Abstract
Despite the titanic efforts of health systems worldwide through the implementation of severe public health measures, the number of patients with the current coronavirus disease 2019 (COVID-19) has been dramatically increasing since December 2019. COVID-19 is a real threat that is currently becoming a major concern worldwide. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has a virulent infection leading to a high mortality rate. Although, the emergency use authorization of COVID vaccines has brought hope to mitigate pandemic of COVID-19, there remains a need for additional effective vaccines to deal with SARS-CoV-2, a virus characterized by its unpredictable nature, high morbidity, and rapid ability to spread and to meet the global demand and address the potential new viral variants. Still there is a significantly increased demand for the development of new therapeutic alternatives to palliate the ongoing pandemic. Actually, treating critical COVID-19 patients is challenging as no specific treatment options against SARS-CoV-2 are available. The main pathologic features of critical COVID-19 were consistent with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Therefore, regenerative, immunomodulatory, and anti-inflammatory properties of mesenchymal stromal cells (MSCs) can reduce the manifestation of cytokine storm and can restore ARDS and ALI, exhibiting an important option to be applied to critical COVID-19 patients. Here we propose MSCs as a potential alternative therapy for COVID-19 patients and discussed specific aspects of this proposed cell therapy.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- ARDS
- CAR-T cells
- Cell therapy
- COVID-19
- Cytokine storm
- Immunomodulation
- Inflammation
- Mesenchymal stromal cells
- Organoids
- SARS-CoV-2
- Stem cells
Introduction
Late in December 2019, an outbreak of atypical pneumonia of unknown etiology was described in Wuhan Province in China. A novel coronavirus named “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)” was then identified as the etiologic agent (Gorbalenya et al. 2020; Wu et al. 2010). Later, the disease was designated COrona VIrus Disease 2019 (COVID-19) (World Health Organization 2020). The rapid expansion of COVID-19 cases in number and geographic distribution prompted the World Health Organization (WHO) to declare a global health emergency. Containment of the disease was hindered by the lack of antiviral treatment, lack of vaccines, and asymptomatic carriers. On March 11, 2020, COVID-19 was officially classified by the WHO as a pandemic.
The WHO has declared coronavirus disease 2019 (COVID-19) a pandemic due to the rapid increase in infections worldwide. The initial outbreak occurred in Wuhan City of Hubei Province of the People’s Republic of China in December 2019 and has since spread to nearly every country and territory globally. As of April 24, 2021, there have been more than 145 million confirmed cases of COVID-19 in the world, including about 3 million deaths, reported to WHO (WHO 2020). However, despite strict worldwide containment strategies and national closures in many countries, prevalence rates continue to increase with significant mortality.
Since COVID-19 affects different people in different ways, most infected people develop mild-to-moderate disease and recover without hospitalization, but a subset of patients progresses to severe illness, with a high mortality rate and limited treatment options. The clinical feature of COVID-19 varies from asymptomatic forms to conditions involving multiorgan and systemic manifestations in terms of septic shock and multiple organ dysfunction syndromes (MODS). The primary pathologic features of critical COVID-19 were consistent with acute lung injuries (ALI) and acute respiratory distress syndrome (ARDS). The majority of infected persons is usually asymptomatic or has mild symptoms, and about 15% are affected by ARDS, of which 5% progress to multiple organ dysfunction syndromes or failure. From the point of view of prevention, evasive carriers still in the early stage of infection, and that therefore do not show any clinical manifestation of the disease, are the most infectious and the least tractable.
This pathology involves direct attacks by the virus on the cells and secondary attacks on the body after activating the immune system. This means that both the virus and the immune response can cause damage to the body, and common complications or secondary infection can occur. At the cellular level, the spike protein (S-protein) of SARS-CoV-2 interacts with cell receptors to infect target cells. SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2), triggering the endocytosis of virus particles. Consequently, ACE2 receptor would represent a potential therapeutic candidate to study SARS-CoV-2 infection mechanisms. Treating COVID-19 patients is challenging as no specific treatment options against SARS-CoV-2 are available. The current supportive but not curative treatments consist of the use of experimental medication. These include remdesivir, hydroxychloroquine, abidol, lopinavir/ritonavir, plasma from convalescent patients, antibody, and other nonspecific vaccines (Haider and Hyder 2020). Currently, remdesivir appears to be the most promising pharmacological intervention for the treatment of pneumonia caused by COVID-19.
The exact pathogenesis of the virus and the dynamics of the disease are not yet fully understood; therefore, the available treatment options are limited. These consist mainly of supportive therapies for symptomatic treatment. Several antiviral drugs (Grein et al. 2020), corticosteroids (Wang et al. 2020b; Al-Rasheed et al. 2021), convalescent plasma (Shen et al. 2020; Verma et al. 2020), and neutralizing monoclonal antibodies (Shanmugaraj et al. 2020) have been tested and have undergone different phases of clinical trials, but none have been approved explicitly for COVID-19. Another alarming fact is the report from some countries of recurrence of infection in recovered as well as vaccinated individuals, which calls into question the efficacy of available treatments (Lan et al. 2020). In the absence of a recommended treatment, observance of general principle of resorting to take preventive measures, including social distancing, hygienic precautions, and use of face masks, remains the preferred strategy (Hyder and Haider 2020).
Within this scenario, investigations have been conducted at a dizzying speed to achieve a vaccine. The pioneering manufacturer’s platforms of vaccines (BioNTech-Pfizer, University of Oxford-AstraZeneca, Moderna, Johnson & Johnson, Sanofi-GlaxoSmithKline, CanSino Biologics, Inovio, Sinovac, Novavax, Gamaleya Research Institute, CureVac, Clover Biopharmaceuticals, Merck & Co.) have been able to ensure their historic and rapid development. According to WHO, as of February 18, 2021, at least seven different vaccines across three platforms have been rolled out in countries. Vulnerable populations in all countries are the highest priority for vaccination. At the same time, more than 200 additional vaccine candidates are in development, of which more than 60 are in clinical development (García-Montero et al. 2021; Yan et al. 2021a).
The first mass vaccination program was initiated in early December 2020, and as of February 15, 2021, 175.3 million vaccine doses have been administered (Hasan et al. 2021). At least seven different vaccines (three platforms) have been developed and administered as part of the worldwide vaccination program. WHO issued an Emergency Use Listing (EUL) for the Pfizer COVID-19 vaccine (BNT162b2) on December 31, 2020. On February 15, 2021, WHO issued EUL for two versions of the AstraZeneca/Oxford COVID-19 vaccine, manufactured by the Serum Institute of India and SKBio. On March 12, 2021, WHO issued an EUL for the COVID-19 vaccine Ad26.COV2.S, developed by Janssen (Johnson & Johnson). WHO is on track to EUL other vaccine products through June.
Although many biotechnology companies have developed different vaccines and millions of people have been vaccinated to date, the complete process of safety evaluation, manufacturing, and scale-up are still under question, and longer follow-up is needed (Yan et al. 2021b; Kadkhoda 2021). As such, the development of feasible, safe, and effective therapies is extremely urgent. Therefore, increasing experimental and clinical evidence has given credibility to the claim that advanced therapies research could change the future of COVID-19 and the forthcoming emergence of virulent viruses. Notably, cell-based therapies will impact, not yet foreseen, on the present and future sequels of COVID-19. In this regard, mesenchymal stem cells (MSCs) have long been associated with the repairing and rejuvenating damaged tissues due to their broad pharmacological effects, including anti-inflammation, immunomodulation, anti-apoptosis, angiogenesis, and trans-differentiation to specific cell types. They also secrete a myriad of soluble factors and vesicles altogether involved in restoring tissue homeostasis and functionality. The efficacy of MSCs and their secretory factors has been proven in successfully reducing inflammation, dampening immune responses, and repairing lung damage in various preclinical and clinical models (Hmadcha et al. 2009). Therefore, the potential of MSC-based therapy as an option for severe or critically ill COVID-19 patients is being explored in the current scenario (Leng et al. 2020; Sánchez-Guijo et al. 2020) (Table 1).
