Abstract
The digestive ontogeny of Trachinotus ovatus from hatch to 32 days post-hatch was reviewed in this chapter. The development of digestive system in T. ovatus can be divided into three stages: stage I starting from hatching and ending at the onset exogenous feeding (3 DPH), stage II starting from first feeding and ending at the formation of gastric glands in fish stomach (15 DPH), and stage III starting from the appearance of gastric glands and continuing onward. The specific activity of lipase, trypsin, and amylase in fish increased rapidly from the exogenous feeding to 5–7 DPH and followed by random fluctuations. Pepsin activity was firstly detected on 15 DPH, and the specific activities increased with fish age. The dynamics of enzyme activity reflected the structural development in fish digestive system. After the formation of gastric glands in the fish stomach, the enzyme activities incline to be stable. According to the development of the digestive system, weaning of T. ovatus larvae can be initiated at 15 DPH. This chapter will improve our understanding of T. ovatus ontogeny during the larval phase and supply the feeding and weaning protocol for this important economic fish in hatcheries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
The larval phase of marine fish has been deemed the main “bottleneck” limiting fingerling production to the grow-out facilities (Tucker 1998; Joan Holt et al. 2007). Inappropriate feed uptake can lead to massive mortality in this stage (Kohno et al. 1997; Hunt von Herbing and Gallager 2000; Yufera and Darias 2007; Ma et al. 2012b). During the initial feeding stage, larval fish experience a period of mixed nutrition and progressively transfer from endogenous nutrition to exogenous nutrition. During this transaction period, fish tissues, organs, and systems undergo structural or functional transformation or development. Therefore, knowledge on fish nutritional requirement, digestive performance, and live food supply will improve survival, growth, and nutrition supplement to fish larvae in hatchery rearing environment (Ma et al. 2012b; Hu et al. 2018).
The digestive system in most of the marine fish larvae is immature at hatching and will undergo a series of developments to have a fully and complete functional digestive system (Canino and Bailey 1995; Chen et al. 2006a). Determine the stage of developmental process when a mature digestive system can be formed is practically important in hatchery practices (Ma et al. 2012a). An accurate understanding of the main developmental events timing in fish larvae could guide us to conclude the proper time to wean fish larvae to formulated diets (Cahu and Zambonino-Infante 2001; Baglole et al. 1997). Furthermore, knowledge on the morphological and developmental differences of the digestive system in the larval stage will promote a further understanding on the species-specific digestive abilities and nutritional physiology of larval fish and can be applied to correspond the physiological developments with feeding practice and rearing protocol (Mai et al. 2005; Chen et al. 2006a, b; Lazo et al. 2011).
Previous studies have revealed that fish larvae generally have plenty of levels of digestive enzymes to digest live feeds at first feeding but probably difficult to digest formulated diet (Cahu and Zambonino-Infante 2001; Kolkovski 2001). Evidence indicates that marine larval fish can swallow formulated diet in their early life, but cannot digest it and mostly die without complete digestion of the diet (Beccaria et al. 1991; People Le Ruyet et al. 1993; Koven et al. 2001). The defeat of adaption of formulated diet in larval fish may be caused by insufficient dietary composition and low digestibility, especially when the digestive system of fish larvae is immature (Govoni et al. 1986; Cahu and Zambonino-Infante 2001; Chen et al. 2006b; Rønnestad et al. 2013). Evidence has proposed that the availability of digestive enzymes is an essential criterion for larval fish to survive on a formulated feed (Yufera et al. 2000; Kolkovski 2001; Yufera and Darias 2007). Without enow number of enzymes in the digestive system, food particles cannot be fully digested. Accordingly, understanding of the ontogenetic development of digestive enzymes in larval fish is critical to choose a proper weaning regime to a formulated diet in larva cultivation (Chen et al. 2006b; Lamarre et al. 2007; Ma et al. 2012a).
The aim of this chapter was to review the structural changes and development of digestive enzymes during early development of indoor cultured T. ovatus from hatch until metamorphosis, focusing on the digestive physiology of fish larvae, and pave the way for the improvement of golden pompano hatchery management level.
