Abstract
Cholesterol is a major component of mammalian cell membranes and plays important structural and functional roles. However, excessive cholesterol accumulation is toxic to cells and constitutes the molecular basis for many diseases, especially atherosclerotic cardiovascular disease. Thus, cellular cholesterol is tightly regulated to maintain a homeostasis. Reverse cholesterol transport (RCT) is thought to be one primary pathway to eliminate excessive cholesterol from the body. The first and rate-limiting step of RCT is ATP-binding cassette (ABC) transports A1 (ABCA1)- and ABCG1-dependent cholesterol efflux. In the process, ABCA1 mediates initial transport of cellular cholesterol to apolipoprotein A-I (apoA-I) for forming nascent high-density lipoprotein (HDL) particles, and ABCG1 facilitates subsequent continued cholesterol efflux to HDL for further maturation. In this chapter, we summarize the roles of ABCA1 and ABCG1 in maintaining cellular cholesterol homoeostasis and discuss the underlying mechanisms by which they mediate cholesterol export.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Cholesterol functions as an important cell membrane component in mammals and is essential for establishing proper membrane permeability and fluidity. Additionally, cholesterol is associated with cell signaling transduction and takes part in the biosynthesis of bile acids, vitamin D, and steroid hormones. Thus, maintaining cholesterol homeostasis is of critical importance for human health. However, cholesterol accumulation can cause cell death and be harmful human body. For instance, hypercholesterolemia has long been regarded as a critical contributor to atherosclerotic cardiovascular disease, the first cause of death globally.
To maintain the dynamic balance of cholesterol, excessive cholesterol must be eliminated from the body. Reverse cholesterol transport (RCT), which is referred to the delivery of cholesterol in arterial wall cells to the liver by high-density lipoprotein (HDL) for further metabolism and excretion, plays a key role in this process [1]. The ATP-binding cassette (ABC) superfamily of transporters belongs to transmembrane proteins and contains a variety of members. Among these, ABCA1 mediates initial transport of free cholesterol (FC) and phospholipid molecules to apolipoprotein A-I (apoA-I) for nascent HDL biogenesis, and ABCG1 promotes subsequent continued efflux of FC to these HDL particles [2]. ABCA1- and ABCG1-dependent cholesterol efflux is the first and rate-limiting step of RCT. There is increasing evidence that upregulation of these two transporter expression facilitates RCT and protects against atherosclerosis in animal models [3,4,5]. This chapter summarizes the role of ABCA1 and ABCG1 in maintaining cellular cholesterol homoeostasis with an emphasis on how they mediate cholesterol efflux from cells.
7.2 ABCA1 and Cholesterol Homeostasis
7.2.1 Structural Features of ABCA1
ABCA1 is a full transporter and an integral membrane protein. Human ABCA1 gene is mapped to chromosome 9q31.1, spans 149 kb, and contains 50 exons and 49 introns. The mature ABCA1 protein is made up of 2261 amino acids, and its molecular mass is 254 kDa. ABCA1 has two transmembrane domains (TMDs) linked covalently, each containing six transmembrane α-helices (TMs). Also, there are two large extracellular domains (ECDs) and multiple membrane-spanning segments. ECD1 resides between TM1 and TM2, and ECD2 resides between TM7 and TM8. They are connected by two intramolecular disulfide bonds and serve as the binding sites for apoA-I. There are two nucleotide-binding domains (NBDs) in its intracellular region. There are three conserved peptide motifs in each NBD, designated as Walkers A, B, and C. ATP is known to bind to Walker A, and Walker B is associated with subsequent hydrolysis. The hydrolysis of ATP in these sites can alter ECD conformation, thereby leading to the binding of apoA-I. The NBD1 includes a PEST sequence that is composed of proline, glutamic acid, serine, and threonine. Calpain degrades ABCA1 through interaction with this sequence. However, whether Walker C signature is involved in nascent HDL biogenesis remains unclear and needs further characterization.
A recent study revealed the single-particle cryoelectron microscopy structure of human ABCA1 protein at 4.1 Å resolution [6]. The overall structure resembles an elongated torch that is about 200 Å high. Both ECDs are the upward flaring flame, and other domains constitute the handle. There is a shallow pocket enclosed by the intracellular segments of TMs 1/2/5. The amino acid residues within these TMs are mostly polar and charged, which are responsible for binding to the polar heads of phospholipid molecules. Both TMD1 and TMD2 constitute a narrow chamber as an intracellular gate, which drives the transport of lipids from the membrane inner leaflet to the membrane outer leaflet. The helical domains in ECD1 and ECD2 together enclose an elongated hydrophobic tunnel that acts as a temporary storage and delivery passage for lipids. These structural features provide an important framework for better understanding of ABCA1-mediated cholesterol efflux.
7.2.2 Expression and Regulation of ABCA1
Almost all tissues and cells can express ABCA1. However, it is highly expressed in cardiovascular system, digestive system, and central nervous system. Liver X receptor α (LXRα), a member of the nuclear receptor superfamily, plays a critical role in stimulating ABCA1 transcription. LXRα combines with retinoid X receptor (RXR), leading to the formation of an obligate heterodimer. The LXRα/RXR complex then binds to the promoter region of ABCA1 gene to induce its expression. The increase in intracellular cholesterol levels has been shown to promote the production of oxysterols, which act as a potent LXRα agonist and upregulate the expression of ABCA1 [7]. Exposure of nobiletin to mouse macrophages J774.1 activates the AMP-activated protein kinase/LXRα pathway, leading to increased ABCA1 expression [8]. In murine bone-marrow-derived macrophages, treatment with DASA-58, an activator of pyruvate kinase M2, downregulates LXRα expression and then decreases ABCA1 levels [9]. Additionally, fargesin facilitates the phosphorylation of CEBPα at Ser21, leading to upregulation of LXRα and ABCA1 expression in macrophages [3].
