Abstract
Proteases are responsible for several processes essential to life and, controlling their activity is naturally important in many specific metabolic events. When a phytophagous insect feeds, the response machinery of a plant leads to the production of protease inhibitors (PIs), which can occur locally or systemically. Upon reaching the insect’s intestine, PIs bind to specific proteases and compromise the insect’s digestibility, reducing the absorption of dietary amino acids. The impaired nutritional balance affects the insect’s development and can lead to death. In this sense, PIs have gained prominence as alternatives in the control of pest insects, minimizing the toxic effects on other animals and the environment. Conversely, insects express multiple isoforms of important digestive enzymes to circumvent the toxic effect of plant PIs. Our research group is dedicated to understanding the biochemical mechanisms involved in plant–pest interaction from an enzymatic, proteomic, and molecular biology point of view. Because of these efforts, dozens of articles were generated, besides PI patented for use as ecologically correct agricultural defensives. This chapter provides an updated overview of advances in PI research applied to insect pest control.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Proteases handle several processes essential to life. This diverse group of enzymes can cleave peptide bonds to finely control protein catabolism, selectively degrade damaged proteins, or promote mass hydrolysis of dietary proteins. Advances in enzymology and proteomics in recent decades have shown that proteases are essential not only for providing free amino acids to the cell but also for modulating important processes, such as the removal of specific segments in zymogens (Stroud et al. 1977; Gorelick and Otani 1999; Donepudi and Grütter 2002; Plainkum et al. 2003) and immature proteins (Peng et al. 1989; Muramatsu and Fukazawa 1993; Khan and James 1998) or the removal of a signal peptide when the protein is already in the appropriate cell compartment (Hussain et al. 1982; Novak and Dev 1988; Friedmann et al. 2004; Lemberg 2011).
Proteolytic activity is also linked to the need to control specific metabolic events, such as the final processing of proteins before they play their role in the cell (Lum and Blobel 1997; Guttentag et al. 2003; Grau et al. 2005; Manolaridis et al. 2013), selectively removing proteins when they are no longer useful or recycling amino acids needed to synthesize new polypeptide chains (Ciechanover 1994; Bochtler et al. 1999). Besides intra- and intercellular processes, proteases mediate several molecular interactions that occur between different organisms in a given environment. Intracellular parasites, for example, secrete proteases that help their interactions and survival in the host cell (Alves and Colli 2007; Knox 2007; Laliberté and Carruthers 2008). Likewise, the hydrolysis of plant proteins in the intestine of herbivorous insects are extracellular processes that aim to provide free amino acids that will be absorbed to later make up new proteins (da Silva Júnior et al. 2020).
During the evolutionary process, insects gained complex protease systems, an essential process to get a better nutritional benefit (Silva-Júnior et al. 2021). If, on the one hand, an arsenal of proteases favors herbivory, co-evolution has selected plants with strategies to overcome the adverse effects of insect proteases (Zhu-Salzman and Zeng 2015; Pilon et al. 2017a; Meriño-Cabrera et al. 2018). Plants challenged by a pathogen or predator, for example, produce protease inhibitors (PI) that bind to proteolytic enzymes and prevent or limit their activity (Ryan 1990; Habib and Fazili 2007); this digestive deficiency implies less free amino acids to be absorbed and used as raw material in the synthesis of proteins necessary for the proper development of the insect. It is already well established that a wide variety of organisms use PIs not only to control endogenous proteolytic functions but also to ensure their protection against herbivory or infection. In this sense, PIs act for the complex set of molecular interactions that occur between different organisms in an ecosystem, acting as regulators of proteolytic events. Therefore, it is not surprising that PIs are being proposed as a tool for the control of herbivores and pathogens (Clemente et al. 2019).
Based on this, pest control strategies using PIs were developed to control nematodes (Turrà et al. 2009), viruses (Masoud et al. 1993) bacteria (Mishra et al. 2020), and phytophagous insects (Senthilkumar et al. 2010). The effects of dietary PIs on the fecundity and growth of herbivorous insects have been described for several species (Thomas et al. 1995; Telang et al. 2003; Jamal et al. 2015; Dantzger et al. 2015; Singh et al. 2020), and the implication of extracellular proteases in pathogenic processes has been documented in several cases (Dunaevsky et al. 2005; Armstrong 2006; Santos and Braga-Silva 2012). For this, the use of PIs of protein origin was proposed to be expressed through transgenics to protect plants from agricultural pests (Gatehouse et al. 1993; De Leo et al. 2002; Zhu-Salzman and Zeng 2015). Today, the expression of PIs by genetically modified plants is a reality in the control of herbivores and plant parasites (Rahbé et al. 2003; Riglietti et al. 2008; Senthilkumar et al. 2010; Khalf et al. 2010). Therefore, this additional protection granted to economically important plants has a powerful appeal from the food, biofuel, textile industry and from the entire production chain that involves them.
6.2 Serine Proteases and Plant Protease Inhibitors
According to the enzymatic classification system created in 1956 (Knight 1962), serine proteases (EC 3.4.21) belong to the class of hydrolases, a sub-class of hydrolases that act on peptide bonds. The family name derives from the nucleophilic Ser residue in the active site of the enzyme, which attacks the peptide carbonyl group of the substrate to form a tetrahedral acyl-enzyme intermediate (Hedstrom 2002; Cox and Nelson 2008). At the end of the peptide bond hydrolysis, the complete organic reaction mechanism of the serine proteases involves the catalytic triad composed of Ser, His, and Asp (Matthews et al. 1967; Blow et al. 1969; Henderson 1971).
Serine proteases are the best studied peptidases and are considered the main responsible for protein digestion in the intestine of important pest insects, such as those belonging to the orders Lepidoptera (Pilon et al. 2017b; Zhao and Ee 2018; Meriño-Cabrera et al. 2018; Zhao et al. 2019; da Silva Júnior et al. 2020) and Coleoptera (Mochizuki 1998; Alarcón et al. 2002; Marshall et al. 2008). At this point, enzymes from the Trypsin-like, Chymotrypsin-like, and Elastase-like families stand out as the main representatives (Kuwar et al. 2015; da Silva Júnior et al. 2020). Because of this importance, several plant serine protease inhibitors have been described and characterized.
Protease inhibitors of protein origin are classified into 99 families according to the homology existing in the amino acid sequence of their representatives, at least in the inhibitory unit. There may also be subfamilies when there is evidence of a very old evolutionary divergence within the family. PIs are also grouped into clans, which represent a group of inhibitors in one or more families that show evidence of an evolutionary relationship from their similar tertiary structures (Rawlings et al. 2018).
Against this background, plant protease inhibitors (PPI) are usually small proteins found in plant storage tissues such as the root, but also in leaves (De Leo et al. 2002). In seeds, tubers, and other plant storage tissues, trypsin inhibitors represent about 10% of the total protein content (Mandal et al. 2002). These high levels of PPI are associated with plant resistance to insects and pathogens (Kim et al. 2009; Dunse et al. 2010). Although high levels of PPI are often found in Leguminosae, Solanaceae, and Gramineae (Brzin and Kidrič 1996; Xu et al. 2001; Sin and Chye 2004), the expression of these PPI depends on the stage of development of the plant, tissue, and presence of stressors, even presenting different isoforms in the same tissue (Sels et al. 2008).
Two very well-studied PPI families are Kunitz and Bowman-Birk. Members of the Kunitz family have in their primary structure some conserved residues, such as the four cysteine residues that form the two intrachain disulfide bonds (Pouvreau et al. 2003), besides being monomeric proteins containing from 150 to 200 amino acid residues and approximately 20 kDa (Norioka et al. 1988). Each molecule contains a unique binding site that interacts strongly with the protease against which the inhibitor is targeted (Salier 1990).
On the other hand, Bowman-Birk PPIs are polypeptide chains of approximately 8 kDa that can form oligomers, ranging from 54 to 133 amino acid residues (Birk 1985; Kennedy 1998). A Bowman–Birk basic unit contains a high proportion of cysteine residues and forms several intrachain disulfide bonds, leading to a rigidly folded conformation (Losso 2008). The monomeric unit contains two binding loops with reactive sites on the enzyme. Therefore, each inhibitor can inhibit up to two proteases with different inhibitory specificities (Qi et al. 2005).
Although PPIs are very well documented as plant defensive compounds, the damage caused to insects and pathogens goes far beyond just decreasing the activity of digestive proteases. The molecular mechanisms are not fully known, but metabolome, transcriptome, proteome, and histology studies have shown several effects on the physiology of insects subjected to PPI (Valueva and Mosolov 2004; Bayés et al. 2006; Quilis et al. 2007; Bobbarala 2009; Sabotič and Kos 2012; Radanovic and Anauate 2013; Quilis et al. 2014; Shao et al. 2016; Cingel et al. 2017; Shamsi et al. 2018).
6.3 Contributions in the Field from Our Research Group
The use of PIs as an agricultural defensive was suggested as far back as 1947, when Mikel and Standish (1947) observed that a soy-based diet limited the development of some insects. Just 25 years later, Green and Ryan (1972) demonstrated that damage to nightshade leaves induced PI synthesis, suggesting the protective role of this compound. In the following decades, the economic and environmental importance of developing alternative strategies for the ecologically correct control of agricultural pests increased interest in the development of IP for this purpose. However, the biochemical mechanisms involved in the interaction between insect physiology and PIs were not well known.
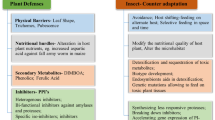
Given this scenario, our research group focused on understanding the biochemical mechanisms involved in the plant–pest interaction from the point of view of biochemistry and molecular biology, exploring techniques of enzymology, proteomics, metabolomics, and transcriptomics. The interaction between soybean (Glycine max) and soybean caterpillar (Anticarsia gemmatalis) was used as a model to validate the biochemical mechanism of plant response via the Lipoxygenase pathway (Fig. 6.1). Soy was chosen for its importance in agricultural production in Brazil, and A. gemmatalis was chosen because, besides being a key soybean pest, it is an insect that is easy to breed and presents a good yield of biological material for studies involving biochemical analyses that require purification and characterization of the enzymes involved in the insect–plant interaction process.
Lipoxygenase pathway. The mechanical damage caused by the biting insect activates a cascade of intracellular messengers and leads to the formation of jasmonic acid. This plant hormone activates transcription factors that end with the production of protease inhibitors, compromising the insect’s digestibility
The study of plant–insect interaction requires a thorough understanding of the arthropod and the plant under properly controlled conditions. In this sense, the determination of the enzymatic profile in the different larval instars of A. gemmatalis was an important step to determine the proteases responsible for the digestive process of the caterpillar throughout its development. For this, da Silva Júnior et al. (2020) showed that the proteolytic profile in the intestine of A. gemmatalis changes during larval development, with a predominance of cysteine protease activity in the third instar and serine protease in the fourth and fifth instars, suggesting modulation in gene expression accompanied by different nutrient demand throughout this internship. Previous studies involving Lepidoptera focused on the physiology and biochemistry of the insect only in the fifth instar, as this is the moment of the greatest voracity of the caterpillar. However, some studies show changes in the morphological profile in the intestine of some insects during development (Chougule et al. 2005; Kipgen and Aggarwal 2014; Zhao et al. 2019). This dataset suggests that both cysteine and serine proteases are important targets for the development of PIs, aiming to compromise larval development in different instars. Insects express an arsenal of isoforms for digestive enzymes, having as an important consequence the attempt to circumvent the negative effects of PIs (Kotkar et al. 2009; Lomate and Hivrale 2011; Crava et al. 2013). Therefore, knowledge of the primary and tertiary structures of proteins is of fundamental importance. Thinking about that, Silva-Júnior et al. (2021) described the proteomic profile of the intestine of A. gemmatalis, showing a large number of functional enzymes, their sequences and post-translational modifications (PTM) through proteomics techniques. The description of a proteomic profile of enzymes is a challenge because of the low concentrations of these hydrolases concerning other abundant proteins present in the sample. However, the conciliation of several proteomic methodologies allowed for high coverage of the intestinal proteome of the caterpillar as shown in the workflow in Fig. 6.2. Furthermore, research by our group showed the energies and points of interaction between enzymes and inhibitors by molecular docking are important information for the rational design of PIs (Meriño-Cabrera et al. 2019, 2020; de Almeida Barros et al. 2021; Silva-Júnior et al. 2021).
A deeper knowledge of the enzymatic kinetics of digestive proteases from A. gemmatalis allows a better understanding of the active centers, the mechanisms of reaction of these enzymes, and the PI that need to be applied as inhibitors of the complex arsenal of digestive proteases of the insect. In this sense, trypsins bound to the intestinal membrane of the soybean caterpillar were partially purified and identified by mass spectrometry (Reis et al. 2012). In addition, our research group also evaluated the contribution of endosymbiotic bacteria in the production of proteolytic enzymes in the intestine of A. gemmatalis (Pilon et al. 2013). The main trypsins of these bacterial isolates were purified and kinetically characterized (Pilon et al. 2017b) and this dataset allowed us to infer that endosymbiont bacteria synthesize trypsin, contributing to the insect’s digestibility.
These works developed by our group brought a look towards the insect and its intestinal enzymology of A. gemmatalis under normal conditions, that is, free from PI treatments. However, the development of PIs, peptides, or organic peptide mimetics with inhibitory activity requires a thorough understanding of the enzyme-inhibitor complex. Inhibition kinetic studies are tools for understanding the multi-mechanistic enzyme system. Thus, we performed the kinetic characterization of trypsin-like inhibition of the insect against natural soybean PI (SKTI and SBBI) and synthetic PI (Benzamidine and Berenil) to understand the inhibition from the physiological structure/function point of view (Patarroyo-Vargas et al. 2020; Silva-junior and de Almeida Oliveira 2021). Our study showed, for the first time, the adaptation of trypsin-like enzymes in the intestine of A. gemmatalis against different inhibitors. The effect of PI was also evidenced when the caterpillar was challenged with Benzamidine (Pilon et al. 2018), Berenyl (Moreira et al. 2011; Paixão et al. 2013), synthetic peptides (Patarroyo-Vargas et al. 2018; de Oliveira et al. 2020; de Almeida Barros et al. 2021), SKTI, SBBI (Mendonça et al. 2020), ILTI and ApTI (Meriño-Cabrera et al. 2020).
If, on the one hand, the in-depth study of the pest insect is important, it is necessary to understand the physiology of the target plant and its response systems against the herbivore. With this in mind, we performed biological assays associated with metabolome analysis in two soybean genotypes contrasting for herbivory resistance in response to A. gemmatalis (Gomez et al. 2018). This approach allowed showing flavonoid profiles from soybean leaf extract and efficiently identifying some new compounds related to resistance. With the metabolic profiles, it was possible to reconstruct the biosynthetic pathways of flavonoids, revealing upregulated glycoconjugate flavonoids in the resistant soybean genotype. These differences in abundance between genotypes suggest they handle resistance to herbivory in these varieties and open the door to a vast field of investigation aimed at increasing soybean resistance against insects. Still from the perspective of how the plant perceives and reacts to damage caused by the herbivore, we show that the response to flavonoids also occurs when the plant suffers artificial mechanical damage (da Silva Júnior et al. 2021). In addition, the deletion of genes in soybean seeds that code for proteins important to plant defense, such as the lipoxygenase enzyme and PI SKTI, does not interfere with the plant’s ability to respond to wounds through the lipoxygenase pathway (da Silva Fortunato et al. 2007). These results have industrial and practical appeal since these proteins are undesirable in the seed, but fundamental in the plant’s defense against agricultural pests. Given the reality of climate change, it is important to foresee how the plant–insect interaction responds to environmental variations. Faustino et al. (2021) showed that soybean subjected to drought reduces herbivory and survival of A. gemmatalis. The group relied on gene expression, enzymatic kinetics, and metabolomic analysis to conclude that the drought signal alone is not enough to promote increased resistance to insect attack. These results generated by our research group in the last decade made it possible to identify the target enzymes and map the active sites, allowing the development of potent peptide PIs to be sprayed, used as models for mimetic peptide production, or even as a model for transgenics in the agricultural pest control. We have developed promising protein inhibitors for agricultural pest control. Part of these contributions is compiled in Fig. 6.3.
Some researches carried out in our group are characterized by the study of the insect–plant interaction. Anticarsia gemmatalis represents the main object of study of our papers. And the plants are crops such as soybeans, tomatoes, and coffee, but mainly soybeans have been used because it is the host plant of A. gemmatalis. In insect pests, the activity of enzymes from the digestive tract of caterpillars has been evaluated and characterized, mainly trypsins, enzymes that catalyze the degradation of proteins, the activity has been determined in crude extracts from the intestine and in samples enriched as trypsin isoforms from affinity purification, as well as two-dimensional electrophoresis associated with mass spectrometry (1). In parallel, in the host plants of these pest insects, a metabolic defense pathway has been evaluated, which is the lipoxygenase pathway that activates the expression of protease inhibitors, as well as the purification and stability analysis of these inhibitors (2). From the results of several studies of insect–plant interaction, we started to evaluate the interaction of protein inhibitors and trypsins through in silico studies (protein modeling, protein–protein docking, and simulations by molecular dynamics) that allow predicting the inhibitory effect and its capacity of trypsin binding complementing the experimental study (in vitro and in vivo) (3). From these studies and analyzes of a region known as interface (protein–protein interaction site) several peptides (patent deposits) have been proposed with bioinsecticide application on Anticarsia gemmatalis and Spodoptera cosmioides (4) corroborated from in vitro and in vivo tests (5)
6.4 Final Considerations
The agricultural ex vivo application of protease inhibitors is still limited because of the large molecular size, which turns them unstable in the environment. We believe that smaller scaffold peptides designed according to the active site of important digestive proteases and reactive domains of protease inhibitors could overcome this bottleneck. Besides that, novel designed peptides have an advantage over vegetable PIs that are not having co-evolved with insects, which might avoid adaptations. To counteract the complex set of proteases that insects possess in their midgut, exposing them to PIs for different classes of proteases could overcome the adaptative mechanisms more efficiently. Despite all the bottlenecks, PIs could be useful in integrated pest management as an alternative/supplementary approach if well explored.
References
Alarcón FJ, Martínez TF, Barranco P, Cabello T, Díaz M, Moyano FJ (2002) Digestive proteases during development of larvae of red palm weevil, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera: Curculionidae). Insect Biochem Mol Biol 32(3):265–274
Alves MJM, Colli W (2007) Trypanosoma cruzi: adhesion to the host cell and intracellular survival. In: IUBMB life. Wiley, New York, pp 274–279
Armstrong PB (2006) Proteases and protease inhibitors: a balance of activities in host-pathogen interaction. Immunobiology 211:263–281
Bayés A, de la Vega MR, Vendrell J, Aviles FX, Jongsma MA, Beekwilder J (2006) Response of the digestive system of Helicoverpa zea to ingestion of potato carboxypeptidase inhibitor and characterization of an uninhibited carboxypeptidase B. Insect Biochem Mol Biol 36(8):654–664
Birk Y (1985) The Bowman-Birk inhibitor. Trypsin- and chymotrypsin inhibitor from soybeans. Int J Pept Protein Res 25:113–131
Blow DM, Birktoft JJ, Hartley BS (1969) Role of a buried acid group in the mechanism of action of chymotrypsin. Nature 221(5178):337–340
Bobbarala V (2009) Antifungal activity of selected plant extracts against phytopathogenic fungi Aspergillus niger F2723. Indian J Sci Technol 2(4):87–90
Bochtler M, Ditzel L, Groll M, Hartmann C, Huber R (1999) The proteasome. Annu Rev Biophys Biomol Struct 28:295–317
Brzin J, Kidrič M (1996) Proteinases and their inhibitors in plants: role in normal growth and in response to various stress conditions. Biotechnol Genet Eng Rev 13(1):421–468
Chougule NP, Giri AP, Sainani MN, Gupta VS (2005) Gene expression patterns of Helicoverpa armigera gut proteases. Insect Biochem Mol Biol 35(4):355–367
Ciechanover A (1994) The ubiquitin-proteasome proteolytic pathway. Cell 79:13–21
Cingel A, Savić J, Lazarević J, Ćosić T, Raspor M, Smigocki A, Ninković S (2017) Co-expression of the proteinase inhibitors oryzacystatin I and oryzacystatin II in transgenic potato alters Colorado potato beetle larval development. Insect Sci 24(5):768–780
Clemente M, Corigliano MG, Pariani SA, Sánchez-López EF, Sander VA, Ramos-Duarte VA (2019) Plant serine protease inhibitors: biotechnology application in agriculture and molecular farming. Int J Mol Sci 20:1345
Cox M, Nelson DL (2008) Lehninger principles of biochemistry, 7th edn. W. H. Freeman and Company, New York
Crava CM, Bel Y, Jakubowska AK, Ferré J, Escriche B (2013) Midgut aminopeptidase N isoforms from Ostrinia nubilalis: activity characterization and differential binding to Cry1Ab and Cry1Fa proteins from Bacillus thuringiensis. Insect Biochem Mol Biol 43(10):924–935
da Silva Fortunato F, de Almeida Oliveira MG, Brumano MHN, Silva CHO, Guedes RNC, Moreira MA (2007) Lipoxygenase-induced defense of soybean varieties to the attack of the velvetbean caterpillar (Anticarsia gemmatalis Hübner). J Pest Sci 80(4):241–247
da Silva Júnior NR, Vital CE, de Almeida Barros R, Faustino VA, Monteiro LP, Barros E, de Oliveira EE, de Oliveira Ramos HJ, de Almeida Oliveira MG (2020) Intestinal proteolytic profile changes during larval development of Anticarsia gemmatalis caterpillars. Arch Insect Biochem Physiol 103(1):e21631
da Silva Júnior NR, Barros RA, Meriño-Cabrera YB, de Almeida Oliveira MG (2021) Does mechanical damage on soybean induces the production of flavonoids? In: Current approaches in science and technology research, vol 3. Book Publisher International, Hooghly, pp 56–66
Dantzger M, Vasconcelos IM, Scorsato V, Aparicio R, Marangoni S, Macedo MLR (2015) Bowman-Birk proteinase inhibitor from Clitoria fairchildiana seeds: isolation, biochemical properties and insecticidal potential. Phytochemistry 118:224–235
de Almeida Barros R, Meriño-Cabrera Y, Vital CE, da Silva Júnior NR, de Oliveira CN, Lessa Barbosa S, Marques Gonçalves Assis JV, Ramos HJO, de Almeida Oliveira MG (2021) Small peptides inhibit gut trypsin-like proteases and impair Anticarsia gemmatalis (Lepidoptera: Noctuidae) survival and development. Pest Manag Sci 77(4):1714–1723
De Leo F, Volpicella M, Licciulli F, Liuni S, Gallerani R, Ceci LR (2002) PLANT-Pls: a database for plant protease inhibitors and their genes. Nucleic Acids Res 30(1):347–348
de Oliveira G, de Almeida Barros R, da Silva Júnior NR, Vital CE, Cordeiro G, da Silva CR, Vargas AMP, Campos WG, de Oliveira Ramos HJ, de Almeida Oliveira MG (2020) Inhibitory effects of tripeptides to enzymatic activity and life cycle parameters of Anticarsia gemmatalis. Phytoparasitica 48(5):823–831
Donepudi M, Grütter MG (2002) Structure and zymogen activation of caspases. Biophys Chem 101–102:145–153
Dunaevsky YE, Elpidina EN, Vinokurov KS, Belozersky MA (2005) Protease inhibitors in improvement of plant resistance to pathogens and insects. Mol Biol 39:608–613
Dunse KM, Stevens JA, Lay FT, Gaspar YM, Heath RL, Anderson MA (2010) Coexpression of potato type I and II proteinase inhibitors gives cotton plants protection against insect damage in the field. Proc Natl Acad Sci U S A 107(34):15011–15015
Faustino VA, de Souza Gouveia A, Coutinho FS, da Silva Júnior NR, de Almeida Barros R, Meriño Cabrera Y, Vital CE, Loriato VAP, Martins LGC, Fontes EPB, de Oliveira Ramos HJ, Oliveira MGA (2021) Soybean plants under simultaneous signals of drought and Anticarsia gemmatalis herbivory trigger gene expression and metabolic pathways reducing larval survival. Environ Exp Bot 190:104594
Friedmann E, Lemberg MK, Weihofen A, Dev KK, Dengler U, Rovelli G, Martoglio B (2004) Consensus analysis of signal peptide peptidase and homologous human aspartic proteases reveals opposite topology of catalytic domains compared with presenilins. J Biol Chem 279(49):50790–50798
Gatehouse AMR, Shi Y, Powell KS, Brough C, Hilder VA, Hamilton WDO, Newell CA, Merryweather A, Boulter D, Gatehouse JA (1993) Approaches to insect resistance using transgenic plants. Philos Trans R Soc Lond Ser B Biol Sci 342(1301):279–286
Gomez JD, Vital CE, Oliveira MGA, Ramos HJO (2018) Broad range flavonoid profiling by LC/MS of soybean genotypes contrasting for resistance to Anticarsia gemmatalis (Lepidoptera: Noctuidae). PLoS One 13(10):e0205010
Gorelick FS, Otani T (1999) Mechanisms of intracellular zymogen activation. Baillieres Best Pract Clin Gastroenterol 13(2):227–240
Grau S, Baldi A, Bussani R, Tian X, Stefanescu R, Przybylski M, Richards P, Jones SA, Shridhar V, Clausen T, Ehrmann M (2005) Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc Natl Acad Sci U S A 102(17):6021–6026
Green TR, Ryan CA (1972) Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science 175(4023):776–777
Guttentag S, Robinson L, Zhang P, Brasch F, Bühling F, Beers M (2003) Cysteine protease activity is required for surfactant protein B processing and lamellar body genesis. Am J Respir Cell Mol Biol 28(1):69–79
Habib H, Fazili KM (2007) Plant protease inhibitors: a defense strategy in plants. Biotechnol Mol Biol Rev 2(3):68–85
Hedstrom L (2002) Serine protease mechanism and specificity. Chem Rev 102(12):4501–4523
Henderson R (1971) Catalytic activity of -chymotrypsin in which histidine-57 has been methylated. Biochem J 124(1):13–18
Hussain M, Ozawa Y, Ichihara S, Mizushima S (1982) Signal peptide digestion in Escherichia coli effect of protease inhibitors on hydrolysis of the cleaved signal peptide of the major outer-membrane lipoprotein. Eur J Biochem 129(1):233–239
Jamal F, Pandey PK, Singh D, Ahmed W (2015) A Kunitz-type serine protease inhibitor from Butea monosperma seed and its influence on developmental physiology of Helicoverpa armigera. Process Biochem 50(2):311–316
Kennedy AR (1998) The Bowman-Birk inhibitor from soybeans as an anticarcinogenic agent. Am J Clin Nutr 68(6 Suppl):1406S–1412S
Khalf M, Goulet C, Vorster J, Brunelle F, Anguenot R, Fliss I, Michaud D (2010) Tubers from potato lines expressing a tomato Kunitz protease inhibitor are substantially equivalent to parental and transgenic controls. Plant Biotechnol J 8(2):155–169
Khan AR, James MNG (1998) Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci 7:815–836
Kim JY, Park SC, Hwang I, Cheong H, Nah JW, Hahm KS, Park Y (2009) Protease inhibitors from plants with antimicrobial activity. Int J Mol Sci 10:2860–2872
Kipgen L, Aggarwal KK (2014) Gut protease profiles of different instars of Helicoverpa armigera (Lepidoptera: Noctuidae). Int J Trop Insect Sci 34(3):172–178
Knight SG (1962) Report of the commission on enzymes of the International Union of Biochemistry. AIBS Bull 12(1):39
Knox DP (2007) Proteinase inhibitors and helminth parasite infection. Parasite Immunol 29:57–71
Kotkar HM, Sarate PJ, Tamhane VA, Gupta VS, Giri AP (2009) Responses of midgut amylases of Helicoverpa armigera to feeding on various host plants. J Insect Physiol 55(8):663–670
Kuwar SS, Pauchet Y, Vogel H, Heckel DG (2015) Adaptive regulation of digestive serine proteases in the larval midgut of Helicoverpa armigera in response to a plant protease inhibitor. Insect Biochem Mol Biol 59:18–29
Laliberté J, Carruthers VB (2008) Host cell manipulation by the human pathogen toxoplasma gondii. Cell Mol Life Sci 65:1900–1915
Lemberg MK (2011) Intramembrane proteolysis in regulated protein trafficking. Traffic 12:1109–1118
Lomate PR, Hivrale VK (2011) Differential responses of midgut soluble aminopeptidases of Helicoverpa armigera to feeding on various host and non-host plant diets. Arthropod Plant Interact 5(4):359–368
Losso JN (2008) The biochemical and functional food properties of the Bowman-Birk inhibitor. Crit Rev Food Sci Nutr 48(1):94–118
Lum L, Blobel CP (1997) Evidence for distinct serine protease activities with a potential role in processing the sperm protein fertilin. Dev Biol 191(1):131–145
Mandal S, Kundu P, Roy B, Mandal RK (2002) Precursor of the inactive 2S seed storage protein from the Indian mustard Brassica juncea is a novel trypsin inhibitor. Characterization, post-translational processing studies, and transgenic expression to develop insect-resistant plants. J Biol Chem 277(40):37161–37168
Manolaridis I, Kulkarni K, Dodd RB, Ogasawara S, Zhang Z, Bineva G, O’Reilly N, Hanrahan SJ, Thompson AJ, Cronin N, Iwata S, Barford D (2013) Mechanism of farnesylated CAAX protein processing by the intramembrane protease Rce1. Nature 504(7479):301–305
Marshall SDG, Gatehouse LN, Becher SA, Christeller JT, Gatehouse HS, Hurst MRH, Boucias DG, Jackson TA (2008) Serine proteases identified from a Costelytra zealandica (White) (Coleoptera: Scarabaeidae) midgut EST library and their expression through insect development. Insect Mol Biol 17(3):247–259
Masoud SA, Johnson LB, White FF, Reeck GR (1993) Expression of a cysteine proteinase inhibitor (oryzacystatin-I) in transgenic tobacco plants. Plant Mol Biol 21(4):655–663
Matthews BW, Sigler PB, Henderson R, Blow DM (1967) Three-dimensional structure of tosyl-α-chymotrypsin. Nature 214(5089):652–656
Mendonça EG, de Almeida Barros R, Cordeiro G, da Silva CR, Campos WG, de Oliveira JA, de Almeida Oliveira MG (2020) Larval development and proteolytic activity of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) exposed to different soybean protease inhibitors. Arch Insect Biochem Physiol 103(1):e21637
Meriño-Cabrera Y, Zanuncio JC, da Silva RS, Solis-Vargas M, Cordeiro G, Rainha FR, Campos WG, Picanço MC, de Almeida Oliveira MG (2018) Biochemical response between insects and plants: an investigation of enzyme activity in the digestive system of Leucoptera coffeella (Lepidoptera: Lyonetiidae) and leaves of Coffea arabica (Rubiaceae) after herbivory. Ann Appl Biol 172(2):236–243
Meriño-Cabrera Y, de Oliveira Mendes TA, Macedo MLR, de Almeida Oliveira MG (2019) Inhibition of digestive trypsins by plant Kunitz proteins reduces the viability of Spodoptera cosmioides larvae. Ann Appl Biol 175(3):336–349
Meriño-Cabrera Y, de Oliveira Mendes TA, Castro JGS, Barbosa SL, Macedo MLR, de Almeida Oliveira MG (2020) Noncompetitive tight-binding inhibition of Anticarsia gemmatalis trypsins by Adenanthera pavonina protease inhibitor affects larvae survival. Arch Insect Biochem Physiol 104(3):e21687
Mikel CE, Standish J (1947) Susceptibility of processed soy flour and soy grits in storage to attack by Tribolium castaneum (Herbst)
Mishra UN, Reddy MV, Prasad DT (2020) Plant serine protease inhibitor (SPI): a potent player with bactericidal, fungicidal, nematicidal and antiviral properties. Int J Chem Stud 8(1):2985–2993
Mochizuki A (1998) Characteristics of digestive proteases in the gut of some insect orders. Appl Entomol Zool 33(3):401–407
Moreira LF, Campos WG, Ribeiro FR, Guedes RNC, Oliveira MGA (2011) Survival and developmental impairment induced by the trypsin inhibitor bis-benzamidine in the velvetbean caterpillar (Anticarsia gemmatalis). Crop Prot 30(10):1285–1290
Muramatsu M, Fukazawa C (1993) A high-order structure of plant storage proprotein allows its second conversion by an asparagine-specific cysteine protease, a novel proteolytic enzyme. Eur J Biochem 215(1):123–132
Norioka N, Hara S, Ikenaka T, Abe J (1988) Distribution of the Kunitz and the Bowman–Birk family proteinase inhibitors in leguminous seeds. Agric Biol Chem 52(5):1245–1252
Novak P, Dev IK (1988) Degradation of a signal peptide by protease IV and oligopeptidase A. J Bacteriol 170(11):5067–5075
Paixão GP, Lourenção AL, Silva CR, Mendonça EG, Silva PL, Oliveira JA, Zanuncio JC, Oliveira MGA (2013) Biochemical responses of anticarsia gemmatalis (Lepidoptera: Noctuidae) in soybean cultivars sprayed with the protease inhibitor berenil. J Agric Food Chem 61(34):8034–8038
Patarroyo-Vargas AM, Merino-Cabrera YB, Zanuncio JC, Rocha F, Campos WG, de Almeida Oliveira MG (2018) Kinetic characterization of Anticarsia gemmatalis digestive serine- proteases and the inhibitory effect of synthetic peptides. Protein Pept Lett 24(11)
Patarroyo-Vargas AM, Cordeiro G, da Silva CR, da Silva CR, Mendonça EG, Visôtto LE, Zanuncio JC, Campos WG, Oliveira MGA (2020) Inhibition kinetics of digestive proteases for Anticarsia gemmatalis. An Acad Bras Cienc 92(Suppl 1):1–12
Peng C, Ho BK, Chang TW, Chang NT (1989) Role of human immunodeficiency virus type 1-specific protease in core protein maturation and viral infectivity. J Virol 63(6):2550–2556
Pilon FM, Visôtto LE, Guedes RNC, Oliveira MGA (2013) Proteolytic activity of gut bacteria isolated from the velvet bean caterpillar Anticarsia gemmatalis. J Comp Physiol B Biochem Syst Environ Physiol 183(6):735–747
Pilon AC, Valli M, Dametto AC, Pinto MEF, Freire RT, Castro-Gamboa I, Andricopulo AD, Bolzani VS (2017a) NuBBEDB: an updated database to uncover chemical and biological information from Brazilian biodiversity. Sci Rep 7(1):7215
Pilon FM, da Rocha Silva C, Visôtto LE, de Almeida Barros R, da Silva Júnior NR, Campos WG, de Almeida Oliveira MG (2017b) Purification and characterization of trypsin produced by gut bacteria from Anticarsia gemmatalis. Arch Insect Biochem Physiol 96(2):e21407
Pilon AM, Campos WG, Silva CR, Cordeiro G, Silva CR, Oliveira MGA (2018) Protease inhibitory, insecticidal and deterrent effects of the trypsin-inhibitor benzamidine on the velvetbean caterpillar in soybean. An Acad Bras Cienc 90(4):3475–3482
Plainkum P, Fuchs SM, Wiyakrutta S, Raines RT (2003) Creation of a zymogen. Nat Struct Biol 10(2):115–119
Pouvreau L, Gruppen H, Van Koningsveld GA, Van Den Broek LAM, Voragen AGJ (2003) The most abundant protease inhibitor in potato tuber (cv. Elkana) is a serine protease inhibitor from the Kunitz family. J Agric Food Chem 51(17):5001–5005
Qi RF, Song ZW, Chi CW (2005) Structural features and molecular evolution of Bowman-Birk protease inhibitors and their potential application. Acta Biochim Biophys Sin Shanghai 37:283–292
Quilis J, Meynard D, Vila L, Avilés FX, Guiderdoni E, San Segundo B (2007) A potato carboxypeptidase inhibitor gene provides pathogen resistance in transgenic rice. Plant Biotechnol J 5(4):537–553
Quilis J, López-García B, Meynard D, Guiderdoni E, San Segundo B (2014) Inducible expression of a fusion gene encoding two proteinase inhibitors leads to insect and pathogen resistance in transgenic rice. Plant Biotechnol J 12(3):367–377
Radanovic M, Anauate M (2013) P4–254: a phenomenological study of realistic picture copy in a case of advanced semantic dementia. Alzheimers Dement 9(4S_Part_20):P799
Rahbé Y, Deraison C, Bonadé-Bottino M, Girard C, Nardon C, Jouanin L (2003) Effects of the cysteine protease inhibitor oryzacystatin (OC-I) on different aphids and reduced performance of Myzus persicae on OC-I expressing transgenic oilseed rape. Plant Sci 164(4):441–450
Rawlings ND, Barrett AJ, Thomas PD, Huang X, Bateman A, Finn RD (2018) The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res 46(D1):D624–D632
Reis DTC, dos Mares-Guia TR, de Oliveira JS, Santos AMC, Santoro MM, Oliveira MGA (2012) Purification of a membrane-bound trypsin-like enzyme from the gut of the velvetbean caterpillar (Anticarsia gemmatalis Hübner). Acta Sci Biol Sci 34(3):263–270
Riglietti A, Ruggiero P, Crecchio C (2008) Investigating the influence of transgenic tobacco plants codifying a protease inhibitor on soil microbial community. Soil Biol Biochem 40(12):2928–2936
Ryan CA (1990) Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Annu Rev Phytopathol 28(1):425–449
Sabotič J, Kos J (2012) Microbial and fungal protease inhibitors—current and potential applications. Appl Microbiol Biotechnol 93:1351–1375
Salier JP (1990) Inter-α-trypsin inhibitor: emergence of a family within the Kunitz-type protease inhibitor superfamily. Trends Biochem Sci 15:435–439
Santos ALS, Braga-Silva LA (2012) Aspartic protease inhibitors: effective drugs against the human fungal pathogen Candida albicans. Mini Rev Med Chem 13(1):155–162
Sels J, Mathys J, De Coninck BMA, Cammue BPA, De Bolle MFC (2008) Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiol Biochem 46:941–950
Senthilkumar R, Cheng CP, Yeh KW (2010) Genetically pyramiding protease-inhibitor genes for dual broad-spectrum resistance against insect and phytopathogens in transgenic tobacco. Plant Biotechnol J 8(1):65–75
Shamsi TN, Parveen R, Ahmad A, Samal RR, Kumar S, Fatima S (2018) Inhibition of gut proteases and development of dengue vector, Aedes aegypti by Allium sativum protease inhibitor. Acta Ecol Sin 38:325–328
Shao J, Zhao M, Tong M, Wei J, Wise MR, Stone P, Chamley L, Chen Q (2016) Increased levels of HMGB1 in trophoblastic debris may contribute to preeclampsia. Reproduction 152(6):775–784
Silva-junior NR, de Almeida Oliveira MG (2021) Cinética De Inibição Das Proteases Digestivas De Anticarsia Gemmatalis:79–95
Silva-Júnior NR, Cabrera YM, Barbosa SL, de Almeida Barros R, Barros E, Vital CE, Ramos HJO, Oliveira MGA (2021) Intestinal proteases profiling from Anticarsia gemmatalis and their binding to inhibitors. Arch Insect Biochem Physiol 107(3):e21792
Sin SF, Chye ML (2004) Expression of proteinase inhibitor II proteins during floral development in Solanum americanum. Planta 219(6):1010–1022
Singh S, Singh A, Kumar S, Mittal P, Singh IK (2020) Protease inhibitors: recent advancement in its usage as a potential biocontrol agent for insect pest management. Insect Sci 27(2):186–201
Stroud RM, Kossiakoff AA, Chambers JL (1977) Mechanisms of zymogen activation. Annu Rev Biophys Bioeng 6:177–193
Telang M, Srinivasan A, Patankar A, Harsulkar A, Joshi V, Damle A, Deshpande V, Sainani M, Ranjekar P, Gupta G, Birah A, Rani S, Kachole M, Giri A, Gupta V (2003) Bitter gourd proteinase inhibitors: potential growth inhibitors of Helicoverpa armigera and Spodoptera litura. Phytochemistry 63(6):643–652
Thomas JC, Adams DG, Keppenne VD, Wasmann CC, Brown JK, Kanost MR, Bohnert HJ (1995) Protease inhibitors of Manduca sexta expressed in transgenic cotton. Plant Cell Rep 14(12):758–762
Turrà D, Bellin D, Lorito M, Gebhardt C (2009) Genotype-dependent expression of specific members of potato protease inhibitor gene families in different tissues and in response to wounding and nematode infection. J Plant Physiol 166(7):762–774
Valueva TA, Mosolov VV (2004) Role of inhibitors of proteolytic enzymes in plant defense against phytopathogenic microorganisms. Biochemist 69:1305–1309
Xu ZF, Qi WQ, Ouyang XZ, Yeung E, Chye ML (2001) A proteinase inhibitor II of Solanum americanum is expressed in phloem. Plant Mol Biol 47(6):727–738
Zhao J, Ee KY (2018) Protease inhibitors. In: Encyclopedia of food chemistry. Elsevier, Amsterdam, pp 253–259
Zhao A, Li Y, Leng C, Wang P, Li Y (2019) Inhibitory effect of protease inhibitors on larval midgut protease activities and the performance of Plutella xylostella (Lepidoptera: Plutellidae). Front Physiol 10:1–9
Zhu-Salzman K, Zeng R (2015) Insect response to plant defensive protease inhibitors. Annu Rev Entomol 60(1):233–252
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Silva-Junior, N.R., Meriño Cabrera, Y.B., de Almeida Barros, R., de Almeida Oliveira, M.G. (2022). Use of Protease Inhibitors as a Promising Alternative for Pest Control. In: Maheshwari, V.L., Patil, R.H. (eds) Natural Products as Enzyme Inhibitors. Springer, Singapore. https://doi.org/10.1007/978-981-19-0932-0_6
Download citation
DOI: https://doi.org/10.1007/978-981-19-0932-0_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0931-3
Online ISBN: 978-981-19-0932-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)