and 6 weeks later the fistula plug loaded with autologous ASCs (named MSCs-MATRIX) was placed during a surgical intervention. Before the surgical procedure, ASCs were thawed and cultured on a Gore Bio-A Fistula Plug for 3–6 days in a polypropylene bioreactor. Each plug contained about 20 × 106 cells.
The primary study objective was to establish the safety and efficacy of autologous MSCs-MATRIX in the treatment of recurrent anal fistulas. The criteria for the secondary endpoint of the study were both clinical and radiographical. The former included: (1) partial response, when the drainage and symptoms reported by a patient were notably reduced, and (2) complete healing, when the drainage was not seen either without any action or with a mild pressure in 6 months after the treatment. The latter criterion included the narrowing and shortening of the fistula tract, as well as the absence of an abscess, as visualized by MRI (T2-weighted hyperintense fistula tract on a T2-weighted fast spin echo image). Quantitatively, the MRI results were presented in percent difference from the baseline and using the Van Assche perianal fistula severity score.
The applied MSCs-MATRIX plug for a fistula did not cause any serious effects during the 6 months of observation. Ten of the twelve patients (83%) in 6 months had clinical and radiographic signs of the complete healing. Thus, the bioabsorbable plugs containing MSCs proved themselves safe and efficient for chronic perianal fistulas.
A recently published systematic review and meta-analysis by Cao et al. have estimated the efficiency of stem cells (MSCs derived from the bone marrow and adipose tissue) in the treatment of CD-associated fistulas of any form (Cao et al. 2021). In total, a total of 29 clinical studies involving 1252 patients were included in the review and analyzed. It was shown that the group of patients with CD-associated fistulas, to whom stem cells were transplanted, demonstrated a higher degree of fistula healing as compared to the placebo-treatment group (61.75% vs. 40.46%, or 2.21, 95% CI 1.19–4.11, P < 0.05). The group of patients who received stem cells in the dose of 3 × 107 cells/mL had a 71.0% acceleration of fistula healing vs. the groups of stem cell treatment with other doses (RR 1.3, 95% CI 0.76–2.22). The percentage of cured patients with perianal and trans-sphincteric fistulas was higher than patients with rectovaginal fistulas (77.95% vs. 76.41%). It is of interest that Crohn’s Disease Activity Index (CDAI) and Perianal Disease Activity Index (PDAI) temporarily increased 1 month after stem cell–based therapy; however, they returned to the initial level 3 months after the treatment. Moreover, the incidence of side effects related to the treatment was significantly lower in the MSCs-treated group than in the placebo-treatment group (RR 0.58, 95% CI 0.30–1.14). The conducted study has shown that the application of stem cells, especially ASC, is a promising approach in the treatment of CD-associated fistulas, based on its higher efficiency and lower incidence of adverse events.
Intravenous MSCs Administration
Systemic (intravenous) administration of MSCs is used mainly in the therapy of luminal (inflammatory) forms of IBD (Ko et al. 2021).
In a randomized placebo-controlled clinical trial (ClinicalTrials.gov: NCT01221428), Hu et al. studied the safety and efficiency of hUC-MSCs in treating moderate and severe UC (Hu et al. 2016). Thirty-four UC patients were included in group I and received an MSCs infusion in addition to the basic treatment, while 36 patients in group II received saline in addition to the basic treatment. One-month post-treatment, the incidence of diffuse and deep ulcers and severe inflammatory processes in the mucosa was essentially reduced in 30 patients of group I. During the following observation, the average score of the Mayo scale and the histological score were decreased in group I, while the IBDQ score was significantly improved as compared to before the treatment and group II (P < 0.05). Furthermore, in comparison with group II, no apparent adverse reactions were observed after MSCs infusion in group I patients. Again, no chronic or long-lasting side effects were observed during the entire observation period. Thus the authors demonstrated that MSCs infusion was a safe and efficient strategy to treat UC.