At the transcriptional level, ABCA1 expression can be regulated in an LXRα-independent manner. Like LXRα, retinoid acid receptor–related orphan receptor α (RORα) is also a nuclear receptor. There is a RORα response element present in the ABCA1promoter. Both CPG52608 and SR1001 have been identified as the specific ligands of RORα. Upon binding to CPG52608 and SR1001 in THP-1 macrophages, RORα exerts a transactivating effect on ABCA1 [10]. Overexpression of prolactin regulatory element-binding (PREB) protein in CRL-2018 cells directly enhances the transcriptional activity of ABCA1 [11]. Heat shock protein 70 impairs ABCA1 expression by preventing Elk-1 from binding to the ABCA1 promoter in THP-1-macrophage-derived foam cells [12]. In contrast, zinc finger protein 202 was shown to directly inhibit ABCA1 transcription [13]. C1q tumor necrosis factor–related protein (CTRP) 1 decreases miR-424-5p levels, which in turn increases Forkhead box O1 expression in the nucleus and inhibits ABCA1 transcription [14]. Overexpression of Kcnq1 overlapping transcript 1, a long noncoding RNA, sponges miR-452-3p to enhance histone deacetylase 3 expression, leading to a significant decrease in the transcriptional activity of ABCA1. Akt is also identified as a transcriptional inhibitor of ABCA1 [15].
At the posttranscriptional level, a variety of agents have been reported to modulate ABCA1 expression. For instance, apoA-I interacts with ABCA1 to suppress the cleavage of ABCA1 protein by calpain. After stimulation with LXRα agonists, syntrophin forms a complex with ABCA1 in macrophages. The resulting complex combines with apoA-I, leading to a significant increase in ABCA1 protein stability [16]. Thus, LXRα can induce ABCA1 expression at the transcriptional and posttranscriptional levels. Tetramethylpyrazine is a biologically active component isolated from the Chinese medicinal plant Ligusticum wallichii Franchat. Administration of tetramethylpyrazine markedly enhances ABCA1 protein stability by inhibiting lysosomal degradation in RAW264.7 macrophages. When apoA-I binding protein (AIBP) combines with apoA-I, the degradation of ABCA1 protein mediated by COP9 signalosome subunit 2 is markedly inhibited [17]. In addition, calmodulin and paeonol were shown to protect ABCA1 from calpain-mediated cleavage [18]. Nucleolin significantly increases the stability of ABCA1 mRNA in ox-LDL-treated RAW264.7 macrophages [19]. A similar effect is also observed when extracellular-signal-regulated kinases 1/2 (ERK1/2) are inactivated. As an RNA-binding protein, human antigen R promotes ABCA1 translation by binding to its 3′-untranslated region (UTR) [20]. MicroRNAs (miRNAs) mediate the degradation of target mRNAs and/or inhibit their translational process by binding to the 3’-UTR, leading to silencing of target gene expression. Dysregulation of miRNAs has been linked to abnormal lipid metabolism and the development of atherosclerosis. Interestingly, ABCA1 has been identified as a putative target of a variety of miRNAs, such as miR-33a/b, miR-20a-5p, miR-27a/b, miR-758, miR-19b, miR-17-5p, miR-93, miR-325, miR-25-5p, and miR-361-5p [21].
7.2.3 Biological Functions of ABCA1
The major biological function of ABCA1 is to mediate the translocation of FC from cells, especially macrophages and vascular smooth muscle cells (VSMCs), to lipid-poor/free apoA-I for nascent HDL particle generation. When mouse peritoneal macrophages laden with cholesterol were treated with diluted human serum, ABCA1 mediates approximately half of cholesterol export and ABCG1 mediated 20% of cholesterol export [22]. This finding suggests that ABCA1 is the most important transporter responsible for cholesterol release from cells. Consistently, when the ABCA1 gene is mutated in humans, Tangier disease occurs. This disease displays extremely low plasma HDL cholesterol (HDL-C) concentration and premature atherosclerosis [23]. Deletion of ABCA1 in mice impairs macrophage-to-feces RCT and promotes atherosclerotic plaque formation [24]; however, overexpression of ABCA1 in mice was shown to increase circulating HDL-C concentration and mitigate atherosclerosis [25]. Thus, targeting ABCA1 is an attractive and promising strategy to prevent and treat atherosclerotic cardiovascular disease.
In addition to taking part in lipid metabolism, ABCA1 has other biological effects. It has been reported that knockout of ABCA1 promotes the expression of proinflammatory cytokines and chemokines in mouse bone marrow–derived macrophages challenged with lipopolysaccharide (LPS) [26]. In contrast, overexpression of ABCA1 significantly attenuates the levels of interleukin-6 and tumor necrosis factor-α in bovine aortic endothelial cells stimulated with LPS [27]. Incubation with AIBP or apoA-I upregulates the expression of ABCA1, thereby leading to decreased secretion of proinflammatory cytokines from THP-derived macrophages challenged with LPS [28]. These observations indicate that ABCA1 exerts an anti-inflammatory action and acts as a critical link between inflammation and lipid metabolism. Additionally, ABCA1 can inhibit retinal ganglion cell apoptosis, promote colorectal cancer cell proliferation, enhance platelet reactivity, and stimulate insulin secretion from pancreatic β-cells [29].
7.2.4 Potential Mechanisms Underlying ABCA1-Dependent Cholesterol Efflux
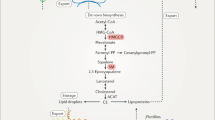
Currently, several models have been presented to clarify the molecular mechanisms underlying ABCA1-dependent cholesterol efflux. The first model involves channel trafficking [6]. It is suggested that a channel is present in between TMD1 and TMD2 of ABCA1, which plays a central role in controlling lipid access. The chamber is initially open at the bottom and close at the top. In the presence of lipid loading, accumulated phospholipids in the inner leaflet of plasma membrane are laterally transferred to the chamber by binding to amino acid residues within TMs 1/2/5. In this process, cholesterol is concurrently accessible to the chamber with the aid of phospholipids. ATP is then recruited to both NBDs, which leads to the dimerization of NBDs and consequently closes the chamber. ABCA1 then flops the trapped lipids to the outer leaflet of plasma membrane. ATP hydrolysis at both NBDs leads to the formation of an ADP-bound intermediate, which alters the conformation of TMD1 and TMD1 to open the chamber at the top. Cholesterol and phospholipids are egressed from the chamber to the elongated hydrophobic tunnel formed by both ECDs. The ECD conformation can be also changed by the hydrolysis of ATP. This allows the interaction of ECDs with apoA-I. Upon binding to ABCA1, apoA-I takes up lipids from the elongated hydrophobic tunnel to assemble nascent HDL particles. These particles are then released from the cell surface to further accept lipids. Following dissociation of ADP from NBDs, the chamber is restored to the initial open status for lipid uptake. Given that this is a structure-based model, more direct evidence needs to confirm its rationality.