On the one hand, recent studies focus on regenerative, immunomodulatory, and anti-inflammatory properties of mesenchymal stromal cells (MSCs) to reduce the manifestation of cytokine storm and to restore ARDS and ALI, exhibiting an important option to be applied to critical COVID-19 patients, or on MSCs secretome to treat COVID-19 pneumonia (Tang et al. 2020; Meng et al. 2020; Li et al. 2020b; Liang et al. 2020; Lanzoni et al. 2021). Other research includes the use of hematopoietic stem cells derived from umbilical cord blood, bone marrow, or mobilized peripheral blood, as well as immune chimeric antigen receptor T cell (CAR-T cell). On the other hand, the understanding of the mechanism of infection and pathogenesis are still limited. In this regard, the use of human pluripotent stem cells, both embryonic stem cells (hESCs) and induced stem cells (hiPSCs), to generate tissue-specific human organoids (lung, intestinal, liver, vascular, heart, and kidney organoids) may provide a next-generation cellular model for investigating viral infection and drug screening. Altogether, the ultimate goal of all these strategies is to achieve a definitive and efficient therapy for COVID-19.
Mechanism of Infection and Immune Response
Several types of coronavirus are known to have the potential for human infection; only six are known to cause disease in humans: HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, Middle East respiratory syndrome coronavirus (MERS-CoV), and SARS-CoV (Skariyachan et al. 2019; Bonilla-Aldana et al. 2020). The 2019-nCoV, a single-stranded RNA virus, is closely related to SARS-CoV that emerged in 2003–2004 and caused an epidemic disease (Racaniello 2016). The virus was provisionally designated 2019-nCoV and later given the official name SARS-CoV-2 (Gorbalenya et al. 2020). SARS-CoV-2 was characterized as a beta-coronavirus and recognized as the seventh discrete coronavirus species capable of causing human disease (Zhu et al. 2020a). The virion nucleocapsid consists of an RNA genome complexed with a nucleoprotein and is enveloped by a phospholipid bilayer. This bilayer is covered by two types of spike proteins: protein S, which is present in all known coronaviruses and forms peplomers on the surface, which gives it a corona solar appearance, and protein hemagglutinin esterase (HE), which is present in only a few types of coronaviruses. The spike S protein interacts with the host angiotensin-converting enzyme 2 (ACE2) receptor protein, resulting in membrane fusion with subsequent release of the viral genome into the cell, or clathrin-dependent and clathrin-independent endocytosis of the virus (Li et al. 2020a).
Moreover, the ACE2 receptor is widely expressed by human cells, particularly in the lungs by type 2 alveolar epithelial cells (AT2s) and capillary epithelial cells, and cells of the heart, kidney, and intestine. In addition, two proteases, transmembrane serine protease 2 (TMPRSS2) and extracellular matrix metalloproteinase inhibitor (EMMPRIN or CD147), have been reported to be essential for virus entry into the host cell (Chen et al. 2020). The damage to the lungs is caused by the virus either directly, by the destruction of AT2s and capillary endothelial cells, which disrupts the renin-angiotensin system, or indirectly, by dampening the immune response (Jin et al. 2020). The precise pathogenesis of this particular virus remains unknown. Most of the information on the cycle of infection and subsequent immune response is primarily derived from the SARS and MERS coronaviruses due to the correlation in the clinical features of patients with COVID-19 with these viral infections (Guan et al. 2020; Huang et al. 2020).
Upon infection, the virus is recognized by the innate immune system through pathogen-associated molecular patterns (PAMPs), which in this case is the genomic RNA of the virion. This leads to activation of the NFkB pathway and the IRF3 pathway, which results in the expression of type I interferon (IFN). IFN then activates the JAK/STAT pathway and induces the expression of IFN-stimulated genes (ISGs) that have antiviral activity (Prompetchara et al. 2020).
Successful viral clearance and amelioration of the clinical manifestations of the disease depend on this effective immune response. However, the virus can evade IFN- and ISG-mediated killing and often results in a delayed IFN response. This results in the infiltration of hyper-inflammatory neutrophils and macrophages into the lung site, along with pro-inflammatory cytokines, mainly IL-1b, IL-2, IL-6, IL-7, IL-8, IL-17, G-CSF, GM-CSF, CCL3, MCP1, and TNF (Cao 2020). This so-called cytokine storm is a result of the innate response (neutrophils and macrophages). Moreover, hyperactivation of T lymphocytes (especially the Th1 response) is actually responsible for pulmonary dysfunction and abnormalities such as pneumonitis, ARDS, respiratory failure, viral sepsis, and organ failure. Elevated pro-inflammatory cytokines also induce the synthesis of hyaluronan synthase 2, which produces hyaluronan in the lungs, leading to the characteristic opacity or fluid accumulation in the lungs (Shi et al. 2020).
In critical cases, the virus can also enter the peripheral blood (viremia) and translocate to other target organs expressing the ACE2 receptor, such as the heart, kidney, and intestines, resulting in multiple organ dysfunctions. Thus, there is a great need to discover the specific virulence mechanisms during which cell and tissue injury occurs. As it is not always possible to capture the underlying mechanisms of pathophysiology in humans, several modeling methods have been developed, including 3D-engineered organoids derived from pluripotent stem cells (ESCs and iPSCs), different types of stem cells, and animals (Sun et al. 2020; Yang et al. 2020; Youk et al. 2020; Zheng et al. 2021; Boudewijns et al. 2020).
The Rationale for the Clinical Use of MSCs for COVID-19 Patients
MSCs administration tends to unbalance the pro-inflammatory cytokines, immune cells, and tissue damage toward an anti-inflammatory and regenerative microenvironment. MSCs have been widely used in cell-based therapy, from basic research to clinical trials (Acosta et al. 2013; Capilla-González et al. 2018; Soria-Juan et al. 2019). Safety and efficacy have been avidly documented in many clinical trials, especially in both systemic and local immune-mediated inflammatory diseases, such as GvHD, Crohn’s complex fistula, and type 2 diabetes complications (Soria-Juan et al. 2019; García-Gómez et al. 2010; Herreros et al. 2012). MSCs play a positive role mainly in two ways: immunomodulatory effects and anti-inflammatory abilities both linked to regeneration (Pacienza et al. 2017; Lee and Kang 2020). MSCs, when activated, can secrete many types of cytokines by paracrine secretion or make direct interactions with immune cells (Leng et al. 2020).
MSCs may act as suppressors of the cytokine storm, specifically through IL-1 blockade. Recent data support that IL-1 receptor antagonist, a naturally occurring antagonist of IL-1α/IL1-β signaling pathways, has been attributed to the immunosuppressive effects of MSCs (Harrell et al. 2020). So, IL-1 blockade seems to activate MSCs toward anti-inflammatory phenotype able of releasing anti-inflammatory cytokines, of increasing Treg, and of favoring polarization of M1 (pro-inflammatory) macrophages into M2 (anti-inflammatory), which could contribute to revascularization and regeneration of lung tissue (Varghese et al. 2017).