Zhang et al. studied the safety and efficiency of hUC-MSCs to treat CD (Zhang et al. 2018). Eighty-two patients with diagnosed CD who had received the supporting steroid therapy for more than 6 months were included in the study. Forty-one patients were randomly assigned for administering four peripheral intravenous infusions of 1 × 106 hUC-MSCs/kg, one infusion per week. The patients were observed in the dynamics for up to 12 months. CDAI, Harvey-Bradshaw Index (HBI), and the dosage of corticosteroids were evaluated. As a result of the treatment, CDAI, HBI, and the dosage of corticosteroids decreased by 62.5 ± 23.2, 3.4 ± 1.2, and 4.2 ± 0.84 mg/day, respectively, in the hUC-MSCs group, and by 23.6 ± 12.4, 1.2 ± 0.58, and 1.2 ± 0.35 mg/day, respectively, as compared to the control group (р < 0.01, p < 0.05, and p < 0.05 for the hUC-MSCs group vs. the control, respectively). Four patients developed fever after the cell-based infusion. No serious adverse events were observed. The researchers concluded that hUC-MSCs were efficient in CD treatment, though occasionally may cause mild side effects.
In one of our studies, 22 patients with exacerbation of moderate and severe UC were treated with allogeneic BM-MSCs (Knyazev et al. 2016). The patients were divided into two groups. Patients of group I (n = 12), in addition to the standard anti-inflammatory therapy, received MSCs according to the following protocol: 0 (first infusion), week 1, and week 26, followed by every 6 months for the subsequent years of observation. Patients of group 2 (n = 10) received the standard anti-inflammatory therapy with 5-aminosalicylic acid (5-АSA) and glucocorticosteroids (GCS). Of group I patients, 58.3% had a severe UC exacerbation, and 41.7% had moderate UC exacerbation; in group II, the severe and moderate UC patients constituted 60% and 40%, respectively. Total colitis was established in 33.3% of group I patients and in 40% of group II patients; left-sided colitis was observed in 66.7% and 60% patients, respectively. The efficiency criterion for the therapy was a no-relapse course of the disease for 12 months. The UC clinical activity was estimated by the Rahmilevich score, endoscopic activity – by the Mayo score. The control over the dynamics of clinical, laboratory, and endoscopic indices was performed in 2, 6, and 12 months, then yearly for 3 years. During the first year of observations, in group I, a UC relapse occurred in two patients (16.7%), in group II – in three patients (30%). The relative risk (RR) was 0.3 (95% CI 0.08–1.36; p = 0.2; χ2 = 1.47). The Rahmilevich Clinical Activity Index was 3.33 ± 0.54 points in group I, 4.4 ± 1.13 points in group 2 (р = 0.81), the Mayo score was 3.1 ± 0.85 and 3.9 ± 1.06, respectively (р = 0.66). In 2 years of observation, the risk of a UC relapse in group I was three times lower than that in group 2 (р = 0.03). The average duration of remission in group I was 22 months, in group II – 17 months (p = 0.049). In 3 years of observation, the duration of remission was 22 and 20 months, respectively (p = 0.66). The Rahmilevich Clinical Activity Index was 4.75 ± 1.13 points in group I, 8.1 ± 1.1 points in group II (р = 0.001). In conclusion, the study reliably demonstrated that MSCs infusions enhanced the efficiency of anti-inflammatory therapy in patients with the acute UC.
In another study (Lazebnik et al. 2010), we used intravenous administration of allogeneic BM-MSCs to treat 39 UC patients and 11 CD patients (with the control groups of 30 UC patients and 10 CD patients). A statistically significant decrease in the indices of the clinical and morphological activities of the inflammatory process was noted after the MSCs transplantation in 39 patients with UC and in 11 patients with CD as compared to the control groups. A clinico-morphological remission occurred in 40 patients (80%). In addition, the use of MSCs made it possible to discontinue GCS in 34 out of 50 patients (68%) with the hormone-dependent and hormone-resistant forms of UC and CD and reduced the dose of prednisolone to 5 mg/day in 7 patients, with administering 5-ASA only.
