Abstract
Access to safe and clean water has become a more challenging task worldwide as water resources are limited, and the population that relies on these limited supplies is expected to grow. The presence of pollutants in water affects human health and hygiene and decreases food safety. The supply of clean water is required for all the phases of food production, including processing, transportation, and consumption. Environmentally viable nanomaterials are being used to purify wastewater because of their distinctive characteristics, like high effectiveness and selectivity, larger surface area, cost-effective, recyclable, and high thermal and mechanical stability. This chapter provides an overall review of nanomaterials and their types and techniques used to eliminate organic and inorganic contaminants from wastewater.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Water is the most critical component for every living creature present in the world. Clean and drinkable water is a primary need for all living things and mankind on this earth. Fresh and clean water supply is a major challenge faced by human society and is expected to increase in the coming years. Availability of drinking water is far from the demand due to the gradual increase in population and various contaminates (Kunduru et al. 2017). A constant supply of safe and reliable water is required for proper sanitation for optimal health. The individual water requirements range from 7.5 L per day for drinking purposes and approximately 20–50 L per day for other daily needs (Khan and Malik 2019).
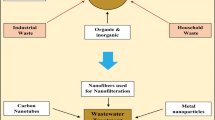
On the other hand, both developed and developing countries have reported water-borne diseases and believe that 50% of people in underdeveloped countries do not have access to clean and safe drinking water. Therefore, in developing countries, the uncontrolled discharge of contaminants that inadequacy of water purification system is the main problem (Figoli et al. 2017a; Borji et al. 2020). The causes of water pollution include improper sewage disposal, mining, industrial waste, oil spills, chemical fertilizers, pesticides, radioactive water discharge, etc. (Fig. 1a) (Kaur et al. 2020). The contaminants of wastewater affect our human health and cause several health problems like diarrhea, jaundice, impaired nerve function, skin infections, brain damage, dysfunction of the liver, etc. (Fig. 1b) (Cínti et al. 2019).
The prospect of the increasing freshwater source is limited because of the competing demands of the world’s growing population. Industrial development improved the lifestyle of humans and damaged our natural resources and aquatic systems. Industries such as leather, food, pharmaceuticals, and packaging generate an enormous daily amount of wastewater which contains heavy metals, organic dyes, and other harmful chemicals. These gallons of wastewater are dumped into our aquatic ecosystem without any meaningful treatment. Thus, the effective removal of such contaminants is needed. Nanotechnology has shown the potential to solve water quality problems effectively due to its environment-friendly and non-toxicity properties for water purification. In particular, the vast range of nanostructures (NSs) such as metal oxide (ZnO, TiO2, ZnS, Fe3O4, etc.) NSs [NPs (NPs), nanorods (NRs), etc.] and their nanocomposites (NCs), Carbon nanotubes (CNTs) or Carbon nanotube (CNT), graphene oxide (GO), reduced graphene oxide (rGO) graphene nanosheets (GNs) helps to develop a more effective treatment in advanced water purification systems (Amin et al. 2014). Unique advantages of nanomaterials like high surface-to-volume ratio, small size, well-organized structure, and ability of filtration make them a potential alternative for water treatment. Although, there are still some major challenges like high production cost, specific selectivity and availability, sustainability, and recyclability. Worldwide, various scientific groups are trying to develop highly effective and environmentally viable nanomaterials at a cost-effective for ecological purification of wastewater which contains hazardous heavy metals ions, dyes, detergents, and chemical waste from industries (Nasrollahzadeh et al. 2021).
In this chapter, we have discussed nanotechnology-enabled technologies that utilize different nanomaterials like silver NPs, metal-based NPs, carbon-based nanomaterials, etc. Their properties and reviewed other technologies for water purification adsorption disinfection, photocatalytic action, membranes, etc. Properties of various nano materials are reviewed in this chapter and varius technologies like water purification adsorption disinfection, photocatalytic action, membranes, etc. are discussed.
1.1 Nanomaterials for Water Purification
1.1.1 Zero-valent Metal NPs
Silver (Ag) is a transition metal (soft and shiny) whose atomic number is 47 (Fig. 2a) and exhibits greater electrical conductivity (~6.3 × 107 m/Ω) and thermal conductivity (~429 W/m K). Ag NPs are the most extensively utilized material because of their low toxicity and can be easily extracted from their salts, such as silver nitrate and silver chloride. Ag NPs have been used for various applications such as antibacterial, thin films, and water purification (Chamoli et al. 2017; Maninder and Baojun 2019). Ag NPs have strong microbial inactivation in water and show excellent antibacterial effects against various micro-organisms such as viruses, bacteria, and fungi. Therefore, it is commonly used for water disinfection due to its antibacterial properties (Lu et al. 2016). In particular, Ag NPs have been synthesized and utilized for the photodegradation of various organic dyes. For example, Pandey et al. synthesized Ag NPs from κ-Carrageenan gum and investigated their photocatalytic activity in the presence of UV with RhB and MB as the target pollutants. They were able to remove up to 100% in a short period of time (Pandey et al. 2020). Similarly, Rajkumar et al. have synthesized Ag NPs by utilizing cell-free extract of Chlorella Vulgaris. The synthesized Ag NPs showed 96.51% of photocatalytic decolorization activity using methylene blue dye (100 ppm) within 3 h incubation time (Rajkumar et al. 2021). However, Gola et al. have synthesized Ag NPs and achieved degradation up to 100% from blue dye and orange dye ~97.4% degradation, respectively. Further, dye mixture studies (orange + blue dye) have been examined and found 100% degradation in just 5 min (Fig. 2b) (Gola et al. 2021). Jain et al. have prepared aqueous Ag NPs (Pa–Ag NPs) using leaf extract of C. papaya and studied dye degradation ability for blue CP and yellow 3RS with degradation ability 90 and 83%, respectively (Jain et al. 2020).
a Crystal structures of Ag and b Ag NPs for orange and blue dye degradation. Reproduced with permission from Gola et al. (2021)
1.1.2 Metal Oxide NSs and NCs
1.1.2.1 TiO2 NSs
Titanium dioxide (TiO2) is a wide bandgap semiconductor with three different crystallographic forms (polymorphs, Fig. 3a), viz., anatase (with a bandgap of 3.1 eV), rutile (3.02 eV), and brookite (2.96 eV) (Haggerty et al. 2017). TiO2-anatase is an extensively used photocatalyst due to its high photocatalytic activity, low price, and good biological and chemical stability. Under the presence of light (UV and visible), TiO2 NSs act as an excellent photocatalyst and successfully degrade various organic contaminants. Primarily, TiO2 produces many reactive oxygen species that can entirely deteriorate pollutants in a short reaction time under ultraviolet irradiation (Ali et al. 2018a).