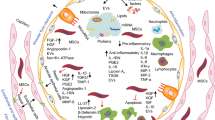
In summary, MSCs tend to unbalance the pro-inflammatory cytokines, immune cells, and tissue damage toward an anti-inflammatory and regenerative microenvironment (Fig. 1).
Schematic of the anti-inflammatory effects of mesenchymal stem cells-derived exosomes, secretome, and combinatory treatment on lung inflammation and tissue damage caused by COVID-19. Abbreviations: Breg, regulatory B cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; IDO, indoleamine-pyrrole 2,3-dioxygenase; IFNγ, interferon gamma; IL-1RA, interleukin-1 receptor antagonist; IL, interleukin; IP-10, interferon gamma-induced protein 10 (or CXCL10, C-X-C motif chemokine ligand 10); M1-macrophages, classically activated macrophages; M2-macrophages, alternatively activated macrophages; MCP, monocyte chemoattractant protein; MIP-1, macrophage inflammatory protein 1; NK, natural killer; PGE2, prostaglandin E2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Treg, regulatory T cell; TGF-β, transforming growth factor-beta; Th1, T helper type 1; Th2, T helper type 2; TNFα, tumor necrosis factor-alpha; TSG-6, stimulated gene-6; VEGF, vascular endothelial growth factor
This is why these cells have been proposed for use in pulmonary sepsis and cystic fibrosis. They are safe when used for ARDS (Wilson et al. 2015). The intravenous route is the most appropriate for the current intensive care unit (ICU) setting. Additionally, MSCs of any origin injected intravenously are rapidly located in the pulmonary microcirculation network because the cells are significant than the diameter of the capillaries, based on previous data in preclinical animal models. They can also be captured by local macrophages, which subsequently stimulate MSCs to produce IL-10, indirectly providing a source of immunosuppressive cytokines (Argibay et al. 2017). Altogether, MSCs have been successfully tested in other inflammatory diseases. Preclinical data suggest that they may be beneficial in patients with COVID-19 with severe pulmonary inflammation and oxygen therapy (mechanical ventilation) without excluding their use in earlier stages of the disease.
Progress in MSC-Based Therapy for COVID-19
MSCs have been widely used for their capacity to differentiate into diverse cell lineages, migration, and secretion of cellular regulators (secretome), together with their immunosuppressive and immunomodulatory potential. Moreover, their isolation is almost easy and presents no major ethical problems, making them the most suitable stem cells among many others (Larijani et al. 2015).
On the one hand, adipose tissue, umbilical cord, bone marrow, and blood are some important sources of MSCs. Although adipose tissue-derived MSCs have been shown to have more exciting results initially, the best source of stem cells still needs to be found (Gentile and Sterodimas 2020; Song et al. 2021). On the other hand, through their impacts on T and B cells, macrophages, and dendritic cells, they help establish a tolerogenic environment leading to an optimal therapeutic condition (Wang et al. 2018; Lee and Song 2018). Consequently, by inhibiting T- and B-cell proliferation and successfully regulating pro-inflammatory cytokines to optimize the microenvironment for intrinsic recovery, MSCs can reduce the cytokine storm. Moreover, MSCs can restrict the infiltration of innate immune system cells, consequently decreasing the secretion of inflammatory cytokines, which may indirectly attenuate the cytokine storm (Zhu et al. 2020b; Ellison-Hughes et al. 2020; Song et al. 2021; Jeyaraman et al. 2021).
Under COVID conditions, a few days after infusion of MSCs, immune cells related to the cytokine storm are shown to decrease. Increased levels of lymphocytes and regulatory dendritic cells along with decreased levels of CRP, IL-1, IL-6, IL-12, IFN-γ, and TNF are also other results of this type of MSC-based therapy. MSCs can also deliver antimicrobial peptides and anti-inflammatory cytokines (Rajarshi et al. 2020; Wang et al. 2020a; Leng et al. 2020). In addition to these anti-inflammatory characteristics, the secretion of IL-10 and some growth factors, together with their regenerative and repair capacity, renders MSCs a potent therapeutic tool for lung repair and treatment of ARDS (Azmi et al. 2020). Administration of MSCs has also been shown to have beneficial effects in conditions like sepsis and septic shock due to their ability to normalize inflammatory biomarkers, oxygen saturation, and pulmonary improvements. For sepsis condition, umbilical cord Wharton’s jelly-derived MSCs are mentioned as the best source of MSCs due to their effectiveness and acceptability (Laroye et al. 2020).
Furthermore, the gene expression profile showed that MSCs were negatively expressed angiotensin-converting enzyme 2 (ACE2-), an essential protein for viral infection along with transmembrane serine protease 2 (TMPRSS2-), which indicated that MSCs are free from COVID-19 infection (Leng et al. 2020; Hernandez et al. 2021). In this regard, the FDA has confirmed the safety and efficacy of MSCs for widespread application for COVID-19 cases (Choudhery and Harris 2020; Kavianpour et al. 2020). Despite the aforementioned benefits on the administration of MSCs, there are still some challenges; MSCs-related characteristics regarding their dosage, route of administration, frequency, and location of MSCs in the damaged sites have posed some limitations. Ethical concerns that remain, along with the lack of standardized protocols in the preparation and isolation processes, are other challenges.
Another primary concern about MSCs therapy is their side effect of increased hypercoagulability (Jeyaraman et al. 2021). Therefore, in accordance with the increased risk of thrombosis, cell-free therapies, including MSCs secretome and MSCs extracellular vesicles, appear to be an interesting treatment approach for COVID-19 having no risk of mutagenesis and/or tumorigenicity. Exosomes harbor different types of microRNAs and mRNAs and various protein components and have a lower risk of escort and lower transmission of infection. However, a clearer understanding of the dose, timing, and route of administration of the cells is needed. Their ability for nebulized administration (Hmadcha et al. 2020; Aguilera et al. 2021) and more extended storage periods also make them promising alternative therapeutic approaches (Maron-Gutierrez and Rocco 2020; Kheirkhah et al. 2021).
One of the first clinical trials to study the efficacy of intravenous infusion of MSCs in ten patients with confirmed moderate, severe, or critical forms of COVID-19 aged 45–75 years was conducted in China (Chinese Clinical Trial Registry – ChiCTR2000029990). Seven patients (one with the critical, four with the severe, and two with the moderate form of the disease) received an intravenous infusion of MSCs; three patients with the severe disease received a placebo. Two days after MSCs infusion, all seven patients showed a significant improvement in lung function and a decrease in the expression of disease symptoms. All seven patients who received MSCs recovered and were discharged 10 days after the procedure. Due to the immunosuppressive properties of MSCs, after infusion, they contributed to a significant decrease in the level of pro-inflammatory cytokines and chemokines in serum, which attracted a lower number of mononuclear cells/macrophages to the damaged lungs. At the same time, MSCs promoted the recruitment of many regulatory dendritic cells (DCs) to the site of inflammation. In addition, there was an increase in IL-10 and vascular endothelial growth factor (VEGF) levels in this trial, which may contribute to lung recovery. Among the three patients in the control group (who received a placebo), one died, the second developed ARDS, and the third remained in severe condition (Leng et al. 2020). Since then and over a year, many clinical trials have been launched with different types of MSCs to treat COVID-19 disease.