Our later study estimated the efficiency of BM-MSCs therapy of CD patients receiving azathioprine (AZA) (Knyazev 2018a). The study included 34 patients with the inflammatory (luminal) CD form. Group I (n = 15) received an anti-inflammatory therapy with the use of MSCs culture in combination with AZA. Group II (n = 19) received MSCs without AZA. The severity of attack was estimated in CDAI points. The blood serum was studied, including immunoglobulins (IgA, IgG, IgM), interleukins (IL) 1β, 4, and 10, tumor necrosis factor-α (TNF-α), interferon-γ (INF-γ), transforming growth factor-1β (TGF-1β), С-reactive protein (CRP), thrombocytes, and erythrocyte sedimentation rate (ESR) in 2, 6, and 12 months from the MSCs therapy beginning. The initial mean CDAI was 337.6 ± 17.1 points in group I and 332.7 ± 11.0 points in group II (p = 0.3). In both groups, a significant decrease in CDAI was noted in two and 6 months from the therapy beginning: in 2 months – to 118.9 ± 12.4 in group I and to 120.3 ± 14.1 in group II (p = 0.7), in 6 months – to 110.3 ± 11.1 in group I and to 114.3 ± 11.8 in group II (p = 0.8). In 12 months, the CDAI was 99.9 ± 10.8 in group I and 100.6 ± 12.1 in group II (p = 0.8); in 24 months – 133.2 ± 28.3 in group I and 120.8 ± 15.5 in group II (p = 0.2); in 36 months – 139.9 ± 23.4 and 141.7 ± 20.8 (p = 0.9) in group I and II, respectively. The IgA, IgG, and IgM levels were significantly lower in the group of patients with a more extended history of the disease and prolonged use of AZA. After the MSCs infusions, in both groups, we observed a tendency to the increase in the pro- and anti-inflammatory cytokines, with a significantly lower level of pro-inflammatory cytokines (INF-γ, TNF-α, and IL-1β) in group I. The latter indicates potentiation of the immunosuppressive effect of MSCs and AZA, which provides a more pronounced anti-inflammatory effect. Moreover, it has been demonstrated that MSCs transplantation stimulates elevation of the initially reduced concentration of immunoglobulins and cytokines in the blood serum and restoring their balance with the setting-in of the clinical remission.
Interesting results were obtained when comparing the effects of combined (local and systemic) administration of BM-MSCs, anti-cytokine therapy (infliximab, IFL) and antibiotic, and immunosuppressive therapy on the healing of CD-associated simple perianal fistulas (Knyazev 2018b). The first group of CD patients aged from 19–58 years (Ме – 29; n = 12) received MSCs systemically according to a scheme and locally. The second group aged from 20–68 years (Ме – 36, n = 10) received IFL according to a scheme. The third group aged from 20–62 years (Ме – 28, n = 14) received antibiotics and immunosuppressants. According to the study results, in 12 weeks the cure of simple fistulas was noted in eight patients (66.6%) of group I, in six patients (60%) of group II, and in one patient (7.14%) of group III. In 6 months, the simple fistulas were still healed in eight patients (66.6%) of group I, in six patients (60%) of group II, and in one patient (7.14%) of group III. In 12 months, the healing was sustained in seven patients (58.3%) of group I, in six patients (60%) of group II, and in two patients (14.3%) of group III. During 24 months follow-up, the closure of fistulas was sustained in five patients (41.6%) of group I, in four patients (40%) of group II, and no patient (0%) in group III. In conclusion, it was demonstrated that combined cell and anti-cytokine therapy of CD with perianal lesions reliably provided more frequent, sustained, and prolonged closure of simple fistulas, as compared to antibiotic and immunosuppressive therapy, and reduction of the relapse incidence as well (Fig. 2).
In their recent publication, Ko et al. have provided an extensive analysis of the safety and efficiency of MSCs-based cell therapy of IBD involving on 24 studies, in 17 of which MSCs were administered locally while in the remaining 7 studies MSCs were administered systemically (Ko et al. 2021). The authors concluded that local MSCs injection-based protocol for fistulizing (extra-luminal) CD form demonstrated long-term efficiency, with the good safety level. However, regarding the efficiency of systemic MSCs infusion, the evidence was ambiguous, in the authors’ opinion. They noted the marked methodological heterogeneity of the studies (first of all, due to different MSCs sources), along with the absence of facts confirming that MSCs reach the colon after an intravenous injection, and found that the safety profile was not always clearly demonstrated. At the same time, in our studies mentioned above, unequivocal pieces of evidence have been obtained for the efficiency of systemic allogeneic MSCs infusions in the IBD therapy (Lazebnik et al. 2010; Knyazev et al. 2016; Knyazev et al. 2018a, b).