1.2 Growth and Development Pattern of T. ovatus
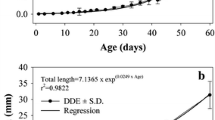
Data described in this chapter derived from our previous study on the digestive functionality in T. ovatus (Ma et al. 2014). On 1 DPH, the standard length of larval T. ovatus averaged at 3.06 mm (Ma et al. 2014). The average growth rate (AGR) and specific growth rate (SGR) were 0.49 mm/day and 5.88%/day, respectively. At hatching, the digestive tract of T. ovatus was a straight tube lying dorsally to the yolk sac, and incipient liver and pancreas were not presented at this stage (Ma et al. 2014). Incipient liver and pancreas come into view and become distinguished on 3 DPH. Mouth opening of fish was observed at the 2 DPH night. First feeding activity was detected on 3 DPH, and rotifers can be easily identified in the midgut of larval fish (Ma et al. 2014). On 4 DPH, yolk sac was completely absorbed, and supranuclear vacuoles (SNV) was formed. Oil globules were completely absorbed on 7 DPH, and the mixed feeding was carried out between 3 and 7 DPH. The standard length between 3 and 7 DPH had no significant difference (P > 0.05).
Gastric glands were firstly found on 15 DPH. The gastric glands consist of single-type secretory cells beneath the epithelium between the cardiac and pyloric regions (Ma et al. 2014). The number of gastric glands increased with the increasing of fish age, and gastric glands were found clustered in the cardiac and fundic regions (Ma et al. 2014). In the meantime, rudimentary pyloric caeca were found on the anterior midgut. Between 17 and 18 DPH, the stomach could be divided into cardiac, fundic, and pyloric regions. With the increasing of fish age, the fundic region was elongated and formed the largest section of the stomach. The number of SNVs in the hindgut decreased and cannot be observed after 19 DPH (Ma et al. 2014). The number of lipid vacuoles increased in posterior midgut on 18 DPH.
Similar to most marine fishes, the digestive system development in T. ovatus can be divided into three stages in accordance with the morphological and histological characters. Stage I began with hatching and finished before commencing exogenous feeding on 2 DPH. At the end of stage I, the yolk sac was partially depleted, the mouth opened, and the intestine was able to adapt to the exogenous feeds. The duration of stage I in T. ovatus is similar to bluefin leatherjacket Thamnaconus modestus, yellowtail kingfish Seriola lalandi, and coral trout Plectropomus leopardus, where nutrition is singly endogenous in the first 2 days after hatching (Chen et al. 2006a; Guan et al. 2013), but is shorter than common dentex Dentex dentex L., where nutrition is singly endogenous in the first 4 days after hatching or even longer (Santamaria et al. 2004; Liu et al. 2013).
Stage II started from onset first feeding (3 DPH) and ended at the formation of gastric glands. The stage II is the momentous stage in fish life as larva needs to quickly develop their feeding ability to accept the exogenous feeding, and failures will affect other physiological functions, leading to malnutrition and even death (Segner et al. 1993; Chen et al. 2006a; Ma et al. 2012b; Xu et al. 2021). In stage II, T. ovatus experienced a mixed nutrition period which fish gradually transferred from endogenous feeding to exogenous feeding. Within this transaction, the digestive system of T. ovatus was developed in structure and function to accommodate to exogenous feeding before yolk-sac reserves were completely absorbed. Thus, the digestive tract of larval fish was further differentiated, and the supranuclear vacuoles and lipid vacuoles appeared in the hindgut and midgut on 4–5 DPH after depletion of yolk residuals. In stage II, T. ovatus fed on Artemia nauplii and rotifers for nutritional resource. Similar to most marine larval fish, significant transformation in the digestive system and speedy depletion of yolk-sac and oil globules may cause starvation or malnutrition if suitable feed is unavailable (Chen et al. 2006a).
Stage III started from the appearance of gastric glands and the pyloric caeca in the stomach of fish. This characteristic can be used as the standard to separate larvae from juveniles in marine fish (Baglole et al. 1997; Rønnestad et al. 2013) and also can be used as the point in time to start weaning fish to formulated diet (Ma et al. 2012b). The formation of this critical organs is species-specific and may also be accomplished with unsynchronized pepsinogen-secretion (reviewed by Rønnestad et al. 2013). For example, it takes 33 days in haddock Melanogrammus aeglefinus L. from hatch to stage III (Hamlin et al. 2000), while it takes 22 days in dover sole Solea solea (Boulhic and Gabaudan 1992), and 15 days in yellowtail kingfish Seriola lalandi (Chen et al. 2006a). Similar to yellowtail kingfish, it took 15 days in T. ovatus to enter stage III, indicating that the T. ovatus larvae belong to fast development species.
1.3 Changes of Digestive Enzyme Activity in Larva T. ovatus
The total activity of trypsin was not observed before 1 DPH and gradually increased with the increasing of fish age, after initial feeding (Ma et al. 2014). The first rapid increase was found on 15 DPH which coincided with the feeding of Artemia nauplii (P < 0.05). The total activity of trypsin reached the maximum at 19 DPH and was not significantly different afterward (P > 0.05). The specific activity of trypsin increased significantly on 7 DPH compared before (P < 0.05). Then, the specific activity of trypsin reduced on 13 DPH and increased significantly on 15 DPH (P < 0.05). Afterward, the specific activity gradually reduced to a low level on 32 DPH, which was similar to the level in newly hatched larvae. The total activity of amylase was not observed before first feeding and remained at low until 13 DPH (Ma et al. 2014). The activity progressively increased from 3 DPH to 17 DPH. The total activity peaked at 26 DPH (P < 0.05) and then kept at this level until the end of the experiment (P > 0.05). The specific activity of amylase increased rapidly on 3 DPH at the initial feeding (P < 0.05). After that, the activity reduced gradually from 5 DPH to 32 DPH (Ma et al. 2014).
The total activity of pepsin was first spotted on 15 DPH (Ma et al. 2014). Afterward, an upward trend was seen in the total activity of pepsin with fish age before 17 DPH. The total pepsin activity increased significantly from 19 DPH to 32 DPH (P < 0.05). The specific activity of pepsin was firstly detected on 15 DPH (Ma et al. 2014). Then, the activity increased with the increasing of fish age and reached to the maximum level on 32 DPH. The total activity of lipase was not significantly different from 1 DPH to 19 DPH. The total activity of lipase gradually increased from 22 DPH to 36 DPH. The specific activity of lipase was increased rapidly from newly hatched larva to 5 DPH (P < 0.05) which was cohering with fish feeding on live feeds. After the first maximum, the specific activity gradually increased until the end of this experiment. On 32 DPH, the specific activity of lipase was 24.7 mU/mg protein, which was 120 times higher than that on 1 DPH (Ma et al. 2014).
Previous studies have demonstrated that the activity of some digestive enzymes in marine fish larvae can be detected before exogenous feeding (Zambonino Infante et al. 2008; Zambonino Infante and Cahu 1994b; Cara et al. 2003; Kolkovski 2001). The development of digestive enzymes in fish larvae can determine the timing of offering exogenous feeds to fish larvae (Gawlicka et al. 2000). In T. ovatus, the specific activity of trypsin, amylase, and lipase was spotted before the onset exogenous feeding, and a rapidly increasing trend was found between 3 and 7 DPH. In Pacific threadfin Polydactylus sexfilis, the amylase activity can’t be quantified at hatch, but can be detected before the first feeding (Kim et al. 2001). The activities of trypsin, amylase, and lipase were significantly stronger than those in the newly hatched larvae in some species such as white sea bream Diplodus sargus and yellowtail kingfish (Cara et al. 2003; Chen et al. 2006b). In larval Eurasian perch Perca fluviatilis, the specific activities of trypsin and amylase can be found after hatch and increased greatly in the first few days after exogenous feeding (Cuvier-Peres and Kestemont 2002). These results suggest that digestive enzymes of fish larvae are at a low level before the start of exogenous feeding, while their activities rise to a high level during exogenous feeding. Similarly, the start points of trypsin, amylase, and lipase in T. ovatus are triggered by internal mechanisms, rather than by dietary stimulation, but reaching a relatively high level may be associated with food stimulation.
The developing patterns of digestive enzymes of T. ovatus like lipase and amylase were strongly associated with the structural changes of the digestive system identified in the histological studies. The significant increase of specific activities of lipase and amylase before exogenous feeding is similar to species like crimson snapper Lutjanus erythropterus and yellowtail kingfish (Chen et al. 2006b; Cui et al. 2018). The fluctuations in specific enzyme activities reflected functional development in the digestive tract and associated glands of fish larvae. After the appearance of gastric glands, the digestive system became functional in acidic digestion, and the specific activities of these digestive enzymes remained constant, while the total enzyme activities increased gradually with age. In crimson snapper, yellowtail kingfish Seriola lalandi, and walleye pollock Gadus chalcogrammus larvae, high specific activities of the trypsin and amylase were corresponded to the transitional period from endogenous to exogenous feeding (Cui et al. 2018; Chen et al. 2006b; Oozeki and Bailey 1995). Evidences have indicated that the increase of the digestive enzyme activities in the early larval phase could be genetically programmed and is not led by external food (Zambonino Infante and Cahu 1994a; Ma et al. 2014). The developmental pattern of the enzyme activity from fluctuation to relatively stable in a late stage may be related to structural and physiological alterations in the larvae during metamorphosis (Chen et al. 2006b). Similar to the findings reported by Chen et al. (2006b), the development of larval T. ovatus shows the concurrence between the digestive enzyme activities and the morphological development of the digestive system.
In T. ovatus, the developmental pattern of trypsin activities varied from other digestive enzymes. Two maximums were recorded on 7 and 15 DPH, and then the specific activity reduced progressively. During the ontogenetic development of T. ovatus, the total enzyme activity showed a progressive increasing trend and followed by a decreasing trend afterward. The pattern of trypsin activities observed in T. ovatus larvae is similar to European sea bass larvae, in which the trypsin-specific activity progressive increased to 23 DPH followed by a rapid decrease but increased again on 40 DPH to the level when the exogenous feeding started (Zambonino Infante and Cahu 1994a). Meanwhile, the development patterns are varied in many other species. In Eurasian perch larvae, both specific and total activity of trypsin sustained to increase as fish larvae grew for 30 days (Cuvier-Peres and Kestemont 2002). In T. ovatus, the maximum specific activity of trypsin occurred on 15 DPH when the gastric glands formed and pepsin activity was detected. Afterward, the specific activity of trypsin is reduced with the increase of the pepsin activity. The sustaining reduction of trypsin activity after metamorphosis and the increase of pepsin activity in T. ovatus indicate that protein digestive enzyme has been transformed from trypsin to pepsin, and the stomach becomes functional (Chen et al. 2006a, b; Segner et al. 1994; Guerreiro et al. 2010). The digestive system of the T. ovatus larvae has the physiological conditions to receive the compound inert diet on 15 DPH, and weaning should begin at this point and afterward.
1.4 Conclusion
In conclusion, T. ovatus belongs to the fast-growing species as the standing of digestive ontogeny of fish larvae phase is relatively short. The specific activities of amylase and lipase were stable, and the total activity of these two enzymes increased gradually from 18 DPH. The relatively low specific of amylase reflects the carnivorous nature of T. ovatus juveniles and the limited capacity to digest carbohydrates.
References
Baglole CJ, Murray HM, Goff GP, Wright GM (1997) Ontogeny of the digestive tract during larval development of yellowtail flounder: a light microscopic and mucous histochemical study. J Fish Biol 51:120–134
Beccaria C, Diaz JP, Connes R, Chatain B (1991) Organogenesis of the exocrine pancreas in the sea bass, Dicentrarchus labrax L., reared extensively and intensively. Aquaculture 99:339–354
Boulhic M, Gabaudan J (1992) Histological study of the organogenesis of the digestive system and swim bladder of the Dover sole, Solea solea (Linnaeus 1758). Aquaculture 102:373–396
Cahu C, Zambonino-Infante J (2001) Substitution of live food by formulated diets in marine fish larvae. Aquaculture 200:161–180
Canino MF, Bailey KM (1995) Gut evacuation of walleye pollock larvae in response to feeding conditions. J Fish Biol 46:389–403
Cara JB, Moyano FJ, Cardenas S, Fernandez-Diaz C, Yufera M (2003) Assessment of digestive enzyme activities during larval development of white bream. J Fish Biol 63:48–58
Chen BN, Qin JG, Kumar MS, Hutchinson W, Clarke S (2006a) Ontogenetic development of the digestive system in yellowtail kingfish Seriola lalandi larvae. Aquaculture 256:489–501
Chen BN, Qin JG, Martin SK, Hutchinson WG, Clarke SM (2006b) Ontogenetic development of digestive enzymes in yellowtail kingfish Seriola lalandi larvae. Aquaculture 260:264–271
Cui K, Fu Z, Cheng D, Yang Q, Ma Z, Qin JG, Hu J (2018) Development of immune functionality in larval and juvenile crimson snapper Lutjanus erythropterus (Bloch 1790). Aquacult Rep 10:1–7
Cuvier-Peres A, Kestemont P (2002) Development of some digestive enzymes in Eurasian perch larvae Perca fluviatilis. Fish Physiol Biochem 24:279–285
Gawlicka A, Parent B, Horn MH, Ross N, Opstad I, Torrissen OJ (2000) Activity of digestive enzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): indication of readiness for first feeding. Aquaculture 184:303–314
Govoni JJ, Boehlert GW, Watanabe Y (1986) The physiology of digestion in fish larvae. Environ Biol Fish 16:59–77
Guan J, Ma Z, Zheng Y, Guan S, Li C, Liu H (2013) Breading and larval rearing of bluefin leatherjaket, Thamnaconus modestus (Gunther, 1877) under commerical scales. Int J Aquacult 3:55–62
Guerreiro I, de Vareilles M, Pousão-Ferreira P, Rodrigues V, Dinis MT, Ribeiro L (2010) Effect of age-at-weaning on digestive capacity of white seabream (Diplodus sargus). Aquaculture 30:194–205
Hamlin HJ, Hunt von Herbing I, Kling LJ (2000) Histological and morphological evaluations of the digestive tract and associated organs of haddock throughout post-hatching ontogeny. J Fish Biol 57:716–732
Hu J, Liu Y, Ma Z, Qin JG (2018) Feeding and development of warm water marine fish larvae in early life. In: Yúfera M (ed) Emerging issues in fish larvae research. Springer, Cham, pp 275–296
Hunt von Herbing I, Gallager SM (2000) Foraging behavior in early Atlantic code larvae (Gadus morhua) feeding on a protozoan (Balanion sp.) and a copepod nauplius (Pseudodiaptomus sp.). Mar Biol 136:591–602
Joan Holt G, Faulk CK, Schwarz MH (2007) A review of the larviculture of cobia Rachycentron canadum, a warm water marine fish. Aquaculture 268:181–187
Kim BG, Divakaran S, Brown CL, Ostrowski AC (2001) Comparative digestive enzyme ontogeny in two marine larval fishes: Pacific threadfin (Polydactylus sexfilis) and bluefin trevally (Caranx melampygus). Fish Physiol Biochem 24:225–241
Kohno H, Ordonio-Aguilar RS, Ohno A, Taki Y (1997) Why is grouper rearing difficult?: an approach from the development of the feeding apparatus in early stage larvae of the grouper, Epinephelus coioides. Ichthyol Res 44:267–274
Kolkovski S (2001) Digestive enzymes in fish larvae and juveniles-implications and applications to formulated diets. Aquaculture 200:181–201
Koven W, Kolkovski S, Hadas E, Gamsiz K, Tandler A (2001) Advances in the development of microdiets for gilthead seabream, Sparus aurata: a review. Aquaculture 194:107–121
Lamarre SG, François NRL, Lemieux H, Falk-Petersen I, Blier PU (2007) The digestive and metabolic enzyme activity profiles of a nonmetamorphic marine fish species: effects of feed type and feeding level. Can J Fish Aquat Sci 64:849–856
Lazo JP, Darias MJ, Gisbert E (2011) Ontogeny of the digestive tract. In: Holt GJ (ed) Larval fish nutrition. Wiley, West Sussex, UK, pp 1–47
Liu CX, Luo Z, Tan XY, Gong SY (2013) Ontogenetic development of the digestive system in agastric Chinese sucker, Myxocyprinus asiaticus, larvae. J World Aquacult Soc 44:350–362
Ma Z, Qin JG, Hutchinson W, Chen BN, Song L (2012a) Responses of digestive enzymes and body lipids to weaning times in yellowtail kingfish Seriola lalandi (Valenciennes, 1833) larvae. Aquac Res 45:973–982
Ma Z, Qin JG, Nie Z (2012b) Morphological changes of marine fish larvae and their nutrition need. In: Pourali K, Raad VN (eds) Larvae: morphology, biology and life cycle. Nova Science Publishers, Inc., New York, pp 1–20
Ma Z, Guo H, Zheng P, Wang L, Jiang S, Qin JG, Zhang D (2014) Ontogenetic developmeng of digestive functionality in golden pompano Trachinotus ovatus (Linnaeus 1758). Fish Physiol Biochem 40:1157–1167
Mai K, Yu H, Ma H, Duan Q, Gisbert E, Infante JLZ, Cahu CL (2005) A histological study on the development of the digestive system of Pseudosciaena crocea larvae and juveniles. J Fish Biol 67:1094–1106
Oozeki Y, Bailey KM (1995) Ontogenetic development of digestive enzyme activities in larval walleye pollock, Theragra chalcogramma. Mar Biol 122:177–186
People Le Ruyet J, Alexandre JC, Thébaud L, Mugnier C (1993) Marine fish larvae feeding: formulated diets or live prey? J World Aquacult Soc 24:211–224
Rønnestad I, Yúfera M, Ueberschär B, Ribeiro L, Sæle Ø, Boglione C (2013) Feeding behaviour and digestive physiology in larval fish: current knowledge, and gaps and bottlenecks in research. Rev Aquacult 5:S59–S98
Santamaria CA, Marin de Mateo M, Traveset R, Sala R, Grau A, Pastor E, Sarasquete C, Crespo S (2004) Larval organogenesis in common dentex Dentex dentex L. (Sparidae): histological and histochemical aspects. Aquaculture 237:207–228
Segner H, Roesch R, Verreth J, Witt U (1993) Larval nutritional physiology: studies with Clarias gariepinus, Coregonus lavaretus and Scophthalmus maximus. J World Aquacult Soc 24:121–134
Segner H, Storch V, Reinecke M, Kloas W, Hanke W (1994) The development of functional digestive and metabolic organs in turbot, Scophthalmus maximus. Mar Biol 119:471–486
Tucker JW (1998) Marine fish culture. Kluwer Academic Publishers, Norwell, MA, pp 299–374
Xu H, Fan S, Wang G, Miao X, Li Y (2021) Transcriptome analysis reveals the importance of exogenous nutrition in regulating antioxidant defenses during the mouth-opening stage in oviparous fish. Fish Physiol Biochem 47:1087–1103
Yufera M, Darias MJ (2007) The onset of exogenous feeding in marine fish larvae. Aquaculture 268:53–63
Yufera M, Fernandez-Diaz C, Pascual E, Sarasquete M, Moyano F, Diaz M, Alarcon F, Garcia-Gallego M, Parra G (2000) Towards an inert diet for first-feeding gilthead seabream Sparus aurata L. larvae. Aquac Nutr 6:143–152
Zambonino Infante JL, Cahu C (1994a) Development and response to a diet change of some digestive enzymes in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem 12:399–408
Zambonino Infante JL, Cahu CL (1994b) Influence of diet on pepsin and some pancreatic enzymes in sea bass (Dicentrachus labrax) larvae. Comp Biochem Physiol 109A:209–212
Zambonino Infante J, Gisbert E, Sarasquete C, Navarro I, Gutiérrez J, Cahu C (2008) Ontogeny and physiology of the digestive system of marine fish larvae. In: Cyrino JEP, Bureau DP, Kapoor BG (eds) Feeding and digestive function of fishes. Science Publishers, Enfield, NH, p 281
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 China Agriculture Press
About this chapter
Cite this chapter
Ma, Z., Yu, G., Qin, J.G. (2022). Ontogenetic Development of the Digestive System in Golden Pompano Trachinotus ovatus. In: Ma, Z., Yu, G., Qin, J.G. (eds) Ontogenetic development of pompano Trachinotus ovatus. Springer, Singapore. https://doi.org/10.1007/978-981-19-1712-7_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-1712-7_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1711-0
Online ISBN: 978-981-19-1712-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)