Other Cell-Based Innovation for COVID-19
Cell therapy is emerging as one of the most promising strategies for regenerating damaged or failed tissues and organs in the healthcare system. In this regard, in addition to the extensive use of MSCs of both autologous and allogeneic origin, there is a wide range of treatments using various cell types (e.g., T cells, NK lymphocytes, and different stem cells). In this context, the adoptive T-cell therapy approach or CAR-T cell therapy as a type of immunotherapy has proven effective against some infections and diseases. In this case, T cells are extracted from the patient’s immune system (autologous source) and sent to the laboratory for genetic modification. The modified cells are then reinfused into the patient (Bonifant et al. 2016; Maus and Levine 2016; Seif et al. 2019). Despite the impressive efficacy of CAR-T cell therapy in treatment, it has several serious side effects, including cytokine release syndrome (CRS) and neurological difficulties. Immediate-onset CRS tends to be a cytokine storm (Chen et al. 2019; Hong et al. 2021). Currently, T-cell therapy has also shown promise in immunosuppressed individuals as a preventive measure against COVID-19. Thus, peripheral blood cells from convalescent subjects who had been at risk for the virus were used (Keller et al. 2020).
Regulatory T-cell-related strategies have also been suggested as treatment approaches for disease management based on their ability to inactivate innate/adaptive immunity through inhibitory molecules (Stephen-Victor et al. 2020). In addition, antigen-specific modified/unmodified T-cell transfer has shown promising results in treating different disorders by reconstituting T-cell subsets (effector/memory cells). In this context, it is mentioned that adoptive T-cell therapy by transferring immune subsets of T cells has therapeutic benefits that may be the same as the characteristics of adult tissue stem cells. However, the required high maintenance of memory T cells and engraftment processes may create some limitations (Busch et al. 2016). In this regard, specific COVID-19-related T cells (within CD45RA memory T cells) have been recognized that can be feasibly received by depleting CD45RA from convalescent donors. These cells can provide a cell population for the condition of lymphopenia along with rapid reactions to infection. Memory T cells can respond quickly to infection and provide long-term immune protection to reduce the severity of COVID-19 symptoms. Also, CD45RA memory T cells confer protection from other pathogens encountered by the donors throughout their life (Ferreras et al. 2021). CD45RA memory T cells also provide immunity against probable secondary infections that may be found in COVID-19 hospitalized individuals (Ferreras et al. 2021). HLA-matched cytotoxic T cells isolated from convalescent patients are other promising approaches for treating COVID-19, as are EBV-specific cytotoxic T cells used for EBV+-related lymphomas (Hanley et al. 2020).
Another promising candidate for a significant advance has been natural killer (NK) cell therapy. In this case, autologous or allogeneic origins can be used to create pure populations of NK cells. The use of allogeneic NK cells as a platform for CAR engineering has been augmented by the limitations of autologous NK cells (such as a diminished effector role and the requirement for a patient-specific stock) (Veluchamy et al. 2017; Daher and Rezvani 2018). Given that the decrease in NK cell numbers may be related to the severity of COVID-19 infection, some clinical trials used engineered NK cells to help combat COVID-19 (Market et al. 2020; van Eeden et al. 2020). However, the use of NK cells also has many drawbacks that may clinically hinder their efficacy. Their short lifespan (due to the lack of cytokine support), low cell number, and vulnerability to immunosuppressed status could limit their trafficking and function (Nayyar et al. 2019; Liu et al. 2021).
Side Effects of Mesenchymal Stem Cell-Based Therapy
Although the safety and efficacy of MSCs infusion have been demonstrated in hundreds of clinical trials, this treatment can lead to potential complications (Acosta et al. 2013; Capilla-González et al. 2018; Soria-Juan et al. 2019), and possibly it is the time for combinatory therapies. Preclinical studies have shown that the lungs act as a filter that retains most of the cells injected intravenously (Zhang et al. 2020). Numerous critically ill COVID-19 patients are in a hypercoagulable procoagulant state. Hence, they are at high risk for disseminated intravascular coagulation, thromboembolism, and thrombotic multiorgan failure, another cause of high lethality of the infection. It remains unclear whether intravenous (IV) infusion is a safe and effective route of MSCs infusion for COVID-19 patients. This information is important as MSC-based products express variable levels of highly procoagulant tissue factor (TF or CD142), which compromises the hemocompatibility and safety profile of the cells. Of potential concern is that intravenous infusions of poorly characterized MSC products with uncontrolled (high) TF (CD142) expression may trigger blood clotting in COVID-19 subjects and other vulnerable patient populations and further promote the risk of thromboembolism.
By contrast, well-characterized products with robust manufacturing procedures and optimized clinical delivery modes hold great promise for improving COVID-19 patients by exerting their beneficial immunomodulatory effects, inducing tissue repair and organ protection. While the need for MSCs therapy for COVID-19 subjects is evident, integrating innate and adaptive immune compatibility testing into current cell, tissue, and organ transplantation guidelines is critical for safe and effective therapies. Thus, it is essential to use only well-characterized and safe MSCs for even the most urgent and experimental treatments (Moll et al. 2020). Because the COVID-19 patients suffer a prothrombotic state, concomitant use of heparin and defibrotide, a drug used in sinusoidal obstructive syndrome (SOS) after hematopoietic stem cell transplantation, has been proposed. Defibrotide is a mixture of single-stranded oligonucleotide aptamers with multi-target pharmacology limiting endothelial cell activation (Pescador et al. 2013). Given its antithrombotic, anti-TNFα, anti-atherosclerotic, etc., the US Food and Drug Administration (FDA) approved its use in SOS. It will be consistent both to block the cytokine storm and prevent pulmonary thromboembolism. With HIV infections, we learned that combinatorial therapies show higher effectiveness in controlling the disease. Case reports, pilot studies, and well-designed clinical trials are needed to fight this pandemic. Now and when it comes back.
Unmet Challenges of Adoptive MSCs Therapy
Despite the promising preliminary results, specific challenges demand attention before MSCs can be adopted at a larger scale in treating coronaviruses-induced infections. These challenges include the study design, source of MSCs, route of administration, dosage requirements, and their laboratory preparation and manipulation (Escacena et al. 2015; Soria-Juan et al. 2019; García-Bernal et al. 2021).
Study Design
We believe that most of the trials that have been registered utilizing MSCs or their derived products do not have any appropriate control to conclusively determine the efficacy of cellular or cell-free therapy (Table 1). In most of these cases, MSCs are used in combination with adjunct antiviral drugs and supportive therapy. In such cases, it would become almost impossible to determine if the observed clinical improvements are actually attributed to MSCs or not. Therefore, a strategy including an appropriate control should be included in the design of such trials along with a greater sample size to validate the clinical efficacy of stem cells technology fully.
Source of Cells
Different tissue sources, whether adult- or fetus-associated tissues, like adipose tissue, umbilical cord, dental pulp, and bone marrow, vary in their capacity to generate MSCs. Therefore, choosing an ideal source for harvesting MSCs and subsequently generating cell-free products is equally important as cell-free therapy is fast emerging as a therapeutic option (Haider and Aslam 2018). MSCs tissue sources, like umbilical cord and adipose tissue, are easily accessible without discomfort to the donor and generate a greater amount of MSCs with equivalent differentiation potential from the same amount of tissue in comparison to bone marrow, which incidentally is more invasive.
Route of Cell Administration
Another major factor to consider here is their route of administration. MSCs and their products can either be systemically or locally injected. There are only limited studies that have compared the different routes of MSCs administration and have reported different outcomes. Therefore, it is difficult to conclude which route can be considered as the safest and most effective (Antunes et al. 2014; Cardenes et al. 2019). For COVID-19 disease, MSCs can be administered both systemically and locally with equal efficacy as both of these routes would result in their delivery first to the lungs only. However, in the case of cell-free products, like exosomes, delivery directly to the lungs via intranasal or intratracheal route is a more tenable option as systemic delivery often leads to a substantial loss in the amount of these products, mainly due to the activity of circulatory proteases and their distribution to the liver and spleen first, thus calling for booster doses (Gardin et al. 2020; Mahajan and Bhattacharyya 2021).