In a larger and a more extensive study with a 5-year follow-up, we compared the safety profile of BM-MSCs and a standard treatment using 5-ASA, GCS, and immunosuppressive agents (Knyazev et al. 2015). The study included 103 IBD patients (56 UC patients and 47 CD patients) who received the MSCs therapy and 208 patients receiving the standard anti-inflammatory therapy (but not anti-TNF therapy). All the participants were similar in their demographic characteristics and disease features. No differences were found in the development of acute posttransfusion toxicity, infectious complications, exacerbation of chronic inflammatory diseases, serious infectious complications, malignancy, and death, with the exception of fever in some patients treated with MSCs. Thus, cell-based therapy was considered safe for the clinical practice.
Proceeding Clinical Studies
Currently (by March 2021), 14 proceeding clinical trials involving MSCs-based cell therapy for the IBD treatment have been registered at Clinicaltrials.gov (Table 2). Included in these clinical studies are autologous MSCs-based cell therapy (two studies) and allogeneic MSCs-based cell therapy (12 studies). BM-MSCs are used in seven studies, MSCs derived from the adipose tissue will be used in five studies, one study will use MSCs derived from the umbilical cord blood, while one study will use Wharton’s jelly–derived cells. Ten clinical trials are dedicated to the treatment of CD, while the other four trials will focus UC treatment. Local MSCs administration protocol will be used in 12 studies while systemic administration will be used in the other two studies.
The mentioned above Mayo Clinic study of MSCs-impregnated plugs for perianal fistulas (Dietz et al. 2017) has a very promising development with young patients (Pediatric MSCs-AFP Sub-Study for Crohn’s Fistula, NCT03449069). A single dose of 20 million autologous MSCs is suggested to use in five pediatric patients aged from 12–17 with CD-associated perianal fistulas. The treatment will begin with a standard therapy of infection drainage and placement of a draining seton. In 6 weeks after the placement of a draining seton, it will be removed and replaced with a fistula plug (MSCs-coated Gore Bio-A Fistula Plug). The follow-up period will be 24 months, with the treatment safety and the fistula response being monitored.
Conclusion
The numerous open and randomized clinical studies on MSCs in the IBD therapy have unequivocally shown the safety of this approach and its potential efficiency, including the traditional treatment-resistant cases. The therapeutic action of MSCs originates from the potent immunomodulating effect resulting in the reduction of the autoimmune inflammation and stimulation of reparative processes in the intestinal mucosa. In turn, it prolongs the duration of remission, decreases the risk of relapses, and the frequency of hospital admissions. Based on the conducted clinical trials, a first medication based on allogeneic MSCs derived from the adipose tissue, Darvadstrocel (Alofisel, Takeda), has been approved in the EU for the therapy of complicated perianal fistulas in patients with luminal CD. A promising approach in the treatment of fistulizing CD is the use of biomaterials as carriers for MSCs (fistula plugs coated with MSCs). Firstly, the donor cell survival is higher on a biomaterial. Secondly, the application of autologous MSCs enhances the therapeutic effect of fistula plugs.
However, presently there is no single established optimal protocol for MSCs transplantation in IBD therapy. Additional studies are warranted on the optimal MSCs source, dosage, delivery method, and optimal treatment frequency. Despite the achievement of positive results, further preclinical and clinical studies are required to enhance the efficiency of both local and systemic MSCs transplantation. Along with BM-MSCs and ASC, the use of MSCs from the placenta appears promising. With the techniques enhancing the efficacy of MSCs production, such as 3D culturing and application of large-volume bioreactors, it may essentially lower the price of MSCs production and make this unique therapy available for a wide circle of patients.