The second model involves ABCA1 dimerization [30, 31]. In this model, ABCA1 monomers constantly transport FC and phospholipids from plasma membrane to their ECDs due to ATP hydrolysis. Following sequestration of sufficient lipids, these monomers undergo conformational changes to dimerize. Subsequently, the membrane-skeletal actin filaments and other stable structure components in plasma membrane are recruited to the lipidated ABCA1 dimers. This interaction is beneficial for apoA-I access. Lipid-poor/free apoA-I combines with the ECDs within the ABCA1 dimers. The lipids reserved by ABCA1 are then delivered to apoA-I. Lipid loading induces a conformational change of apoA-I, leading to its dissociation from ABCA1 and nHDL production. Upon release of the reserved lipids, the ABCA1 dimers transform into monomers, entering next cycle to receive lipids. This model suggests that ABCA1 dimerization is of critical importance to cholesterol export from cells. Promoting the conversion of ABCA1 monomers to dimers may be another effective strategy for inhibiting intracellular lipid accumulation.
The third model demonstrates that ABCA1 promotes the efflux of intracellular cholesterol to apoA-I through a two-step process [32, 33]. In this model, ABCA1 first facilitates the delivery of phospholipids from the inner membrane leaflet to the outer membrane leaflet. TP is recruited to both NBDs of ABCA1. Then, the hydrolysis of ATP induces a conformational change of ABCA1. This allows an interaction of apoA-I with ABCA1. Phospholipids are loaded onto apoA-I to form a complex, which is a much better acceptor for cholesterol than apoA-I itself. The phospholipid/apoA-I complexes then enter the caveolae, 50–100 nm cellular membrane invaginations enriched in lipids. FC in the caveolae is transported to these complexes in an ABCA1-independent autocrine or paracrine fashion for nHDL production. It is noteworthy that when cells are pretreated with cyclodextrin to deplete intracellular cholesterol, phospholipid efflux and apoA-I binding are not affected, while cholesterol removal disappears [34]. This suggests that both steps are independent of each other. However, a later study showed that phospholipid efflux is tightly coupled with cholesterol efflux [18]. Thus, additional work is required to determine what causes the conflicting findings.
The forth model involves apoA-I-free vesicle [35]. Two apoA-I monomers form a half-circle dimer as revealed by the crystal structure, which is necessary for lipid binding. Interestingly, dimerized apoA-I can acquire lipids from not only cellular membrane but also membrane-derived vesicles shed by ABCA1 during nascent HDL biogenesis. In this process, ABCA1 first translocates FC and phospholipids to the outer leaflet independent of apoA-I. Higher phospholipid levels in this region create mushroom-like protrusions in close proximity to ABCA1 for alleviating surface tension. These protrusions are released from plasma membrane as apoA-I-free vesicles, representing apoA-I-independent lipid efflux. Subsequently, apoA-I docks to plasma membrane through its hydrophobic C-terminus and binds to the ECDs of ABCA1 via its N-terminal domain. The interaction between both molecules leads to the unfolding of apoA-I N-terminus, allowing it to form a dimer. A large amount of FC and phospholipid molecules transported by ABCA1 are loaded onto dimerized apoA-I, leading to the production and release of nascent HDL particles. It is worth noting that these nascent HDL particles can continue to gain lipids from apoA-I-free vesicles to form larger particles.
The fifth model involves retroendocytosis [36, 37]. In addition to plasma membrane, the endosomes are regarded as an important reservoir of cellular cholesterol. ABCA1 resides not only on the cell surface, but also in the endosomal compartments. Consistently, the apoA-I lipidation can occur at these two sites. Several lines of evidence have demonstrated that apoA-I can be internalized to the endosomes for nHDL assembly in a pathway called retroendocytosis. In this model, apoA-I binds to ABCA1 on the cell surface to form a complex, which then enters clathrin-coated pits. The complexes are endocytosed to early endosomes in a Rab5-dependent manner. ABCA1 located in the early endosomal membrane translocates lipids to the endosomal lumen for apoA-I lipidation. ApoA-I is further lipidated when early endosomes become late endosomes. This results in the biogenesis of nascent HDL particles. These particles are then transferred to cycling endosomes that return to the cell surface with the help of Rab4. After fusion with plasma membrane, nHDL is secreted into the extracellular space, and ABCA1 recycles to plasma membrane. This model suggests that stimulating apoA-I internalization provides an alternative approach to enhance ABCA1-dependent cholesterol efflux.
7.3 ABCG1 and Cholesterol Homeostasis
7.3.1 Structural Features of ABCG1
Human ABCG1 gene is located in chromosome 21q22.3. There are 23 exons in human ABCG1 gene. This gene comprises alternative start codons, which leads to the generation of multiple transcripts in a tissue-specific manner. The alternative splicing can generate eight different ABCG1 isoforms with 644–785 amino acids in length. The ABCG1 protein is composed of one NBD at the N-terminus and one transmembrane domain containing six α helices. Thus, ABCG1 belongs to a half-transporter. ABCG1 must be dimerized to exert its biological functions.
7.3.2 Expression and Regulation of ABCG1
Like ABCA1, highly expressed ABCG1 is found in arterial wall cells, including macrophages, VSMCs, and endothelial cells. It is worth noting that in addition to cardiovascular system, other organs such as the spleen, lung, kidney, and brain can express ABCG1. At the subcellular levels, ABCG1 predominantly locates in plasma membrane and endosomes.
Similar to ABCA1, LXRα plays a central role in stimulating ABCG1 transcription. Biochanin A is a dietary isoflavone that is extracted from red clover and cabbage and has a cardiovascular protective property. Administration of biochanin A was shown to upregulate ABCG1 expression and inhibit foam cell formation in THP-1-derived macrophages, at least in part, by activating the peroxisome-proliferator-activated receptor γ (PPARγ)/LXRα signaling pathway [38]. CTRP12 is a highly conserved paralog of adiponectin. Overexpression of CTRP12 through lentiviral vector in THP-1-derived macrophages dramatically diminishes miR-155-5p levels, which in turn increases LXRα expression and elevates the transcriptional activity of ABCG1. TP53-induced glycolysis and apoptosis regulator (TIGAR) is a new p53-inducible protein and plays an important role in protecting against oxidative stress. Increased TIGAR expression is observed in macrophage foam cells and atherosclerotic lesions. Importantly, silencing of TIGAR by short hairpin RNA restrains ABCG1 expression and promotes lipid accumulation through the reactive oxygen species/27-hydroxylase/LXRα signaling cascade in THP-1-derived macrophages. In addition, ox-LDL inhibits ABCG1 expression through the mitogen-activated protein kinase/ERK1/2/LXRα pathway in INS-1 cells.
Many agents have been reported to modulate ABCG1 expression at the posttranscriptional level. For example, treatment of Raw264.7 cells with 12-O-tetradecanoylphorbol-13-acetate activates protein kinase C and then phosphorylates ABCG1, leading to a significant increase in its stability and subsequent cholesterol efflux. Activation of adenosine monophosphate–activated protein kinase robustly enhances the stability of ABCG1 mRNA by binding to its 3’-UTR in human and bovine aortic endothelial cells. Treatment with coenzyme Q10 elevates the mRNA and protein levels of ABCG1 through the activator protein-1/miR-378 pathway in peritoneal macrophages of apolipoprotein-E-deficient (apoE−/−) mice [39]. In plasma and macrophages of apoE−/− mice with atherosclerosis, miR-23a-5p expression is upregulated. Knockdown of miR-23a-5p promotes cholesterol efflux in macrophages and reduces atherosclerotic burden in apoE−/− mice by directly upregulating ABCG1 expression. Also, miR-33a/b, miR-320b, miR-581, and miR-128-2 have been proven to directly target ABCG1.
7.3.3 Biological Functions of ABCG1
The major biological effect of ABCG1 is to promote the efflux of intracellular FC to HDL for further maturation. There is growing evidence that promoting ABCG1 expression inhibits lipid accumulation and foam cell formation in THP-1 macrophages as well as increases RCT efficiency and protects against atherosclerosis in apoE−/− mice. Conversely, prevention of ABCG1 accelerates macrophage foam cell formation and the development of atherosclerosis in animal models [40, 41]. Although the majority of studies indicated that ABCG1 plays a beneficial effect on atherogenesis, some reports showed conflicting results. It has been reported that ABCG1 overexpression in apoE−/− mice does not alter atherosclerotic plaque burden. Macrophage-specific ablation of ABCG1 reduces atherosclerotic lesion area in hyperlipidemic low-density lipoprotein receptor-deficient (Ldlr−/−) mice. In addition. Deletion of ABCG1 in Ldlr−/− mice promotes early atherosclerotic lesion formation and slows down the progression of advanced atherosclerotic lesions [42]. Therefore, further research will be required to clarify the precise role of ABCG1 in the development of atherosclerosis.
Given the critical role of ABCA1 and ABCG1 in mediating intracellular cholesterol export, the combined upregulation of these two transporter expression may be more effective in suppressing foam cell formation and atherosclerosis development. As expected, the simultaneous overexpression of ABCA1 and ABCG1 promotes FC efflux from macrophage in a synergistic manner. In contrast, silencing of ABCA1 and ABCG1 leads to more FC release from macrophage and RCT when compared with ABCG1 silencing alone [43]. In line with this study, transplantation of bone marrow lacking ABCA1 and ABCG1 into Ldlr−/− mice results in increased foam cell accumulation and atherosclerotic plaque burden as compared to single knockout of ABCA1 or ABCG1 [44]. Loss of ABCA1 and ABCG1 in endothelial cells of mice displays a similar effect [45]. Thus, targeting these two transporters simultaneously may be a more promising approach to improve plasma lipid profiles and treat atherosclerosis-associated diseases.
7.3.4 Potential Mechanisms Underlying ABCG1-Dependent Cholesterol Efflux
Currently, three models have been available to reveal how ABCG1 drives cholesterol translocation. The first model proposed that ABCG1 promotes the delivery of FC from the inner membrane leaflet to the outer membrane leaflet, which makes FC easier to be removed by HDL [46]. The second model demonstrated that ABCG1 functions as an intracellular cholesterol transporter localized in the endocytic vesicles [47]. In this model, ABCG1 first transports FC to the inner leaflet of these vesicles. Subsequently, these vesicles fuse with plasma membrane. Finally, HDL accepts FC from plasma membrane. The third model suggests that ABCG1 takes up HDL for entry into late endosomes, where HDL binds to FC [48]. Thereafter, FC-bound HDL is secreted into extracellular space. Of note, these models can not fully explain the mechanisms by which ABCG1 promotes intracellular cholesterol efflux. Additional work is still needed to precisely clarify the underlying mechanisms.
7.4 ApoA-I and Cholesterol Homeostasis
It is well known that apoA-I accepts cholesterol exported by ABCA1, which is essential for nascent HDL particle formation. Thus, it is not surprising that apoA-I acts as the core and major component of HDL. It is estimated that apoA-I accounts for about 70% of total HDL proteins. ApoA-I is predominantly produced by hepatocytes and enterocytes. Human apoA-I protein is made up of 243 amino acids with a molecular mass of 28 kDa. The last 199 amino acids comprise a series of ten repeating amphipathic α-helices. Among these, eight α-helices contain 22 amino acids, and two α-helices contain 11 amino acids [49]. The ninth and tenth α-helices are localized to the C-terminal region and have the highest affinity for lipid binding [50, 51]. Thus, these two α-helices are of critical importance for FC transport. Following synthesis, apoA-I is secreted into the circulation and exists in lipid-free, poor, and bound states. Lipid-free/poor apoA-I has a stronger ability to accept FC than lipid-bound apoA-I. Therefore, targeting lipid-free/poor apoA-I is more effective for the therapeutic intervention of hypercholesterolemia and atherosclerosis-associated diseases.
There are two natural mutations of apoA-I in humans, designated as L141RPisa and L159RFIN. When L141RPisa or L159RFIN is expressed in mice, circulating HDL levels are significantly decreased and atherosclerosis occurs at the early stage [52]. In hyperlipidemic mice, dysfunctional HDL with L159RFIN leads to a significant increase in atherosclerotic lesion area. ApoA-I Mytilene is a truncation form of apoA-I and results from a heterozygous nonsense mutation. It has been reported that apoA-I Mytilene leads to a marked decrease in plasma apoA-I concentration and the occurrence of premature coronary artery disease. OSBPL1A p.C39X is a loss-of-function variant of human apoA-I gene. It has been shown that this variant dramatically diminishes circulating HDL-C concentration, inhibits cholesterol efflux from macrophages, and impairs RCT. Cathepsin B is known to mediate the cleavage of apoA-I at Ser228 in the C-terminal domain. Overexpression of cathepsin B was shown to impair the atheroprotective effect of apoA-I. On the other hand, injection of recombinant HDL reconstituted with the gain-of-function mutant apoA-I N74C inhibits the development of atherosclerosis in apoE−/− mice fed with a high-fat diet [53]. Local vascular gene therapy with apoA-I not only decreases atherosclerotic lesion area at the early stage but also contributes to the regression of atherosclerotic plaques at the advanced stage in a rabbit model of atherosclerosis [54, 55]. Additionally, AIBP suppresses promotes cholesterol efflux and inhibits lipid accumulation in macrophages as well as is protective against atherosclerosis in mice by interacting with apoA-I. Collectively, the above findings suggest apoA-I as a valuable target for the prevention and treatment of atherosclerosis.
7.5 HDL and Cholesterol Homeostasis
7.5.1 Components and Functions of HDL
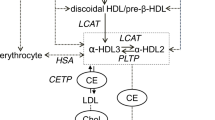
As a type of highly heterogeneous lipoprotein particles, HDL has various densities, shapes, sizes, and components. There are about 90 proteins in the HDL particles [56]. In addition to apoA-I, apoA-II, apoE, enzymes, and lipid transfer proteins are identified as the major protein components of HDL. HDL also contents many lipids, including phospholipids, sphingolipids, triglyceride, cholesterol esters, and FC. The major function of HDL is to deliver lipids for the metabolism in the liver. In this process, both cholesterol and phospholipids are transported to apoA-I, resulting in the formation of nascent discoid HDL, namely, pre-β HDL. Subsequently, pre-β HDL is further lipidated to form larger HDL particles, named HDL3. Lecithin:cholesterol acyltransferase promotes the transformation of FC to cholesterol esters, which enter the hydrophobic core of HDL [57]. This results in the generation of mature, spherical, α-migrating HDL particles, termed HDL2. Of note, pre-β HDL is easy to be eliminated through the kidney, whereas the turnover of HDL3 and HDL2 is slower than pre-β HDL [58].
HDL-bound cholesterol is able to be removed from the body through three pathways. Firstly, scavenger receptor class B type I (SR-BI) located in hepatocytes mediates the uptake of cholesterol esters within HDL for hepatobiliary secretion. Secondly, cholesterol esters within HDL are transported to apoB-containing lipoproteins by cholesteryl ester transfer protein in exchange for triglyceride. Cholesterol esters within the apoB particles are taken up into hepatocytes by hepatic low-density lipoprotein receptor. Finally, cholesterol esters within HDL and is directly secreted into the intestinal lumen. This process is termed transintestinal cholesterol excretion. Of note, HDL also has anti-inflammatory, antioxidative, and antithrombotic effects [59,60,61].
7.5.2 Role of HDL in Cardiovascular Disease
The basic studies and clinical trials have shown that HDL has an atheroprotective role. For instance, injection of CRE-001, a novel HDL-mimetic, into Ldlr−/− mice was shown to increase macrophage-to-feces RCT and decrease atherosclerotic lesion area [62]. Conversely, subcutaneous immunization with heat shock protein 65 in apoE−/− mice causes HDL dysfunction and consequently accelerates the development of atherosclerosis [63]. Administration of miR-19b precursor markedly reduces circulating HDL-C concentration, impairs the whole-body RCT, and aggravates atherosclerosis in apoE−/− mice. When plasma HDL-C levels elevate by 1 mg/dL, the risk of coronary heart disease decreases by 2% in men and 3% in women, respectively. In comparison with the subjects with plasma HDL-C concentration less than 35 mg/dL, the subjects with plasma HDL-C concentration more than 35 mg/dL diminish the risk of coronary artery disease by 70% during a follow-up of 6 years [64]. In addition, the efficacy of statin therapy is better in coronary heart disease patients with high HDL-C levels than those with low HDL-C levels [65].
Even though the majority of clinical studies have demonstrated an inverse correlation between circulating HDL-C levels and cardiovascular disease risk, the elevation of circulating HDL-C levels does not certainly translate into clinical benefits. It has been reported that single-nucleotide polymorphisms can lead to a significant increase in circulating HDL-C concentration, but this increase has no effect on the incidence of myocardial infarction. During a median follow-up of 3.1 years, coronary heart disease patients with low HDL-C (<40 mg/dL) and high HDL-C (≥70 mg/dL) have significantly higher risk of all-cause and cardiovascular disease mortality than those with HDL-C between 40 and 49 mg/dL, thereby revealing a U-shape relationship between HDL-C and adverse cardiovascular events [66]. Combined treatment with simvastatin and niacin to treat dramatically increases circulating HDL-C concentration but fails to show an additive clinical efficacy as compared to simvastatin monotherapy in coronary heart disease patients with low LDL-C concentration. Dalcetrapib is an inhibitor of cholesteryl ester transfer protein. Treatment of acute coronary syndrome patients with dalcetrapib does not decrease the risk of recurrent cardiovascular events despite elevated HDL-C levels [67]. A similar effect is seen when fenofibrate is used to treat type 2 diabetes mellitus [68]. In postinfarction patients with high HDL-C and C-reactive protein levels, the risk of recurrent events is still high [69].
Although HDL possesses complex functions, cholesterol efflux capacity (CEC) from peripheral cells is thought to be a key function of HDL. Moreover, CEC has a stronger protective effect on cardiovascular disease than traditional HDL-C concentration. It has been demonstrated that circulating apoA-I levels in individual HDL subpopulations are better to predict the risk of cardiovascular events than simple HDL-C levels [70, 71]. The macrophage-derived CEC is negatively correlated with carotid intima-media thickness and the likelihood of angiographic coronary artery disease, and this correlation is independent of HDL-C [72,73,74]. The small lipid-poor preβ-1-HDL particles are known to accept cholesterol delivered by ABCA1; however, the large lipid-rich α-1 and α-2 HDL particles predominantly accept cholesterol transported by SR-BI [75]. In healthy subjects, there are strong positive correlations between ABCA1-mediated cholesterol export and preβ-1-HDL levels, and between SR-BI-mediated cholesterol export and α-1/2 HDL levels [76]. Further analysis demonstrated that coronary heart disease patients has increased preβ-1-HDL levels as compared with controls, but their functionality (preβ-1-HDL concentration normalized ABCA1-dependent cholesterol efflux) is significantly decreased [77]. On the other hand, although large HDL levels in these patients are lower than those in controls, their functionality (α-1/2 HDL concentration normalized SR-BI-dependent cholesterol efflux) is significantly increased. These observations indicate that CEC is dependent on individual HDL particle concentration and their functional properties. Therefore, measurement of the concentration in combination with functionality of HDL particles may be more valuable than traditional HDL-C concentration in predicting the risk of cardiovascular events in the general population. It is worth noting that net cholesterol flux in the body is dependent not only on HDL, but also is associated with ABCA1, ABCG1, and SR-BI. It is not surprisingly that these transporters are also the important determinants of HDL concentration and functionality, while their relative contributions to the functional changes of individual HDL particles are still required to be defined in the future studies.
7.6 Conclusion
ABCA1, ABCG1, apoA-I, and HDL are the critical players of RCT and play important roles in promoting cholesterol homeostasis. At present, some drugs to enhance the functions of these proteins, such as LXRα activators, RVX-208, apoA-I mimetic peptides and reconstituted HDL, are available. The monotherapy of these drugs or combination with statins has shown considerable promise for reducing the incidence of cardiovascular events. Nevertheless, our current understanding of how ABCA1 and ABCG1 mediate cholesterol efflux is still limited. This leads to a delay for therapeutic intervention targeting these agents. As CEC is a better predictor of cardiovascular risk than traditional HDL-C levels, it is also important to establish a standard method to measure CEC in clinic. Collectively, a better understanding of the role of ABCA1, ABCG1, apoA-I, and HDL in regulating cholesterol homeostasis will certainly help us to develop lipid-lowering therapies and improve the prognosis of atherosclerosis-associated disease patients in the future.
References
Yu XH, Zhang DW, Zheng XL et al (2019) Cholesterol transport system: an integrated cholesterol transport model involved in atherosclerosis. Prog Lipid Res 73:65–91
Yu XH, Fu YC, Zhang DW et al (2013) Foam cells in atherosclerosis. Clin Chim Acta 424:245–252
Wang G, Gao JH, He LH et al (2020) Fargesin alleviates atherosclerosis by promoting reverse cholesterol transport and reducing inflammatory response. Biochim Biophys Acta Mol Cell Biol Lipids 1865(5):158633
Luo J, Wang X, Jiang X et al (2020) Rutaecarpine derivative R3 attenuates atherosclerosis via inhibiting NLRP3 inflammasome-related inflammation and modulating cholesterol transport. FASEB J 34(1):1398–1411
Yin K, You Y, Swier V et al (2015) Vitamin D protects against atherosclerosis via regulation of cholesterol efflux and macrophage polarization in hypercholesterolemic swine. Arterioscler Thromb Vasc Biol 35(11):2432–2442
Qian H, Zhao X, Cao P et al (2017) Structure of the human lipid exporter ABCA1. Cell 169:1228–1239
Tang CK, Yi GH, Yang JH et al (2004) Oxidized LDL upregulated ATP binding cassette transporter-1 in THP-1 macrophages. Acta Pharmacol Sin 25(5):581–586
Tsuboi T, Lu R, Yonezawa T et al (2020) Molecular mechanism for nobiletin to enhance ABCA1/G1 expression in mouse macrophages. Atherosclerosis 297:32–39
Kumar A, Gupta P, Rana M et al (2020) Role of pyruvate kinase M2 in oxidized LDL-induced macrophage foam cell formation and inflammation. J Lipid Res 61(3):351–364
Coban N, Gulec C, Ozsait-Selcuk B et al (2017) CYP19A1, MIF and ABCA1 genes are targets of the RORalpha in monocyte and endothelial cells. Cell Biol Int 41(2):163–176
Nishiuchi Y, Murao K, Imachi H et al (2010) Transcriptional factor prolactin regulatory element-binding protein-mediated gene transcription of ABCA1 via 3′,5′-cyclic adenosine-5′-monophosphate. Atherosclerosis 212(2):418–425
Zhao ZW, Zhang M, Chen LY et al (2018) Heat shock protein 70 accelerates atherosclerosis by downregulating the expression of ABCA1 and ABCG1 through the JNK/Elk-1 pathway. Biochim Biophys Acta Mol Cell Biol Lipids 1863(8):806–822
Porsch-Ozcurumez M, Langmann T, Heimerl S et al (2001) The zinc finger protein 202 (ZNF202) is a transcriptional repressor of ATP binding cassette transporter A1 (ABCA1) and ABCG1 gene expression and a modulator of cellular lipid efflux. J Biol Chem 276(15):12427–12433
Zhang ZZ, Chen JJ, Deng WY et al (2021) CTRP1 decreases ABCA1 expression and promotes lipid accumulation through the miR-424-5p/FoxO1 pathway in THP-1 macrophage-derived foam cells. Cell Biol Int 45(11):2226–2237
Nagao S, Murao K, Imachi H et al (2006) Platelet derived growth factor regulates ABCA1 expression in vascular smooth muscle cells. FEBS Lett 580(18):4371–4376
Tamehiro N, Park MH, Hawxhurst V et al (2015) LXR agonism upregulates the macrophage ABCA1/syntrophin protein complex that can bind apoA-I and stabilized ABCA1 protein, but complex loss does not inhibit lipid efflux. Biochemistry 54(46):6931–6941
Zhang M, Li L, Xie W et al (2016) Apolipoprotein A-1 binding protein promotes macrophage cholesterol efflux by facilitating apolipoprotein A-1 binding to ABCA1 and preventing ABCA1 degradation. Atherosclerosis 248:149–159
Li X, Zhou Y, Yu C et al (2015) Paeonol suppresses lipid accumulation in macrophages via upregulation of the ATPbinding cassette transporter A1 and downregulation of the cluster of differentiation 36. Int J Oncol 46(2):764–774
Li Y, Jiang B, Liang P et al (2017) Nucleolin protects macrophages from oxLDL-induced foam cell formation through up-regulating ABCA1 expression. Biochem Biophys Res Commun 486(2):364–371
Ramirez CM, Lin CS, Abdelmohsen K et al (2014) RNA binding protein HuR regulates the expression of ABCA1. J Lipid Res 55(6):1066–1076
Fernández-Tussy P, Ruz-Maldonado I, Fernández-Hernando C (2021) MicroRNAs and circular RNAs in lipoprotein metabolism. Curr Atheroscler Rep 23(7):33
Adorni MP, Zimetti F, Billheimer JT et al (2007) The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res 48:2453–2462
Oram JF (2000) Tangier disease and ABCA1. Biochim Biophys Acta 1529:321–330
Wang MD, Franklin V, Marcel YL (2007) In vivo reverse cholesterol transport from macrophages lacking ABCA1 expression is impaired. Arterioscler Thromb Vasc Biol 27:1837–1842
Singaraja RR, Fievet C, Castro G et al (2002) Increased ABCA1 activity protects against atherosclerosis. J Clin Invest 110(1):35–42
Yvan-Charvet L, Welch C, Pagler TA et al (2008) Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 118(18):1837–1847
Stamatikos A, Dronadula N, Ng P et al (2019) ABCA1 overexpression in endothelial cells in vitro enhances apoAI-mediated cholesterol efflux and decreases inflammation. Hum Gene Ther 30(2):236–248
Zhang M, Zhao GJ, Yin K et al (2018) Apolipoprotein A-1 binding protein inhibits inflammatory signaling pathways by binding to apolipoprotein A-1 in THP-1 macrophages. Circ J 82(5):1396–1404
Li L, Xu L, Chen W et al (2018) Reduced annexin A1 secretion by ABCA1 causes retinal inflammation and ganglion cell apoptosis in a murine glaucoma model. Front Cell Neurosci 12:347
Nagata KO, Nakada C, Kasai RS et al (2013) ABCA1 dimer-monomer interconversion during HDL generation revealed by single-molecule imaging. Proc Natl Acad Sci U S A 110(13):5034–5039
Ishigami M, Ogasawara F, Nagao K et al (2018) Temporary sequestration of cholesterol and phosphatidylcholine within extracellular domains of ABCA1 during nascent HDL generation. Sci Rep 8(1):6170
Fielding PE, Nagao K, Hakamata H et al (2000) A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein A-1. Biochemistry 39(46):14113–14120
Wang N, Silver DL, Thiele C et al (2001) ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem 276(26):23742–23747
Smith JD, Le Goff W, Settle M et al (2004) ABCA1 mediates concurrent cholesterol and phospholipid efflux to apolipoprotein A-I. J Lipid Res 45(4):635–644
Liu M, Mei X, Herscovitz H et al (2019) N-terminal mutation of apoA-I and interaction with ABCA1 reveal mechanisms of nascent HDL biogenesis. J Lipid Res 60(1):44–57
Takahashi Y, Smith JD (1999) Cholesterol efflux to apolipoprotein AI involves endocytosis and resecretion in a calcium-dependent pathway. Proc Natl Acad Sci U S A 96(20):11358–11363
Chen W, Sun Y, Welch C et al (2001) Preferential ATP-binding cassette transporter A1-mediated cholesterol efflux from late endosomes/lysosomes. J Biol Chem 276(47):43564–43569
Yu XH, Chen JJ, Deng WY et al (2020) Biochanin a mitigates atherosclerosis by inhibiting lipid accumulation and inflammatory response. Oxidative Med Cell Longev 2020:8965047
Wang D, Yan X, Xia M et al (2014) Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arterioscler Thromb Vasc Biol 34(9):1860–1870
Mo ZC, Xiao J, Tang SL et al (2014) Advanced oxidation protein products exacerbates lipid accumulation and atherosclerosis through downregulation of ATP-binding cassette transporter A1 and G1 expression in apolipoprotein E knockout mice. Circ J 78(11):2760–2770
Zhao GJ, Tang SL, Lv YC et al (2014) NF-kappaB suppresses the expression of ATP-binding cassette transporter A1/G1 by regulating SREBP-2 and miR-33a in mice. Int J Cardiol 171(3):e93–e95
Meurs I, Lammers B, Zhao Y et al (2012) The effect of ABCG1 deficiency on atherosclerotic lesion development in LDL receptor knockout mice depends on the stage of atherogenesis. Atherosclerosis 221(1):41–47
Wang X, Collins HL, Ranalletta M et al (2007) Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest 117(8):2216–2224
Yvan-Charvet L, Ranalletta M, Wang N et al (2007) Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest 117(12):3900–3908
Westerterp M, Tsuchiya K, Tattersall IW et al (2016) Deficiency of ATP-binding cassette transporters A1 and G1 in endothelial cells accelerates atherosclerosis in mice. Arterioscler Thromb Vasc Biol 36(7):1328–1337
Vaughan AM, Oram JF (2005) ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J Biol Chem 280(34):30150–30157
Tarling EJ, Edwards PA (2011) ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc Natl Acad Sci U S A 108(49):19719–19724
Neufeld EB, O’Brien K, Walts AD et al (2014) Cellular localization and trafficking of the human ABCG1 transporter. Biology (Basel) 3(4):781–800
Getz GS, Reardon CA (2011) Apolipoprotein A-I and A-I mimetic peptides: a role in atherosclerosis. J Inflamm Res 4:83–92
Palgunachari MN, Mishra VK, Lund-Katz S et al (1996) Only the two end helixes of eight tandem amphipathic helical domains of human apo A-I have significant lipid affinity. Implications for HDL assembly. Arterioscler Thromb Vasc Biol 16(2):328–338
Mishra VK, Palgunachari MN, Datta G et al (1998) Studies of synthetic peptides of human apolipoprotein A-I containing tandem amphipathic alpha-helixes. Biochemistry 37(28):10313–10324
Tiniakou I, Kanaki Z, Georgopoulos S et al (2015) Natural human apoA-I mutations L141RPisa and L159RFIN alter HDL structure and functionality and promote atherosclerosis development in mice. Atherosclerosis 243(1):77–85
Zhang X, Zhu X, Chen B (2010) Inhibition of collar-induced carotid atherosclerosis by recombinant apoA-I cysteine mutants in apoE-deficient mice. J Lipid Res 51(12):3434–3442
Flynn R, Qian K, Tang C et al (2011) Expression of apolipoprotein A-I in rabbit carotid endothelium protects against atherosclerosis. Mol Ther 19(10):1833–1841
Wacker BK, Dronadula N, Zhang J et al (2017) Local vascular gene therapy with apolipoprotein A-I to promote regression of atherosclerosis. Arterioscler Thromb Vasc Biol 37(2):316–327
Mo ZC, Ren K, Liu X et al (2016) A high-density lipoprotein-mediated drug delivery system. Adv Drug Deliv Rev 106(Pt A):132–147
Jonas A (2000) Lecithin cholesterol acyltransferase. Biochim Biophys Acta 1529(1–3):245–256
Rye KA, Barter PJ (2004) Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler Thromb Vasc Biol 24(3):421–428
Gordon SM, Remaley AT (2017) High density lipoproteins are modulators of protease activity: implications in inflammation, complement activation, and atherothrombosis. Atherosclerosis 259:104–113
Choi HY, Hafiane A, Schwertani A, Genest J (2017) High-density lipoproteins: biology, epidemiology, and clinical management. Can J Cardiol 33:325–333
Hu J, Xi D, Zhao J, Luo T, Liu J, Lu H et al (2016) High-density lipoprotein and inflammation and its significance to atherosclerosis. Am J Med Sci 352:408–415
Tardy C, Goffinet M, Boubekeur N et al (2014) CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis 232(1):110–118
Sun H, Shen J, Liu T et al (2014) Heat shock protein 65 promotes atherosclerosis through impairing the properties of high density lipoprotein. Atherosclerosis 237(2):853–861
Assmann G, Schulte H, von Eckardstein A et al (1996) High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 124(Suppl):S11–S20
Barter P, Gotto AM, LaRosa JC et al (2007) HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 357(13):1301–1310
Ding D, Li X, Qiu J et al (2014) Serum lipids, apolipoproteins, and mortality among coronary artery disease patients. Biomed Res Int 2014:709756
Schwartz GG, Olsson AG, Abt M et al (2012) Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 367(22):2089–2099
Keech A, Simes RJ, Barter P et al (2005) Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366(9500):1849–1861
Corsetti JP, Ryan D, Rainwater DL et al (2010) Cholesteryl ester transfer protein polymorphism (TaqIB) associates with risk in postinfarction patients with high C-reactive protein and high-density lipoprotein cholesterol levels. Arterioscler Thromb Vasc Biol 30(8):1657–1664
Asztalos BF, Cupples LA, Demissie S et al (2004) High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham offspring study. Arterioscler Thromb Vasc Biol 24(11):2181–2187
Asztalos BF, Collins D, Cupples LA et al (2005) Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the veterans affairs HDL intervention trial. Arterioscler Thromb Vasc Biol 25(10):2185–2191
Khera AV, Cuchel M, de la Llera-Moya M et al (2011) Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364(2):127–135
Saleheen D, Scott R, Javad S et al (2015) Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol 3(7):507–513
Rohatgi A, Khera A, Berry JD et al (2014) HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 371(25):2383–2393
Asztalos BF, de la Llera-Moya M, Dallal GE et al (2005) Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res 46(10):2246–2253
Asztalos BF, Horvath KV, Mehan M et al (2017) Influence of HDL particles on cell-cholesterol efflux under various pathological conditions. J Lipid Res 58(6):1238–1246
Asztalos BF, Horvath KV, Schaefer EJ (2018) HDL (high-density lipoprotein) particles, cell-cholesterol efflux, and coronary heart disease risk: the prebeta-1 paradox. Arterioscler Thromb Vasc Biol 38(9):2007–2015
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yu, XH., Tang, CK. (2022). ABCA1, ABCG1, and Cholesterol Homeostasis. In: Zheng, L. (eds) HDL Metabolism and Diseases. Advances in Experimental Medicine and Biology, vol 1377. Springer, Singapore. https://doi.org/10.1007/978-981-19-1592-5_7
Download citation
DOI: https://doi.org/10.1007/978-981-19-1592-5_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1591-8
Online ISBN: 978-981-19-1592-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)