Dosage Strategies
The number of MSCs required per dose and the total number of doses required are quite crucial in determining the treatment outcome. Based on the previous studies, it is estimated that approximately 4 × 108 MSCs are required for every patient regardless of the clinical indication (Olsen et al. 2018). The trials registered for COVID-19 have reported varied dosages, with an average of 1 × 106 cells/kg body weight up to 2–5 times to this average dose, but the actual number of MSCs required to produce such doses is not mentioned in any report, which needs to be optimized. Furthermore, while MSCs can be injected directly, the products like exosomes need to be prepared into stable formulations for their delivery to the patients (Gardin et al. 2020; Mahajan and Bhattacharyya 2021).
Risks Associated with Stem Cell Therapy
While MSCs and their products have proven beneficial in the current scenario, we should not overlook the risks associated with stem cell therapy. Many stem cells clinics have opened up in recent years, marketing unethical and unauthorized stem cell treatment for various ailments, including COVID-19, by feeding on people’s fears and anxieties (Turner 2020). These less than clinical-grade stem cells and unlicensed stem cell treatments are potentially dangerous to the general public and undermine the efforts at determining stem cells efficacy in clinical trials. Undoubtedly, the use of MSCs and their factors has proven to be quite promising in the current scenario but is mainly dependent on the functional quality and integrity of stem cells. Therefore, stringent regulatory control should be maintained for the manufacturing and distribution of these products (see Chap. 3, “Considerations for Clinical Use of Mesenchymal Stromal Cells” of this book for review on clinical use of MSCs).
Concluding Remarks
Considering the prevalence of COVID-19 and its complications, such as cytokine storm, which is followed by ARDS and death of patients, finding a way to treat and improve patients is of great importance. As previously mentioned, currently available vaccines are not a cure for COVID-19; there is no specific therapy for this virus, and supportive therapies as well as nonspecific antiviral drugs are mainly used for this purpose. Cell-based therapy is a modern method to treat various diseases. Recently, a number of studies have been conducted to treat COVID-19 using stem cells, suggesting the application of MSCs or immune cells such as T, CAR-T, or NK cells. Accordingly, the safety and immunomodulatory role of MSCs in ARDS has been approved. The MSCs can secrete factors that improve the pulmonary microenvironment, inhibit immune system over-activation, promote tissue repair, rejuvenate alveolar epithelial cells, inhibit counteract pulmonary fibrosis, or improve function in lung tissue damaged by SARS-CoV-2 infection.
Nonetheless, many issues related to the application of MSCs, such as the ideal dose and optimal timing of administration, need further study. Of note, in several animal models of human disease, the use of MSC-secreting exosomes has been claimed to mimic the beneficial effects of MSCs in antiviral therapy against the influenza virus by reducing virus replication in the lungs and virus-induced release of pro-inflammatory cytokines, which highlights the great potential of cell-free-based therapies. In addition, considering the impossibility of studying the detailed mechanism of pathogenicity and the sequence of suggested drugs or vaccine candidates in human beings, these significant steps toward cell-based therapies in the SARS-CoV-2 field of study should be continued. Ongoing experimental studies and randomized trials will play an essential role in elucidating the therapeutic potential of MSCs, leading to a better understanding of how MSCs interact with SARS-CoV-2-infected lung tissue. Although current progress on COVID-19 vaccinations is promising, the world population will have to continue to adapt to the “new normal” and practice social distancing and hygienic measures, at least until an effective cure is available to the general public.
Abbreviations
- 3D:
-
Three-dimensional
- ACE2:
-
Angiotensin-converting enzyme 2
- ALI:
-
Acute lung injuries
- Ang-1:
-
Angiopoietin-1
- ARB:
-
Angiotensin receptor blocker
- ARDS:
-
Acute respiratory distress syndrome
- AT2s:
-
Type 2 alveolar epithelial cells
- CAR-T:
-
Chimeric antigen receptor T cells
- CCN1:
-
CCN family number 1
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- CLDN1:
-
Claudin1
- COVID-19:
-
Coronavirus disease 2019
- CPE:
-
Cytopathologic effect
- Cyr61:
-
Cysteine-rich protein 61
- DEX:
-
Dexamethasone
- ECM:
-
Extracellular matrix
- EMMPRIN:
-
Extracellular matrix metalloproteinase inhibitor (or CD147)
- FDA:
-
Food and Drug Administration
- GFP:
-
Green fluorescent protein
- GI:
-
Gastrointestinal
- Gsis:
-
γ-Secretase inhibitors
- HACE2:
-
Human angiotensin-converting enzyme 2
- HCQ:
-
Hydroxychloroquine
- HE:
-
Heme agglutinin esterase
- HESCs:
-
Human embryonic stem cells
- HiPSCs:
-
Human-induced pluripotent stem cells
- HPSC:
-
Human pluripotent stem cell
- Hrsace2:
-
Human recombinant soluble ACE2
- Hs-cTnI:
-
Highly sensitive troponin-I
- HSV1:
-
Herpes simplex virus-1
- ICU:
-
Intensive care units
- IFN:
-
Interferon
- Ifnar1-/-:
-
C57BL/6 mice with a genetic ablation of their type I interferon receptors
- IL-1α:
-
Interleukin-alpha
- IL1-β:
-
Interleukin-beta
- IL-2:
-
Interleukin-2
- Il28r-/-:
-
C57BL/6 mice with a genetic ablation of their type III interferon receptors
- Il2rg:
-
Interleukin-2 receptor gamma chain
- Isgs:
-
Interferon-stimulated genes
- KGF:
-
Keratinocyte growth factor
- KRT18:
-
Cytokeratin 18
- LPS:
-
Lipopolysaccharide
- MAS:
-
Macrophage activation syndrome
- Mascp6:
-
Mouse-adapted strain at passage 6
- MERS:
-
Middle East respiratory syndrome
- MODS:
-
Multiple organ dysfunction syndromes
- MPA:
-
Mycophenolic acid
- MSCs:
-
Mesenchymal stem cells
- NPC:
-
Neural progenitor cells
- NSCs::
-
Neural stem cells
- NSG mouse:
-
NOD-SCID with null mutation in the gene encoding the il2rgl
- PAMPs:
-
Pathogen-associated molecular patterns
- PDGFb:
-
Platelet-derived growth factor subunit b
- PMN:
-
Polymorphonuclear
- QNHC:
-
Quinacrine dihydrochloride
- RIG-I:
-
Retinoic acid-inducible gene-I-like
- RLU:
-
Relative luciferase units
- RM:
-
Regenerative medicine
- SARS-cov-2:
-
Severe acute respiratory syndrome coronavirus-2
- SFTPC:
-
Surfactant protein-C
- SLC10A2:
-
Solute carrier family 10 member 2
- SOS:
-
Sinusoidal obstructive syndrome
- S-protein:
-
Spike protein
- TF:
-
Tissue factor (or CD142)
- TMPRSS2:
-
Transmembrane serine protease 2
- TNFα:
-
Tumor necrosis factor alpha
- WHO:
-
World Health Organization
- WT:
-
Wild type
References
Acosta L, Hmadcha A, Escacena N, Pérez-Camacho I, de la Cuesta A, Ruiz-Salmeron R, Gauthier BR et al (2013) Adipose mesenchymal stromal cells isolated from type 2 diabetic patients display reduced fibrinolytic activity. Diabetes 62(12):4266–4269. https://doi.org/10.2337/db13-0896