Abbreviations
- 5-АSA:
-
5-Aminosalicylic acid
- APC:
-
Antigen-presenting cells
- ASC:
-
Adipose tissue–derived stem cells
- AZA:
-
Azathioprine
- BM:
-
Bone marrow
- CCL-2:
-
С-С-chemokine ligand 2
- CD:
-
Crohn’s disease
- CDAI:
-
Crohn’ Disease Activity Index
- CI:
-
Confidence interval
- DC:
-
Dendritic cells
- DSS:
-
Dextran sodium sulfate
- GCS:
-
Glucocorticosteroids
- GI:
-
Gastrointestinal
- HBI:
-
Harvey-Bradshaw Index
- HGF:
-
Hepatocyte growth factor
- HLA-G:
-
Human leukocyte antigen-G
- hUC:
-
Human umbilical cord
- I/A:
-
Intra-arterial
- I/V:
-
Intravenous
- IBD:
-
Inflammatory bowel disease
- IBDQ:
-
Inflammatory Bowel Disease Questionnaire
- ICG:
-
Lindocyanine green
- IDO:
-
Indoleamine-2,3-dioxygenase
- IFN:
-
Interferon
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- LPS:
-
Lipopolysaccharide
- MHC:
-
Major histocompatibility complex
- miR:
-
MicroRNA
- MMP:
-
Matrix metalloproteinases
- MSCs:
-
Mesenchymal stem cells
- NK-cells:
-
Natural killer cells
- PDAI:
-
Perianal Disease Activity Index
- PGE2:
-
Prostaglandin E2
- PSC:
-
Primary sclerosing cholangitis
- RR:
-
Relative risk
- SMA:
-
Smooth muscle actin
- SVF:
-
Stromal vascular fraction
- TGF-β:
-
β-transforming growth factor
- TNBS:
-
Trinitrobenzenesulfonic acid
- TNF:
-
Tumor necrosis factor
- UC:
-
Ulcerative colitis
References
Ahluwalia B, Moraes L, Magnusson MK, Öhman L (2018) Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol 53:379–389. https://doi.org/10.1080/00365521.2018.1447597
Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T et al (2012) Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 10(5):544–555. https://doi.org/10.1016/j.stem.2012.03.007
Barnhoorn MC, Wasser MNJM, Roelofs H, Maljaars PWJ, Molendijk I, Bonsing BA, Oosten LEM et al (2020) Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J Crohns Colitis 14(1):64–70. https://doi.org/10.1093/ecco-jcc/jjz116
Bernardo ME, Fibbe WE (2013) Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13(4):392–402. https://doi.org/10.1016/j.stem.2013.09.006
Buccini S, Haider KH, Ahmed RPH, Jiang S, Ashraf M (2012) Cardiac progenitors derived from reprogrammed mesenchymal stem cells contribute to angiomyogenic repair of the infarcted heart. Basic Res Cardiol 107(6):301–314
Cao Y, Su Q, Zhang B, Shen F, Li S (2021) Efficacy of stem cells therapy for Crohn’s fistula: a meta-analysis and systematic review. Stem Cell Res Ther 12(1):32. https://doi.org/10.1186/s13287-020-02095-7
Caplan AI (2016) MSCss: the sentinel and safe-guards of injury. J Cell Physiol 231(7):1413–1416. https://doi.org/10.1002/jcp.25255
Caplan AI, Correa D (2011) The MSCs: an injury drugstore. Cell Stem Cell 9(1):11–15. https://doi.org/10.1016/j.stem.2011.06.008
Cassinotti A, Passamonti F, Segato S (2012) Cell therapy in inflammatory bowel disease. Pharmacol Res 163:2021,105247. https://doi.org/10.1016/j.phrs.2020.105247
CCFA (The Crohn’s & Colitis Foundation of America) (2014) The facts about inflammatory bowel diseases. https://www.crohnscolitisfoundation.org/sites/default/files/2019-02/Updated%20IBD%20Factbook.pdf
Chen QQ, Yan L, Wang CZ, Wang WH, Shi H, Su BB, Zeng QH et al (2013) Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol 19(29):4702–4717. https://doi.org/10.3748/wjg.v19.i29.4702
Cho YB, Park KJ, Yoon SN, Song KH, Kim DS, Jung SH, Kim M et al (2015) Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med 4(5):532–537. https://doi.org/10.5966/sctm.2014-0199
de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A (2013) Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Color Dis 28(3):313–323. https://doi.org/10.1007/s00384-012-1581-9
Dias CB, Milanski M, Portovedo M, Horita V, Ayrizono Mde L, Planell N, Coy CS et al (2014) Defective apoptosis in intestinal and mesenteric adipose tissue of Crohn's disease patients. PLoS One 9(6):e98547. https://doi.org/10.1371/journal.pone.0098547
Dietz AB, Dozois EJ, Fletcher JG, Butler GW, Radel D, Lightner AL, Dave M et al (2017) Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology 153(1):59–62.e2. https://doi.org/10.1053/j.gastro.2017.04.001
GBD 2017 Inflammatory Bowel Disease Collaborators (2020) The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 5(1):17–30. https://doi.org/10.1016/S2468-1253(19)30333-4
Ghannam S, Bouffi C, Djouad F, Jorgensen C, Noël D (2010) Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther 1(1):2. https://doi.org/10.1186/scrt2
Gronthos S, Arthur A, Bartold PM, Shi S (2011) A method to isolate and culture expand human dental pulp stem cells. Methods Mol Biol 698:107–121. https://doi.org/10.1007/978-1-60761-999-4_9
Guindi M, Riddell RH (2004) Indeterminate colitis. J Clin Pathol 57(12):1233–1244. https://doi.org/10.1136/jcp.2003.015214
Haider KH (2018) The aging stem cells and cardiac reparability: lesson learnt from clinical studies is that old is not always gold. Regen Med 13(4):457–475
Haider KH, Aramini B (2020) Mircrining the injured heart with stem cell-derived exosomes: an emerging strategy of cell-free therapy. Stem Cell Res Ther 11(1):23. https://doi.org/10.1186/s13287-019-1548-7
Haider KH, Jiang S, Niagara MI, Ashraf M (2008) IGF-I over expressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circ Res 103:1300–1308
Haider KH, Igura K, Ashraf M (2011) Revascor, an injectable formulation of allogeneic, adult mesenchymal precursor cells for the treatment of cardiovascular diseases. Curr Opin Mol Ther 2011
Han X, Ding S, Jiang H, Liu G (2021) Roles of macrophages in the development and treatment of gut inflammation. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2021.625423
Herreros MD, Garcia-Olmo D, Guadalajara H, Georgiev-Hristov T, Brandariz L, Garcia-Arranz M (2019) Stem cell therapy: a compassionate use program in perianal fistula. Stem Cells Int 6132340. https://doi.org/10.1155/2019/6132340
Hidalgo-Garcia L, Galvez J, Rodriguez-Cabezas ME, Anderson PO (2018) Can a conversation between mesenchymal stromal cells and macrophages solve the crisis in the inflamed intestine? Front Pharmacol 9:179. https://doi.org/10.3389/fphar.2018.00179
Hosseini SM, Sani M, Haider KH, Dorvash MR, Ziaee SM, Karimi A (2018) Concomitant use of mesenchymal stem cells and neural stem cells for treatment of spinal cord injury: a combo cell therapy approach. Neurosci Lett 668:138–146
Hu J, Zhao G, Zhang L, Qiao C, Di A, Gao H, Xu H (2016) Safety and therapeutic effect of mesenchymal stem cell infusion on moderate to severe ulcerative colitis. Exp Ther Med 12(5):2983–2989. https://doi.org/10.3892/etm.2016.3724
Irhimeh MR, Cooney J (2016) Management of inflammatory bowel disease using stem cell therapy. Curr Stem Cell Res Ther 11(1):72–77. https://doi.org/10.2174/1574888x10666150728121738
Jiang S, Haider KH, Niagara MI, Salim A, Ashraf M (2006) Supportive interaction between cell survival signaling and angio-competent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res 99:776–784
Jo H, Eom YW, Kim HS, Park HJ, Kim HM, Cho MY (2018) Regulatory dendritic cells induced by mesenchymal stem cells ameliorate dextran sodium sulfate-induced chronic colitis in mice. Gut Liver 12(6):664–673. https://doi.org/10.5009/gnl18072
Khor B, Gardet A, Xavier RJ (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474(7351):307–317. https://doi.org/10.1038/nature10209
Knyazev OV, Parfenov AI, Konoplyannikov AG, Kagramanova AV, Churikova AA, Ruchkina IN, Lishinskaya AA et al (2015) Safety of mesenchymal stem cells therapy in patients with inflammatory bowel diseases – 5 year follow-up. J Biotechnol Biomater 5:192. https://doi.org/10.4172/2155-952X.1000192
Knyazev OV, Parfenov AI, Konoplyannikov AG, Boldyreva ON (2016) Use of mesenchymal stem cells in the combination therapy of ulcerative colitis. Ter Arkh 88(2):44–48. https://doi.org/10.17116/terarkh201688244-48
Knyazev OV, Kagramanova AV, Fadeeva NA, Lishchinskaya AA, Boldyreva ON, Noskova KK, Gudkova RB et al (2018a) Mesenchymal stromal cells of bone marrow and azathioprine in Crohn’s disease therapy. Ter Arkh 90(2):47–52. https://doi.org/10.26442/terarkh201890247-52
Knyazev OV, Fadeeva NA, Kagramanova AV, Belyakov NI, Orlova NV, Lishchinskaya AA, Konoplyannikov AG et al (2018b) Stem cell therapy for perianal Crohn’s disease. Ter Arkh 90(3):60–66. https://doi.org/10.26442/terarkh201890360-66
Ko JZ, Johnson S, Dave M (2021) Efficacy and safety of mesenchymal stem/stromal cell therapy for inflammatory bowel diseases: an up-to-date systematic review. Biomol Ther 11(1):82. https://doi.org/10.3390/biom11010082
Lazebnik LB, Konopliannikov AG, Kniazev OV, Parfenov AI, Tsaregorodtseva TM, Ruchkina IN, Khomeriki SG et al (2010) Use of allogeneic mesenchymal stem cells in the treatment of intestinal inflammatory diseases. Ter Arkh 82(2):38–43
Lei Y, Haider KH, Sim EKW (2006) Adult stem cells for cardiac repair: a choice between skeletal myoblasts and bone marrow stem cells. Exp Biol Med 231:8–11
Liu J, Liu Q, Chen X (2020) The immunomodulatory effects of mesenchymal stem cells on regulatory B cells. Front Immunol 11:1843. https://doi.org/10.3389/fimmu.2020.01843
Lopez-Santalla M, Hervas-Salcedo R, Fernandez-Garcia M, Bueren JA, Garin MI (2020) Cell therapy with mesenchymal stem cells induces an innate immune memory response that attenuates experimental colitis in the long term. J Crohn’s Colitis 14(10):1424–1435. https://doi.org/10.1093/ecco-jcc/jjaa079
Lv FJ, Tuan RS, Cheung KM, Leung VY (2014) Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32(6):1408–1419. https://doi.org/10.1002/stem.1681
Maacha S, Sidahmed H, Jacob S, Gentilcore G, Calzone R, Grivel JC, Cugno C (2020) Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int 2020:4356359. https://doi.org/10.1155/2020/4356359
Magro F, Portela F (2010) Management of Inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies. BioDrugs 24:3–14. https://doi.org/10.2165/11586290-000000000-00000
Mao F, Wu Y, Tang X, Kang J, Zhang B, Yan Y, Qian H et al (2017) Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int:5356760. https://doi.org/10.1155/2017/5356760
Mendt M, Rezvani K, Shpall E (2019) Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant 54(Suppl 2):789–792. https://doi.org/10.1038/s41409-019-0616-z
Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, Mirzaei H et al (2019) Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther 10(1):340. https://doi.org/10.1186/s13287-019-1445-0
Ocansey DKW, Qiu W, Wang J, Yan Y, Qian H, Zhang X, Xu W, Mao F (2020) The achievements and challenges of mesenchymal stem cell-based therapy in inflammatory bowel disease and its associated colorectal cancer. Stem Cells Int. 7819824, 18 pp. https://doi.org/10.1155/2020/7819824
Ommer A, Herold A, Joos A, Schmidt C, Weyand G, Bussen D (2012) Gore BioA fistula plug in the treatment of high anal fistulas – initial results from a German multicenter-study. Ger Med Sci 10:Doc13. https://doi.org/10.3205/000164
Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A et al (2016) Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet 388(10051):1281–1290. https://doi.org/10.1016/S0140-6736(16)31203-X
Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A et al (2018) Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn’s disease. Gastroenterology 154(5):1334–1342.e4. https://doi.org/10.1053/j.gastro.2017.12.020
Prockop DJ (2009) Repair of tissues by adult stem/progenitor cells (MSCss): controversies, myths, and changing paradigms. Mol Ther 17(6):939–946. https://doi.org/10.1038/mt.2009.62
Sairenji T, Collins KL, Evans DV (2017) An update on inflammatory bowel disease. Prim Care 44(4):673–692. https://doi.org/10.1016/j.pop.2017.07.010
Samoylova EM, Kalsin VA, Bespalova VA, Devichensky VM, Baklaushev VP (2017) Exosomes: from biology to clinics. Genes Cells XII(4):7–19. https://doi.org/10.23868/201707024
Scott LJ (2018) Darvadstrocel: a review in treatment-refractory complex perianal fistulas in Crohn’s disease. BioDrugs 32(6):627–634. https://doi.org/10.1007/s40259-018-0311-4
Sisakhtnezhad S, Alimoradi E, Akrami H (2017) External factors influencing mesenchymal stem cell fate in vitro. Eur J Cell Biol 96(1):13–33. https://doi.org/10.1016/j.ejcb.2016.11.003
Siyu L, Fei L, You Z, Baeku J, Qiang S, Shu G (2020) Immunosuppressive property of mscs mediated by cell surface receptors. Front Immunol 11:1076. https://doi.org/10.3389/fimmu.2020.01076
Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L (2008) Mesenchymal stem cells inhibit natural killer–cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111(3):1327–1333. https://doi.org/10.1182/blood-2007-02-074997
Terai S, Tsuchiya A (2017) Status of and candidates for cell therapy in liver cirrhosis: overcoming the “point of no return” in advanced liver cirrhosis. J Gastroenterol 52(2):129–140. https://doi.org/10.1007/s00535-016-1258-1
Tolar J, Le Blanc K, Keating A, Blazar BR (2010) Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells 28(8):1446–1455. https://doi.org/10.1002/stem.459
Wang S, Miao Z, Yang Q, Wang Y, Zhang J (2018) The dynamic roles of mesenchymal stem cells in colon cancer. Can J Gastroenterol Hepatol 7628763:8 pp. https://doi.org/10.1155/2018/7628763
Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG (1999) Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg 65(1):22–26
Yabana T, Arimura Y, Tanaka H, Goto A, Hosokawa M, Nagaishi K, Yamashita K et al (2009) Enhancing epithelial engraftment of rat mesenchymal stem cells restores epithelial barrier integrity. J Pathol 218(3):350–359. https://doi.org/10.1002/path.2535
Yang R, Huang H, Cui S, Zhou Y, Zhang T, Zhou Y (2020) IFN-γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death Dis 11(7):603. https://doi.org/10.1038/s41419-020-02788-0
Zhang J, Lv S, Liu X, Song B, Shi L (2018) Umbilical cord mesenchymal stem cell treatment for Crohn’s disease: a randomized controlled clinical trial. Gut Liver 12(1):73–78. https://doi.org/10.5009/gnl17035
Zhao T, Sun F, Liu J, Ding T, She J, Mao F, Xu W et al (2019) Emerging role of mesenchymal stem cell-derived exosomes in regenerative medicine. Curr Stem Cell Res Ther 14(6):482–494. https://doi.org/10.2174/1574888X14666190228103230
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228. https://doi.org/10.1089/107632701300062859
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Konoplyannikov, M., Knyazev, O., Timashev, P., Baklaushev, V. (2022). Mesenchymal Stem Cell Therapy for Inflammatory Bowel Disease. In: Haider, K.H. (eds) Handbook of Stem Cell Therapy. Springer, Singapore. https://doi.org/10.1007/978-981-19-2655-6_8
Download citation
DOI: https://doi.org/10.1007/978-981-19-2655-6_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2654-9
Online ISBN: 978-981-19-2655-6
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences