Abstract
Accurate identification of species is fundamental to both basic and applied research. Classification and identification of various life forms, particularly insects, has been a major challenge to the scientific community with the dwindling interest in taxonomy and fund availability. In quarantine and plant protection activities, their immature stages are met with and diagnosis of these is important to foster a rapid, accurate species identification that is crucial in various spheres of pest management like biological control, insecticide resistance management, preventing the entry of invasive and alien species, and insect vector management that include identification of biotypes, cryptic species. With the advent of molecular biology and molecular tools, identification of life forms including insects has become quick, precise, and easy. Deoxyribonucleic acid (DNA) barcoding is an alternative way to accurately identify species, which also complements conventional taxonomy. DNA barcoding enables even a non-specialist to identify a species even using immature stages like egg, larva, nymph, or pupa. The mitochondrial cytochrome c oxidase subunit I (mtCO-I) region marker was used in the species diagnosis and genetic diversity research. The polymerase chain reaction (PCR) method developed effectively identified biotypes of insect pests. Molecular identification is applied to a great extent in sucking pests including thrips, mealybugs, whiteflies, aphids, and leafhoppers, besides fruit flies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Insects are one of the numerous life forms that have captured the attention of human beings since ancient times. In the same context, proper classification and identification of life forms has been a challenge, and a plausible method of classification was established by Carolus Linnaeus, a Swedish botanist who published Systema Naturae in 1758. However, the Linnaeus system of classification was not based on evolutionary relationships among the target groups. Later, Darwin’s The Origin of Species in 1859 changed the way life forms were classified, where the identification, description, and explanation of the diversity of the organisms had come to be known as systematics. Insects are the largest and most diverse group of organisms on Earth. In this context, identification of insects has been a monumental task, which calls for the availability of more specialists and funding. But with the dwindling interest in taxonomy and fund availability, the classification and identification of various life forms, particularly insects, has been a major challenge to the scientific community. With the advent of molecular biology and molecular tools, the identification of life forms, including insects, has become quick, precise, and easy. The development of species-specific markers enables even a non-specialist to identify insects to the species level.
2 Methods of Classification and Identification
2.1 Linnaean System
Taxonomists assess the physical characteristics that a set of species share and selects the most representative species to be the “type” for each genus, and the most representative genus to be the type of the family, and so on. Individual specimens are deposited in museums to serve as a reference for that species and genus. When new species are found with similar traits, they are categorized as part of a known species, as a new species, or as a new genus, depending on how closely the new specimens resemble the type. The reliance on type has resulted in dramatic changes if a taxonomist re-evaluates a group and decides that some members do not belong and suggest that the group name must be changed.
2.2 Cladistics
During the 1980s, another classification method called cladistics, which is based on the evolutionary histories of organisms, was proposed. This method is based on phylogeny, whereas the Linnaean system is not.
2.3 PhyloCode
In this system, the genus name is removed, and species name is shortened and hyphenated with their former genus name or given numeric identification.
3 Shortfalls in Morphological Identification
Current estimates suggest that the earth may have anywhere from 10 to more than 40 million species of organisms, but only about 1.7 million of them have actually been described. It includes over 750,000 insects, and it took 250 years for taxonomists to categorize all 1.7 million species, which comprise only 10% of the total species on earth (Hebert & Gregory, 2005). Further, we need about 15,000 taxonomists working for centuries to complete this monumental task of classifying the remaining 90% of the unidentified organisms. Economic development and increased international commerce are leading to higher extinction rates and introduction of invasive pest species. Therefore, there is a need for faster species identification and information about their biodiversity for conserving them before they vanish from the face of the earth. Undoubtedly, the contribution of morphological taxonomy is enormous, but it also has some drawbacks, such as the following:
-
1.
Incorrect identification due to both phenotypic plasticity and genetic variability in the characters employed for species recognition.
-
2.
There are many morphologically cryptic taxa that are common in many groups.
-
3.
Morphological examination is time consuming, and is often effective only for a particular life stage or gender of the insects. As a result, many cannot be identified.
-
4.
Moreover, the use of morphological taxonomic keys often demands a high level of expertise that often leads to misidentification.
-
5.
Taxonomists have always looked for discontinuous character variations that could signal divergence between species. The debate on threshold values employing molecular identification for interspecific divergence is also true in the case of morphology-based identification.
-
6.
Early identification of new invasions is an important aspect in preventing the spread. Rapid and accurate identification of many cryptic species of insects is not easily accomplished with conventional taxonomy. Taxonomy separation of many species occurring together can be difficult, particularly for the immature stages that are primarily involved.
-
7.
The effectiveness of morphological keys may also be affected by geographic variations or by the loss of some morphological characters, such as color patterns, as a result of preservation processes.
Thus the limitations in morphology-based identification systems and the dwindling pool of taxonomists urgently require a new robust approach for taxon recognition. Due to the difficulties associated with morphological identification of insects, it became necessary to resort to other identification tools, such as deoxyribonucleic acid (DNA) barcoding, where the mitochondrial cytochrome c oxidase subunit I (mtCO-I) molecular marker is commonly used. Hence, there is a need for an adjunct tool that facilitates rapid identification of species where molecular identification, popularly called “DNA barcoding,” becomes handy. The concept of DNA barcoding was proposed by Hebert et al. (2003a, b) as a rapid and precise way for species discrimination of a broad range of biological specimens using a selected 658-bp fragment of the 5′ end of the mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene.
4 DNA Barcoding of Insects
“DNA barcoding” is a method based on DNA sequencing of a standard gene region. In 2003, Paul Hebert, from University of Guelph in Ontario, Canada, proposed “DNA barcoding” as an alternative way to accurately identify species that also complements conventional taxonomy. Barcoding uses a very short genetic sequence from a standard part of the mitochondrial genome. The standard sequence employed for this purpose is the 5′ region of the mitochondrial cytochrome c oxidase subunit I (mtCO-I). Presently, DNA barcoding has been defined as the molecular identification of a species based on the reference sequence with the lowest genetic distance. It can be helpful in species diagnosis because sequence divergences are usually much lower among individuals of a species than between closely related species. Hebert et al. (2003b) focused this discussion by proposing that a DNA barcoding system for animal life could be based upon sequence diversity in the mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene.
In molecular identification, DNA sequences are considered as unique characters in the same way that morphological differences are used in conventional or matrix-based keys to separate relatedness of the specimens. DNA code is an arrangement of adenine (A), guanine (G), cytosine (C), and thymine (T) described as letters. The sequence in which these four letters occur over and over again remains unique enough to separate one organism from another and every species of insects possess this unique DNA code. These sequences can be used in a comparative way to know the degree of relatedness of a new or unknown specimen if compared to a reference library of sequence. Generally, these sequence verbatim the term marker. Genetic marker defines the genetic differences between the organisms or species. These markers would be a coding or non-coding stretches of DNA, which is generally conserved across the insects. Any mutation at these markers region in an evolutionary time scale would generally lead to a variation in the sequence within that region. All that we need is conserved sequences and a dissimilarity within them to deduce the lineage and unique patters between two individuals. The combination of four different nucleotide bases in DNA is enough to serve this purpose.
Advantages of DNA Barcoding
-
1.
DNA barcoding can identify a species from bits and pieces of insects. The effectiveness of morphological keys may also be affected by the loss of some morphological characters as a result of preservation processes. Also, species discrimination is possible with DNA barcoding for the damaged and archival specimens, which cannot be identified by conventional taxonomy.
-
2.
Developmental stage is not a limiting factor in molecular species identification. Some insects, especially beetles, take long time to reach adult stage in which morphological identification is done. DNA barcoding can identify a species in all its stages, namely the egg, larva/nymph, pupa, and adult.
-
3.
DNA barcoding has appeared to be a useful tool in resolving issues like significant morphological similarities found within or in between species of insects making reliable taxonomic identification difficult. In this context, developing species-specific markers for the species in question will even enable a non-specialist to identify the species without the need for going in for sequencing. This also helps in discriminating closely related species and also in situations where two or more species co-occur on a crop. Barcoding can distinguish among species that look alike, uncovering dangerous organisms masquerading as harmless ones.
-
4.
It is necessary to identify the invasive exotic species of the insect without ambiguity. Written as a sequence of four discrete nucleotides—CATG—along a uniform locality on genomes, a barcode of life provides a digital identifying feature, supplementing whether abundant or rare, native or invasive, engendering appreciation of biodiversity, locally and globally.
-
5.
Accurate identification of species is fundamental to both basic and applied research. DNA barcoding proved to be an effective tool that can be employed for accurate species identification. DNA barcode method enables better quantitative analysis, provides more information for detecting and assessing false positives and false negatives, and uses a data set that can be easily shared and accessed by the greater research community. Currently, international consortium for barcode of life (iBOL) advocates the use of CO-I for species identification, as it exhibits reliable interspecific variation.
-
6.
Several insect pest species like thrips, whiteflies, aphids, and leafhoppers are minute in size, and also show cryptic behavior. At this juncture, molecular identification of species based on CO-I comes handy.
-
7.
Increased transboundary movement of horticultural produce resulted in the chance introduction of many invasive species. On the other hand, in plant consignments, rapid identification is important to prevent the introduction of new pests into non-infested areas. Quick and authentic identification of exotic and potentially invasive taxa with capability of causing high economic losses or detriments is essential pre-requisite for effective plant quarantine. Correct and quick identification of the insect up to species level is important from the plant quarantine view, where morphological identification has limited role, as it requires presence of adult specimens, availability of specialists, the lack of taxonomic keys for immature stages for many species. DNA barcoding is going to play a vital role in the quick identification of insect pests at the port of entry. At this juncture, molecular identification of species based on CO-I comes handy.
-
8.
Success in classical biological control programs depend critically on accuracy of exotic species discrimination and identification. Molecular method confirms the morphological identification of many invasive species so that correct bio-management practices can be advised. DNA barcoding offers the potential not only for enhanced recognition and thereby control of harmful species in the immediate term, but also it promises to help us to understand the global movement of species in a level of detail never before attainable.
-
9.
Another area where molecular markers are likely to have an increasingly important role is in biosecurity. One of the crucial guidelines of the biosecurity framework is the rapid and accurate diagnosis of potentially invasive species and biotypes. Many government agencies are adopting risk analysis and management programs that will serve to prevent “alien species” from entering and establishing into new environments. All these issues can be solved with molecular identification.
-
10.
DNA barcoding is generally considered to be reliable, cost-effective, and an easy molecular identification tool with a wide applicability across animal taxa. As such, it could be very useful to routinely identify difficult taxa of economic importance such as insects that comprise large numbers of serious pest species or disease vectors. The morphological method of identification needs large number of experts and time but the molecular techniques are much efficient in proper identification within short period of time.
-
11.
Variation and polymorphism is common between insect species; nevertheless, it is often ignored by taxonomists. Molecular studies have the potential for detection of genetic polymorphism within species. Using random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR), genetic polymorphisms and genetic diversity in natural populations between the insect species have been studied. Molecular techniques include restriction fragment length polymorphism (RFLP), random amplified polymorphic DNA (RAPD), arbitrary fragment length polymorphism (AFLP). In this connection, molecular identification employing CO-I barcoding has an advantage of not being limited by polymorphism, sexual form (asexual/sexual), and life stages of the target species.
-
12.
Some insect species like Bemisia tabaci are complex, composed of at least 41 biotypes. This makes the taxonomic identity of existing biotype difficult and sometimes ambiguous. Molecular techniques help in accurately identifying different biotypes that differ in their biology, host plant preference, insecticide susceptibility, and ability to transmit plant pathogenic viruses.
-
13.
Insect mitochondrial DNA (mtDNA) analysis is a powerful tool for the study of population genetics and phylogenetics. Molecular markers have paved the way for vital data in species identification and phylogenetic studies. RAPD marker is well suited for use in large samples throughout systems required for population genetics. Recently developed molecular marker techniques provide an important tool that ease the assessment of genetic diversity and facilitate genotyping, classification, inventorying, and phylogenetic studies. Molecular markers have paved the way for vital data in species identification and phylogenetic studies. Genetic differentiation and gene flow between the species can be analyzed by using various molecular markers, like RAPD, microsatellite, mitochondrial, and ribosomal markers, which facilitate basic biodiversity inventories like molecular phylogenetics for assembling tree of life and identifying clades and evolutionary relationships. Current applications of molecular genetics and genomics play an important role in the study of invertebrate pest invasions and outbreaks.
-
14.
Barcoding opens the way for an electronic hand-held field guide, the Life Barcoder: Barcoding links biological identification to advancing frontiers in DNA sequencing, miniaturization in electronics, and computerized information storage. Photo-documentation can be easily created for any future reference for the specimen under study.
-
15.
Barcoding demonstrates value of collections: Compiling the library of barcodes begins with the multimillion specimens in museums, herbaria, zoos and gardens, and other biological repositories.
-
16.
Barcoding speeds up writing the encyclopedia of life: Compiling a library of barcodes linked to the voucher specimens and their binomial names will enhance public access to biological knowledge, helping to create an online encyclopedia of life on earth, with a webpage for every species of plant and animal.
-
17.
Barcoding democratizes access: A standardized library of barcodes will empower many more people to call by name the species around them. It will make possible the identification of species characterized for each taxonomic group (2–12%), above which groups of individuals do not belong to the same species but form supraspecies taxon. Therefore, unknown individuals could be assigned to a species level.
-
18.
The development of simple diagnostics and their use alongside classical and molecular techniques for the early detection of resistant populations are of great importance for pest management strategies. The practical implications of molecular diagnostics are discussed in light of control of whitefly and other pests.
In brief, DNA barcoding proved to be an effective tool that can be employed for accurate species identification, elucidation of cryptic species and biotypes, and also in the discovery of new species. Molecular studies will be useful in the study of population genetics, evolutionary biology, evolutionary biology, biodiversity and conservation biology, ecology, vector transmission, insecticide resistance, and biological control and quarantine. All these kinds of data or information can lead to formulating correct strategies of insect pest control.
5 Mitochondrial DNA
Mitochondrial DNA (mtDNA) has a long history of use at the species level; recent analyses suggest that the use of a single gene, particularly mitochondrial, is unlikely to yield data that are balanced, universally acceptable, or sufficient in taxonomic scope to recognize many species lineages. Mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene sequence is suitable for this role because its mutation rate is often fast enough to distinguish closely related species, and also because its sequence is conserved among conspecifics and a lack of recombination. Mitochondrial cytochrome c oxidase subunit I gene sequence differences are too small to be detected between closely related species; more than 2% sequence divergence has been detected between such organisms, proving the barcode effective. However, the rate of evolution of CO-I is very slow.
5.1 Genomic DNA Isolation
Total genomic DNA can be extracted from individual insects using a non-destructive method (Hajibabaei et al., 2006), while voucher specimens are required to be mounted on glass slides and deposited with any of the National Insect Repository such as the National Pusa Collection (NPC) or the Indian Agricultural Research Institute (IARI), Delhi. Various DNA isolation protocols are available, namely (1) direct TNES buffer method, (2) spot-PCR method, (3) phenol/chloroform method, and (4) salting-out method.
5.1.1 Direct Buffer Method
A single insect can be crushed in 50–200 μL YNES (50 mM Tris-HCI, pH 7.5, 0.4 M NaCI, 20 mM EDTA, 0.5% SDS), STE (0.1 M NaCI, 10 mM Tris, pH 8.61 mM EDTA), GES (0.1 M glycine, pH 9, 50 mM NaCI, 1 mM EDTA, 1% β-mercaptoethanol, 0.5% Triton X-100), or CTAB (100 mM Tris-HCL, pH 8, 1.4 M NaCI, 20 mM EDTA, 2% CTAB, 0.2% β-mercaptoethanol) buffer. The sample is to be incubated at 94 °C for 12 min, with the cell debris to be precipitated by spinning it at 13,000 rpm for 1 min. The extracted DNA is to be stored at −20 °C.
5.1.2 Spot-PCR Method
A single insect should be crushed on a positively charged nylon membrane soaked in a 50 mM NaOH and 2.5 mM EDTA solution, and then allowed to dry. A small portion (ca. 3 mm 2) of the spotted membrane is to be cut out and placed in 10–50 μL TNES, STE, GES, or CTAB buffer (described above). The sample can then be incubated at 95 °C for 10 min and cooled on ice. Extracted DNA can be stored at −20 °C.
5.1.3 Phenol/Chloroform Method
DNA from a single insect can be extracted using the modification of a general procedure for extraction with phenol. The insect is to be crushed and incubated at 40 °C in 0.6 mg/mL proteinase K and 300 μL TNES buffer for 4–18 h. DNA can then be purified by washing with organic solvents: once with a chloroform:isoamyl mix (24:1 v/v); once with a chloroform:phenol mix (1:1 v/v), and once with chloroform only. DNA can then be precipitated with absolute ethanol. Extracted DNA can be stored at −20 °C.
5.1.4 Salting-out Method
DNA from a single whole insect can be extracted using the protocol of Sunnucks and Hales (1996) with minor adjustments, including the following: the insect can be incubated at 40 °C in 0.6 mg/mL proteinase K and TNES buffer, and the samples can be left for at least 1 h at −20 °C during precipitation of the DNA with absolute ethanol. Extracted DNA can be stored at −20 °C.
5.2 Polymerase Chain Reaction
Polymerase chain reaction (PCR) was developed by Kary B. Mullis (Mullis & Faloona, 1987) and has radically changed molecular research and diagnostics. PCR involves the in vitro synthesis of large amounts of DNA copies from a single starting molecule and employs short single strands of DNA (18–30 nucleotides) called oligomers or primers to select a region of specific interest from the DNA. Once the primers are annealed to the DNA, Taq DNA polymerase builds a complementary strand extending from the primer by incorporating free deoxynucleoside triphosphate (dNTP: base + deoxyribose sugar + phosphate) molecules in the reaction mix. Two primers that anneal on complementary strands are used, with the Taq extending the region between them. The reaction mixture is cycled between different temperature optima for the different stages of reaction of denaturation, annealing, and elongation. This process is repeated in a number of cycles (usually 30–40), and the DNA thus produced increases exponentially.
5.3 Sequence Analyses and Submission
The amplified products can be eluted using an extraction kit according to the manufacturer’s protocol, and the sequencing can be done in an automated sequencer (ABI prism® 3730 XLDNA Analyzer; Applied Biosystems, USA) using PCR-specific primers, both in forward and reverse directions. Homology search and sequence alignment can be performed employing the NCBI BLAST and BioEdit versions 7.0 and 9.0, respectively. All the sequences generated in the respective studies need to be deposited in the NCBI GenBank and the Barcode of Life Data (BOLD) systems.
5.4 Nuclear Copies of Mitochondrial Genes
There is a possibility that a pseudogene is being amplified if the study encounters the following anomalies (Zhang & Hewitt, 1996):
-
1.
More than one bands, or different bands, are constantly produced during PCR amplification.
-
2.
Background peaks or sequence ambiguities are constantly found when sequencing.
-
3.
The DNA sequence contains data that will unexpectedly change the polymerase translation of the sequence, such as unusual frame shifts, insertion/deletion, or stop codons.
-
4.
The DNA sequence is particularly more divergent than expected.
-
5.
Phylogenetic analysis results in unusual, unexplained, or contradictory tree topology.
In the recent past, DNA barcoding has gained importance in the species diagnosis of animal species, but has some difficulty with certain insects. This is probably due to its inconsistency in amplifying the 5′-mtCO-I region.
5.5 Advantages of Using Mitochondrial Genome
-
1.
Haploid mode of inheritance and it supports less recombination.
-
2.
Mitochondrial genome does not have introns.
-
3.
Universal primers are robust, which can amplify 5′ end in most of the animals, including insects.
-
4.
Rapid evolution allows the discrimination of not only closely related species but also phylographic groups within a single species.
-
5.
In animal mitochondrial genome, the 13 protein coding genes are better targets because of rare insertions and deletions (indels).
-
6.
By identifying amino acid substitution patterns of mtCO-I, it is possible to assign any undefined organisms to a higher taxonomic group before examining nucleotide substitutions to determine its species identity.
6 Other Targets for Molecular Identification of Insects
6.1 Ribosomal DNA
Ribosomes are the major components of cells that are involved in translating the messenger ribonucleic acid (mRNA) into proteins. Ribosomes consist of both proteins and RNAs. The ribosomal RNA (rRNA) regions that are conserved and more variable regions can serve as both slow and fast clocks in identifying and unraveling the molecular phylogeny. In eukaryotes (including insects), the genes encoding both 18S and 28S rRNA are clustered as tandem repeats in the nucleolus; in most animals, there are 100–500 copies of ribosomal DNA (rDNA) in the nuclear genome in tandemly repeated transcription units. The repeated transcription unit is composed of a leader promoter region known as external transcribed spacer (ETS) region, 18S rDNA coding region, internal transcribed spacer (ITS) region, 28S rDNA coding region, and an internal non-coding transcribed spacer (IGS) region. In addition to the above, R1 and R2 retrotransposable elements are found in specific locations. Different portions of the repeated transcription units evolve at different rates in the nuclear genome; a higher degree of polymorphism is found in the non-coding segments (IGS, ITS, ETS), and the most variable part of the repeated unit is IGS, which contains reiterated sub-repeats ranging from 50 to several hundred base pairs in length. The coding regions of the repeated unit change relatively less and can be used for systematic studies of higher taxa or for ancient lineages. Ribosomal RNA genes undergo concerted evolution so that the sequence similarity of the members of an RNA family is expected to be greater within species than between species. In addition to the above retrotransposons, R1 and R2 have been in the 28S rRNA genes of most insects, are associated with arthropods, and are usually precisely located at the same nucleotide position within the 28S rRNA gene. Most of the R2 elements are located about 74 bp upstream from the site of R1 insertions. R1 and R2 do not have long terminal repeats and block the production of functional rRNA, since there are many rRNA genes, and R2 are kept from invading by microRNA/small interfering RNA (miRNA/siRNA). Usually, R1 and R2 do not have accumulated mutations that would make them inactive.
6.2 Satellite DNA
Satellite DNA may consist of a large fraction of the total DNA in an insect. Microsatellites are usually species specific, and evolve at very high rates. Satellite DNA can also be used for species identification and analysis of populations.
6.3 Nuclear Protein Coding Genes
A variety of protein coding loci have been used in molecular systematics, and some of them are listed below: (1) alpha amylase, (2) acetyl choline esterase, (3) actin, (4) alcohol dehydrogenase, (5) arylphorin, (6) cecropin, (7) chorin, (8) DOPA carboxylase, (9) elongation factor 1 alpha, (10) esterase, (11) glycerol 3 phosphate, (12) glycerol 6 phosphate dehydrogenase, (13) guanylate cyclase, (14) Globin family genes, (15) histones 1 and 4, (16) hunch back, (17) Krüppel, (18) luciferase, (19) lysozyme intron, (20) myosin alkali light chain intron, (21) nullo and (22) opsin.
7 Applications of Molecular Identifications
Uses of molecular tools to discriminate insect populations, and insects’ adaptation to various stresses are wider in applications. However, the use of DNA barcoding databases is of a considerable advantage only when these databases are large enough to cover the range of intra- and interspecific genetic diversity observed in the field.
7.1 Mealybugs
Mealybugs are under a strict regulation at foreign trades of horticultural produce because they are one of the most economically damaging groups of insects on several horticultural crops. Morphological identification of mealybug species is usually time consuming, requires a high level of taxonomic expertise, and usually only adult females can be identified. DNA-barcoding-based approaches were proved to resolve problems related with morphological identification of mealybugs, particularly early life stages, and can provide valuable information for investigating mealybug associations and interactions with natural enemies.
-
1.
The relationship of six mealybug species (Planococcus citri, Planococcus ficus, Planococcus ovae, Pseudococcus longispinus, Pseudococcus viburni, and Phenacoccus aceris) was studied using randomly amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR) in Turkey. Cluster analyses of RAPD data clearly separated the species into two groups (Serce et al., 2007).
-
2.
Seven species of mealybugs (Pseudococcus maritimus, P. viburni, P. longispinus, Pseudococcus calceolariae, Planococcus ficus, P. citri, and Ferrisia gilli) were identified using a multiplex PCR based on the mitochondrial cytochrome c oxidase subunit I gene (Daane et al., 2011).
-
3.
There was a slight difference in morphological characters in the populations of Planococcus ficus, indicating that there are two different populations of the same species in Tunisian vineyards. Likewise, in the molecular analyses, two separate clades were revealed in the neighbor-joining (NJ) phylogenetic tree, supporting the morphological studies and suggesting there are two distinct populations of grape vine in Tunisia, which might be two different biotypes (Mansour et al., 2012).
-
4.
The PCR method effectively identified five mealybug species of economic interest on grape in Brazil: Dysmicoccus brevipes, P. citri, P. viburni, Phenacoccus solenopsis, and P. ficus. Planococcus citri, D. brevipes, and P. viburni were the most frequently collected species. Ferrisia terani and Ferrisia meridionalis were reported for the first time in Brazil. This multiplex PCR proved useful for the rapid and cost-efficient identification of the above mealybug species (Pacheco da Silva et al., 2014).
-
5.
Pseudococcus jackbeardsleyi is a native species of the neotropical region. P. jackbeardsleyi infesting Musa sp. in Costa Rica was identified by molecular method (Jiménez & Meneses, 2016). Molecular identification by sequencing the 5′ mitochondrial cytochrome oxidase confirmed its identity as Jack Beardsley mealybug Pseudococcus jackbeardsleyi for the first time in India. Successful and timely identification of the invasive pest P. jackbeardsleyi helped in emergency pest management plans, including biological control, by which further spread into other states were hindered or delayed to avoid economic losses (Mani et al., 2013).
-
6.
There are a number of species in the Dysmicoccus genus, but Dysmicoccus brevipes is the most similar to Dysmicoccus neobrevipes, native to tropical America. In the molecular data, BLAST hits from GenBank, it was possible to associate it to the species Dysmicoccus brevipes. Until the date, this species had been associated to pineapple crop. Therefore, this study provided insight into the ability of dissemination of this polyphagous pest, present in Costa Rica in a different uncommon crop (Palma-Jiménez & Blanco-Meneses, 2017).
-
7.
Molecular methods (mitochondrial genes for cytochrome c oxidase subunit I [CO-I]) confirmed morphological identification of the invasives Phenacoccus solenopsis and Paracoccus marginatus in mainland China. Identification information of these invasive species helped to strengthen quarantine programs so that inspectors and identifiers were able to determine the species at Chinese ports to make control decisions (Wu et al., 2014).
-
8.
The genetic variation of cassava mealybug (Phenacoccus manihoti) populations collected from 28 major cassava-growing areas within 18 provinces in Thailand was determined using mitochondrial and nuclear DNA sequence-based analysis. Although seven mitochondrial CO-I and six nuclear ITS1 haplotypes were found, low genetic-diversity indices were detected. These results suggested a high potential for population reproduction in this species (Rattanawannee & Chongrattanameteekul, 2016).
-
9.
Two cassava mealybug species namely Phenacoccus herreni and Phenacoccus manihoti have high level of similarity in appearance, and it is difficult to differentiate them at species level. Two RAPDs were used to rapidly distinguish P. herreni from P. manihoti at Cali, Colombia (Cuervo et al., 2002). Both of RAPD (random amplified polymorphic DNA) analyses using the operon primers H9 and H16 are useful for making a clear distinction among P. herreni, P. manihoti, and Phenacoccus madeirensis, especially in areas of Brazil (Calatayud & Le Rü, 2006).
-
10.
The mealybugs infesting vineyards in four regions of Chile were accurately characterized by DNA sequencing for two markers (cytochrome c oxidase subunit I and internal transcribed spacer 2 [ITS2]). Pseudococcus viburni was the most common species, followed by Pseudococcus meridionalis and Pseudococcus cribata. A comparison of haplotypes of P. viburni worldwide provides support for a recent hypothesis that this species is native to South America, a finding with direct consequences for management (Correa et al., 2012).
-
11.
By generating amplification products of different sizes, the three species-specific primers, along with universal CO-I primers, were successfully used in multiplex PCR tests to identify accurately all three mealybug species, namely P. citri, P. viburni, and Pseudococcus comstocki, infesting ornamental plants in Guilan Province (Iran) in a single reaction (Hosseini & Hajizadeh, 2011).
-
12.
Molecular identification using fragment of mitochondrial cytochrome c oxidase subunit I revealed the presence of six mealybug species, namely Phenacoccus madeirensis, P. solenopsis, Saccharicoccus sacchari, P. citri, Paracoccus burnerae, and Phenacoccus solani, in the Lubombo, Highveld, Middleveld, and Lowveld regions of Swaziland. There is a high diversity of mealybugs in crops, ornamentals, and wild host plant species in Swaziland. This first DNA-based characterization of mealybugs from Swaziland helped in decision-making while considering biological control programs (Assefa & Malindzisa, 2018).
-
13.
A polymerase chain reaction-based method for species identification was developed for six mealybug species known to infest Korean pears including two regulated insects, Planococcus kraunhiae and Crisicoccus matsumotoi. This molecular method has facilitated trade and export requirements, as well as identification of the species at any stage of mealybug being intercepted (Park et al., 2010).
-
14.
Identification of principal mealybug species namely Phenacoccus solani, Phenacoccus solenopsis, and Planococcus citri, infesting the major pumpkin-producing regions in Egypt, was confirmed by molecular and morphological characterization (Dewer et al., 2018).
-
15.
For an easy, user-friendly molecular laboratory technique, the mitochondrial DNA cytochrome c oxidase subunit I (mtDNA CO-I) gene was developed to accurately identify mealybug eggs and crawlers to species level (Planococcus citri, Paracoccus burnerae, Pseudococcus longispinus, P. calceolariae, and P. viburni). The molecular method has facilitated export consignments of citrus fruits from South Africa to the USA, South Korea, and China, previously regularly refused based on the presence of unidentifiable mealybug nymphs or eggs (Pieterse et al., 2010).
-
16.
Morphological identification of the mealybug species Planococcus citri and Planococcus minor is often complicated by the existence of intermediate forms and a lack of knowledge of the intraspecific variation that occurs in each species in California. The mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene, in combination with morphological and geographical data, has helped to accurately identify morphologically similar species, namely P. citri and P. minor. Molecular identification was used to accurately identify the P. minor clade, the P. citri clade, and the clade from the Hawaiian Islands in most cases (Rung et al., 2008).
-
17.
Their high degree of morphological similarity makes Planococcus citri and Planococcus ficus difficult to distinguish. With a simple and fast PCR-based method with the use of a short DNA extraction method and species-specific primer pairs, Planococcus citri and P. ficus can be distinguished at any developmental stages within 3 h. Molecular diagnosis has served as a promising tool for distinguishing the two closely related species of P. citri and P. ficus (Tóbiás et al., 2012).
-
18.
The single-step multiplex PCR developed here, based on the mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene, is rapid, reliable, sensitive, accurate, and simple. The entire identification of three mealybug species (P. ficus, P. citri, and Pseudococcus longispinus) associated with grapevine in South Africa using the protocol (including DNA extraction, PCR, and electrophoresis) was completed in approximately 4 h (Saccaggi et al., 2008).
-
19.
Four diverse methods (best close match [BCM], neighbor-joining [NJ] tree, Barcoding with LOGic [BLOG] formulas, Poisson Tree Process [PTP] Species Delimitation Method) were employed using two molecular markers (mitochondrial cytochrome c oxidase subunit I [mtCO-I] and large ribosomal subunit gene [28S]) for the identification of 54 mealybug species that commonly occur in China. This study corroborates the utility of the CO-I and 28S genes in the rapid identification of mealybugs, and the barcode library provided will create an effective identification system for mealybug pest management in China (Wang et al., 2016).
7.2 Scale Insects
Armored scale insects (Diaspidids) display extreme sexual dimorphism; however, males are difficult to collect because of their brief time as adults and the lack of males in parthenogenetic species on which morphological identification is done. The paucity and delicate nature of morphological characters traditionally used to diagnose armored scales often require careful preparation of slide-mounted specimens and expert knowledge of the group, for their accurate identification. When scale insects are intercepted on imported produce, they must be rapidly and accurately identified, using morphology-based keys. This is time consuming, and requires extensive taxonomic experience. In addition, intercepted specimens are often immature or damaged, making morphological identification difficult or impossible. A reliable complementary tool is needed for identification. DNA barcoding is of great value for this purpose.
-
1.
Two species of giant scale, namely Drosicha mangiferae and Drosicha stebbingi, lack diagnostic morphological differentiation between their nymphal instars. Sequence analysis of 18S rDNA and CO-I genes did not suggest the presence of two species with differing host plant preferences. We conclude that D. mangiferae and D. stebbingi are simply host races of the same species (Ashfaq et al., 2011).
-
2.
Four Indian populations of giant scales (mango, litchi, guava from Gurdaspur, and mango from Jalandhar) were analyzed. The mtCO-I region was amplified, cloned, and the nucleotide sequences were determined and analyzed. All the four species were found to be D. mangiferae. Genetic diversity in giant scale population was quite less over a large geographical area (Banta et al., 2016).
-
3.
Molecular technique based on amplification of the internal transcribed spacer 2 of ribosomal DNA, by using the polymerase chain reaction (PCR), revealed the presence of six diaspine species Abgrallaspis aguacatae, Hemiberlesia lataniae, Hemiberlesia sp. near latania, Hemiberlesia rapax, Acutaspis albopicta, and Pinnaspis strachani on Avocado, imported into California from Mexico. Two additional species, Diaspis miranda and Diaspis sp. near miranda, also are separated from the others. DNA-based method presented here allows quick and accurate identification of eight species of armored scale, resident on Mexican Hass avocado, regardless of size, life stage, or sex of the specimen (Rugman-Jones et al., 2009).
-
4.
Genetic structure of the outbreak populations of the coconut scale insect pest, Aspidiotus rigidus using mitochondrial CO-I and nuclear EF-1α markers indicated clear differentiation among the A. rigidus populations separating the north from the southern regions of the Philippines. This study provides valuable information on the genetic differentiation of the two A. rigidus groups that would be useful for developing and implementing biological control strategies against this pest in the Philippines (Serrana et al., 2019).
-
5.
Molecular identification revealed the presence of three diapine scales namely Lepidosaphes pistaciae, Suturaspis davatchi, and Melanaspis inopinata infesting pistachio in Kerman Province in Iran. A new species, Melanaspis pistaciae sp. n., is also described. Phylogenetic trees based on molecular analysis of CO-I and 28S rDNA fragments placed all the species in separated clades and confirmed M. pistaciae as a new taxon. Melanaspis pistaciae sp. n. has spread to most cultivated pistachio areas in Iran and has probably been misidentified as M. inopinata in the past. This study may lead to development of more effective approaches for controlling this pest (Hosseininaveh et al., 2018).
-
6.
Randomly amplified polymorphic DNA (RAPD-PCR) was employed to identify six species under the genus Quadraspidiotus including the San-José Scale (SJS) Quadraspidiotus perniciosus, a quarantine pest in Switzerland. This key was able to identify males caught on pheromone traps in the field and to assess the species-specificity of the SJS-pheromone (Frey & Frey, 1995).
-
7.
Mitochondrial cytochrome c oxidase subunit I (mtCO-I) and the D2–D3 expansion segments of 28S rDNA were used for accurate identification of these two morphologically similar species, Eulecanium giganteum and Eulecanium kuwanai, infesting ornamental plants and fruit trees from 19 different locations in China. Differentiating between E. giganteum and E. kuwanai was challenging when using ecological and morphological traits. In contrast, identification using DNA diagnostics appears to be very effective, especially when slide-mounted specimens are difficult to obtain (Deng et al., 2016).
-
8.
The immature stages of greedy scale Hemiberlesia rapax and latania scale Hemiberlesia lataniae cannot be easily distinguished morphologically. A molecular diagnostic test that employs rapid DNA extraction using prepGEM® Insect followed by multiplex PCR utilizing sequence variation in the cytochrome c oxidase subunits I and II (CO-I and CO-II) genes for all the life stages of these three armored scale insects allows hundreds of samples to be processed in a day and has provided a detailed picture of the distribution and abundance of these pests across green and gold kiwifruit in orchards throughout New Zealand (Edwards et al., 2008).
-
9.
With the nuclear regions 18S and 28S as complementary DNA barcodes to the mitochondrial CO-I gene, ten scale insect species under the families namely Asterolecaniidae, Coccidae, Dactylopiidae, Diaspididae, Eriococcidae, Kerriidae, Lecanodiaspididae, Margarodidae, Ortheziidae, and Pseudococcidae were identified. Combining multiple criteria, our results indicate that the concatenation of CO-I and 28S greatly improves the identification success rate of scale insects to 91.5%, demonstrating the utility of DNA barcoding in pest management (Sethusa et al., 2014).
7.3 Whiteflies
Whiteflies are inadvertently, but commonly, transported in international plant trade. Rapid, accurate identification is the essential first step when such insects are intercepted by quarantine authorities. Whitefly taxonomy, and identification, is almost entirely based on the fourth-larval instar or puparium, but often only the eggs, early larval instars, or adults are detected. This makes them excellent candidates for identification using DNA barcoding. Whitefly species like Bemisia tabaci contains morphologically indistinguishable biotypes or cryptic species or genetic groups making them difficult and sometimes ambiguous in identification. Genetic differentiation of different populations in the species complex was analyzed mainly based on the ribosomal internal transcribed spacer 1 (rITS1) and mitochondrial cytochrome c oxidase subunit I (mtCO-I) sequences worldwide.
-
1.
Mitochondrial cytochrome c oxidase subunit I sequences were employed to determine the prevalence of genetic groups Bemisia tabaci on 30 host plants from different locations in India. Results revealed the existence of five genetic groups of B. tabaci in Karnataka, India, identified as Asia-I, Asia-II-7, Asia-II-8, MEAM-1, and a previously unreported genetic group, MEAM-K. This work will help in rapid and accurate identification of these putative genetic groups of B. tabaci, which in turn will help in further elucidating the epidemiology and management of Gemini viruses, and be of value in the operation of quarantines (Roopa et al., 2015).
-
2.
Phylogenetic diversity analysis using two well-known markers, such as mitochondrial CO-I gene and the ribosomal ITS1, confirmed the presence of four putative species of Bemisia tabaci such as Asia-I, Asia-II-1, Asia-II-5, and Asia-II-8 in India. The Asia-I genetic group was found as most widely distributed and shows relatively polyphagus, which has mtCO-I consensus sequence identity of 84.32–86.76% with Asia-II subgroups. This work has shown the genetic boundary of B. tabaci, which helped in understanding host specificity across Karnataka, India. The patterns of spread and impacts on species diversity with host plant species will provide useful insights into the invasion process and in the discovery of newly evolving biotypes that would help in the management of pest (Ellango et al., 2014).
-
3.
The whitefly B. tabaci A-biotype was previously the predominant biotype in most regions of the Mediterranean (MED) and Middle East. Many of these populations had been displaced by the B-biotype. A new Q-biotype of whitefly spread rapidly into several states of the USA. Molecular techniques clearly indicated the dominance of B-biotype of the whitefly on crops grown in greenhouses in Al-Ahsa region of Saudi Arabia (Alhudaib et al., 2014).
-
4.
Nuclear markers and mtCO-I barcoding sequences of different populations of African cassava whitefly B. tabaci associated with epidemics of two viral diseases did not support the “invader” hypothesis. Our evidence shows that no new species or new population were found in 20 years; instead, the distribution of already present genetic clusters composing Sub-Saharan Africa 1 (SSA1) species have changed over time and that this may be in response to several factors including the introduction of new cassava varieties or climate changes. The practical implications are that cassava genotypes possessing both whitefly and disease resistances are needed urgently (Hadija et al., 2019).
-
5.
Sequences of mitochondrial DNA cytochrome c oxidase subunit I (mtDNA CO-I) revealed the presence of six distinct genetic groups of B. tabaci, including three non-cassava haplotypes (Mediterranean [MED], Indian Ocean [IO], and Uganda) and three cassava haplotypes (Sub-Saharan Africa 1 subgroup 1 [SSA1-SG1], SSA1-SG3, and SSA2) in B. tabaci, infesting sweet potato and cassava in South Sudan. MED predominated on sweet potato and SSA2 on cassava in all the sampled locations. The Uganda haplotype was also widespread, occurring in five of the sampled locations. This study provides important information on the genetic diversity, geographical distribution, population dynamics, and host range of B. tabaci species in South Sudan, which is vital for its effective management (Misaka et al., 2020).
-
6.
Molecular studies through RAPD-PCR technique revealed the presence of B-biotype of whitefly Bemisia tabaci in the districts namely Rangareddy, Medak, and Chittoor districts in Andhra Pradesh with similar banding pattern to whiteflies (B-biotype) collected from Kolar district of Karnataka. There is every possibility that this biotype may spread to other parts of the state and may cause substantial losses to vegetable production (Rajasri et al., 2016).
-
7.
Molecular study with the mitochondrial DNA gene, cytochrome c oxidase subunit I (CO-I) revealed the predominance of B-biotype in B. tabaci, infesting eggplant and squash and tomato in Philippines. Two other biotypes namely Asia biotypes I and II-6, were also found in B. tabaci samples infesting eggplant. This is the first report on the presence of these B. tabaci biotypes in the Philippines (Sanchez & Caoili, 2016).
-
8.
Molecular study with PCR followed by an RFLP assay revealed the presence of insecticide-resistance-prone biotype Q whiteflies on poinsettia imported into Finland and Sweden, both protected zones for B. tabaci, emphasizing the importance of preserving the quarantine status of the pest to prevent permanent establishment (Lemmetty & Vänninen, 2014).
-
9.
Phylogenetic analysis of the whitefly mtCO-I sequence indicated the presence of the invasive B and Q biotypes of Bemisia tabaci in Japan. The Q-biotype was found at four locations: Mihara in Hiroshima, Nishigoshi in Kumamoto, and Miyanojo and Okuchi in Kagoshima prefectures; the remaining eight collections were identified as the B-biotype. This is the first report of the introduction of Q-biotype in Japan (Ueda & Brown, 2006).
-
10.
Analysis with random amplified polymorphic DNA-polymerase chain reaction (RAPD-PCR) markers confirmed that the cassava populations of B. tabaci populations were distinct from non-cassava populations of Sub-Saharan Africa. Results revealed that cassava-associated populations were restricted to cassava only, whereas B. tabaci from other hosts were polyphagous but did not colonize cassava. Hence, populations of B. tabaci from cassava in Africa represent a distinct group (Abdullah et al., 2003).
-
11.
Where morphological separation of two species is sometimes inconclusive, or impossible, identification can be achieved using four real-time PCR assays, designed and validated to distinguish between the four species. The assays are generic in their setup and can be multiplexed to form two reactions allowing discrimination of Bemisia afer and B. tabaci in one well, and Trialeurodes ricini and Trialeurodes vaporariorum in another (Malumphy et al., 2009).
-
12.
DNA barcoding using mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene and the internal transcribed spacer (ITS) sequences of ribosomal DNA among various populations of T. vaporariorum clearly indicated that there are no cryptic species or biotypes in T. vaporariorum in Karnataka, Tamil Nadu, and Andhra Pradesh, India, in contrast to the studies of B. tabaci in which there is evidence for many biotypes. The phylogenetic analyses comprised of four Trialeurodes species showed two clades. Clade I is comprised of T. vaporariorum and Trialeurodes abutilonea, which are genetically close. Clade II consists of the remaining two species, viz., Trialeurodes lauri and T. ricini. Also, the current study provided evidence of the suggested emergence of biotypes T. ricini. Quick and accurate identification of whitefly vectors in the early life stages is important from the point of view of understanding the epidemiology of Crinivirus transmitted by Trialeurodes spp. and in their management and quarantine (Roopa et al., 2012).
-
13.
Molecular identification of whitefly adults sampled from the affected cassava field revealed the presence of a new whitefly species, Paraleyrodes bondari, infesting cassava in Uganda. This provides great impetus for a Uganda-wide survey to establish the host range, distribution, and pest status of this species, and is critical to understanding the threat to cassava posed by this pest and designing a suitable management strategy (Omongoa et al., 2018).
-
14.
The level and patterns of genetic variability in populations of exotic spiraling whitefly Aleurodicus dispersus in India was studied using the simple sequence repeat-polymerase chain reaction (SSR-PCR) technique. About 66.0% of alleles were polymorphic in A. dispersus populations. The SSR survey clearly detected moderate levels of polymorphism among the whitefly populations; these populations from the Maharashtra and Tamil Nadu populations were distinct from each other (Boopathi et al., 2014).
-
15.
Molecular characterization of mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene of Aleurodicus sp. collected from coconut indicated 100% similarity with that of mitochondrial CO-I sequence of Aleurodicus rugioperculatus reported from Florida, USA, thus confirming the molecular taxonomic identity as A. rugioperculatus in confirmation with species-specific morphological characters. On the other hand, Aleurodicus sp. collected from guava identified as Aleurodicus dispersus based on morphological taxonomic keys showed 100% similarity with CO-I sequences of A. dispersus, thus confirming the molecular taxonomic identity as A. dispersus. It is observed that A. dispersus and A. rugioperculatus are widely separated by molecular phylogeny; however, genetic closeness in having a common parasitoid Encarsia guadeloupae suppressing both the whitefly species is worth experimenting (Chandrika et al., 2017).
-
16.
A DNA barcoding cocktail to amplify the 5′ end of the CO-I mitochondrial gene from fig whitefly (FW) Singhiella simplex, rugose spiraling whitefly (RSW) Aleurodicus rugioperculatus, and Bondar’s nesting whitefly (BNW) Paraleyrodes bondari species was developed. Besides FW, RSW, and BNW, two additional species of whiteflies were detected in collections, namely Paraleyrodes pseudonaranjae and a species provisionally designated Aleurodicinae sp1. RSW and BNW clustered with congeners within the phylogeny, and FW was resolved as a possible sister taxa to the genus Bemisia. The barcoding cocktail should allow sequencing of 5′ CO-I from multiple genera and both subfamilies of whiteflies, and the primers developed for each species, will facilitate rapid identification of these three invasive whiteflies (Dickey et al., 2015).
-
17.
The development of simple diagnostics and their use alongside classical and molecular techniques for the early detection of resistant populations are of great importance for pest management strategies. Molecular assays were used to investigate the frequency of known resistance mutations. The practical implications of our results are discussed in light of whitefly (Trialeurodes vaporariorum) control (Kapantaidaki et al., 2018).
7.4 Thrips
Morphological identification of thrips using both adult and larvae is challenging due to their tiny size and cryptic behavior. Difficulty in identification of thrips not only exists in the developmental stage, but also between polyphagous thrips species; e.g., Thrips flavus is found to be morphologically very similar to Thrips palmi. Morphological examination of thrips to species level is restricted to adult specimens, as there are no adequate keys for identification of egg, larvae, or pupae. Morphological identification of thrips vectors is often a stumbling block in the absence of a specialist, and limited by polymorphism, sex, stage of development, etc. Molecular identification, on the other hand, is not hampered by the above factors, and can easily be followed by a non-specialist with a little training. The mitochondrial cytochrome c oxidase subunit I (mtCO-I) exhibits reliable interspecies variations as compared to other markers. Molecular studies can complement its morphological distinctions as it could be applied for identification of its intraspecific populations. Large-scale DNA barcode data for economically important taxa like Thysanoptera can provide a common platform to researchers from wide array of biological studies such as taxonomy, ecology, behavior, life histories, pest management, vector–virus relationship, etc.
-
1.
Molecular identification of cardamom thrips Sciothrips cardamomi based on cytochrome c oxidase subunit I revealed the molecular diversity deciphered among the 45 intraspecific populations from ecotypes of cardamom, viz., Vazhukka, Malabar, and Mysore. The populations of S. cardamomi from various locations analyzed belong to a single species. There are no significant variations among these intraspecific populations occurring in cardamom. Such results on S. cardamomi show that there are no appreciable nucleotide differences in its intraspecific populations (Asokan et al., 2012d).
-
2.
Molecular identification of Scirtothrips dorsalis based on internal transcribed spacer 2 (ITS2) sequences revealed that moderate variations among populations of S. dorsalis from the southern states of India, and the thrips populations have shown close evolutionary relationship with the Asian group. This marker provided a rapid and reliable molecular identification of S. dorsalis and was also valuable in understanding the molecular diversity and phylogeny (Latha et al., 2015).
-
3.
DNA barcoding of 151 species of thrips based on the mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene sequences revealed the existence of cryptic species in Thrips hawaiiensis and Scirtothrips perseae for the first time, along with previously reported cryptic species such as Thrips palmi, Thrips tabaci, Frankliniella occidentalis, and Scirtothrips dorsalis. This will in turn help in elucidation of the epidemiology of Tospoviruses, and in their management, and serve as a potentially valuable tool in quarantine at ports of entry (Rebijith et al., 2014).
-
4.
There were differences in the mtCO-I partial sequence of morphologically identified specimens of Thrips tabaci and T. palmi collected from onion and watermelon, respectively. Phylogenetic analyses showed that both T. tabaci and T. palmi formed different clades as compared to other NCBI accessions. The implication of these variations in vector efficiency has to be investigated further. The result of this investigation is useful in the quick identification of T. tabaci and T. palmi, a critical factor in understanding the epidemiology of the Tospoviruses and their management and also in quarantine (Asokan et al., 2007).
-
5.
Two species of thrips, Thrips palmi and Scirtothrips dorsalis, can be differentiated based on the PCR amplicon size. The phylogenetic analysis showed that there are two major groups in CO-I among 21 populations of T. palmi collected from Karnataka, India: one is clearly associated with Indian population of T. palmi, and the second is associated with the remaining countries (Japan, Thailand, Dominican Republic, China, and the UK). Our studies clearly refute the general belief that T. palmi is a single cosmopolitan and polyphagous species. On the contrary, by the standards of genetic and ecological differentiation in other species groups, the recognition of geographically associated and distinct T. palmi subspecies may be considered, similar to what has been observed in T. tabaci. Such similar results have been observed for S. dorsalis, where Indian and Chinese population of S. dorsalis form separate groups (Rebijith et al., 2011).
-
6.
The phylogenetic analysis showed that thrips populations, collected from thrips insects collected from nine crops, viz., tomato, chili, onion, cabbage, cucumber, watermelon, Ethiopian mustard, French bean, and peanut in different countries, clustered with five distinct species groups designated as Thrips palmi group, T. tabaci group, Frankliniella occidentalis group, Scirtothrips dorsalis group, and an unclassified group. Higher intraspecific genetic variation was observed in S. dorsalis and T. palmi followed by T. tabaci and F. occidentalis. Thus, it was confirmed that the cytochrome c oxidase subunit I (CO-I) gene could be useful in grouping different thrips species and genera that coexist in a particular cropping system. The study demonstrated that partial CO-I sequences provide a simple and accurate means of identifying four major thrips species (T. palmi, T. tabaci, S. dorsalis, and F. occidentalis). In addition to identification, this method was useful in grouping completely unknown thrips species and their populations collected from different vegetable and field crops (Kadirvel et al., 2013).
-
7.
Results of molecular identification of thrips species of citrus orchards with ITS-RFLP technique of the amplified internal transcribed spacer regions of ribosomal DNA revealed the presence of four species, namely Heliothrips haemorrhoidalis, Frankliniella occidentalis, Pezothrips kellyanus, and Thrips tabaci, in the Mediterranean Region, whereas three species, Frankliniella bispinosa, Scirtothrips aurantii, and Scirtothrips citri, are considered quarantine species for the European Union (EU) territories. This study has shown that the use of genetic markers can be a valid alternative for quarantine workers and for epidemiological researchers, for whom the correct identification of pest species through classic morphological methods could be either difficult or time consuming or visually impossible (De Grazia et al., 2016).
-
8.
DNA barcoding confirmed presence of Thrips parvispinus in papaya plantations. Haplotyping data suggested that Indonesia may be a probable source of invasion of this pest to India (Tyagi et al., 2015).
-
9.
The results on DNA barcoding initiative on 370 sequences of 89 thrips morphospecies including 104 novel sequences from 39 morphospecies revealed that the type specimens of four species from multiple species delimitation methods (BIN, ABGD, GMYC, and bPTP) were consistent for 73 species (82%) with their morphological identifications. We detected more than one MOTU in 14 morphospecies indicating to have cryptic diversity, including two major vector species (Frankliniella schultzei and Thrips palmi). However, four morphospecies (Thrips moundi, Thrips carthami, Haplothrips andersi, and Haplothrips gowdeyi) showed low genetic distances between them with overlapping in barcode gap. Simultaneous use of multiple delimitation methods is advantageous for detection and identification of cryptic species (Tyagi et al., 2017).
-
10.
Molecular method based on nucleotide sequencing analysis of the mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene for the identification of T. tabaci collected from four different sites of Mashhad, Iran, had revealed that phylogenetic analyses conducted by the neighbor-joining method yielded almost identical phylogenetic reconstructions of trees that separated thrips based on the geographic origin. Molecular data indicate that different thrips species are located in distinct groups. These results show that molecular keys can be a useful method to provide much-needed information on thrips identification for pest management officers and quarantine purposes (Karimi et al., 2010).
-
11.
Mitochondrial CO-I (mtCO-I) region was sequenced from population of Thrips tabaci collected from different locations of Hungary. Genetic analysis of the T. tabaci species complex based on mtCO-I gene confirmed the three well-known biotypes (L1, L2, T) and a new biotype because the new molecular evidence presented in this study suggests T-biotype of T. tabaci forming two distinct (sub)clades (T1 and T2). The results demonstrated that the new marker effectively identifies the different T. tabaci biotypes. We believe that our reliable genotyping method will be useful in further studies focusing on T. tabaci biotypes and in pest management by scanning the composition of sympatric T. tabaci populations (Farkas et al., 2019).
-
12.
The rapid detection and differentiation between more and less harmful Frankliniella species on the quarantined list of the European Plant Protection Organization is important in order to combat the pests at the time of their appearance. The protocol is based on PCR amplification of ITS1 rDNA fragments of these insects using universal primers pair giving products of slightly distinct length for studied insects. The method was shown to be species-specific and sensitive. Even single specimens in either the larvae or adult stage could be distinguished (Przybylska et al., 2016).
-
13.
In southern Africa, a molecular identification tool, based on nucleotide sequencing analysis of the mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene, allowed a rapid, accurate, and simple means of identifying the nine thrips species namely Frankliniella occidentalis, F. schultzei, Fulmekiola serrata, Haplothrips nigricornis, Haplothrips clarisetis, Heliothrips sylvanus, Scirtothrips aurantii, Synaptorthrips psoraleae, and Thrips tabaci. The molecular key will provide much-needed information on thrips’ identification for South African pest management officers and quarantine purposes. Much of this economic damage may be prevented by an accurate system for identifying pest thrips species (Timm et al., 2008).
-
14.
DNA barcoding of two Scirtothrips species and distinctive clustering in BA phylogeny with high genetic divergence confirmed the presence of cryptic diversity in both S. dorsalis and Scirtothrips oligochaetus. These humble contributions of barcode data in global database also represent three major pest species and one vector species of thrips (Chakrabortya et al., 2019).
-
15.
Molecular analysis with mtCO-I gene sequences confirmed the presence of Frankliniella occidentalis infesting chrysanthemum in the Nilgiris and Salem districts of Tamil Nadu by molecular markers. The confirmation of presence of F. occidentalis in India is of paramount importance considering its role as active vector of Tospoviruses present in the important ornamental high-valued crops (Suganthy et al., 2017).
-
16.
In Iran, five primers used to simultaneously amplify a specific region of the mitochondrial DNA and produce species-specific fragments were capable of detecting four species, namely Thrips tabaci, Thrips palmi, Frankliniella intonsa, and Frankliniella occidentalis. This method is simple enough to be implemented by non-experts and also can be extended to any organism for which quick and reliable identification is needed (Sabahi et al., 2017).
7.5 Leafhoppers
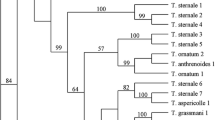
Identification of leafhopper species requires dissection and examination of the male genitalia. Species differentiation is comparatively tedious as the only reliable morphological character is the structure of male genitalia. Some taxonomically problematic species apparently exhibit substantial intraspecific variation in male genital structures, and this causes confusion among taxonomists. The CO-I sequence is an effective tool to identify the leafhoppers in any stage of its life cycle. In addition, DNA barcoding is used to provide putative species identities for morphologically indistinct nymph specimen. The mitochondrial cytochrome c oxidase subunit I (mtCO-I) region marker was used in the species diagnosis and genetic diversity research.
-
1.
Species diagnosis of mango leafhoppers by the conventional taxonomy is limited by the morphological similarity among the various species namely Amritodus atkinsoni and Amritodus brevistylus, and Idioscopus clypealis and Idioscopus nagpurensis. Alternatively, species diagnosis of mango leafhoppers could be achieved employing CO-I, by which even a non-specialist could easily identify the species in question. Additionally, phylogenetic information could also be derived from the CO-I sequences. DNA barcoding employing a 658 bp fragment of 5′ region of the mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene is an effective tool in addressing the rapid, accurate, and timely identification of mango leafhoppers (Asokan et al., 2015).
-
2.
Molecular characterization by using cytochrome c oxidase subunit I (CO-I) gene confirmed that Idioscopus nitidulus of Raichur, Bramhavar, and Hyderabad population showed 99% similarity, A. atkinsoni of Dharwad and Shivamoga showed 99% similarity, and I. nagpurensis of Kerala showed 98% similarity. It is inferred that there was a considerable molecular diversity among the leafhopper populations of major mango-growing areas. The maximum identity of I. nitidulus and I. nagpurensis showed 91–99% variation indicating a higher genetic diversity in these two species, and in A. atkinsoni the variation was 97–99% (Manjunatha et al., 2018).
-
3.
With use of the mitochondrial cytochrome c oxidase subunit I (mtCO-I) region marker, six different leafhopper (China Aster, Marigold, and Chrysanthemum) species namely Sogatella furcifera, Homalodisca insolita, Amrasca biguttula, Balclutha incise, Balclutha abdominalis, and Japanagallia trifurcate were identified. This research contributes valuable knowledge to molecular biology and recognizes leafhopper species that serve as major phytoplasma vectors (Mahadevaswamy et al., 2019a, b).
-
4.
The phylogenetic tree was prepared for mtCO-I gene sequences of different populations (number) of A. atkinsoni (1), A. brevistylus (2), Idioscopus niveosparus (1), I. nagpurensis (3), and I. clypealis (1) and populations from Punjab. The tree revealed two clades, i.e., first corresponding to A. atkinsonii and A. brevistylus, while, clade 2 consisted of three species of Idioscopus with three sub-clusters for each I. clypealis, I. niveosparus, and I. nagpurensis. Nucleotide pairwise distances ranged from 0.002 to 0.199. The analysis revealed very low genetic variations among the South and North Indian populations of A. atkinsoni (Vikas et al., 2016).
7.6 Aphids
The complex life cycles, significant polymorphism, immature taxonomy, and absence of trained manpower make the morphological identification of the aphids difficult. The identification of immature aphids is often difficult or impossible. In addition, their small size, presence of cryptic species, and damaged specimens dictate the need for a strategy that will ensure timely and accurate identification. A reliable, quick, accurate, and life-stage-independent method of identification of vectors such as Aphis gossypii and Myzus persicae is important with respect to virus transmission, insecticide resistance, and biological control. DNA barcoding is useful to identify many aphid species.
-
1.
Molecular diversity analyses using both mitochondrial and nuclear markers showed that neither A. gossypii nor M. persicae has as much genetic variability as expected. An outcome of this investigation is the development of a technique that is useful for the quick identification of A. gossypii and M. persicae, a critical factor in understanding the epidemiology and management of the Potyviruses, and also in facilitating quarantines of these two pests (Rebijith et al., 2012a).
-
2.
RFLP markers were developed for the identification of five aphid species, which are among the most damaging pest of vegetables in Kenya. DNA barcoding identified the morphologically indistinguishable Aphis craccivora and Aphis fabae and separated two subspecies of A. fabae. Our DNA barcoding results contribute to the growing database of DNA barcodes of aphid species in the world. With the availability of quick and accurate identification tools, monitoring and detection of potentially invasive species could be heightened, facilitating successful pest management strategies and contributing to effective phytosanitary management systems in Kenya and beyond (Kinyanjui et al., 2016).
-
3.
Comparing mitochondrial gene sequences of rose aphids with extant sequences in gene bank shows high diversity of them, and then studied samples from various places of Isfahan (Iran) were classified in four groups: Aphis gossypii, Ericaphis scammelli, Macrosiphum rosae, and Wahlgreniella nervata. According to results, W. nervata is new for Isfahan aphid’s fauna and E. scammelli is new for Iran’s rose aphids. There were a little E. scammelli extant among samples and it seems that rose is not its main host in this region. This is the first report of this aphid on rose (Jalalizanda et al., 2012).
-
4.
Molecular analysis based on a fragment of the mitochondrial DNA containing the 5′ region of the cytochrome c oxidase 1 (mtCO-I) confirmed the presence of the invasive aphid species Wahlgreniella nervata in Bengaluru, India. The invasive species compendium developed by CAB International, 2013, has listed W. nervata as invasive in nature (Joshi et al., 2014).
-
5.
Both molecular approaches, namely DNA mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene barcoding and microsatellite flanking region sequences, clearly distinguished two groups corresponding to the morphologically defined species, namely the green apple aphid (Aphis pomi) and the spirea aphid (Aphis spiraecola). Among Aphis pomi samples, microsatellite flanking region sequences were relatively uniform, whereas A. spiraecola exhibited much variability, which suggests that North American populations of the latter species are genetically much more complex (Foottit et al., 2009).
-
6.
CO-I mitochondrial region as a variable region between species is able to differentiate between 25 aphid species that are commonly found in horticultural crops in Victoria, Australia. The restriction enzymes HpyCH4 IV, DraI, HinfI, TaqI, and SspI characterized 26 haplotypes that corresponded to 25 aphid species commonly found in southern Australian aphid surveys, including the currant-lettuce aphid Nasonovia ribisnigri that has recently invaded Australia, presumably from New Zealand. Overseas specimens of Aulacorthum solani and N. ribisnigri showed no significant sequence difference when compared with their Australian counterparts. The CO-I gene provides a useful marker for diagnostic aphid surveys (Valenzuela et al., 2007).
-
7.
DNA barcode was developed to identify 33 species of aphids infesting several horticultural crops in India (Asokan et al., 2011a, b).
-
8.
Pentalonia nigronervosa samples on banana and Zingiberaceae and Araceae species from Micronesia and Hawaii, Florida, and Australia exhibit fixed differences in DNA sequence in mitochondrial cytochrome c oxidase subunit I. Molecular identification confirmed presence of Pentalonia nigronervosa feeding on banana, and Pentalonia caladii feeding on the plants belonging to Zingiberaceae and Araceae (Foottit et al., 2010).
7.7 Fruit Flies
Increased transboundary movement of horticultural produce has resulted in the chance introduction of many invasive species including fruit flies mainly at immature stages. At quarantine checkpoints, fruit flies are most commonly intercepted at the larval stage; however, larvae have few diagnostic morphological features. At this juncture, molecular species diagnostics based on CO-I have become handy, because diagnosis is not limited by developmental stages. Polymerase chain reaction-based methods such as DNA barcoding and restriction fragment length polymorphism are being used for the identification of various fruit flies, their biodiversity, and genetic diversity.
-
1.
Phylogenetic relationships among five subgenera, viz., Austrodacus, Bactrocera, Daculus, Notodacus, and Zeugodacus, have been resolved employing the 5′ region of CO-I (1490–2198), where CO-I sequences for Bactrocera dorsalis, Bactrocera tau, Bactrocera correcta, and Bactrocera zonata from India were compared with other NCBI-GenBank accessions. Phylogenetic analysis employing Maximum Parsimony (MP) and Bayesian phylogenetic (BP) approaches showed that the subgenus Bactrocera is monophyletic. CO-I was very useful for the quick and accurate species diagnoses of eggs, larvae, pupae, and adults of Bactrocera zonata, B. tau, and B. dorsalis. Furthermore, the utility of species-specific markers in differentiating B. zonata (500 bp) and B. tau (220 bp) was shown (Asokan et al., 2011a, b).
-
2.
The phylogram for the Bactrocera spp. suggests that B. tau is phylogenetically distant from B. dorsalis, B. zonata, and B. correcta. Moreover, B. dorsalis, B. zonata, and B. correcta had maximum sequence identity (98%) with very few variable sites in the 28S rDNA sequences. It is inferred that 28S rDNA region will have high reliability for species identification in these species studied (Asokan et al., 2013).
-
3.
PCR analysis using mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene-based primers CO-I gene revealed the presence of three species, B. dorsalis, B. correcta, and B. zonata, on guava and mango orchards in Punjab (Pakistan). The sequencing results and phylogenetic analysis of collected Punjab specimens indicated that sequences of B. dorsalis, Bactrocera cucurbitae, and B. zonata have 99–100% similarity with fruit flies reported from other countries (Ahmad et al., 2019).
-
4.
The RAPD-PCR of 31 DNA samples of B. dorsalis collected in different districts of Karnataka has revealed polymorphism varying from 33.33 to 100%. UPGMA dendrogram generated by RAPD data indicated the segregation of populations into three clusters. The recorded genetic differences in terms of DNA profiles for the better understanding of the genetic diversity among B. dorsalis populations and information obtained will be useful for tracking the movement of this pest, and its analysis would provide knowledge in developing and improving management strategies (Rashmi et al., 2016).
-
5.
The study on the taxonomic status of Bactrocera spp. using the cytochrome oxidase I gene of mitochondrial DNA and its phylogenic has shown that sequence (435 bp) of the Bactrocera sp. had highest similarity to B. cucurbitae (100%) and 96% homology with Bactrocera calumniata. The phylogenetics clearly showed that Bactrocera sp. have the same common ancestor that came from Switzerland: B. cucurbitae (Indriyanti et al., 2017).
-
6.
Molecular characterization of fruit fly species (collected from four different agroclimatic zones of Bihar) associated with cucurbitaceous crops revealed the presence of Bactrocera cucurbitae, B. tau, Bactrocera caudata, Bactrocera nigrofemoralis, Bactrocera diversa, and Dacus ciliatus. Significant achievements of the study were: Bactrocera nigrofemoralis will be the first report from Bihar as a new fruit fly species, and it also identified new hosts D. ciliatus and B. diversa from pointed gourd and flowers of Cucurbita moschata, respectively. The present study provided a platform to make aware the proper management practices of different species of fruit flies and also provided a level of biodiversity of Tephritids in Bihar (Singh, 2017).
-
7.
By using restriction enzyme Alul and Msel, five Bactrocera species namely B. correcta, Bactrocera verbascifoliae, Bactrocera dorsalis, Bactrocera papayae, and B. zonata showed different restriction patterns in their DNA sequences. Using PCR amplification of DNA sequences, we can be able to show species identification from DNA sequences that will be helpful in quarantine work irrespective of their growth stage (Ukey et al., 2017).
-
8.
DNA-based barcode using mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene was very useful to identify and confirm the new report of Bactrocera occipitalis as exotic fruit fly in Bali, Indonesia, and B. dorsalis specimens in fruit orchards in Europe.
-
9.
Ten fruit fly species of the genus Bactrocera was genetically characterized by using standard DNA barcoding region of CO-I gene. The characterization and identification of eight species were straightforward. Phylogenetic analysis formed separate clades for fruit and vegetable infesting fruit flies. Bactrocera aethriobasis, Bactrocera thailandica, and Bactrocera tuberculata have been reported for the first time from northeastern India. The information generated from this study would certainly have implications for pest management, taxonomy, quarantine, and trade (Manger et al., 2018).
-
10.
Considering the speed, specificity, as well as sensitivity of the assay, Taqman real-time PCR can be used as a swift and specific method for the peach fruit fly Bactrocera zonata (including various life stages) at ports of entry (Koohkanzade et al., 2018).
-
11.
The species-specific PCR analysis using mitochondrial DNA cytochrome c oxidase subunit I (mtDNA CO-I) barcoding genes proved to be a robust single-step molecular technique for the diagnosis of guava fruit fly Bactrocera correcta, an invasive pest of fruit and vegetable crops in Southeast Asia (Jiang et al., 2013).
-
12.
Mitochondrial DNA cytochrome c oxidase subunit I (mtDNA CO-I) gene sequence has confirmed the presence of four species of fruit flies, namely Bactrocera minax, B. dorsalis, Bactrocera tsuneonis, and Bactrocera ruiliensis, infesting citrus fruits in China. In the regions surveyed, B. minax is the dominant fruit fly species that damages citrus fruits. The results of this study are helpful in monitoring and controlling citrus fruit flies (Zhong-Yi et al., 2020).
-
13.
Molecular genetic method using restriction enzyme digestions of PCR products from the mitochondrial gene, cytochrome c oxidase subunit I (CO-I), provides a simple diagnostic marker for Korean fruit flies, namely pumpkin fruit fly Bactrocera depressa and pumpkin flower fruit fly Bactrocera scutellata, and widespread oriental fruit fly B. dorsalis and medfly Ceratitis capitata. The simplicity and relatively low cost of this molecular approach will facilitate rapid quarantine decisions concerning exotic fruit flies (HanSong et al., 2000).
-
14.
A PCR amplification technique was used to successfully establish RFLP patterns of the CO-I coding gene to differentiate the exotic Bactrocera invadens (syn. Bactrocera dorsalis) from the native Ceratitis species infesting sweet oranges in Nigeria. The molecular method will enhance easy monitoring, early detection of species involved, and implementation of appropriate management programs that effectively reduce yield losses in citrus production in Nigeria (Onah et al., 2015).
-
15.
In the Campania Region (southern Italy), molecular characterization with CO-I confirmed the presence of Bactrocera dorsalis. This is the first record of B. dorsalis specimens in fruit orchards in Europe; this finding can strongly affect both the production in Italian orchards and crops and the commercial exchanges of Italian fruits in Europe due to the existing quarantine measures (Nugnes et al., 2018).
7.8 Tea Mosquito Bugs (Helopeltis Spp.)
The species of Helopeltis closely resemble each other. Many oriental species of Helopeltis are often misidentified due to the variations in size, coloration, and the scutellar process. The problematic immature stages are most often encountered in import consignments. Identification is further complicated by the lack of morphological keys for immature stages, i.e., eggs, larvae, and pupae. Molecular species diagnostics based on CO-I have become handy in identifying various life stages, i.e., egg, nymph, and adult. Further molecular diversity analyses were also employing CO-I partial sequences for Helopeltis antonii to elucidate if biotypes or cryptic species exist.
-
1.
The molecular identification has helped in quick, accurate, and timely identification of Helopeltis antonii and Helopeltis theivora, a critical factor in understanding the epidemiology of the crop losses in cashew, resistance management, and also in quarantine. The phylogenetic analysis did not show any geographic or host-associated genetic differences in H. antonii, which were collected on different host plants (Asokan et al., 2012c).
-
2.
The usefulness of CO-I is measured for the species discrimination of mirids in India, viz., Helopeltis antonii, H. theivora, Helopeltis bradyi, and Pachypeltis maesarum, in their various life stages. Analysis of CO-I gene revealed <1% intraspecific divergence for all four species examined, whereas the interspecific distances ranged from 7% to 13%. This study showed that the DNA barcode and species-specific markers will aid the identification of mirids in India and will stand as a decisive tool in formulating integrated pest management (IPM) strategy, and quick identification of invasive and cryptic species, haplotypes, biotypes, and other factors, if any (Rebijith et al., 2012).
-
3.
Molecular identification and diversity characterization of Helopeltis collected from tea-growing regions of southern and northern India was done using cytochrome c oxidase subunit I (CO-I) gene of mitochondrial (mt) DNA. This report will provide basic information for diffusion pattern, population dynamics, and chemical application (Suganthi et al., 2016).
-
4.
Both marker (multilocus DNA fingerprinting and morphometry) systems indicated that genetic variability within Helopeltis theivora populations of Assam was significantly high. Appropriate management programs are supported by the knowledge of patterns of population connectivity and historical demography (Bhau et al., 2014).
-
5.
DNA isolated from tea mosquito bug Helopeltis theivora, collected in upper Assam, separated on the basis of pronatum color, shows polymorphism using RAPD-PCR with seven primers. The study indicates that the population consists of discontinuous phenotypes among individuals within a freely interbreeding population that has many of its hosts in the vicinity. Genetic variation among the phenotypes within a population focuses on some evolutionary mechanisms that may resist the effect of pesticide (Sarmah & Bandyopadhyay, 2009).
-
6.
This study showed that 5′-anchored PCR technique was suitable and efficient for isolating single-locus DNA microsatellites for Helopeltis theivora, known as a pest of cocoa, tea, cashew, mangoes, and ornamental plants in Peninsular Malaysia. A total of six polymorphic microsatellite markers were isolated successfully. This study is important in the quest to provide a sufficient number of codominant genetic markers for studying the population genetic structure of H. theivora in Asia (Latip et al., 2010).
7.9 Psyllids
In view of the invasiveness of psyllids, an effective, rapid, and timely identification has to be carried out so that the epidemic of the pest can be managed. The technique of molecular species identification based on mitochondrial cytochrome c oxidase subunit I (mtCO-I) is very useful for rapid identification of psyllids infesting horticultural crops.
-
1.
Mitochondrial cytochrome c oxidase subunit I (mtCO-I) specific primer was employed for molecular identification of citrus psylla Diaphorina citri from different regions of India through DNA barcoding. The technique uses 51 regions of mitochondrial cytochrome c oxidase subunit I for reliable and accurate species identification. Further, our study revealed that among 794 bp, there were 23 variable nucleotides with 7 nucleotides being Parsimony informative; also, overall Transition (ti)/Transversion (tv) bias was found to be R = 4.95. In addition, the neighbor-joining (NJ) tree showed clear clades with an outgroup Pachypsylla venusta (NCBI accession number AY278317). Data procured in current study provide information that can be used for identification, management, and quarantine purposes (Chaitanya et al., 2016).
-
2.
The following species have been successfully sequenced at the CO-I region: Diaphorina citri, Trioza remota, Trioza albiventris, Trioza anthriscii, and Trioza apicalis. The following species have been sequenced at the ITS2 for this project: Bactericera cockerelli, Bactericera albiventris, Bactericera curvatinervis, Cacopsylla pulchra, Cacopsylla brunneipennis, Cacopsylla melanoneura, D. citri, T. remota, and T. albiventris. The ITS2 region appears to be highly variable among psyllid species and is therefore ideal for species-specific primer design. Its variability has been used to discriminate the Cacopsylla pruni complex, revealing the existence of two biologically significant species (Sjölund et al., 2016).
-
3.
DNA sequence analyses of the 16S rDNA and cytochrome c oxidase subunit I (CO-I) DNA regions showed that Cacopsylla chinensis was found in most pear orchards in China, but Cacopsylla qianli was found in only the cities of Guiyang (Guizhou Province) and Xiangyang (Hubei Province). The results revealed that C. chinensis lineage I was found in most provinces of China, while C. chinensis lineage II samples were mainly found in the Bohai rim region of China, and lineage III samples were found in Northeast China. The results of this study will provide information to pear producers regarding effective control measures to prevent further damage from pear psyllids (Chen et al., 2018).
-
4.
Sequencing of the CO-I gene confirmed the presence of psyllid Bactericera cockerelli feeding on potatoes in South America (Ecuador). Molecular tests on a mitochondrial gene confirmed the population from Ecuador as central haplotype. This suggests that the psyllid populations found in Ecuador might come from North or Central America, probably along with agricultural products or other plant material. Nonetheless, there is a risk of introduction of psyllid yellows and ZC into South America. This will help to develop strategies to control the psyllid and make recommendations for local potato growers (Castillo et al., 2019).
7.10 Lepidopterans
Key to making correct management decisions is the rapid and accurate identification of lepidopterans at all life stages. Lepidopterous pests are morphologically identical in early instars, making identification difficult and time consuming. Consequently, accurate identification requires rearing these immature larvae to adult stage. Molecular technique is used for accurate and rapid identification of the lepidopterans at all life stages.
-
1.
The California almond and pistachio in California are attacked by a variety of lepidopteran pests. Molecular tests (diagnostic PCR) are employed to rapidly and inexpensively identify lepidopteran pests, namely fruit moth (Grapholita molesta), carob moth (Ectomyelois ceratoniae), filbert worm (Cydia latiferreana), Indian meal moth (Plodia interpunctella), and raisin moth (Cadra figulilella) of tree nuts. PCR is used to rapidly differentiate multiple species in a single reaction (Rohith, 2018).
-
2.
With the molecular identification based on DNA barcode using mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene, the South American Tomato moth, Tuta absoluta, has been reported as a new invasive pest on tomatoes from Ludhiana and Patiala districts of Punjab, India, during 2016–2017. The report indicated that there is a need for constant vigil on the pest, for preventing its further spread within and the adjoining the states of Punjab (Sandeep et al., 2017). Molecular analysis has confirmed the morphological identification and thus confirming first report of Tuta absoluta in Botswana. This is to promote coordinated efforts among stakeholders, research specialists, and extension officers in Botswana and across the southern African region in monitoring and managing the pest (Mutamiswa et al., 2017).
-
3.
Taxonomic identification of leaf miner species of Liriomyza (Liriomyza trifolii, Liriomyza sativae, and Liriomyza huidobrensis) is very complex due to morphological resemblance, and, consequently, species-level identification is often done incorrectly by mistaking one species for another. In Meghalaya state, the species-level identity of leaf miner was confirmed as L. sativae at the molecular level by using mitochondrial cytochrome oxidase gene. Moreover, the information on the correct distribution, seasonal incidence, and basic biological attributes at different stages of L. sativae infesting tomato crop is reported (Firakea et al., 2018).
-
4.
As molecular variability of brinjal Leucinodes orbonalis population is an important consideration for constant monitoring, it is essential to study the possible development of resistance. Molecular characterization of nine different populations belonging to various brinjal-growing regions was done using cytochrome c oxidase subunit I (CO-I) gene. This study revealed that there was no significant molecular diversity within 21 populations of L. orbonalis collected from Karnataka, Andhra Pradesh, New Delhi, and Uttar Pradesh in India (Murali et al., 2017).
-
5.
Molecular sequencing in reporting species of Thylacoptila as insect pest is an important contribution to the annals of insect–pest–plant interaction, and more importantly in medicinally important plant species Senna alata (Savitha et al., 2019).
-
6.
Molecular CO-I barcode sequences confirm diagnostic morphology of cardamom shoot and capsule borer Conogethes sahyadriensis sp. nov. The new species is delineated from closely related and superficially similar species of Conogethes. Precise identification would then be utilized for developing realistic practices for the management of Conogethes sp. in diversified cultivated ecosystems (Shashank et al., 2018).
-
7.
Phylogenetic relationship of Helicoverpa armigera inferred from mitochondrial cytochrome c oxidase subunit I (mtCO-I) revealed that there were no significant variations in the CO-I sequences of H. armigera collected on various hosts and geographical locations in India (Asokan et al., 2012b). Phylogenetic analysis concluded that these intraspecific populations of H. armigera from eight H. armigera populations from India formed a major clade. This analysis will be useful for delineating genetic relationships among the intraspecific populations and estimating genetic diversity, thereby gaining insight into genetic structure of population. Therefore, understanding the sequence polymorphism of such genes in its intraspecific populations would help in downregulation of these in reducing the resistance development (Asokan et al., 2012a). The study using ten microsatellite simple sequence repeat (SSR) markers of the genetic variability of H. armigera populations from six different host plants with nine SSR primers indicated high variability across the different host-associated populations, with polymorphism ranging from 75% to 100% and high similarity among populations collected from vegetable crops while more variability among the population collected from the cotton crops. This phenomenon indicates a strong genetic variability among H. armigera populations collected from different host plants (Subramanian & Mohankumar, 2006).
-
8.
Differentiation of Helicoverpa armigera and Helicoverpa zea is difficult, since larvae and adults are morphologically similar. A rapid and inexpensive procedure for obtaining amplifiable genomic DNA from H. armigera and H. zea was developed. The high-resolution melt analysis combined with rapid DNA extraction could be used as an inexpensive method to genetically differentiate large numbers of H. armigera and H. zea using readily available reagents (Omaththage et al., 2015).
-
9.
Simulations on spatial distribution patterns show that the detection of rare and/or the absence of dominant mtDNA haplotypes in southern H. armigera populations are inconsistent with genetic signatures observed in northern and central Brazil. Incursions of H. armigera into the New World are therefore likely to have involved independent events in northern/central Brazil, and southern Brazil/Uruguay-Argentina-Paraguay. This study demonstrates the significant biosecurity challenges facing the South American continent, and highlights alternate pathways for introduction of alien species into the New World (Arnemann et al., 2019).
-
10.
Our mitochondrial DNA sequence data support the single-species status of H. armigera across Africa, Asia, and Australia. The finding of high genetic similarity between Old World H. armigera and New World H. zea emphasizes the need to consider work on both pests when building pest management strategies for either. The current study does not suggest that H. armigera and H. zea are a single species but does show their close relationship and indicates a fairly recent (<1.5 mya) divergence of H. zea from a parental H. armigera stock (Behere et al., 2007).
-
11.
The UPGMA dendrogram showed that H. armigera population from Bangladesh had 25–45% similarity, and in its Indian population the similarity remained within this range (Rahman et al., 2014).
-
12.
Maximum-likelihood molecular analyses of Spodoptera litura, Spodoptera exigua, and Spodoptera mauritia reveal that there exist significant variations among these. Spodoptera exigua showed intraspecific variations with respect to different geographic locations. Present study proves the utility of CO-I for identification of S. litura and S. exigua irrespective of their life stages, and also draws inferences on the phylogenetic relationships between the three pest species, solving intra and interspecific complexities (Shashank et al., 2015).
7.11 Coleopterans
Several beetle pests take long time (up to 1 year) in some species to reach adult stage, in which morphological identification is done. This fact, together with lack of morphological traits to identify the species from the larval stage, poses obstacles for the quick taxonomic identification and characterization of the beetles. It is also very difficult to make reliable identifications from structurally damaged samples where key morphological characteristics are absent or compromised, or to distinguish among members of a cryptic species complex. To solve this matter, random amplified polymorphic DNA (RAPD) molecular markers have been applied to determine species boundaries. Rapid DNA extraction (N10) is recommended due to the shorter time required. Molecular identification can be done even when very little DNA is recovered.
-
1.
The Asiatic palm weevils in Indonesia exhibit a wide range of color polymorphisms, namely the Asiatic palm weevil with red stripe, the Asiatic palm weevil with rusty red, and the Asiatic palm weevil with intermediate color between red stripe and rusty red morphs. Molecular identification has confirmed the presence of two coconut palm weevil species Rhynchophorus vulneratus and Rhynchophorus bilineatus in Indonesia, as well as Rhynchophorus ferrugineus in Saudi Arabia. CO-I analysis revealed high intraspecific variability in palm weevils from Indonesia and Saudi Arabia (Sukirno et al., 2018).
-
2.
Coconut rhinoceros beetle Oryctes rhinoceros and oriental flower beetle Protaetia orientalis are nearly indistinguishable until they have grown to their later life stages, making early morphological detection difficult. A new genetic-testing method using UH identified genetic markers in the beetles’ DNA was used for differentiation via the test, which promises to be much faster than the existing morphological identification method. The new molecular method will ensure that eradication efforts are being directed at coconut rhinoceros beetle and not oriental flower beetle in Hawaii (Watanabe & Melzer, 2017).
-
3.
Molecular identification confirmed the first report of citrus long-horned beetle, Anoplophora chinensis, on Acer negundo in Antalya Province, Turkey. The sequence alignment of the mitochondrial cytochrome oxidase I gene region showed that Antalya population showed 99% similarity with A. chinensis. According to the phylogenetic analysis, populations of A. chinensis from Antalya and China were placed in the same subgroup and therefore it is postulated that the origin of the Antalya population is China (Topakci et al., 2017).
-
4.
By cloning and sequencing the 5′ mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene, the beetle’s molecular identification confirmed its identity as Xylosandrus compactus. There were no molecular differences between space and time, collected coffee shot, and hole borers; thus all the specimens described were X. compactus infesting both robusta and arabica coffee. For its effective management, accurate, timely, and rapid identification of species is critical (Kiran et al., 2019).
-
5.
White grubs species namely Leucopholis lepidophora, Leucopholis burmeisteri, and Leucopholis coneophora collected from various locations of Karnataka and Kerala were successfully identified by employing CO-I gene (Mahadevaswamy et al., 2019a, b).
-
6.
Random amplified polymorphic DNA (RAPD) as molecular markers revealed that the Madeira population of banana weevil Cosmopolites sordidus is phylogenetically and geographically closer to the Canary Islands populations, suggesting that it is the most likely source of the insects introduced in the Canary Islands (Magan et al., 2007).
-
7.
CO-I sequences of populations of banana leaf and fruit-scarring beetles Basilepta subcostata from Assam and Uttar Pradesh showed 98–100% homology, indicating that these populations are conspecific and that CO-I sequences can be used for rapid species determination. CO-I sequences will be useful for economic entomologists and non-taxonomists in identifying these insects (Prathapan et al., 2019).
-
8.
Genetic divergences of mitochondrial cytochrome c oxidase subunit I (mtCO-I) genes have been used in insect species identification of Xyleborus. The results showed that the mean interspecific divergence values (23.6%) were 15-fold higher than the observed intraspecific divergence (1.6%), except Xyleborus affinis. The results supported the inference that the barcode variation within species of insects is somewhat higher than interspecific ones. Thus, this study validated the effectiveness of barcoding for the identification of Xyleborus species (Chang et al., 2013).
-
9.
Molecular identification was also done on the basis of the 5′ region of the mitochondrial cytochrome c oxidase subunit I (mtCO-I) gene, obtained from a specimen of granulate ambrosia beetle from Argentina. Results from the sequence analyses confirm the identity of Xylosandrus crassiusculus, and for the first time, documenting expansion of this exotic species in South America into Argentina and Uruguay in South America (Landi et al., 2017).
8 Limitations of DNA Barcoding Employing mtCO-I
-
1.
Limitations in resolving species at species boundaries in some groups where nuclear ribosomal regions are suitable.
-
2.
Mitochondrial cytochrome c oxidase subunit I (mtCO-I) does not show much variation in plants, except for some algae.
-
3.
Introgression: mtCO-I is largely maternally inherited, and usually as a single copy. Hence, it has one-fourth the population size of other nuclear genes, has a different inheritance pattern, and is more sensitive than nuclear genes to population bottlenecks. mtDNA introgression confounds the boundaries between otherwise distinct lineages; such introgression between species could lead to inaccurate identifications.
-
4.
Maternal inheritance: The full effect of maternal inheritance on rates of molecular divergence in mtDNA is not predictable, and therefore the failure rate of DNA barcoding is also unpredictable. mtDNA is inherited maternally, but not in bivalve mollusks that display double unpatented mtDNA inheritance. It is also evident in a wide range of the taxa in frequent paternal inheritance.
-
5.
Low recombination: The general absence of recombination will lead to the persistence of population structure long after the barriers that created the structures are removed and gene flow is restored. Therefore, it is not possible to estimate species boundaries that would have been estimated from a broader data set.
-
6.
Mutation rate: For the DNA barcode to be used as standalone, there should be a consistent mutation rate, such as the proposed 2–3% divergence to correlate with species limit on a consistent basis. Speciation, uniquely driven by changes in mtDNA or speciation event, necessarily alters the mtDNA haplotypes.
-
7.
Heteroplasmy: This refers to the classical view of mitochondria functionally haploid with multiple identical copies. However, single nucleotide differences are common in some species and are also abundant in some, especially at the restriction sites.
-
8.
Compounding genetic factors: Coinheritance factors that bias single mitochondrial inheritance and are most obvious include: (1) mitochondrial selection either on the barcoding gene itself or on the other linked genes; and (2) cytoplasmically inherited bacteria like Wolbachia and some Rickettsia, which alter the inheritance factors.
-
9.
Identification depends on the intra- and interspecific genetic variations.
-
10.
Difficult to resolve, recently diverged species that arose through hybridization.
-
11.
No single gene is conserved in all domains of life and exhibit enough sequence divergence for species discrimination.
9 Limitations of Nuclear Protein Coding Genes
-
1.
May be heterozygous and present in low copy numbers.
-
2.
Many genes contain large introns that make it difficult to amplify more than one exon.
-
3.
Many single-copy loci are actually present in more than one copy.
-
4.
Pseudogenes may create problem if comparisons are made inadvertently.
References
Abdullah, I., Winter, S., Atiri, G. I., & Thottappilly, G. (2003). Molecular characterization of whitefly, Bemisia tabaci (Hemiptera:Aleyrodidae) populations infesting cassava. Bulletin of Entomological Research, 93(2), 97–106.
Ahmad, J. N., Sharif, T., Samina, J. N., Maqsood, S., & Zafar, F. (2019). Molecular identification and characterization of fruit flies of genus Bactrocera (Diptera: Tephritidae) in Pakistan. Pakistan Journal of Zoology, 51, 2275–2280.
Alhudaib, K. A., Sabour Rezk, A. A., Abdel-Banat, B. M. A., & Soliman, A. M. (2014). Molecular identification of the biotype of whitefly (Bemisia tabaci) inhabiting the eastern region of Saudi Arabia. Journal of Biological Sciences, 14, 494–500.
Arnemann, J. A., Roxburgh, S., Walsh, T., Guedes, J., Gordon, K., Smagghe, G., & Tay, W. T. (2019). Multiple incursion pathways for Helicoverpa armigera in Brazil show its genetic diversity spreading in a connected world. Scientific Reports, 9, 19380. https://doi.org/10.1038/s41598-019-55919-9
Ashfaq, M., Ara, J., Raza, N. A., Hebert, P. D. N., & Mansoor, S. (2011). Molecular phylogenetic analysis of a scale insect (Drosicha mangiferae; Hemiptera: Monophlebidae) infesting mango orchards in Pakistan. European Journal of Entomology, 108, 553–559.
Asokan, R., Chaitanya, B. N., Rebijith, K. B., Krishna Kumar, N. K., Viraktamath, C. A., & Ramamurthy, V. V. (2015). COI based molecular identification of mango leaf hoppers (Hemiptera: Cicadellidae) in India. Indian Journal of Biotechnology, 14, 260–263.
Asokan, R., Nageshaa, S. N., Manamohana, M., Krishnakumar, N. K., Mahadevaswamya, H. M., Rebijitha, K. B., Prakasha, M. N., & Sharath, C. G. (2012a). Molecular diversity of Helicoverpa armigera Hubner (Noctuidae:Lepidoptera) in India. Oriental Insects, 46(2), 130–143.
Asokan, R., Rebijith, K. B., & Krishna Kumar, N. K. (2012b). Genetic diversity of tomato fruit borer, Helicoverpa armigera Hübner (lepidoptera: Noctuidae) inferred by mitochondrial cytochrome oxidase-I (mtCO-I). Pest Management in Horticultural Ecosystems, 18, 65–70.
Asokan, R., Rebijith, K. B., Krishna, V., Krishna, K. N. K., Jacob, T. K., Devasahayam, S. D., Tyagi, K., & Sujeesh, E. S. (2012d). Molecular diversity of cardamom thrips, Sciothrips cardamomi (Ramakrishna) (Thripidae: Thysanoptera). Oriental Insects, 47, 55–64.
Asokan, R., Rebijith, K. B., Singh, S. K., & Ramamurthy, V. V. (2013). Life stage independent identification of fruit flies (Diptera: Tephritidae) using 28S rDNA sequences. The Bioscan, 8, 1–10.
Asokan, R., Rebijith, K. B., Singh, S. K., Sidhu, A. S., Siddharthan, S., Karanth, P. K., Elango, R., & Ramamurthy, V. V. (2011a). Molecular identification and phylogeny of Bactrocera species (Diptera: Tephritidae). Florida Entomologist, 94(4), 1026–1035.
Asokan, R., Rebijith, K. B., Srikumar, K. K., Shivarama, B. P., & Ramamurthy, V. V. (2012c). Molecular identification and diversity of Helopeltis antonii and Helopeltis theivora (Hemiptera: Miridae) in India. Florida Entomologist, 95(2), 350–358.
Asokan, R., Joshi, S., Krishna Kumar, N. K. and Rebijith, K. B. 2011b. DNA barcode for important sap sucking insect pests of horticultural crops. IIHR Tech Bulletin, 57p.
Asokan, R., Krishna Kumar, N. K., Kumar, V., & Ranganath, H. R. (2007). Molecular differences in mitochondrial cytochrome oxidase I (mtCOI) gene and development of a species-specific marker for onion thrips, Thrips tabaci Lindeman and melon thrips, T. palmi Karny (Thysanoptera: Thripidae), vectors of tospoviruses (Bunyaviridae). Bulletin of Entomological Research, 97, 461–470.
Assefa, Y., & Malindzisa, N. (2018). Molecular identification of mealybugs (Hemiptera: Pseudococcidae) on cultivated, ornamental and wild host plants in Swaziland. Asian Research Journal of Agriculture, 9(1), 1–10.
Banta, G., Jindal, V., Mohindru, B., Sharma, S., Kaur, J., & Gupta, V. K. (2016). Molecular phylogenetic analysis of mango mealybug, Drosicha mangiferae from Punjab. Journal of Environmental Biology, 37, 49–55.
Behere, G. T., Tay, W. T., Russell, D. A., Heckel, D. G., Appleton, B. R., Kranthi, K. R., & Batterham, P. (2007). Mitochondrial DNA analysis of field populations of Helicoverpa armigera (lepidoptera: Noctuidae) and of its relationship to H. zea. BMC Evolutionary Biology, 7, 117. https://doi.org/10.1186/1471-2148-7-117
Bhau, B. S., Mech, J., Borthakur, S., Bhuyan, M., & Bhattacharyya, P. R. (2014). Morphological and genetic diversity studies among populations of tea mosquito bug, Helopeltis theivora from Assam, India. Molecular Biology Reports, 41(12), 7845–7856.
Boopathi, T., Mohankumar, S., Karuppuchamy, P., Kalyanasundaram, M., Ravi, M., Preetha, B., Aravintharaj, R., & Manju, T. (2014). Genetic evidence for diversity of spiraling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae) populations in India. Florida Entomologist, 97(3), 1115–1122.
Calatayud P.A. and Le Rü B (Eds). 2006. Cassava and mealybugs. IRD Éditions Institut De Recherche Pour Le Développement, Paris, 2006, pp. 11–22.
Castillo, C., Zhen, F. U., & Burckhardt, D. (2019). First record of the tomato potato psyllid Bactericera cockerelli from South America Carmen. Bulletin of Insectology, 72(1), 85–91.
Chaitanya, B. N., Asokan, R., Rebijith, K. B., Rao, C. N., Ram, K. P., Krishna, K. N., & K. (2016). Molecular identification and diversity of Asian citrus psyllids using mtCoI gene sequences. Pest Management in Horticultural Ecosystems, 22(1), 51–57.
Chakrabortya, R., Singhaa, D., Kumar, V., Pakrashia, A., Shantanu, K., Chandraa, K., Patnaikb, S., & Tyagia, K. (2019). DNA barcoding of selected Scirtothrips species (Thysanoptera) from India. Mitochondrial DNA Part B, 4(2), 2710–2714.
Chandrika M. Josephrajkumar, A., Babu M, Prathibha, P. S., Krishnakumar, V., Hegde, V. and Chowdappa, P. (2017). Invasive rugose spiralling whitefly on coconut, Technical Bulletin No. 117, (Centenary Series 60), ICAR-CPCRI, Regional Station, Kayamkulam, 16 p.
Chang H, Liu Q, Hao D., Liu Y., An Y, Qian L. and Yang X.2013. DNA barcodes and molecular diagnostics for distinguishing introduced Xyleborus (coleoptera: Scolytinae) species in China, Mitochondrial DNA, 25:1, 63–69, DOI: 10.3109/19401736.2013.779260.
Chen, P., Liu, Q. Z., Qiao, X. F., Wang, J. W., & Zhang, T. (2018). Identification and phylogenetic analysis of pear psyllids (Hemiptera: Psyllidae) in Chinese pear orchards. Journal of Economic Entomology, 111(6), 2908–2913.
Correa, M. C., Germain, J. F., Malausa, T., & Zaviezo, T. (2012). Molecular and morphological characterization of mealybugs (Hemiptera: Pseudococcidae) from Chilean vineyards. Bulletin of Entomological Research, 102(5), 524–530.
Cuervo, I. M., Calatayud, P. A., Múnera, S. D., Bellotti, A. C., & Calvert, L. A. (2002). Molecular identification of cassava mealybugs [poster] (p. 1). Centro Internacional de Agricultura Tropical (CIAT).
Daane, K. M., Middleton, M. C., Sforza, R., Cooper, M. L., Walton, V. A., Walsh, D. B., Zaviezo, T., & Almeida, P. P. (2011). Development of a multiplex PCR for identification of vineyard mealybugs. Molecular Ecology and Evolution, 40(6), 1595–1603.
De Grazia, A., Marullo, R., & Moritz, G. (2016). Molecular diagnosis of native and quarantine pest thrips of southern European citrus orchards. Bulletin of Insectology, 69(1), 1–6.
Deng, J., Li, H., Wang, X., Yu, F., Zhang, Y., & Wu, S. (2016). Molecular identification of two morphologically similar Eulecanium species: E. giganteum and E. kuwanai (Hemiptera: Coccidae). The Canadian Entomologist, 148(1), 1–7.
Dewer, Y., Abdel-Fattah, R. S., & Schneider, S. A. (2018). Molecular and morphological identification of the mealybug, Phenacoccus solani Ferris (Hemiptera: Pseudococcidae): First report in Egypt. Bulletin OEPP EPPO Bulletin, 48(1), 155–159.
Dickey, A. M., Stocks, I. C., Smith, T., Osborne, L., & McKenzie, C. L. (2015). DNA barcode development for three recent exotic whitefly (Hemiptera: Aleyrodidae) invaders in Florida. Florida Entomologist, 98(2), 473–478.
Edwards, R., Carraher, C., Poulton, J., Sandanayaka, M., Todd, J. H., Dobson, S., Mauchline, N., Hill, G., MCkenna, C., & Newcomb, R. D. (2008). DNA diagnostics of three armored scale species on kiwifruit in New Zealand. Journal of Economic Entomology, 101, 1944–1949.
Ellango, R., Asokan, R., & Mahmood, R. (2014). Host specific molecular variations in Bemisia tabaci (G.) as revealed by using mitochondrial and ribosomal marker. The Bioscan, 9, 461–466.
Farkas, P., György, Z., Tóth, A., Sojnóczki, A., & Fail, J. (2019). A simple molecular identification method of the Thrips tabaci (Thysanoptera: Thripidae) cryptic species complex. Bulletin of Entomological Research, 1–9. https://doi.org/10.1017/S0007485319000762. (n.d.).
Firakea, D. M., Sankarganeshb, E., Sharmaa, B., Firakea, P., Gajanan, D., & Beherea, T. (2018). DNA barcoding confirmed the occurrence of invasive vegetable leafminer, Liriomyza sativae Blanchard (Diptera:Agromyzidae) in Northeast India. Journal of Asia-Pacific Biodiversity, 11, 56–60.
Foottit, R. G., Lowery, D. T., Maw, H. E. L., Smirle, M. J., & Lushai, G. (2009). Identification, distribution, and molecular characterization of the apple aphids Aphis pomi and Aphis spiraecola (Hemiptera: Aphididae: Aphidinae). Canadian Entomologist, 141(5), 478–495.
Foottit, R. G., Maw, H. E. L., Pike, K. S., & Miller, R. H. (2010). The identity of Pentalonia nigronervosa Coquerel and P. caladii van der Goot (Hemiptera: Aphididae) based on molecular and morphometric analysis. Zootaxa, 2358, 25–38.
Frey, J., & Frey, E. B. (1995). Molecular identification of six species of scale insects (Quadraspidiotus sp.) by RAPD-PCR: Assessing the field-specificity of pheromone traps. Molecular Ecology, 4(6), 777–778.
Hadija, M. A., Hamss, H. E., Simiand, C., Maruthi, M. N., Colvin, J., Christopher, A. O., & Delatte, H. (2019). What has changed in the outbreaking populations of the severe crop pest whitefly species in cassava in two decades? Scientific Reports, 9, 14796. https://doi.org/10.1038/s41598-019-50259-0
Hajibabaei, M., Janzen, D. H., Burns, J. M., Hallwachs, W., & Hebert, P. D. N. (2006). DNA barcodes distinguish species of tropical lepidoptera. Proceedings of the National Academy of Sciences of the United States of America, 103, 968–971.
HanSong, Y., HeungKim, T., & Roderick, G. K. (2000). Molecular genetic diagnosis of four fruit fly species (Tephritidae). Journal of Asia-Pacific Entomology, 3(2), 89–94.
Hebert, P. D. N., & Gregory, T. R. (2005). The promise of DNA barcoding for taxonomy. Systematic Biology, 54, 852–859.
Hebert, P. D. N., Ratnasignham, S., & deWaard, J. R. (2003b). Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society of London Series B, 270(Suppl. 1), S96–S99.
Hebert, P. D. W., Cywinska, A., Ball, S. L., & deWaard, J. R. (2003a). Biological identification through DNA barcodes. Proceedings of the Royal Society of London Series B, 270, 313–321.
Hosseini, R., & Hajizadeh, J. (2011). Molecular identification of three of the most important mealybug species (Hemiptera: Sternorrhyncha: Coccoidea: Pseudococcidae) on ornamental plants in Guilan province, Iran. Zootaxa, 3009, 46–54.
Hosseininaveh, F., Nozari, J., Kaydan, M. B., & Hosseininaveh, V. D. (2018). Molecular and morphological identification of pistachio armored scale insects (Hemiptera: Diaspididae), with description of a new species. https://doi.org/10.11646/zootaxa.4200.4.5
Indriyanti, D. R., Suputa, S., & Jannah, S. N. (2017). Molecular identification of Bactrocera sp. fruit fly from Muria forest, Central Java, Indonesia. ARPN Journal of Engineering and Applied Sciences, 12(9), 2954–2961.
Jalalizanda, A. R., Mirhendib, H., Karimi, A., Modaresi, M., & Mahmoodia, E. (2012). Morphological and molecular identification aphids of Rosae. APCBEE Procedia, 4, 12–15.
Jiang, F., Li, Z., Deng, Y., Wu, J., Liu, R., & Buahom, N. (2013). Rapid diagnosis of the economically important fruit fly, Bactrocera correcta (Diptera: Tephritidae) based on a species-specific barcoding cytochrome oxidase I marker. Bulletin of Entomological Research, 103(3), 363–371.
Jiménez, M. P., & Meneses, M. B. (2016). First record of morphological and molecular identification of mealybug Pseudococcus Jackbeardsleyi (Hemiptera: Pseudococcidae) in Costa Rica. Universal Journal of Agricultural Research, 4(4), 125–133.
Joshi, S., Lokeshwari, D., Krishna, K. N. K., Manjunatha, H., Verghese, A., & Jalali, S. K. (2014). Wahlgreniella Nervata (Hemiptera: Aphididae), a new pest of rose in India. Florida Entomologist, 97(1), 162–165.
Kadirvel, P., Srinivasan, R., Hsu, Y. C., Su, F. C., & Pena, R. D. L. (2013). Application of cytochrome oxidase I sequences for phylogenetic analysis and identification of thrips species occurring on vegetable crops. Journal of Economic Entomology, 106(1), 408–418.
Kapantaidaki, D. E., Sadikoglou, E., Tsakireli, D., Kampanis, V., Stavrakaki, M., Schorn, C., Ilias, A., Riga, M., Tsiamis, G., Nauen, R., Skavdis, G., Vontas, J., & Tsagkarakou, A. (2018). Insecticide resistance in Trialeurodes vaporariorum populations and novel diagnostics for kdr mutations. Pest Management Science, 74(1), 59–69. https://doi.org/10.1002/ps.4674. Epub 2017 Sep 11.
Karimi, J., Hassani-Kakhki, M., & Awal, M. M. (2010). Identifying thrips (Insecta: Thysanoptera) using DNA barcodes. Journal of Cell and Molecular Research, 2(1), 35–41.
Kinyanjui, G., Khamis, F. M., Mohamed, S., Ombura, L. O., Warigia, M., & Ekesi, S. (2016). Identification of aphid (Hemiptera: Aphididae) species of economic importance in Kenya using DNA barcodes and PCR-RFLP-based approach. Bulletin of Entomological Research, 106, 63–72.
Kiran, V. S., Asokan, R., Revannavar, R., Mahadeva Swamy, H. M. M., & Ellango, R. (2019). Genetic characterization and DNA barcoding of coffee shot-hole borer, Xylosandrus compactus (Eichhoff) (Coleoptera: Curculionidae: Scolytinae). Mitochondrial DNA Part A. https://doi.org/10.1080/24701394.2019.1659249
Koohkanzade, M., Zakiaghl, M., Dhami, M. K., Fekrat, L., & Namaghi, H. S. (2018). Rapid identification of Bactrocera zonata (Dip.: Tephritidae) using TaqMan real-time PCR assay. PLoS One, 13(10), e0205136. https://doi.org/10.1371/journal.pone.0205136
Landi, L., Gómez, D., Braccini, C. L., Pereyra, V. A., Smith, S. M., & Marvaldi, A. E. (2017). Morphological and molecular identification of the invasive Xylosandrus crassiusculus (coleoptera: Curculionidae: Scolytinae) and its South American range extending into Argentina and Uruguay. Annals of the Entomological Society of America, 110(3), 344–349.
Latha, K. R., Krishna, K. N. K., Mahadevaswamy, H. M., Asokan, R., Ranganath, H. R., & Mahmood, R. (2015). Molecular identification and diversity of chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) employing ITS2 marker. Pest Management in Horticultural Ecosystems, 21, 16–26.
Latip, S. N. H., Muhamad, R., Manjeri, G., & Tan, S. G. (2010). Development of microsatellite markers for Helopeltis theivora Waterhouse (Hemiptera: Miridae). African Journal of Biotechnology, 9(28), 4478–4481.
Lemmetty, A., & Vänninen, I. (2014). Bemisia tabaci biotype Q determined for the first time on poinsettia crops in Finland and Sweden. Annales Zoologici Fennici, 51, 501–506.
Magan, C., Beroiz, B., Hernandez-Crespo, P., Montes de Oca, M., Carnero, A., Ortego, F., & Castan, P. (2007). Population structure of the banana weevil, an introduced pest in the Canary Islands, studied by RAPD analysis. Bulletin of Entomological Research, 97, 585–590.
Mahadevaswamy, H. M., Asokan, R., Hanamant, G., Samuel, D. K., Kumar, R., & Tejaswini, P. (2019a). DNA barcoding and phylogenetic analysis of leafhoppers associated with aster yellow disease on China aster, marigold and chrysanthemum. Mitochondrial DNA Part A. https://doi.org/10.1080/24701394.2020.1735378
Mahadevaswamy, H. M., Asokan, R., Kalleshwaraswamy, C. M., & Adarsh, S. K. (2019b). Arecanut white grubs Leucopholis species (Melolonthinae:Scarabaeidae:Coleoptera) morphological, molecular identification and phylogenetic analysis. Journal of Asia-Pacific Entomology, 22, 880–888.
Malumphy, C., Walsh, K., Suarez, M. B., Collins, D. W., & Boonham, N. (2009). Morphological and molecular identification of all developmental stages of four whitefly species (Hemiptera: Aleyrodidae) commonly intercepted in quarantine. Zootaxa, 2118(1), 1–29.
Manger, A., Behere, G. T., Firake, D. M., Sharma, B., Deshmukh, N. A., Firake, P. D., Azad Thakur, N. S., & Ngachan, S. V. (2018). Genetic characterization of Bactrocera fruit flies (Diptera: Tephritidae) from Northeastern India based on DNA barcodes. Mitochondrial DNA A DNA Mapping Seqencing and Analysis, 29(5), 792–799.
Mani, M., Joshi, S., Kalyanasundaram, M., Shivaraju, C., Krishnamoorthy, A., Asokan, R., & Rebijith, K. B. (2013). A new invasive Jack Beardsley mealybug Pseudococcus jackbeardsleyi Gimpel and Miller on papaya in India. Florida Entomologist, 96, 242–245.
Manjunatha, R., Viraktamath, S., & Halikatti, G. (2018). Detection of genetic variation in mango leafhoppers based on mitochondrial marker cytochrome oxidase I (COI) and their phylogenetic relationships of south Indian populations. Journal of Entomology and Zoology Studies, 6(6), 165–171.
Mansour, R., Cavalleri, V., Mazzeo, G., Lebdi, K. G., & Russo, A. (2012). A morphological and molecular characterization of vine populations (Hemiptera, Pseudococcidae) from Tunisia. Journal of Entomology Acarology Research, 44(e5), 24–27.
Misaka, B. C., Wosula, E. N., Marchelo-d’Ragga, P. W., Hvoslef-Eide, T., & Legg, J. P. (2020). Genetic diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) colonizing sweet potato and cassava in South Sudan. Journal Reference. Insects, 2020(11), 58. https://doi.org/10.3390/insects11010058
Mullis, K. B., & Faloona, F. A. (1987). Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods in Enzymology, 155, 335–335.
Murali, S., Jalali, S. K., Shylesha, A. N., Shivalinga Swamy, T. M., Reeta, B., & Prathibha, M. (2017). Molecular characterization and their phylogenetic relationship based on mitochondrial cytochrome oxidase I of brinjal shoot and fruit borer, Leucinodes orbonalis (Guenée) (lepidoptera: Pyralidae). International Journal of Current Microbiology Applied Science, 6(7), 2527–2539.
Mutamiswa, R., Machekano, H., & Nyamukondiwa, C. (2017). First report of tomato leaf miner, Tuta absoluta (Meyrick) (lepidoptera: Gelechiidae), in Botswana. Agricultural & Food Security, 6, 49. https://doi.org/10.1186/s40066-017-0128-2
Nugnes, F., Russo, E., Viggiani, G., & Bernardo, U. (2018). First record of an invasive fruit fly belonging to Bactrocera dorsalis Complex (Diptera: Tephritidae) in Europe. Insects, 9, 182.
Omaththage, P. P., Allen, K. C., Jain, D., Purcell, M., Little, N. S., & Luttrell, R. G. (2015). Rapid identification of Helicoverpa armigera and Helicoverpa zea (lepidoptera: Noctuidae) using ribosomal RNA internal transcribed spacer 1. Journal of Insect Science, 15(1), 155. https://doi.org/10.1093/jisesa/iev137
Omongoa, C. A., Namuddua, A., Okao-Okujaa, G., Alicai, T., Brunschot, S., Ouvrardc, D., & Colvin, J. (2018). Occurrence of Bondar’s nesting whitefly, Paraleyrodes bondari (Hemiptera: Aleyrodidae), on cassava in Uganda Christopher. Revista Brasileira de Entomologia, 62, 257–259.
Onah, I. E., Eyo, J. E., & Taylor, D. (2015). Graduate molecular identification of tephritid fruit flies (Diptera: Tephritidae) infesting sweet oranges in Nsukka Agro-ecological zone, Nigeria, based on PCR-RFLP of COI gene and DNA barcoding. African Entomology, 23(2), 342–347.
Pacheco da Silva, V. C., Bertin, A., Blin, A., Germain, J. F., Bernardi, D., Rignol, G., Botton, M., & Malausa, T. (2014). Molecular and morphological identification of mealybug species (Hemiptera: Pseudococcidae) in Brazilian vineyards. PLoS One, 9(7), 1–13.
Palma-Jiménez, M., & Blanco-Meneses, M. (2017). Morphological and molecular identification of Dysmicoccus brevipes (Hemiptera: Pseudococcidae) in Costa Rica. Journal of Entomology and Zoology Studies, 5(2), 1211–1218.
Park, D. S., Leem, Y. J., Hahn, K. W., Suh, S. J., Hong, K. J., & Oh, H. W. (2010). Molecular identification of mealybugs (Hemiptera: Pseudococcidae) found on Korean pears. Journal of Economic Entomology, 103(1), 25–33.
Pieterse, W., Muller, D. L., & Van Vuuren, B. J. (2010). A molecular identification approach for five species of mealybug (Hemiptera: Pseudococcidae) on citrus fruit exported from South Africa. African Entomology. Available at https://scholar.sun.ac.za/handle/10019.1/8604
Prathapan, K. D., Poorani, J., Amritha, K. S., Anuradha, C., Padmanaban, B., & Thanigairaj, R. (2019). Species composition and diagnoses of leaf- and fruit scarring beetles (coleoptera, Chrysomelidae) infesting bananas and plantains (Zingiberales, Musaceae) in the Indian subcontinent. Deutsche Entomologische Zeitschrift, 66(2), 179–202.
Przybylska, A., Fiedler, Ż., & Obrępalska-Stęplowska, A. (2016). PCR-RFLP method to distinguish Frankliniella occidentalis, Frankliniella intonsa, Frankliniella pallida and Frankliniella tenuicornis. Journal of Plant Protection Research, 56(1), 60–66.
Rahman, A. K. M. Z., Haque, M. A., Alam, S. N., Yasodha, P., & Balasubramani, V. (2014). Genetic diversity of fruit borer, Helicoverpa armigera (lepidoptera: Noctuidae) based on random amplified polymorphic DNA- polymerase chain reaction. Bangladesh Journal of Agricultural Research, 39(2), 263–271.
Rajasri, M., Vijaya Lakshmi, K., & Sujatha, M. (2016). Survey and biotype identification of whitefly, Bemisia tabaci transmitting tomato leaf curl virus in Andhra Pradesh, India. Journal of Agricultural Technology, 12(7.1), 1687–1694.
Rashmi, M. A., Verghese, A., Kamala Jayanthi, P. D., Chakravarthy, A. K., Kirubha, A., & Subhash, B. K. (2016). Molecular diversity of oriental fruit fly, Bactrocera dorsalis Hendel (Diptera:Tephritidae) in Karnataka. Pest Management in Horticultural Ecosystems, 22(1), 12–19.
Rattanawannee, A., & Chongrattanameteekul, W. (2016). Genetic variation of cassava mealybug, Phenacoccus manihoti (Hemiptera: Pseudococcidae), based on DNA sequences from mitochondrial and nuclear genes. Walailak J Sci & Tech., 13(2), 123–132.
Rebijith, K. B., Asokan, R., Krishna, K. N. K., Sreekumar, K. K., Ramamurthy, V. V., & Shivarama Bhat, P. (2012). DNA barcoding and development of species-specific markers for the identification of tea mosquito bugs (Miridae: Heteroptera) in India. Environmental Entomology, 41, 1239–1245.
Rebijith, K. B., Asokan, R., Krishna, K. N., & K.. (2011). Development of species- specific markers and molecular differences in mtDNA of Thrips palmi Karny and Scirtothrips dorsalis Hood (Thripidae: Thysanoptera), vectors of tospoviruses (Bunyaviridae) in India. Entomological News, 122, 201–213.
Rebijith, K. B., Asokan, R., Krishna, V., Krishna, K. N., & K and Ramamurthy V. V. (2012a). Development of species-specific markers and molecular differences of Aphis gossypii Glover and Myzus persicae (Sulzer), vectors of potyviruses (Potyviridae). Florida Entomologist, 95(3), 674–682.
Rebijith, K. B., Asokan, R., Krishna, V., Ranjitha, H. H., Krishna, K. N. K., & Ramamurthy, V. V. (2014). DNA barcoding and cryptic diversity in thrips (Thysanoptera). Florida Entomologist, 97, 1028–1047.
Rohith V. (2018). Development of molecular tests (Diagnostic PCR) for the identification of Lepidopteran Pests in Almonds and Pistachios. Available at https://www.semanticscholar.org/paper/Development-of-Molecular-Tests-(Diagnostic-PCR)-for-Vulchi/1b8afb1d743f861e5079ece99ccaf59a6b698db1.
Roopa, H. K., Krishna, K. N. K., Asokan, R., Rebijith, K. B., Mahmood, R., & Verghese, A. (2012). Phylogenetic analysis of Trialeurodes spp. (Hemiptera: Aleyrodidae) from India based on differences in mitochondrial and nuclear DNA. Florida Entomologist, 95, 1086–1094.
Roopa, K. H., Asokan, R., Rebijith, K. B., Ranjitha, H. H., Mahmood, R., & Krishna Kumar, N. K. (2015). Prevalence of a new genetic group, MEAM-K of the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae) in Karnataka, India, as evident from mtCOI sequences. Florida Entomologist, 98(4), 1062–1071.
Rugman-Jones, P. F., Morse, J. G., & Stouthamer, R. (2009). Rapid molecular identification of Armored scale insects (Hemiptera: Diaspididae) on Mexican ‘Hass’ avocado. Journal of Economic Entomology, 102(5), 1948–1953.
Rung, A., Scheffer, S. J., Evans, G., & Miller, D. (2008). Molecular identification of two closely related species of mealybugs of the genus Planococcus (Homoptera: Pseudococcidae). Annals of the Entomological Society of America, 101(3), 525–532.
Sabahi, S., Fekrat, L., & Zakiagh, M. (2017). A simple and rapid molecular method for simultaneous identification of four economically important thrips species. Journal Agricultural Science Techniques, 19(6), 279–1290.
Saccaggi, D. L., Kruger, K., & Pietersen, G. (2008). A multiplex PCR assay for the simultaneous identification of three mealybug species (Hemiptera: Pseudococcidae). Bulletin of Entomological Research, 98, 27–33.
Sanchez, J. L., & Caoili, B. L. (2016). Identification of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biotypes using the molecular marker, cytochrome c oxidase 1 (CO1) gene. AGRIS, 29(1), 136–156.
Sandeep, K. S., Sridhar, V., Sharma, A., & Asokan, R. (2017). Report on the occurrence of south American tomato moth, Tuta absoluta (Meyrick) in Punjab, India as evident from trap catches and molecular diagnosis. Pest Management in Horticultural Ecosystems, 23, 85–87.
Sarmah, M., & Bandyopadhyay, T. (2009). Colour variation and genetic diversity in tea mosquito bug [Helopeltis theivora (Hemiptera: Miridae)] population from Badlabeta tea estate, upper Assam, India. Journal of Entomology, 6, 155–160.
Savitha, V., Ramamoorthy, D., & Sudhakaran, M. (2019). Mitochondrial cytochrome oxidase (COI) sequence-based identification of pod borer of Thylacoptila sp. AA isolated from Senna alata (L.) Roxb. for the first time in India. Mitochondrial DNA Part A, 30(3), 407–413. https://doi.org/10.1080/24701394.2018.1532415
Serce, C. U., Kaydan, M. B., Kilincer, A. N., & Ertunce, F. (2007). Investigation of mealybug (Hemiptera: Coccoidea: Pseudicoccidae) species from Turkey by RAPD. Phytoparasitica, 35(3), 232–238.
Serrana, J. M., Ishitani, N., Carvajal, T. M., Almarinez, B., Barrion, A. T., Amalin, D. M., & Watanabe, K. (2019). Unravelling the genetic structure of the coconut scale insect Pest (Aspidiotus rigidus Reyne) outbreak populations in the Philippines. Insects, 10(11), 374. https://doi.org/10.3390/insects10110374
Sethusa, M. T., Millar, I. M., Yessoufou, K., Jacobs, A., van der Bank, M., & van der Bank, H. (2014). DNA barcode efficacy for the identification of economically important scale insects (Hemiptera: Coccoidea) in South Africa. African Entomology, 22(2), 257–266.
Shashank, P. R., Kammar, V., Mally, R., & Chakravarthy, A. K. (2018). A new Indian species of shoot and capsule borer of the genus Conogethes (lepidoptera: Crambidae), feeding on cardamom. Zootaxa, 4374, 2. Available at https://www.mapress.com/j/zt/article/view/zootaxa.4374.2.3
Shashank, P. R., Ojha, R., Venkatesan, T., Jalali, S. K., & Bhanu, K. R. M. (2015). Molecular characterization of brinjal shoot and fruit borer, Leucinodes orbonalis (Guenée) (lepidoptera: Crambidae) based on mitochondrial marker cytochrome oxidase I and their phylogenetic relationship. Indian Journal of Experimental Biology, 52, 51–55.
Singh, M. P. (2017). Morphological identification of cucurbitaceous fruit flies in Bihar and genetic diversity of Bactrocera cucurbitae. M.Sc.(Ag) thesis, Bihar Agricultural University, Sabour, 70p.
Sjölund M J, Ouvrard D, Kenyon D and Highet F. 2016. Developing AN RT-PCR assay for the identification of psyllid species. Proceedings Crop Protection in Northern Britain 2016. Available at https://www.researchgate.net/publication/303881533.
Subramanian, S., & Mohankumar, S. (2006). Genetic variability of the bollworm, Helicoverpa armigera, occurring on different host plants. Journal of Insect Science, 6, 26–34.
Suganthi, M., Chandrashekara, K. N., Arvinth, S., & Raj, K. R. (2016). Molecular characterization of tea mosquito bug from tea growing regions of India. Mitochondrial DNA Part A, 27(5), 3504–3506.
Suganthy, M., Rageshwari, S., Senthilraja, C., Nakkeeran, S., Malathi, V. G., Ramaraju, K., & P. Renukadevi K. (2017). New record of Western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) in South India. International Journal of Environment, Agriculture and Biotechnology, 1(4), 857–867.
Sukirno, S., Muhammad, T., Rasool, K. G., & Abdulrahman, S. A. (2018). Undescribed color polymorphism of the Asiatic palm weevil, Rhynchophorus vulneratus panzer (coleoptera: Curculionidae) in Indonesia: Biodiversity study based on COI gene. Florida entomologist, 101(4), 642–648.
Sunnucks, P., & Hales, D. F. (1996). Numerous transposed sequences of mitochondrial cytochrome oxidase I-II in aphids of the genus Sitobion (Hemiptera: Aphididae). Molecular Biology and Evolution, 13, 510–524.
Timm A.E., Stiller M. and Frey J.E. 2008.A Molecular identification key for economically important thrips species (Thysanoptera: THRIPIDAE) in Southern Africa. African Entomology 16(1):68–75.
Tóbiás, I., Kozár, F., & Kaydan, M. B. (2012). Molecular diagnosis of two closely related mealybug species (Hemiptera: Pseudococcidae). Acta Phytopathologica et Entomologica Hungarica, 47(1), 51–54.
Topakci, N., Yükselbaba, U., & Gocmen, H. (2017). Detection and identification of citrus long-horned beetle, Anoplophora chinensis (Forster, 1771) (coleoptera: Cerambycidae) a new pest in Antalya Province, Turkey by sequencing of mtCOI region1. Türkish Journal of Entomology, 41(3), 325–331.
Tyagi, K., Kumar, V., Singha, D., & Chakraborty, R. (2015). Morphological and DNA barcoding evidence for invasive pest thrips, Thrips parvispinus (Thripidae: Thysanoptera), newly recorded from India. Journal of Insect Science, 15(1), 1–4.
Tyagi, K., Kumar, V., Singha, S. D., Chandra, K., Amin, L. B., Shantanu, K., Rajasree, C., & Sumantika, C. (2017). DNA barcoding studies on thrips in India: Cryptic species and species complexes. Scientific Reports, 7, 4898. https://doi.org/10.1038/s41598-017-05112-7
Ueda, S., & Brown, J. K. (2006). First report of the Q biotype of Bemisia tabaci in Japan by mitochondrial cytochrome oxidase I sequence analysis. Phytoparasitica, 34, 405–411.
Ukey, N., Chandele, A., Khandare, S., & Chimote, V. (2017). Molecular identification of fruit flies, Bactrocera spp. (Diptera: Tephritidae) infesting guava fruits by using mitochondrial COI gene. Journal of Entomology and Zoology Studies, 5(2), 1503–1507.
Valenzuela, I., Hoffmann, A. H., Malipatil, M. B. R., Peter, M. R., & Weeks, A. R. (2007). Identification of aphid species (Hemiptera: Aphididae: Aphidinae) using a rapid polymerase chain reaction restriction fragment length polymorphism method based on the cytochrome oxidase subunit I gene. AGRIS, 46(4), 305–312.
Vikas, J., Geetika, B., & Singh, M. (2016). Molecular identification of mango hoppers infesting mango trees in Punjab through DNA barcoding. Indian Journal of Horticulture, 73(2), 192–196.
Wang, X. B., Zhang, J. T., Deng, J., Zhou, Q. S., Zhan, Y. Z., & Wu, S. A. (2016). DNA barcoding of mealybugs (Hemiptera: Coccoidea: Pseudococcidae) from mainland China. Annals of the Entomological Society of America, 109(3), 438–446.
Watanabe, S., & Melzer, M. J. (2017). A multiplex PCR assay for differentiating coconut rhinoceros beetle (coleoptera: Scarabaeidae) from oriental flower beetle (coleoptera: Scarabaeidae) in early life stages and excrement. Journal of Economic Entomology, 110(2), 678–682.
Wu, F., Liu, Z., Shen, H., Fei, Y. U., Jun Ma, H. X., & Zeng, L. (2014). Morphological and molecular identification of Paracoccus marginatus (Hemiptera: Pseudococcidae) in Yunnan, China. Florida Entomologist, 97(4), 1469–1473.
Zhang, D. X., & Hewitt, G. D. (1996). Nuclear integrations: Challenges for mitochondrial DNA markers. Trees, 11, 247–251.
Zhong-Yi, C., Qiong, Z., Peng, L. Y., Pin-Fa, S. I., & Yang, W. (2020). Molecular identification of citrus fruit flies and genetic diversity analysis of Bactrocera minax (Diptera: Tephritidae) populations in China based on mtDNA COI gene sequences. Acta Entomologica Sinica, 63(1), 85–96.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mani, M., Venkatesan, T., Chethan, B.R. (2022). Molecular Identification of Insect Pests of Horticultural Crops. In: Mani, M. (eds) Trends in Horticultural Entomology . Springer, Singapore. https://doi.org/10.1007/978-981-19-0343-4_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-0343-4_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0342-7
Online ISBN: 978-981-19-0343-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)