Aguilera Y, Mellado-Damas N, Olmedo-Moreno L, López V, Panadero-Morón C, Benito M, Guerrero-Cázares H et al (2021) Preclinical safety evaluation of intranasally delivered human mesenchymal stem cells in juvenile mice. Cancers (Basel) 13(5):1169. https://doi.org/10.3390/cancers13051169
AL-Rasheedi M, Alhazmi Y, Almaqwashi N, Alreshidi MA, Kardam A, Sharaf M, Haider KH (2021) Corticosteroid therapy for 2019-nCoV infected patients: a case series of eight mechanically ventilated patients. Clin Case Rep 9(5):e04066. https://doi.org/10.1002/ccr3.4066
Antunes MA, Abreu SC, Cruz FF, Teixeira AC, Lopes-Pacheco M, Bandeira E, Olsen PC et al (2014) Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res 15(1):118. https://doi.org/10.1186/s12931-014-0118-x
Argibay B, Trekker J, Himmelreich U, Beiras A, Topete A, Taboada P, Pérez-Mato M et al (2017) Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Sci Rep 7:40758. https://doi.org/10.1038/srep40758
Azmi NU, Puteri MU, Lukmanto D (2020) Cytokine storm in COVID-19: an overview, mechanism, treatment strategies, and stem cell therapy perspective. Pharm Sci Res 7(4):1. https://doi.org/10.7454/psr.v7i4.1092
Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ (2016) Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 3:16011. https://doi.org/10.1038/mto.2016.11
Bonilla-Aldana DK, Quintero-Rada K, Montoya-Posada JP, Ramírez-Ocampo S, Paniz-Mondolfi A, Rabaan AA, Sah R et al (2020) SARS-CoV, MERS-CoV and now the 2019-novel CoV: have we investigated enough about coronaviruses? – a bibliometric analysis. Travel Med Infect Dis 33:101566. https://doi.org/10.1016/j.tmaid.2020.101566
Boudewijns R, Thibaut HJ, Kaptein SJF, Li R, Vergote V, Seldeslachts L, Van Weyenbergh J et al (2020) STAT2 signaling restricts viral dissemination but drives severe pneumonia in SARS-CoV-2 infected hamsters. Nat Commun 11(1):5838. https://doi.org/10.1038/s41467-020-19684-y
Busch DH, Fräßle SP, Sommermeyer D, Buchholz VR, Riddell SR (2016) Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol 28(1):28–34. https://doi.org/10.1016/j.smim.2016.02.001
Cao X (2020) COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 20(5):269–270. https://doi.org/10.1038/s41577-020-0308-3
Capilla-González V, López-Beas J, Escacena N, Aguilera Y, de la Cuesta A, Ruiz-Salmerón R, Martín F et al (2018) PDGF restores the defective phenotype of adipose-derived mesenchymal stromal cells from diabetic patients. Mol Ther 26(11):2696–2709. https://doi.org/10.1016/j.ymthe.2018.08.011
Cardenes N, Aranda-Valderrama P, Carney JP, Sellares Torres J, Alvarez D, Kocyildirim E, Wolfram Smith JA et al (2019) Cell therapy for ARDS: efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respir Res 6(1):e000308. https://doi.org/10.1136/bmjresp-2018-000308
Chen H, Wang F, Zhang P, Zhang Y, Chen Y, Fan X, Cao X et al (2019) Management of cytokine release syndrome related to CAR-T cell therapy. Front Med 13(5):610–617. https://doi.org/10.1007/s11684-019-0714-8
Chen Y, Liu Q, Guo D (2020) Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 92(4):418–423. https://doi.org/10.1002/jmv.25681
Choudhery MS, Harris DT (2020) Stem cell therapy for COVID-19: possibilities and challenges. Cell Biol Int 44(11):2182–2191. https://doi.org/10.1002/cbin.11440
Daher M, Rezvani K (2018) Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering. Curr Opin Immunol 51:146–153. https://doi.org/10.1016/j.coi.2018.03.013
Ellison-Hughes GM, Colley L, O'Brien KA, Roberts KA, Agbaedeng TA, Ross MD (2020) The role of MSC therapy in attenuating the damaging effects of the cytokine storm induced by COVID-19 on the heart and cardiovascular system. Front Cardiovasc Med 7:602183. https://doi.org/10.3389/fcvm.2020.602183
Escacena N, Quesada-Hernández E, Capilla-Gonzalez V, Soria B, Hmadcha A (2015) Bottlenecks in the efficient use of advanced therapy medicinal products based on mesenchymal stromal cells. Stem Cells Int 2015:895714. https://doi.org/10.1155/2015/895714
Ferreras C, Pascual-Miguel B, Mestre-Durán C, Navarro-Zapata A, Clares-Villa L, Martín-Cortázar C, De Paz R et al (2021) SARS-CoV-2-Specific memory T lymphocytes from COVID-19 convalescent donors: identification, biobanking, and large-scale production for adoptive cell therapy. Front Cell Dev Biol 9:620730. https://doi.org/10.3389/fcell.2021.620730
García-Bernal D, García-Arranz M, Yáñez RM, Hervás-Salcedo R, Cortés A, Fernández-García M, Hernando-Rodríguez M et al (2021) The current status of mesenchymal stromal cells: controversies, unresolved issues and some promising solutions to improve their therapeutic efficacy. Front Cell Dev Biol 9:650664. https://doi.org/10.3389/fcell.2021.650664
García-Gómez I, Elvira G, Zapata AG, Lamana ML, Ramírez M, Castro JG, Arranz MG et al (2010) Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther 10(10):1453–1468. https://doi.org/10.1517/14712598.2010.519333
García-Montero C, Fraile-Martínez O, Bravo C, Torres-Carranza D, Sanchez-Trujillo L, Gómez-Lahoz AM, Guijarro LG et al (2021) An updated review of SARS-CoV-2 vaccines and the importance of effective vaccination programs in pandemic times. Vaccines (Basel) 9(5):433. https://doi.org/10.3390/vaccines9050433
Gardin C, Ferroni L, Chachques JC, Zavan B (2020) Could mesenchymal stem cell-derived exosomes be a therapeutic option for critically Ill COVID-19 patients? J Clin Med 9(9):2762. https://doi.org/10.3390/jcm9092762
Gentile P, Sterodimas A (2020) Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia. Expert Opin Biol Ther 20(7):711–716. https://doi.org/10.1080/14712598.2020.1761322
Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, Haagmans BL et al (2020) Severe acute respiratory syndrome-related coronavirus: the species and its viruses – a statement of the coronavirus study group. bioRxiv. https://doi.org/10.1101/2020.02.07.937862
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T et al (2020) Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 382(24):2327–2336. https://doi.org/10.1056/NEJMoa2007016
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720. https://doi.org/10.1056/NEJMoa2002032
Haider KH, Aslam M (2018) Cell-free therapy with stem cell secretions: protection, repair and regeneration of the injured myocardium. In: Stem cells: from Hype to Real Hope. Kh. Husnain Haider and Salim Aziz (Eds.) Medicine & life sciences. DE GRUYTER, Geithner Straße13- 10785 Berlin, Germany. (Published, 2018). https://doi.org/10.1515/9783110642438
Haider KH, Hyder Q (2020) Combating 2019-nCoV amidst the pandemic scare. Open J Regen Med 9:15–19. https://www.scirp.org/journal/ojrm
Hanley B, Roufosse CA, Osborn M, Naresh KN (2020) Convalescent donor SARS-COV-2-specific cytotoxic T lymphocyte infusion as a possible treatment option for COVID-19 patients with severe disease has not received enough attention till date. Br J Haematol 189(6):1062–1063. https://doi.org/10.1111/bjh.16780
Harrell CR, Markovic BS, Fellabaum C, Arsenijevic N, Djonov V, Volarevic V (2020) The role of Interleukin 1 receptor antagonist in mesenchymal stem cell-based tissue repair and regeneration. Biofactors 46(2):263–275. https://doi.org/10.1002/biof.1587
Hasan T, Beardsley J, Marais BJ, Nguyen TA, Fox GJ (2021) The implementation of mass-vaccination against SARS-CoV-2: a systematic review of existing strategies and guidelines. Vaccines (Basel) 9(4):326. https://doi.org/10.3390/vaccines9040326
Hernandez JJ, Beaty DE, Fruhwirth LL, Lopes Chaves AP, Riordan NH (2021) Dodging COVID-19 infection: low expression and localization of ACE2 and TMPRSS2 in multiple donor-derived lines of human umbilical cord-derived mesenchymal stem cells. J Transl Med 19(1):149. https://doi.org/10.1186/s12967-021-02813-6
Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D, FATT Collaborative Group (2012) Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Dis Colon Rectum 55(7):762–772. https://doi.org/10.1097/DCR.0b013e318255364a
Hmadcha A, Dominguez-Bendala J, Wakeman J, Arredouani M, Soria B (2009) The immune boundaries for stem cell based therapies: problems and prospective solutions. J Cell Mol Med 13(8A):1464–1475. https://doi.org/10.1111/j.1582-4934.2009.00837.x
Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V (2020) Therapeutic potential of mesenchymal stem cells for cancer therapy. Front Bioeng Biotechnol 8:43. https://doi.org/10.3389/fbioe.2020.00043
Hong R, Hu Y, Huang H (2021) Biomarkers for chimeric antigen receptor T cell therapy in acute lymphoblastic leukemia: prospects for personalized management and prognostic prediction. Front Immunol 12:627764. https://doi.org/10.3389/fimmu.2021.627764
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Hyder Q, Haider KH (2020) The ongoing battle against COVID-19. Iberoam J Med 2(4):360–366. https://doi.org/10.5281/zenodo.3987277
Jeyaraman M, John A, Koshy S, Ranjan R, Anudeep TC, Jain R, Swati K et al (2021) Fostering mesenchymal stem cell therapy to halt cytokine storm in COVID-19. Biochim Biophys Acta Mol basis Dis 1867(2):166014. https://doi.org/10.1016/j.bbadis.2020.166014
Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G (2020) Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 12(4):372. https://doi.org/10.3390/v12040372
Kadkhoda K (2021) Post-adenoviral-based COVID-19 vaccines thrombosis: a proposed mechanism. J Thromb Haemost. https://doi.org/10.1111/jth.15348
Kavianpour M, Saleh M, Verdi J (2020) The role of mesenchymal stromal cells in immune modulation of COVID-19: focus on cytokine storm. Stem Cell Res Ther 11(1):404. https://doi.org/10.1186/s13287-020-01849-7
Keller MD, Harris KM, Jensen-Wachspress MA, Kankate VV, Lang H, Lazarski CA, Durkee-Shock J et al (2020) SARS-CoV-2-specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood 136(25):2905–2917. https://doi.org/10.1182/blood.2020008488
Kheirkhah AH, Shahcheraghi SH, Lotfi M, Lotfi M, Raeisi S, Mirani Z (2021) Mesenchymal stem cell derived-exosomes as effective factors in reducing cytokine storm symptoms of COVID-19. Protein Pept Lett. https://doi.org/10.2174/0929866528666210222150347
Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, Xu H (2020) Positive RT-PCR test results in patients recovered from COVID-19. JAMA 323(15):1502–1503. https://doi.org/10.1001/jama.2020.2783
Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, Alvarez Gil A et al (2021) Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med 10(5):660–673. https://doi.org/10.1002/sctm.20-0472
Larijani B, Aghayan H, Goodarzi P, Mohamadi-Jahani F, Norouzi-Javidan A, Dehpour AR, Fallahzadeh K et al (2015) Clinical grade human adipose tissue-derived mesenchymal stem cell banking. Acta Med Iran 53(9):540–546. https://acta.tums.ac.ir/index.php/acta/article/view/4290
Laroye C, Gibot S, Huselstein C, Bensoussan D (2020) Mesenchymal stromal cells for sepsis and septic shock: lessons for treatment of COVID-19. Stem Cells Transl Med 9(12):1488–1494. https://doi.org/10.1002/sctm.20-0239
Lee BC, Kang KS (2020) Functional enhancement strategies for immunomodulation of mesenchymal stem cells and their therapeutic application. Stem Cell Res Ther 11:397. https://doi.org/10.1186/s13287-020-01920-3
Lee DK, Song SU (2018) Immunomodulatory mechanisms of mesenchymal stem cells and their therapeutic applications. Cell Immunol 326:68–76. https://doi.org/10.1016/j.cellimm.2017.08.009
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G et al (2020) Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 11(2):216–228. https://doi.org/10.14336/AD.2020.0228
Li X, Geng M, Peng Y, Meng L, Lu S (2020a) Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 10(2):102–108. https://doi.org/10.1016/j.jpha.2020.03.001
Li Z, Niu S, Guo B, Gao T, Wang L, Wang Y, Wang L et al (2020b) Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif 53(12):e12939. https://doi.org/10.1111/cpr.12939
Liang B, Chen J, Li T, Wu H, Yang W, Li Y, Li J et al (2020) Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine (Baltimore) 99(31):e21429. https://doi.org/10.1097/MD.0000000000021429
Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J (2021) NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol 14(1):7. https://doi.org/10.1186/s13045-020-01014-w
Mahajan A, Bhattacharyya S (2021) A brief review on potential application of mesenchymal stem cell and secretome in combating mortality and morbidity in COVID-19 patients. Biomed J 44(1):63–73. https://doi.org/10.1016/j.bj.2020.09.003
Market M, Angka L, Martel AB, Bastin D, Olanubi O, Tennakoon G, Boucher DM et al (2020) Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front Immunol 11:1512. https://doi.org/10.3389/fimmu.2020.01512
Maron-Gutierrez T, Rocco PRM (2020) Cell-Free therapies: novel approaches for COVID-19. Front Immunol 11:583017. https://doi.org/10.3389/fimmu.2020.583017
Maus MV, Levine BL (2016) Chimeric antigen receptor T-Cell therapy for the community oncologist. Oncologist 21(5):608–617. https://doi.org/10.1634/theoncologist.2015-0421
Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y, Yang T et al (2020) Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther 5(1):172. https://doi.org/10.1038/s41392-020-00286-5
Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk HD, Reinke P (2020) MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol 11:1091. https://doi.org/10.3389/fimmu.2020.01091
Nayyar G, Chu Y, Cairo MS (2019) Overcoming resistance to natural killer cell based immunotherapies for solid tumors. Front Oncol 9:51. https://doi.org/10.3389/fonc.2019.00051
Olsen TR, Ng KS, Lock LT, Ahsan T, Rowley JA (2018) Peak MSC-Are we there yet? Front Med (Lausanne) 5:178. https://doi.org/10.3389/fmed.2018.00178
Pacienza NA, Santa-Cruz D, Marcos M, Lemus G, Robledo O, Bertolotti A, Yannarelli G et al (2017) Anti-inflammatory effect of mesenchymal stem cells facilitates lung preservation. J Heart Lung Transplant 36(4):S91–S92. https://doi.org/10.1016/j.healun.2017.01.231
Pescador R, Capuzzi L, Mantovani M, Fulgenzi A, Ferrero ME (2013) Defibrotide: properties and clinical use of an old/new drug. Vasc Pharmacol 59(1-2):1–10. https://doi.org/10.1016/j.vph.2013.05.001
Prompetchara E, Ketloy C, Palaga T (2020) Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 38(1):1–9. https://doi.org/10.12932/AP-200220-0772