For example, Gautam et al. have prepared TiO2 (both anatase and rutile) NPs and successfully degraded MB dye in the presence of UV (8 W) light irradiation, and the degradation efficiency (DE, ~88%) is obtained by using anatase NPs in 150 min (Gautama et al. 2016). Tayeb et al. have synthesized TiO2 NPs and effectively degraded MB dye under UV (15 W) light irradiation with degradation efficiency (DE) of ~ 98% in 90 min (Tayeb and Hussein 2015). Sathiyan et al. have prepared TiO2 NPs and effectively degraded MB dye up to 88% in 180 min in the presence of UV–visible light (Sathiyana et al. 2020). Chamoli et al. used Mangifera indica leaf extract to make TiO2 tripods (TiTPs) via a rapid microwave (180 s, 100 W) green method. TiTPs have shown excellent photocatalytic ability against MB, achieving dye degradation of ~75% (under visible light in 75 min) and 96% (under UV light in 9 min). Moreover, TiTPs have exhibited good adsorbent capabilities, with a maximum adsorption capacity ~17.54 mg/g based on the Langmuir model owing to their porous nature (Fig. 3b) (Chamoli et al. 2021a).
1.1.2.2 ZnO NPs
ZnO is a compound semiconductor material of group-II-VI. The majority of the materials in this group-II-VI are cubic zinc-blende or hexagonal wurtzite structures (four cations surround each anion at the corners of a tetrahedron). ZnO is a 3.37 eV broad bandgap semiconductor with the structure of wurtzite (B4), zinc-blende (B3), and rock salt (B1) (Fig. 4a), and its ionicity is intermediate between covalent and ionic semiconductors (Özgür et al. 2005). ZnO is extensively employed in materials because of its ease of production and low toxicity. It is useful in wastewater remediation due to its distinctive features, such as direct and wide bandgap in the near-ultraviolet spectral region, high oxidation ability, and enhanced photocatalytic performance.
a Stick and ball representation of ZnO crystal structures: cubic rock salt (B1), cubic zinc-blende (B3), and hexagonal wurtzite (B4). The shaded gray and black spheres denote Zn and O atoms, respectively. Reproduced with permission from Özgür et al. (2005). b GZnTPs for photocatalysis and adsorption of RhB dye, Reproduced with permission from Chamoli et al. (2021b)
For example, Fan et al. have synthesized zinc oxide-reduced graphene oxide (ZnO/rGO) NCs for photocatalytic degradation of MB, MO, and RhB in the presence of ultraviolet (UV) light irradiation (150 W) and showed degradation efficiency (DE) of ~99% in 30 min (Fan et al. 2015). Ravi et al. have produced ZnO/rGO NCs for photocatalytic degradation of Congo red (CR) and eosin yellow (EY) in the presence of UV light and found 98% removal of the dyes in 90 min (Ravi et al. 2018). Jabeen et al. have attained 68% removal of methylene blue (MB) dye in 120 min in the presence of UV light (500 W) by employing ZnO/rGO NCs (Jabeen et al. 2017). Furthermore, for the removal of RhB, ternary ZnO/CuO/rGO NCs have also been produced, with 99% degradation efficiency (DE) obtained in 20 min in the presence of visible light (150 W) (Kumaresan et al. 2020). Chamoli et al. have synthesized grapheme—ZnO tetrapods (GZnTPs) and investigated their potential to photodegrade RhB, MO, and MB dyes in the presence of UV and visible (both 125 W) light irradiation. Upon UV light irradiation, GZnTPs behave as an outstanding photocatalyst for RhB, with such a higher degradation efficiency of 91.6% (Chamoli et al. 2021b). Compared to numerous semiconductor metallic oxides, ZnO NPs can adsorb a much broader solar spectral range and more light quanta. Like TiO2 NPs, the light absorption of ZnO NPs is limited to the UV range due to its large band energy. In addition, the utility of ZnO particles is hindered by photo-corrosion, leading to the fact that the photo-generated charges recombine rapidly, resulting in lower photocatalytic efficiency.
1.1.2.3 Zinc Sulfide (ZnS) NSs
ZnS (II-VI group semiconductor) is an important photocatalyst studied at the nanoscale because of its outstanding physical properties and peculiar photocatalytic properties. Cubic (sphalerite) and hexagonal (wurtzite) are two main crystalline forms of ZnS with coordination geometry at Zn and S are of tetrahedral having 3.72 and 3.77 eV bandgaps for cubic and hexagonal ZnS, respectively, (Lee and Wu 2017). This large bandgap of ZnS enables candidacy as an important photocatalyst for various dye degradation and wastewater remediation. For example, Maji et al. have prepared ZnS NCs (rod and sphere) using ethylenediamine and hexadecylamine, which show effective photocatalytic activity against rose bengal dye (RB) under light irradiation. The DE has been achieved ~93% at 225 min (Maji et al. 2011). Zhang et al. have successfully prepared ZnS microcrystals (polyhedron, fan-shaped sheet, hexagonal rectangle, and missing angle rectangle) using a simple hydrothermal method against MB. The degradation efficiency (DE) has been achieved ~91% at 60 min (Zhang 2014). However, metal (Pb, Cu, Ni)-doped ZnS photocatalyst obtained by various methods, and also conjugated ZnS complexes obtained through polyreaction have been shown to have the potential to defluorinate hexafluorobenzene by visible light (Lee and Wu 2017). Thio-glycerol and uncapped ZnS NPs have been produced to use a substantial component of solar energy for dye degradation. Although ultraviolet irradiation is excellent at degrading dyes, naturally occurring solar radiation is also beneficial in dye degradation. As a result, it could be an effective approach for the safe disposal of textile waste into waterways (Sharma et al. 2012), while Lee et al. have been summarized ZnS-assisted photocatalytic degradation of pollutants and water splitting under various conditions (Lee and Wu 2017).
1.1.2.4 Fe3O4 NSs
In recent years, people have been more interested in using iron oxide NPs due to their simplicity and easy accessibility to degrade organic dyes and remove heavy metals from wastewater. Iron oxide NPs have different forms (Fig. 5) and are commonly used as nanoadsorbents such as magnetic magnetite (Fe3O4), magnetic maghemite (\(\gamma \) Fe2O4), and non-magnetic hematite \((\alpha \) Fe2O4). Separating and recovering them from wastewater during photocatalytic degradation is a challenging task. Thus, Fe3O4 and Fe2O4 can be easily be separated and recovered by using an external magnetic field from the wastewater system (Xu et al. 2012). Therefore, iron oxides are widely used in the purification of water.
Different forms of iron-based NPs: oxides, hydroxides, and oxyhydroxides (Aragaw et al. 2021)
For example, Bhuiyan et al. have synthesized α-Fe2O3 NPs from hexahydrate ferric chloride (FeCl3.6H2O) using papaya (Carica papaya) leaf extract and obtained ~76.6% RR dye degradation after 6 h (Bhuiyan et al. 2020). Balu et al. have prepared iron oxide Fe2O3 NPs by Raphanus sativus leave extract to degrade MB and MR. Both the dyes have undergone complete degradation (100%) in 1 h (Balu et al. 2020). However, functionalization of iron oxide NPs with various ligands such as mercaptobutyric acid or polymers boosts absorption efficiency and removes interference from other metal ions (Aragaw et al. 2021). The majority of iron oxide NCs are amorphous NPs with an average size of about 5 nm, which is a suitable size. These distinctive characteristics of amorphous NPs of iron oxide provide a large surface area for (FeOx-GO-80) with an iron oxide content of 80 wt% with a prominent mesoporous structure, resulting in increased adsorption sites and, as a result, increased adsorption capacity for the removal of heavy metal pollutants from wastewater (Su et al. 2017; Rashida et al. 2021). A comparative study has been done for dye degradation using various metal oxides NPs and NSs and tabulated in Table 1.
1.1.3 Carbon-based Nanomaterials
Carbon nanomaterials are attractive materials for various applications because of their strength and capability to make bonds with other elements. Various allotropes of carbon like fullerene, graphene, etc., are CNTs (Fig. 6) and are utilized for wastewater treatment (Selvaraj et al. 2020).
Graphene as a building block of other forms. An illustration of different allotropes of carbon emerging from a graphene sheet. Redrawn from Geim and Novoselov (2007)
1.1.3.1 Fullerenes
In 1985, Fullerene was discovered and gained considerable attraction because of its remarkable photochemical and photophysical features (Selvaraj et al. 2020). Fullerenes formed a cage-like structure (Fig. 6) with twelve 5-member rings and an unspecified number of 6-member rings. Fullerenes are most commonly found in the form of hexagonal rings with carbon atoms organized inside, but sometimes they also contain pentagonal rings. It is prominent that structures with lesser hexagons show sp3 bonding, high strain energy. Several research studies have shown that fullerene behaves as an adsorbent to adsorb organic waste and heavy metal ions from wastewater. For example, in wastewater, hydrophobic organic compounds such as naphthalene could be adsorbed by adsorbent C60 fullerene, which was coated as a thin film and disseminated in water by magnetic mixing. As a result of its hydrophobic surface, C60 fullerene is projected to be an ideal adsorbent for a wide range of organic compounds present in contaminated water (Selvaraj et al. 2020; Geim and Novoselov 2007).
1.1.3.2 Carbon Nanotubes (CNTs)
CNTs are made up of graphene sheets wrapped into cylinders with diameters ranging from 1 to 100 nm. Their unusual structure and electrical properties make nanotubes attractive for basic research and various applications like the adsorption process (Selvaraj et al. 2020). Its advantages in treating wastewater are due to (i) the ability to adsorb various types of pollutants, (ii) fast adsorption kinetics, (iii) greater specific surface area, and (iv) selectivity to aromatics. CNTs are very effective in eliminating bacterial pathogens. It has been extensively used to remove biological impurities and has received special attention because of its excellent capability to eliminate biological contaminants from wastewater. It has antibacterial properties against various micro-organisms, including bacteria like Escherichia coli and Salmonella. Compared with carbon-based adsorbents, the adsorption of cyanobacterial toxins on carbon nanotubes is also greater, mainly because of the larger specific surface area, larger outer diameter, and large mesoporous volume of CNTs. Carbon nanotubes have extraordinary structures and unique characteristics, making them a good candidate for adsorption phenomena, such as metal removal. Single-walled carbon nanotubes (SWCNT) and multi-walled carbon nanotubes (MWCNT) are two types of carbon nanotubes. Both single-walled and multi-walled carbon nanotubes have unique features, such as greater surface area available for adsorption, accessibility of adsorption sites, light mass density. Compared with activated carbon, they also have high adsorption efficiency, which is currently used as the main adsorbent in the purification of water (Rashida et al. 2021).
1.1.3.3 Graphene
Graphene is a sp2-bonded carbon sheet that can be single or multi-layered. It is a hexagonal lattice of carbon atoms that is only one atom thick. It is another type of carbon nanomaterial. It has many excellent properties in physics and chemistry (Selvaraj et al. 2020; Geim and Novoselov 2007; Duklan et al. 2020). Due to its distinctive 2D structure and exceptional mechanical, thermal, and electrical capabilities, graphene, which is made up of a few atomic layered graphites, has also been used to analyze the adsorption of pollutants of wastewater. Graphene oxide nanosheets manufactured from graphite using an improved Hummers method are reported to be used as adsorbents to remove CO2+, Cd2+ from many aqueous solutions. The adsorption of metal ions on graphene oxide nanosheets was highly reliant on pH and weakly reliant on ionic strength, as seen by manipulating variables such as pH, ionic strength, and humic acid on CO2+, Cd2+. When the pH is less than 100 °C, the presence of humic acid decreases the adsorption of CO2+, Cd2+, on graphene oxide nanosheets. At pH 6.0, the maximum adsorption capacity of CO2+, Cd2+ on graphene nanosheets is approximately 106.3 and 68.0 mg/gm, and the temperature is approximately 303 K (Thines et al. 2017). According to this research, graphene is considered the main material for the purification of water if they were manufactured at a larger scale at an affordable cost. Several studies have shown that graphene can be used not only to adsorb heavy metals but also to adsorb fiber dyes for the purification of water (Das et al. 2020). It has been reported that graphite is used for adsorption of dye from an aqueous solution after oxidizing the graphite by using the Hummers-Offeman method (Geim and Novoselov 2007).
2 Removal Techniques
Hazardous pollutants like heavy metals, organic pollutants, and anions are introduced into the system of freshwater supply through industrial and agricultural waste. The types of new organic pollutants cannot be deteriorated by the chemical, biological, and photolytic processes in the environment, including pesticides, drugs, hormones, types of various aromatic compounds, etc. (Thines et al. 2017). The traditional methods for purification of wastewater cannot remove all the contaminants, and even the very low concentration of pollutants will cause to form dangerous disinfection by-products (DBP). The presence of contaminants affects health and hygiene and reduces food safety. The availability of clean water is necessary for food production, including preparation, distribution, and consumption. Due to the increase in the number of water-borne diseases caused by the number of inorganics, organic hazardous waste, the new and innovation of effective treatment processes are vital. Nanotechnology has shown excellent results in the removal of the aforementioned type of pollutants. Many studies have been conducted on wastewater treatment processes supported by nanotechnology, many of which have shown superior performance over traditional technologies (Figoli et al. 2017b). According to the types of nanomaterials, wastewater treatment is divided into three categories: (i) nanoadsorption, (ii) nanocatalyst, and (iii) nanomembrane.
2.1 Nanoadsorption
Adsorption is a surface phenomenon in which contaminants are adsorbed onto a solid surface. Adsorption occurs in all physical forces, but it can also be linked to weak chemical bonding in specific cases. Nanoadsorption is generally used in wastewater treatment to remove organic and inorganic pollutants. The distinctive characteristics of the nanoadsorbent, such as small size, high catalytic potential, high reaction activity, high surface area, ease of separation, and a high number of active sites for interaction with various pollutants, are helpful for wastewater treatment (Table 2).
2.2 Carbon-based Nanoadsorbents
Carbon-based nanoadsorbents are, such as graphene, graphene oxide, and carbon nanotubes. Carbon nanotubes can be divided into single-walled nanotubes (SWNT) and multi-walled nanotubes (MWNT) as advanced water purifiers. These materials are rarely used in their pure form. Still, the most common composition, either dispersed in a polymer or decorated with metal NPs, such as silver (Ag) and iron oxide (Fe3O4) NPs (Smith and Rodrigues 2015). Due to their hydrophobic surface, the carbon nanotubes dissolve the bundle in an aqueous medium, which reduces the active surface area. These aggregates are high-energy locations for adsorbing organic pollutants in the water. The reason for the adsorption is as follows: (i) the availability of larger pores in carbon nanotubes bundles and (ii) greater accessible adsorption sites.
2.3 Metal-based Nanoadsorbents
In the water purification process, metal-based nanoadsorbents, like zinc oxide, titanium oxide, iron oxide, are employed to remove heavy metals. These adsorbents are both efficient and cost-effective. The oxygen in metallic oxides forms a compound with the heavy metals found in wastewater. That is how they work. Magnetic nanoadsorbents, for example, maghemite (\(\gamma \) Fe2O3), hematite (\(\alpha \)-Fe2O3), and spinel ferrite (M2+Fe2O4, Where M2+: Co2+, Fe2+, etc.) are very excellent adsorption materials used to collect and eliminate toxic and carcinogenic pollutants from wastewater (Ahmad et al. 2021). The environmental advantage is reflected in its magnetism. They can be easily detached from the reaction medium by applying an external magnetic field. Various studies proved that metal-based nanoadsorbents remove various elements from wastewater, for example, ionic forms of lead, nickel, arsenic, chromium, cobalt, etc. ZnO nanoadsorbents are employed for the removal of Zn2+, Cd2+, and Hg2+ ions from aqueous solutions in work published in the literature. Metals ions are adsorbed onto ZnO NPs at discrete amounts. Because Hg2+ has the smallest hydrated ionic radius among metal ions, it has the highest adsorption capacity (Ahmad et al. 2021). Alumina nanoadsorbents can also be manufactured because of their large surface area, good thermal stability, and relatively inexpensive. They are used to remove metal ions, like cadmium, chromium, copper, lead, and mercury. Metal-based nanoadsorbents are employed for the elimination of heavy metals in water and high-efficiency nanoadsorbents in wastewater because of their merits, like large adsorption capacity, faster kinetics, etc. (Ahmad et al. 2021).
2.4 Polymer-based Nanoadsorbents
Polymer-based nanoadsorbents have recently acquired attraction toward wastewater treatment. They are used as a structure that can insert nanoscale inorganic materials or a bed or template for preparing NPs. Polymer-based magnetic nanoparticles efficiently removed heavy metal ions like Zn2+, Cd2+, Pb2+ from aqueous solutions and have a high maximum capacity of adsorption of pH 5.5 (Ahmad et al. 2021; Kumar et al. 2011). This nanoadsorbent can be reused for at least four cycles. In recent studies, bi-metal micro- and nano-multifunctional polymeric adsorbents were developed for the elimination of fluoride and arsenic (V). Suspension polymerization is used to make the polymer. To make bi-metal-doped nanoadsorbents, aluminum and iron salts are added during the polymerization process. When compared to fluoride, iron-doped nanoadsorbents had excellent adsorption for arsenic, while aluminum-doped nanoadsorbents had excellent adsorption for fluoride (Kumar et al. 2011). Hence, polymer-based nanoadsorbents are magnificent materials due to their structures, pore sizes, and tunable functional groups; making them selective for a specific contaminant is challenging to remove heavy metal ions from wastewater. The adsorptive capacity is quite low, and regeneration is required when CO is high.
2.5 Nanocatalysis
Nanocatalysis is a rapidly emerging technology in which nanomaterials are used as catalysts in various applications such as reduced global warming, wastewater treatment. Different types of NPs are used as the catalyst for eliminating organic contaminants such as pesticides, dyes, fertilizers, oil grease and inorganic pollutants such as calcium, potassium, chloride, sulfate, nitrate. Nowadays, NPs of titanium oxide (TiO2) have emerged as an attractive photocatalyst for water treatment (Adesina 2004). TiO2 is highly adaptable; they can be used in various applications and can act as an oxidizing and reducing catalyst for the removal of organic and inorganic contaminants from wastewater. The addition of NPs of TiO2 substantially improved the deterioration of organic pollutants in wastewater in the presence of ultraviolet radiation (Fig. 7) (Nawaz et al. 2020). It is reported that NPs of TiO2 effectively (i) deteriorate organic pollutants like chlorinated alkanes and benzene, dioxins, furans, etc. and (ii) remove toxic metal ions like Cr6+, Pt2+ in aqueous solutions under ultraviolet light. When ultraviolet light in the range of 200–390 nm is irradiated on TiO2, electron–hole pairs are photoexcited. They move to the conduction and valence bands, leading to the separation of charge for an efficient photocatalytic function that depends on the substrate’s redox potential. As a result, in a pretreatment phase, the biodegradability of decomposable elements can be improved. Primarily, steady mixtures, like anti-microbial or other micro-contaminants, might be removed by photocatalysis polishing (Ahmad et al. 2021).
Schematic of degradation of MB organic dyes. Redrawn and reprinted with permission from Nawaz et al. (2020)
TiO2 is activated by ultraviolet light, but daylight or apparent light lamps are additionally allowed. KRONO clean 7000, a photocatalyst bandgap moved toward smaller energy: This contributes toward using a broader spectrum in sunlight (Gehrke et al. 2015). Modified technology has been explored to enhance the photocatalytic performance of titanium dioxide, including activity-enhancing or redshift for saving of energy, such as the combination of nano-silica (good thermal and chemical stability) and nano-titanium dioxide (nano-semiconductor) creates new surface-active sites. The catalytic performance of the silica/titanium dioxide nanocomposite is highly dependent on the content and distribution of TiO2. Photocatalysis has a promising future as a long-term, eco-friendly, and cost-effective water purification technique. However, there are several technical hurdles to overcome before it can be used on a wider scale, for example, (i) catalyst tuning to enhance quantum yield or to use visible light, (ii) designing an effective photocatalytic reactor, and (iii) upgrade reaction selectivity.
2.6 Membranes for Water Purification
A nanomembrane is a permeable thin-layered membrane having pores sizes of 1–10 nm that enables water molecules to pass through it while preventing bacteria, viruses, heavy metals, pesticides, etc., from passing through them. The membrane’s operation is dependent whether on pressure-driven or electrical technology. Pressure-driven membrane technique is an excellent approach for wastewater treatment (Kumar et al. 2014). Membrane filtration procedures have become more sophisticated techniques of industrial wastewater treatment. Membranes segregate materials based on the size of the pore and the molecule. It is a sustainable and systematic method for the purification of wastewater. The membrane material determines the membrane system’s efficacy. Membrane permeability, theoretical resistance, thermal and mechanical stabilities are enhanced by incorporating functional nanomaterials into membranes. Surface-functionalized membranes and nanocomposite membranes, which can be produced from mixed materials, are practical filtration units. Nanofillers are used in mixed matrix membranes, and the majority of them are inorganic. They have a large surface area and are incorporated into polymeric or inorganic oxide matrix (Sarkar et al. 2014). Hydrophobic membranes (Fig. 8) are used in various industries for industrial wastewater treatment. For these various hydrophilic metal oxides, nanomaterials are used, such as Al2O3, TiO2, and zeolites. Ag NPs, CNTs, bimetallic NPs are also used for membrane filtration (Karthiga Devi et al. 2016).
GO-based porous nanofibrous membrane for water treatment. Reproduced with permission from Wang et al. (2016)
NCs membranes are made up of nanofillers, which are thin-film polymeric grids made up of a sequential arrangement of mesoporous carbons. They are semi-permeable, and reverse osmosis uses the top surface. Hydrophobic mesoporous carbons are transformed to hydrophilic carbons by atmospheric pressure plasma. A small amount of hydrophilic carbon increases hydrophilicity; this leads to an increase in the permeability of pure water. Thin-film nanocomposites were composed of polyamide and nano-NaX zeolite of 40–150 nm and are covered by interfacial polymerization using trimethyl chloride and m-phenylenediamine monomers over polyethersulfone. This membrane has a high permeability to purify freshwater, leaving pollutants behind the membrane (Fathizadeh et al. 2011). Electrospinning is a useful strategy for modifying the surface characteristics of nanomaterials, and various nanofibers have been successfully employed for wastewater treatment. These nanofibers have a large surface area and porosity, resulting in a nanofiber mat with a complex pore structure. Nanofibers are highly active against water pathogens, have low toxicity, and minimize health hazards. It is extremely simple to dope functional nanomaterials to form a filtration membrane, which has greater reactivity and selectivity to various pollutants.
3 Conclusion
In the present era, wastewater treatment techniques that would provide high-quality freshwater, eliminate organic and inorganic pollutants, and enhance industrial activities are very important. The opportunity is provided by nanotechnology; the distinctive characteristics of nanoparticles, such as greater surface area, size, shape, and dimensions, make them an excellent choice for water purification. Nanoparticles can be used to remove metal ions, anions, organic chemicals, and micro-organisms. Because the nanoparticle doses required for water purification are minimal, their use is reasonably cost-effective. This chapter highlights various nanotechnologies such as nanoadsorption, nanocatalysis, and nanomembranes, among others. Under UV and solar irradiation, photocatalytic processes successfully remove various types of water impurities, including organic and inorganic pollutants. As reactive oxygen species have a limited lifetime, surface modifications may boost the photocatalytic activity of selected compounds and enhance the affinity of modified nanomaterials toward several rising water pollutants. Bimetallic nanomaterials have also been proven to be useful in the treatment of wastewater contaminants. The prospects of NPs in water treatment are promising, but it will take a combined effort from scientific and corporate resources to develop a rapid, environmentally friendly, and practical system for the purification of wastewater. It will be achievable if everyone works with each other to overcome the problem of worldwide water contamination.
References
A.A. Adesina, Industrial exploitation of photocatalysis: progress, perspectives and prospects. Catal. Surv. Asia 8(4), 265–273 (2004)
I. Ahmad, Inexpensive and quick photocatalytic activity of rare earth (Er, Yb) co-doped ZnO nanoparticles for degradation of methyl orange dye. Sep. Purif. Technol. 227, 115726 (2019)
N.A. Ahmad, P.S. Goh, A.K. Zulhairun, T.W. Wong, A.F. Ismail, Chapter 1: the role of functional nanomaterials for wastewater remediation, in Functional Hybrid Nanomaterials for Environmental Remediation (2021), pp. 1–28
M. Akter, F.B.A. Rahman, M.Z. Abedin, S.M.F. Kabir, Adsorption characteristics of banana peel in the removal of dyes from textile effluent. Textiles 1(2), 361–375 (2021)
A.A. Alghandi, A.B. Al-Odayni, W.S. Saeed, M.S. Almutairi, Adsorption of azo dye methyl orange from aqueous solutions using alkali-activated polypyrrole-based graphene oxide. J. MDPI 24, 3685 (2019)
I. Ali, M. Suhail, Z.A. Alothman, A. Alwarthan, Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 8, 30125–30147 (2018a)
I. Ali, C. Peng, Z.M. Khan, M. Sultan, I. Naz, Green synthesis of phytogenic magnetic nanoparticles and their applications in the adsorptive removal of crystal violet from aqueous solution. Arab. J. Sci. Eng. 43, 6245–6259 (2018b)
M.T. Amin, A.A. Alazba, U. Manzoor, A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 825910 (2014)
S. Arabi, M. Reza Sohrabi, Removal of methylene blue, a basic dye, from aqueous solutions using nano-zerovalent iron. J. Water Sci. Tech. 70(1), 24–31 (2014)
T.A. Aragaw, F.M. Bogale, B.A. Aragaw, Iron-based nanoparticles in wastewater treatment: a review on synthesis methods, applications, and removal mechanisms. J. Saudi Chem. Soc. 25(8), 101280 (2021)
A. Balcha, O.P. Yadav, T. Dey, Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 23(24), 25485–25493 (2016)
P. Balu, I.V. Asharani, D. Thirumalai, Catalytic degradation of hazardous textile dyes by iron oxide nanoparticles prepared from Raphanus sativus leaves’ extract: a greener approach. J Mater Sci. Mater Electron 31, 10669–10676 (2020)
Md.S.H. Bhuiyan, M.Y. Miah, S.C. Paul, T.D. Aka, O. Saha, M.M. Rahaman, M.J.I. Sharif, O. Habiba, M. Ashaduzzamane, Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon, 6(8), e04603 (2020)
I. Bibi, N. Nazar, S. Ata, M. Sultan, A. Ali, A. Abbas, M. Iqbal, Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J. Market. Res. 8(6), 6115–6124 (2019)
H. Borji, G.M. Ayoub, R. Bilbeisi, N., Nassar, L. Malaeb, How effective are nanomaterials for the removal of heavy metals from water and wastewater? Water Air Soil Pollut. 231(7), 1–35 (2020)
P. Chamoli, M.K. Das, K.K. Kar, Green synthesis of silver-graphene nanocomposite-based transparent conducting film. Physica E 90, 76–84 (2017)
P. Chamoli, R.K. Shukla, A.N. Bezbaruah, K.K. Kar, K.K. Raina, Rapid microwave growth of mesoporous TiO2 nano-tripods for efficient photocatalysis and adsorption. J. Appl. Phys. 130, 164901 (2021a)
P. Chamoli, R.K. Shukla, A. Bezbaruah, K.K. Kar, K.K. Raina, Microwave-assisted rapid synthesis of honeycomb core-ZnO tetrapods nanocomposites for excellent photocatalytic activity against different organic dyes. Appl. Surf. Sci. 555, 149663 (2021b)
Z. Cheng, J. Liao, B. He, F. Jhang, X. Huang, L. Zhou, One-step fabrication of graphene oxide enhanced magnetic composite gel for highly efficient dye adsorption and catalysis. J. ACS Sustain. Chem. Eng. 3(7), 1677–1685 (2015)
B.L. Cínti, Z.F. PolonioJoão, A. Pamphile, J.C. Polonio, Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 3(2), 275–290 (2019)
X. Cui, Y. Zheng, M. Tian, Z. Dong, Novel yolk–shell-structured Fe3O4@γ-AlOOH nanocomposite modified with Pd nanoparticles as a recyclable catalyst with excellent catalytic activity. J. Appl. Sci. Surf. 416, 103–111 (2017)
K. Cui, B. Yan, Y. Xie, H. Qian, X. Wang, Q. Huang, Y. He, S. Jin, H. Zeng, Regenerable urchin-like Fe3O4@ PDA-Ag hollow microspheres as catalyst and adsorbent for enhanced removal of organic dyes. J. Hazard. Mat. 350, 66–75 (2018)
T.K. Das, T.S. Sakthivel, A. Jeyaranjan, S. Seal, A.N. Bezbaruaha, Ultra-high arsenic adsorption by graphene oxide iron nanohybrid: removal mechanisms and potential applications. Chemosphere 253, 126702 (2020)
N. Duklan, P. Chamoli, K.K. Raina, R.K. Shukla, Dye dispersed lyotropic liquid crystals: soft materials with high ionic conductivity and self-sustained adsorbents for dye sequestration. Inorg. Chem. Commun. 116, 107924 (2020)
S. Dutta, S. Sarkar, C. Ray, T. Pal, Benzoin derived reduced graphene oxide (rGO) and its nanocomposite: application in dye removal and peroxidase-like activity. RSC Adv. 3(44), 21475–21483 (2013)
A. Eyasu, O.P. Yadav, R.K. Bachheti, Photocatalytic degradation of methyl orange dye using Cr-doped ZnS nanoparticles under visible radiation. Int. J. Chem. Tech. Res. 5(4), 1452–1461 (2013)
F. Fan, X. Wang, Y. Ma, K. Fu, Y. Yang, Enhanced photocatalytic degradation of dye wastewater using ZnO/reduced graphene oxide hybrids. Fuller. Nanotub. Car. Nanostruct. 23, 917–921 (2015)
M. Fathizadeh, A. Aroujalian, A. Raisi, Effect of added NaX nano-zeolite into polyamide as a top thin layer of membrane on water flux and salt rejection in a reverse osmosis process. J. Membr. Sci. 375(1–2), 88–95 (2011)
A. Figoli, M.S.S. Dorraji, A.R. Amani-Ghadim, Application of nanotechnology in drinking water purification (Academic Press, Water purification, 2017b), pp. 119–167
A. Figoli, M.S.S. Dorraji, A.R. Amani-Ghadim, Application of nanotechnology in drinking water purification, in Water Purification (Academic Press, 2017a), pp. 119–167
A. Gautama, A. Kshirsagara, R. Biswasa, S. Banerjee, P.K. Khann, Photodegradation of organic dyes based on anatase and rutile TiO2 nano-particles. RSC Adv. 6, 2746–2759 (2016)
I. Gehrke, A. Geiser, A. Somborn-Schulz, Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 8, 1–17 (2015)
A.K. Geim, K.S. Novoselov, The rise of graphene. Nat. Mater. 6, 183–191 (2007)
A.H. Gemeay, E.F. Aboelfetoh, R.G. El-Sharkawy, Immobilization of green synthesized silver nanoparticles onto amino-functionalized silica and their application for indigo carmine dye removal. Water Air Soil Pollut. 229, 16 (2018)
M. Ghaedi, F. Mohammdi, A. Ansari, Gold nanoparticles loaded on activated carbon as novel adsorbent for kinetic and isotherm studies of methyl orange and sunset yellow adsorption. J. Dispersion Sci. Tech. 36, 652–659 (2015)
D. Gola, A. kritia, N. Bhatt, M. Bajpai, A. Singh, A. Arya, N. Chauhan, S.K. Srivastava, P.K. Tyagi, Y. Agrawal, Silver nanoparticles for enhanced dye degradation. Curr. Res. Green Sustain. Chem. 4, 100132 (2021)
J.E.S. Haggerty, L.T. Schelhas, D.A., Kitchaev, High-fraction brookite films from amorphous precursors. Sci. Rep. 7, 15232 (2017)
E.T. Helmy, A. El Nemr, M. Mousa, E. Arafa, S. Eldafrawy, Photocatalytic degradation of organic dyes pollutants in the industrial textile wastewater by using synthesized TiO2, C-doped TiO2, S-doped TiO2 and C, S co-doped TiO2 nanoparticles. J. Water Environ. Nanotechnol. 3(2), 116–127 (2018)
L.H. Huang, C. Sun, Y.L. Liu, Pt/N-codoped TiO2 nanotubes and its photocatalytic activity under visible light. Appl. Surf. Sci. 253, 7029–7035 (2007)
M. Jabeen, M. Ishaq, W. Songz, L. Xu, I. Maqsood, Q. Deng, UV-assisted photocatalytic synthesis of ZnO-reduced graphene oxide nanocomposites with enhanced photocatalytic performance in degradation of methylene blue. J. Solid State Sci. Technol. 6, 36–43 (2017)
A. Jain, F. Ahmad, D. Gola, A. Malik, N. Chauhan, P. Deye, P.K. Tyagic, Multi dye degradation and antibacterial potential of Papaya leaf derived silver nanoparticles. Environ. Nanotechnol. Monit. Manage. 14, 100337 (2020)
K. Jyoti, A. Singh, Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J. Genet. Eng. Biotech. 14(2), 311–317 (2016)
R.D. Kale, P.B. Kane, Decolourization by PVP stabilized Fe-Ni nanoparticles of reactive black 5 dye. J. Environ. Chem. Eng. 6(5), 5961–5969 (2018)
G. Karthiga Devi, P. Senthil kumar, K. Sathish Kumar, Green synthesis of novel silver nanocomposite hydrogel based on sodium alginate as an efficient biosorbent for the dye wastewater treatment: prediction of isotherm and kinetic parameters. J. Desalin. Water Treat. 57, 27686–27699 (2016)
G. Kaur, M. Kaur, A. Thakur, A. Kumar, FeS2 pyrite nanostructures: an efficient performer in photocatalysis, in Green Methods for Wastewater Treatment, ed. by M. Naushad, S. Rajendran, E. Lichtfouse (Springer Nature Switzerland AG, 2020), p. 55
S.T. Khan, A. Malik, Engineered nanomaterials for water decontamination and purification: from lab to products. J. Hazard. Mater. 363, 295–308 (2019)
H. Kolya, P. Maiti, A. Pandey, T. Tripathy, Green synthesis of silver nanoparticles with antimicrobial and azo dye (Congo red) degradation properties using Amaranthus gangeticus Linn leaf extract. J. Anal. Sci. Tech. 6, 33–40 (2015)
S. Kumar, W. Ahlawat, G. Bhanjana, S. Heydarifard, M.M. Nazhad, N. Dilbaghi, Nanotechnology-based water treatment strategies. J. Nanosci. Nanotechnol. 14(2), 1838–1858 (2014)
V. Kumar, N. Talreja, D. Deva, N. Sankararamakrishnan, A. Sharma, N. Verma, Development of bi-metal doped micro-and nano multi-functional polymeric adsorbents for the removal of fluoride and arsenic (V) from wastewater. Desalination 282, 27–38 (2011)
N. Kumaresan, M.M.A. Sinthiya, K. Ramamurthi, R.R. Babu, K. Sethuraman, Visible light driven photocatalytic activity of ZnO/CuO nanocomposites coupled with rGO heterostructures synthesized by solid-state method for RhB dye degradation. Arab. J. Chem 13(2), 3910–3928 (2020)
K.R. Kunduru, M. Nazarkovsky, S. Farah, R.P. Pawar, A. Basu, A.J. Domb, Nanotechnology for water purification: applications of nanotechnology methods in wastewater treatment, in Water Purification (2017), pp. 33–74
G.J. Lee, J.J. Wu, Recent developments in ZnS photocatalysts from synthesis to photocatalytic applications—a review. Powder Technol. 318, 8–22 (2017)
E.R. Leon, E.L. Rodriguez, C.R. Beas, G.P. Villa, R.A.I. Palomares, Study of methylene blue degradation by gold nanoparticles synthesized within natural zeolites. J. Nanomater. 1–10 (2016)
Y. Liu, X. Chen, J. Li, C. Burda, Photocatalytic degradation of azo dyes by nitrogen-doped TiO2 nanocatalysts. Chemosphere 61(1), 11–18 (2005)
D. Liu, G. Wong, J. Lin, Y. He, X. Li, Z. Li, Photocatalysis using zero-valent nano-copper for degrading methyl orange under visible light irradiation. Opti. Mate. 53, 155–159 (2016)
Y. Liu, Y. Zhang, Q. Kou, Y. Chen, D. Han, D. Wang, Z. Lu, L. Chen, J. Yang, S. Xing, Eco-friendly seeded Fe3O4-Ag nanocrystals: a new type of highly efficient and low-cost catalyst for methylene blue reduction. RSC Ad. 8, 2209–2218 (2018)
D. Ljubas, G. Smoljanić, H. Juretić, Degradation of methyl orange and Congo red dyes by using TiO2 nanoparticles activated by the solar and the solar-like radiation. J. Environ. Manage. 161, 83–91 (2015)
H. Lu, J. Wang, M. Stoller, T. Wang, Y. Bao, H. Hao, An overview of nanomaterials for water and wastewater treatment. Adv. Mater. Sci. Eng. 2016 (2016)
T.V. Madhukar Sreekanth, P.C. Nagajyothi, G.R. Reddy, J. Shim, K. Yoo, Urea assisted ceria nanocubes for efficient removal of malachite green organic dye from aqueous system. Sci. Rep. 9, 14477 (2019)
S.K. Maji, A.K. Dutta, D.N. Srivastava, P. Paul, A. Mondal, B. Adhikarya, Effective photocatalytic degradation of organic pollutant by ZnS nanocrystals synthesized via thermal decomposition of single-source precursor. Polyhedron 30(15), 2493–2498 (2011)
M. Maninder, X. Baojun, A critical review on anti-diabetic and anti-obesity effects of dietary resistant starch. Crit. Rev. Food Sci. Nutr. 59(18), 3019–3031 (2019)
M. Nasrollahzadeh, M. Sajjadi, S. Iravani, R.S. Varma, Carbon-based sustainable nanomaterials for water treatment: state-of-art and future perspectives. Chemosphere 263, 128005 (2021)
A. Nawaz, A. Khan, N. Ali, N. Ali, M. Bilal, Fabrication and characterization of new ternary ferrites-chitosan nanocomposite for solar-light driven photocatalytic degradation of a model textile dye. Environ. Technol. Innov. 20, 101079 (2020)
Ü. Özgür, Ya.I. Alivov, C. Liu, A. Teke, M.A. Reshchikov, S. Doğan, V. Avrutin, S.-J. Cho, H. Morkoç, A comprehensive review of ZnO materials and devices. J. Appl. Phys. 98, 041301 (2005)
S. Pandey, J.Y. Do, J. Kim, M. Kanga, Fast and highly efficient catalytic degradation of dyes using κ-carrageenan stabilized silver nanoparticles nanocatalyst. Carbohydr. Polym. 230(15), 115597 (2020)
Q.I. Rahman, M. Ahmad, S.K. Misra, M. Lohani, Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater. Lett. 91, 170–174 (2013)
R. Rajkumar, G. Ezhumalai, M. Gnanadesigan, A green approach for the synthesis of silver nanoparticles by Chlorella vulgaris and its application in photocatalytic dye degradation activity. Environ. Technol. Innov. 21, 101282 (2021)
S. Rani, M. Aggarwal, M. Kumar, S. Sharma, D. Kumar, Removal of methylene blue and rhodamine B from water by zirconium oxide/graphene. J. Water Sci. 30, 51–60 (2016)
U.S. Rashida, T.K. Dasa, T.S. Sakthivel, S. Seal, A.N. Bezbaruaha, GO-CeO2 nanohybrid for ultra-rapid fluoride removal from drinking water. Sci. Total Environ. 793, 148547 (2021)
K. Ravi, B.S. Mohan, G.S. Sree, I.M. Raju, K. Basavaiah, B.V. Rao, ZnO/RGO nanocomposite via hydrothermal route for photocatalytic degradation of dyes in presence of visible light. Int. J. Chem. Stud. 6, 20–26 (2018)
C. Sarkar, C. Bora. S.K. Dolui, Selective dye adsorption by pH modulation on amine-functionalized reduced graphene oxide–carbon nanotube hybrid. J. Ind. Eng. Chem. Res. 53(42), 16148–16155 (2014)
K. Sathiyana, R. Bar-Zivb, O. Mendelson, T. Zidkia, Controllable synthesis of TiO2 nanoparticles and their photocatalytic activity in dye degradation. Mater. Res. Bull. 126, 110842 (2020)
M. Selvaraj, A. Hai, F. Banata, M.A. Haija, Application and prospects of carbon nanostructured materials in water treatment: a review. J. Water Process Eng. 33, 100996 (2020)
M. Sharma, T. Jain, S. Singh, O.P. Pandey, Photocatalytic degradation of organic dyes under UV–visible light using capped ZnS nanoparticles. Sol. Energy 86(1), 626–633 (2012)
R. Singh, P.B. Barman, D. Sharma, Synthesis, structural and optical properties of Ag doped ZnO nanoparticles with enhanced photocatalytic properties by photo degradation of organic dyes. J. Mater. Sci. Mater. Electron. 28(8), 5705–5717 (2017)
S.C. Smith, D.F. Rodrigues, Carbon-based nanomaterials for removal of chemical and biological contaminants from water: a review of mechanisms and applications. Carbon 91, 122–143 (2015)
H. Su, Z. Ye, N. Hmidi, High-performance iron oxide–graphene oxide nanocomposite adsorbents for arsenic removal. Colloids Surf. A 522, 161–172 (2017)
N. Sykam, V. Madhavi, G. Mohan Rao, Rapid and efficient green reduction of graphene oxide for outstanding supercapacitors and dye adsorption applications. J. Environ. Chem. Eng. 6(2), 3223–3232 (2018)
A.M. Tayeb, D.S. Hussein, Synthesis of TiO2 nanoparticles and their photocatalytic activity for methylene blue. Am. J. Nanomater. 3, 57–63 (2015)
R.K. Thines, N.M. Mubarak, S. Nizamuddin, J.N. Sahu, E.C. Abdullah, P. Ganesan, Application potential of carbon nanomaterials in water and wastewater treatment: a review. J. Taiwan Inst. Chem. Eng. 72, 116–133 (2017)
J. Wang, P. Zhang, B. Liang, Y. Liu, T. Xu, L. Wang, B. Cao, K. Pan, Graphene oxide as an effective barrier on a porous nanofibrous membrane for water treatment. ACS Appl Mater. Interfaces 8(9), 6211–6218 (2016)
P. Xu, G.M. Zeng, D.L. Huang, C.L. Feng, S. Hu, M.H. Zhao, C. Lai, Z. Wei, C. Huang, G.X. Xie, Z.F. Liu, Use of iron oxide nanomaterials in wastewater treatment: a review. Sci. Total Environ. 424, 1–10 (2012)
Z. Xu, M. Zhang, J. Wu, J. Liang, L. Zhou, B. Lu, Visible light-degradation of azo dye methyl orange using TiO 2/β-FeOOH as a heterogeneous photo-Fenton-like catalyst. Water Sci. Tech. 68(10), 2178–2185 (2013)
Z. Ye, L. Kong, F. Chen, Z. Chen, Y. Lin, C. Liu, A comparative study of photocatalytic activity of ZnS photocatalyst for degradation of various dyes. Optik 164, 345–354 (2018)
M.L. Yola, T. Eren, N. Atar, S. Wang, Adsorptive and photocatalytic removal of reactive dyes by silver nanoparticle-colemanite ore waste. J. Chem. Eng. 242, 333–340 (2014)
S. Zhang, Preparation of controlled-shape ZnS microcrystals and photocatalytic property. Ceram. Int. 40(3), 4553–4557 (2014)
H.E. Zhong, Y.A.N.G. Shaogui, J.U. Yongming, S.U.N. Cheng, Microwave photocatalytic degradation of Rhodamine B using TiO2 supported on activated carbon: mechanism implication. J. Environ. Sci. 21(2), 268–272 (2009)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Twinkle, T. et al. (2022). Nanomaterials and Purification Techniques for Water Purification and Wastewater Treatment. In: Katiyar, J.K., Panwar, V., Ahlawat, N. (eds) Nanomaterials for Advanced Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-19-1384-6_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-1384-6_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-1383-9
Online ISBN: 978-981-19-1384-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)