Racaniello V (2016) Moving beyond metagenomics to find the next pandemic virus. Proc Natl Acad Sci U S A 113(11):2812–2814. https://doi.org/10.1073/pnas.1601512113
Rajarshi K, Chatterjee A, Ray S (2020) Combating COVID-19 with mesenchymal stem cell therapy. Biotechnol Rep (Amst) 26:e00467. https://doi.org/10.1016/j.btre.2020.e00467
Sánchez-Guijo F, García-Arranz M, López-Parra M, Monedero P, Mata-Martínez C, Santos A, Sagredo V et al (2020) Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine 25:100454. https://doi.org/10.1016/j.eclinm.2020.100454
Seif M, Einsele H, Löffler J (2019) CAR T cells beyond cancer: hope for immunomodulatory therapy of infectious diseases. Front Immunol 10:2711. https://doi.org/10.3389/fimmu.2019.02711
Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W (2020) Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol 38(1):10–18. https://doi.org/10.12932/AP-200220-0773
Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F et al (2020) Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA 323(16):1582–1589. https://doi.org/10.1001/jama.2020.4783
Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, Bucci E et al (2020) COVID-19 infection: the perspectives on immune responses. Cell Death Differ 27(5):1451–1454. https://doi.org/10.1038/s41418-020-0530-3
Skariyachan S, Challapilli SB, Packirisamy S, Kumargowda ST, Sridhar VS (2019) Recent aspects on the pathogenesis mechanism, animal models and novel therapeutic interventions for middle east respiratory syndrome coronavirus infections. Front Microbiol 10:569. https://doi.org/10.3389/fmicb.2019.00569
Song N, Wakimoto H, Rossignoli F, Bhere D, Ciccocioppo R, Chen KS, Khalsa JK et al (2021) Mesenchymal stem cell immunomodulation: in pursuit of controlling COVID-19 related cytokine storm. Stem Cells 39(6):707–722. https://doi.org/10.1002/stem.3354
Soria-Juan B, Escacena N, Capilla-González V, Aguilera Y, Llanos L, Tejedo JR, Bedoya FJ et al (2019) Cost-effective, safe, and personalized cell therapy for critical limb ischemia in type 2 diabetes mellitus. Front Immunol 10:1151. https://doi.org/10.3389/fimmu.2019.01151
Stephen-Victor E, Das M, Karnam A, Pitard B, Gautier JF, Bayry J (2020) Potential of regulatory T-cell-based therapies in the management of severe COVID-19. Eur Respir J 56(3):2002182. https://doi.org/10.1183/13993003.02182-2020
Sun J, Zhuang Z, Zheng J, Li K, Wong RL, Liu D, Huang J et al (2020) Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell 182(3):734–743.e5. https://doi.org/10.1016/j.cell.2020.06.010
Tang L, Jiang Y, Zhu M, Chen L, Zhou X, Zhou C, Ye P et al (2020) Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med 14(5):664–673. https://doi.org/10.1007/s11684-020-0810-9
Turner L (2020) Preying on public fears and anxieties in a pandemic: businesses selling unproven and unlicensed “Stem Cell Treatments” for COVID-19. Cell Stem Cell 26(6):806–810. https://doi.org/10.1016/j.stem.2020.05.003
van Eeden C, Khan L, Osman MS, Cohen Tervaert JW (2020) Natural killer cell dysfunction and its role in COVID-19. Int J Mol Sci 21(17):6351. https://doi.org/10.3390/ijms21176351
Varghese J, Griffin M, Mosahebi A, Butler P (2017) Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther 8(1):45. https://doi.org/10.1186/s13287-017-0483-8
Veluchamy JP, Kok N, van der Vliet HJ, Verheul HMW, de Gruijl TD, Spanholtz J (2017) The rise of allogeneic natural killer cells as a platform for cancer immunotherapy: recent innovations and future developments. Front Immunol 8:631. https://doi.org/10.3389/fimmu.2017.00631
Verma HK, Farran B, Bhaskar LVKS (2020) Convalescent plasma transfusion a promising therapy for coronavirus diseases 2019 (COVID-19): current updates. Antib Ther 3(2):115–125. https://doi.org/10.1093/abt/tbaa010
Wang M, Yuan Q, Xie L (2018) Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int 2018:3057624. https://doi.org/10.1155/2018/3057624
Wang HC, Wang X, Long X (2020a) Stem cell transplantation therapy: a potential method for treating cytokine storm syndromes induced by COVID-19. Cell Transplant 29:963689720965980. https://doi.org/10.1177/0963689720965980
Wang Y, Jiang W, He Q, Wang C, Liu B, Zhou P, Dong N et al (2020b) Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. medRxiv. https://doi.org/10.1101/2020.03.06.20032342
WHO Coronavirus Disease (COVID-19) Dashboard. Retrieved November 16, 2020 from https://covid19.who.int/
Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K et al (2015) Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 3(1):24–32. https://doi.org/10.1016/S2213-2600(14)70291-7
World Health Organization. (2020). Novel Coronavirus (2019-nCoV): situation report, 22. World Health Organization. https://apps.who.int/iris/handle/10665/330991
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y et al (2010) A new coronavirus associated with human respiratory disease in China. Nature 579(7798):265–269. https://doi.org/10.1038/s41586-020-2008-3
Yan Y, Pang Y, Lyu Z, Wang R, Wu X, You C, Zhao H et al (2021a) The COVID-19 vaccines: recent development, challenges and prospects. Vaccines (Basel) 9(4):349. https://doi.org/10.3390/vaccines9040349
Yan ZP, Yang M, Lai CL (2021b) COVID-19 vaccines: a review of the safety and efficacy of current clinical trials. Pharmaceuticals (Basel) 14(5):406. https://doi.org/10.3390/ph14050406
Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X et al (2020) Human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell 27(1):125–136.e7. https://doi.org/10.1016/j.stem.2020.06.015
Youk J, Kim T, Evans KV, Jeong YI, Hur Y, Hong SP, Kim JH et al (2020) Three-Dimensional human alveolar stem cell culture models reveal infection response to SARS-CoV-2. Cell Stem Cell 27(6):905–919.e10. https://doi.org/10.1016/j.stem.2020.10.004
Zhang Y, Ding J, Ren S, Wang W, Yang Y, Li S, Meng M et al (2020) Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther 11(1):207. https://doi.org/10.1186/s13287-020-01725-4
Zheng J, Wong LR, Li K, Verma AK, Ortiz ME, Wohlford-Lenane C, Leidinger MR et al (2021) COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 589(7843):603–607. https://doi.org/10.1038/s41586-020-2943-z
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X et al (2020a) China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–733. https://doi.org/10.1056/NEJMoa2001017
Zhu Y, Geng S, Li Q, Jiang H (2020b) Transplantation of mesenchymal stem cells: a potential adjuvant therapy for COVID-19. Front Bioeng Biotechnol 8:557652. https://doi.org/10.3389/fbioe.2020.557652
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Hmadcha, A., Smani, T., Sempere-Ortells, J.M., Zhao, R.C., Soria, B. (2022). Mesenchymal Stromal Cells for COVID-19 Critical Care Patients. In: Haider, K.H. (eds) Handbook of Stem Cell Therapy. Springer, Singapore. https://doi.org/10.1007/978-981-19-2655-6_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-2655-6_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2654-9
Online ISBN: 978-981-19-2655-6
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences