Abstract
Intensive use of agrochemicals has adverse effects on humans and environmental conditions. Increased consumer demands for safe food have triggered research on the development of safe and eco-friendly biopesticides. Fungi-based biocontrol agents help minimize disease(s) pressure, safer food and feeds, with minimum undesirable impact on human health and environmental conditions. The use of antagonistic endophytes and Trichoderma as biocontrol agents is recently drawing particular attention to managing some of the major plant diseases. Trichoderma spp. has been widely used against many plant pathogens. It produces different secondary metabolites and enzymes such as chitinase, proteases, and β-1,3-glucanase and helps induce plant growth defense, systemic resistance and competes against plant pathogens. Fungi, including species of Trichoderma, Gliocladium, Aspergillus, Fusarium, and Paecilomyces species, antagonize plant pathogens, mycoparasitic pathogens, and trigger systemic acquired resistance. Research related to genetic manipulation to enhance virulence-based biocontrol agents is increasing, but it is not as widely explored as in bacteria-based biocontrol agents. The combination of fungi-based biocontrol agents and biofertilizers contributes to sustainable agriculture, not necessarily with some challenges.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The recent challenge in the agricultural sector is to increase yield and decrease plant disease to a minimum level. Traditional methods such as fungicides, nematicides, herbicides, and fertilizer are among general methods in plant disease management. Although these mechanisms can control plant disease and suppress plant pathogens, they are not eco-friendly. Diseases caused by plant pathogens adversely affect global crop productivity and account for 20–40% yield losses annually in various agricultural and horticultural crops. In India, 57,000 metric tons of synthetic pesticides were used during 2016–2017 to control plant pathogens and insect pests, whereas biopesticide consumption was only 6340 metric tons. The development of resistance due to the continuous use of pesticides in modern farming and the increased availability of pesticide residues in vegetables, cereals, and grains has generated many problems. Moreover, the unregulated and indiscriminate use of chemical pesticides causes pollution of soil, water, and air and decreases the soil microflora and fauna. Beneficial rhizosphere microorganisms could be exploited to provide sustainable solutions in reducing the application of pesticides for agricultural crop production (Sehrawat and Sindhu 2019). Growing public concerns on the overuse of pesticides in agriculture and their effects on the environment have led to research on safe and eco-friendly options for managing pests and pathogens. Different approaches have been used for the mitigation of plant diseases. Beyond good agronomic and horticultural practices, producers often rely heavily on chemical fertilizers and pesticides. However, the environmental pollution caused by undesirable use and unintended misuse of agrochemicals and pesticides has culminated in considerable changes in people’s approaches toward pesticides in agriculture (Jyoti and Singh 2016). Today, there are strict regulations on chemical pesticides, and there is a radical move to remove the most hazardous chemicals from the market.
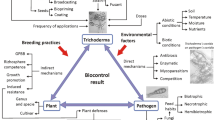
The terms "biological control" and its abbreviated synonym “biocontrol” have been used in different fields of biology, most notably in entomology and plant pathology. The term biocontrol can be defined as a reduction of inoculum density or disease-producing activity of a pathogen or a parasite in its active or dormant state by one or more organisms accomplished naturally or through manipulation of the environment of host or antagonist by mass introduction of one or more antagonists (Baker and Cook 1974). Biological control is a highly supportive approach for disease management, and it is precious to make an eco-friendly environment. Biological control plays a vital role in managing plant disease without disturbing flora and fauna and increasing soil fertility. There is an increasing trend for the exploitation of fungi to control plant diseases, and the most exploited are Trichoderma, Gliocladium, and Paecilomyces. Fungal biological control is an exciting and rapidly developing research area with implications in plant productivity. Hence, this chapter aims to review the method of activity of fungal biocontrol agents with particular reference to most potential bioagent Trichoderma and Paecilomyces for the control of plant diseases.
2 Fungal Based Biocontrol Agents, Formulations, Mechanisms with Some Success Stories
2.1 Trichoderma spp.
Trichoderma spp. are free-living fungi that are common in soil and root ecosystems. Trichoderma has long been recognized as biocontrol agents for controlling plant diseases and enhancing root growth and development, crop productivity, resistance to abiotic stresses, uptake, and use of nutrients. Many successful products based on different species of Trichoderma have been commercialized in India (Kumar et al. 2014).
The first description of the genus Trichoderma was made by Persoon (1794). Tulasne and Tulasne (1860) suggested the sexual state of a Hypocrea species in 1865. Cook and Baker (1983) described genus Trichoderma as a common soil inhabitant, and the conidiophores are terminating in phialides. This fungus acts as a potential biocontrol agent because it stimulates plant resistance, plant growth, and development, resulting in increased crop production and antagonistic activity, viz. mycoparasitism, antibiotics, competition for nutrients, and also induces systemic resistance in plants (Harman et al. 2004).
The different species of the genus Trichoderma/Hypocrea were challenging to be distinguished morphologically. It was even proposed to reduce taxonomy to only a single species, T. viride (Schuster and Schmoll 2010). Nowadays, new species can be easily identified due to the development of TrichOKEY, where an oligonucleotide barcode and TrichoBLAST became a search tool (Druzhinina and Kubicek 2005; Kopchinskiy et al. 2005). Recently, according to Kamala et al. (2015) Trichoderma species belongs to the division Ascomycota, subdivision Pezizomycotina, class Sordariomycetes, subclass Hypocreomycetidae, order Hypocreales, and family Hypocreaceae. Chaverri et al. (2015) have reidentified the ubiquitous Trichoderma harzianum into 14 new species with various characteristics.
2.1.1 Mechanism of Trichoderma spp. Against Plant Pathogens
Trichoderma spp. are ubiquitous soil fungi. By their ability to decompose organic matter, they are free-living in the soil as Saprophytes. However, these species can also live on other fungi and colonize plant roots in the rhizosphere. Trichoderma spp. produce a range of hydrolytic enzymes that make them useful in industry (Mach and Zeilinger 2003). These fungi can parasitize some of the major plant pathogenic fungi that make them useful as biofungicides (Hjeljord and Tronsmo 1988; Mukhopadhyay et al. 1992; Mukhopadhyay and Mukherjee 1996; Chet et al. 1998; Harman and Bjorkmann 1998). Trichoderma spp. produce various kinds of secondary metabolites in abundance, including antibacterial and antifungal antibiotics (Zeilinger et al. 2005). Some of the species/strains of Trichoderma spp. are plant growth promoters and inducers of SAR in plants (Harman et al. 2004). Mechanisms of Trichoderma spp. based biocontrol agents are mycoparasitism, antagonism, antibiosis, and competition for nutrients or space, among others which may operate independently or together to suppress plant pathogens (Harman et al. 2004).
2.1.2 Mycoparasitism
Haran et al. (1996) proposed the mycoparasitic activity (hyperparasitism) of Trichoderma spp. as one of the major mechanisms. Mycoparasitism implies the direct strike of one fungal species on another and is among the most critical antagonistic mechanisms expressed by Trichoderma spp. Firstly, the identification between Trichoderma and the target fungus is mediated by the binding of carbohydrates present in the cell wall of Trichoderma to the lectins of the other one. This is followed by hyphal twirling and appressoria development, which encompasses many osmotic compounds like glycerol. After successful penetration, Trichoderma attacks the host’s cellular machinery by generating numerous fungitoxic cell wall degrading enzymes (CWDEs), such as glucanases, chitinases, and proteases (Harman et al. 2004). The cumulative action of these compounds causes the dissolution of the host cell walls, which ultimately results in parasitism of the target fungus. It has been observed that gaps can be generated at the location of appressoria formation, which facilitates the direct access of Trichoderma hyphae into the lumen of the target fungus, which then proceeds to kill the pathogenic fungus (Kumar 2013). Furthermore, biocontrol agents degrade the cell wall of the target fungus and inactivate its enzymes (e.g., pectinases, etc.), which are essential for pathogenic fungus to colonize and penetrate the plant tissues (Harman et al. 2004). As we know, fungal cell walls are mainly composed of chitin and 1,3-glucan. Chitinases and 1,3-glucanases lytic enzymes synthesized by Trichoderma spp. are supposed to be responsible for their mycoparasitic actions leading to the degradation of phytopathogenic fungal cell walls (De La Cruz et al. 1992; Geremia et al. 1993). In addition, other CWDEs, including those hydrolyzing minor polymers (like proteins, 1,6-glucans, 1,3-glucans, etc.), further ensure the complete and adequate disintegration of fungal mycelial or conidial walls by Trichoderma spp. (Geremia et al. 1993). A chitin-induced subtilisin-type serine proteinase was previously depicted in a Trichoderma harzianum mycoparasitic strain (De La Cruz et al. 1992).
The first Trichoderma secretome analyzed under confrontation with R. solani was that of T. harzianum EST 323. Two-dimensional gels (2-DE) and liquid chromatography mass spectrometry (LC-MS/MS) analysis identified seven CWDEs, including a chitinase, a cellulase, xylanase, a β-1,3-glucanase, a β-1,6-glucanase, a mannanase, and a protease. Enzyme activity staining showed that the xylanase, β-1,3-glucanase, β-1,6-glucanase activities only occurred in media containing R. solani. Eight out of 43 spots on the 2-DE were identified by LC-MS/MS. These proteins included two proteases, two β-glucosidases, two glycoside hydrolases, one endochitinase, and one amino acid oxidase (Tseng et al. 2008). Proteomic analysis to determine the changes on the secretome of T. harzianum in response to glucose, a mixture of glucose and deactivated Botrytis cinerea mycelia, deactivated B. cinerea mycelia, or deactivated T. harzianum mycelia was performed. Ninety-one out of 100 excised proteins were analyzed, and some of them were sequence identified. Two endochitinases and one l-amino acid oxidase (LAAO) were exclusively induced in media containing deactivated B. cinerea mycelia as the sole carbon source. Media containing deactivated B. cinerea mycelia showed high CWDE activities, including chitinase, cellulase, xylanase, β-1,3-glucanase, β-1,6- glucanase, and protease, than in other media. These results confirm the critical role of CWDE in the antagonism by Trichoderma and indicate that cell walls are the primary target of Trichoderma during mycoparasitism (Tseng et al. 2008; Yang et al. 2009).
2.1.3 Antibiosis
Antibiosis is one of the critical attributes in deciding the saprophytic ability of the fungus. A range of antibiotics produced by species of Trichoderma and Gliocladium, which has been suggested as a mode of action of both fungi against plant pathogens, was reported by Weindling (1934). Manibhusanrao et al. (1989) reported that antibiotics like trichodermin, suzukacillin, and alamethicin produced by T. harzianum influence morphological or physiological sequences leading to its successful penetration. Trichoderma spp. and Gliocladium spp. inhibited the growth of a broad range of soilborne fungi, viz. genera of Fusarium, Macrophomina, Pythium, Phytophthora, Rhizoctonia, Sclerotinia, Sclerotium, and Verticillium (Zaher et al. 2013; Ragab et al. 2015; Chen et al. 2016). Do Nascimento Silva et al. (1998) demonstrated the in vitro antagonistic potential of 3 Trichoderma spp. against Colletotrichum gloeosporioides on passion fruit. Bhagat and Pan (2010) screened 12 isolates of Trichoderma spp. in vitro against R. solani Kuhn. causing root and collar rot of French bean (Phaseolus vulgaris L.) by dual culture tests and production of volatile and non-volatile antibiotics, and it was found that all the isolates significantly inhibited the mycelial growth of R. solani.
It is well known that Trichoderma spp. produce a plethora of nonpolar compounds of low molecular weight cataloged as secondary metabolites, including pyrones, terpenoids, steroids, and polyketides. Trichoderma spp. also produces siderophores and many peptaibiotics known as peptaibols, which contain high-frequency nonstandard amino acids (Degenkolb et al. 2006), which play an important role in signaling process development.
2.1.4 Competition
Bioagents compete for nutrients and space with pathogens, and thus, it is the injurious effect of one microorganism on another due to the utilization or removal of some resources from the environment. Competition between iron-containing siderophore of Trichoderma and wood decay Basidiomycete’s fungi was investigated (Srinivasan et al. 1995).
Starvation is the most common cause of death for microorganisms, so that competition for limiting nutrients results in biological control of fungal phytopathogens (Chet et al. 1997). For instance, in most filamentous fungi, iron uptake is essential for viability. Under iron starvation, most fungi excrete low molecular weight ferric iron specific chelators termed siderophores to mobilize environmental iron (Eisendle et al. 2004). Subsequently, iron from the ferrisiderophore complexes is recovered via specific uptake mechanisms. In Aspergillus fumigatus and Aspergillus nidulans, the carbon source negatively regulates siderophore biosynthesis (Eisendle et al. 2004). In Ustilago maydis, gene products related to iron uptake affect plant disease development (Mcintyre et al. 2004). Some Trichoderma BCAs produce highly efficient siderophores that chelate iron and stop the growth of other fungi (Chet and Inbar 1994).
For this reason, soil composition influences the biocontrol effectiveness of Pythium by Trichoderma according to iron availability. In addition, T. harzianum T35 controls Fusarium oxysporum by competing for rhizosphere colonization and nutrients, with biocontrol becoming more effective as the nutrient concentration decreases (Tjamos et al. 1992). Competition has proved to be particularly important for the biocontrol of phytopathogens such as Botrytis cinerea, the primary pathogenic agent during the pre-and post-harvest in many countries (Latorre et al. 2001). The extraordinary genetic variability of this fungus makes it possible for new strains to become resistant to essentially any novel chemical fungicide it is exposed to (Latorre et al. 2001). The advantage of using Trichoderma to control B. cinerea is the coordination of several mechanisms simultaneously, thus making it practically impossible for resistant strains to appear. Among these mechanisms, the most important is nutrient competition since B. cinerea is particularly sensitive to the lack of nutrients. Trichoderma has a superior capacity to mobilize and take up soil nutrients compared to other organisms. The efficient use of available nutrients is based on the ability of Trichoderma to obtain ATP from the metabolism of different sugars such as those derived from polymers widespread in fungal environments: cellulose, glucan, and chitin, among others, all of them rendering glucose (Chet et al. 1997). The key components of glucose metabolism include assimilation enzymes and permeases and proteins involved in membrane and cell wall modifications. While the role of the glucose transport system remains to be discovered, its efficiency may be crucial in competition (Delgado-Jarana et al. 2003) as supported by the isolation of a high-affinity glucose transporter Gtt1, in Trichoderma harzianum CECT 2413. This strain is present in environments very poor in nutrients, and it relies on extracellular hydrolases for survival. Gtt1 is only expressed at very low glucose concentrations, i.e., when sugar transport is expected to be limiting in nutrient competition transformant derivative that carried an additional copy of the transporter gene. Only two other genes encoding glucose transporters have been described in filamentous fungi (Franken et al. 2002), one of them in Uromyces fabae. This basidiomycete has an ATPase and a proton-coupled glucose transport system that is expressed during the infection of Vicia faba. This suggests an additional, antagonistic role for Gtt1, allowing the fungus to obtain energy from hydrolyzed polymers and rapidly transport sugar into the cells. Consequently, transformants able to transport glucose more rapidly than the wild-type (Delgado-Jarana et al. 2003) should be more efficient BCAs. This would serve as an advantageous mechanism of nutrient competition during mycoparasitism interactions.
2.1.5 Molecular Mechanisms of Biocontrol by Trichoderma spp.
Trichoderma spp. produce several types of chitinases, both endo, and exo that exhibit mucolytic activities. Many new chitinases are being discovered, and a genome-wide search would reveal more. The hydrolytic enzymes are generally induced by specific substrates (like chitin, glucan, fungal cell wall) and repressed by glucose. Lorito et al. (1996) observed that mycoparasitic interaction relieves the Cre1 catabolite repressor protein's binding to promoter sequences of the ech42 gene in T. harzianum. Expression of ech42 gene of T. atroviride under carbon starvation is antagonized via a BrlA-like cis-acting element (Brunner et al. 2003). The regulation of expression of two major chitinase genes (ech42 and nag1, encoding CHIT73) of T. atroviride is triggered by different regulatory signals (Mach et al. 1999). The direct evidence on the role of hydrolytic enzymes in biocontrol came from the gene knockout studies, where a gene is selectively deleted through homologous recombination or inactivated by antisense/RNAi. Disruption of ech42 in T. harzianum resulted in almost no endochitinase42 activity, whereas strains carrying multicopy of this gene exhibited up to a 42-fold increase in enzyme activity (Carsolio et al. 1999). Woo et al. (1999) disrupted ech42 in T. harzianum P1 (T. atroviride) and showed reduced biocontrol activity against B. cinerea on bean leaves. However, interestingly, the biocontrol activities of the disruptants were enhanced against R. solani and remained unaltered against P. ultimum. The observation that Trichoderma spp. colonize plant roots and induce systemic resistance against a wide range of fungal, bacterial, and viral pathogens can be considered a breakthrough in biocontrol research (Harman et al. 2004). Inoculation of roots of cucumber seedlings with conidia of T. harzianum T-203 (T. asperellum) in an aseptic hydroponic system resulted in induction of defense responses (Yedidia et al. 2003). Electron microscopy of ultrathin sections from Trichoderma treated roots revealed penetration of the mycoparasite into the roots, restricted mainly to the epidermis and outer cortex. Trichoderma colonization strengthened the epidermal and cortical cell walls and deposition of newly formed barriers, these typical host reactions being found even beyond the sites of potential fungal penetration. The inoculation of Trichoderma initiated increased peroxidase and chitinase activities, both in roots and leaves. Later on, the authors showed that inoculation of cucumber roots with Trichoderma induced an array of PR proteins (Yedidia et al. 2000). Inoculation of cucumber roots with T. asperellum reduced the inoculum load of Pseudomonas syringe pv lachrymans up to 80% when inoculated on leaves (Yedidia et al. 2003), thus providing direct evidence on induced defense-mediated protection of crop plants in response to Trichoderma inoculation. The protection afforded by the biocontrol agent was associated with the accumulation of mRNA of two defense-related genes: the phenylpropanoid pathway gene encoding phenylalanine ammonia-lyase (PAL) and the lipooxygenase pathway gene encoding hydroxyperoxidase lyase (HPL). Recently using the gene knockout approach, a hydrophobin TasHyd1 has been demonstrated in root colonization by T. asperellum (Viterbo and Chet 2006). In a significant finding, Shoresh et al. (2006) identified a MAPK (TIPK—Trichoderma induced MAPK) in cucumber, antisense-mediated silencing of this gene made plants susceptible even after inoculation of roots with T. asperellum. It was thus proved that Trichoderma exerts its positive effects on plants through the activation of a MAPK gene involved in signaling the pathway of defense response A definite role of phytoalexin induction in biocontrol has recently been demonstrated by Howell and Puckhaber (2005), who showed that the “P” strains of T. virens failed to stimulate phytoalexin synthesis in cotton and were ineffective as biocontrol, while the “Q” strains that stimulated phytoalexin biosynthesis were effective. This difference was attributed to the ability of “Q” strains to produce the 18-kDa elicitor protein. Recently, three groups independently identified a homolog of SnodProt proteins, variously named as SnodProt1 (GV Sible and PK Mukherjee, unpublished; GenBank Acc. no. DQ494198), (Djonovic et al. 2006) from T. virens, and Epl1 (Seidl et al. 2006) from T. atroviride. Olson and Benson (2007) have studied three root-colonizing fungi, binucleate Rhizoctonia (BNR) isolates BNR621 and P9023 and T. hamatum isolate 382 (T382), for suppression of Botrytis blight in geraniums by induction of host systemic resistance. Resistance to Botrytis blight was observed in geraniums transplanted into potting mix amended with formulations of P9023 and T382 2 weeks before inoculation with B. cinerea when grown under environments either highly or less conducive to disease development. Restriction of lesion development may play a role in the suppression of Botrytis blight in geraniums. This may be the first to demonstrate induced systemic resistance by BNR fungi to a foliar pathogen and support additional research into the use of T382 in an integrated management program for B. cinerea (Olson and Benson 2007). Research on the specific effects of induced systemic resistance should be continued with additional pathogens since there is some indication of pathogen specificity in the suppression method.
Mukherjee et al. (2004) studied the role of the G-proteins TgaA and TgaB in T. virens. Deleting these genes individually did not affect the hyphal coiling of R. solani, but TgaA was involved in the parasitism of sclerotia of S. rolfsii. Deletion of the MAPK TmkA in T. virens resulted in attenuation of sclerotial parasitism of S. rolfsii and R. solani. In contrast, the hyphal parasitism was unaltered. Reithner et al. (2007) had examined the function of the tmk1 gene encoding a MAPK during fungal growth, mycoparasitic interaction, and biocontrol was examined in T. atroviride. Dtmk1 mutants exhibited altered radial growth and conidiation and displayed de-regulated infection structure formation without a host-derived signal. In confrontation assays, tmk1 deletion caused reduced mycoparasitic activity, although attachment to R. solani and B. cinerea hyphae was comparable to the parental strain. Under chitinase-inducing conditions, nag1 and ech42 transcript levels and extracellular chitinase activities were elevated in a Dtmk1 mutant. In contrast, upon direct confrontation with R. solani or B. cinerea, a host-specific regulation of ech42 transcription was found, and nag1 gene transcription was no more inducible over an elevated basal level. These findings strongly suggest the presence of further, still unknown, mycoparasitism related factors that are missing in our Dtmk1 mutants and therefore affected by a signaling pathway involving Tmk1.
2.1.6 Trichoderma: Formulations and Application Methods
The success of biological control of plant pathogens using Trichoderma spp. does not rely solely on effective antagonists but also the methods of applications such as seed treatments (solid/liquid coating), in-furrow soil application, and or foliar applications. The following are examples of effective modes of delivery and application of Trichoderma.
2.1.7 Success Stories of Seed Treatments from the Indian Agricultural Perspective
Seed treatments or seed coating is one of the most effective methods of application of Trichoderma spp. in the agricultural system (Mathre et al. 1999). Trichoderma spp. is delivered in the infection court (surface of seed coat) as a protectant at planting. This method should limit the growth of competitive microflora and provide conducive growth for the biocontrol agent (Cumagun 2014). Seed treatments using seed dressing formulations, Pusa 5SD has been proven more effective than soil application formulations, Pusa Biogranule 6 (PBG 6) and Pusa Biopellet 16G (PBP 16G) in managing wet root rot of mung bean caused by R. solani (Dubey et al. 2011).
Seed coating with Trichoderma spp. is one of the easy and effective methods of delivering the antagonist to manage seed/soilborne diseases. Seed is coated with dry powder/dust of Trichoderma just before sowing. For commercial purposes, dry powder of antagonist is used at 3–10 g/kg seed based on seed size (Mukhopadhyay et al. 1992). Propagules of biocontrol agents germinate on the seed surface and colonize the roots of germinated seedlings and rhizosphere (Tiwari 1996; Kumar et al. 2009). T. harzianum, T. virens, and T. viride are effective seed protectants against Pythium spp. and R. solani (Mukherjee and Mukhopadhyay 1995). The rice seed treated with two antagonistic fungi, viz. T. viride, and T. harzianum, were found effective in controlling the sheath blight of rice and increasing the crop’s yield (Das and Hazarika 2000). In another study, T. viride was an efficient agent to control the R. solani toxin activity against the same disease (Sriram et al. 2000). Singh and Maheshwari (2001) reported that seed treatment with bioagents like T. viride, T. harzianum, and G. virens was found helpful in combating the loose smut of wheat. Trichoderma, being a growth-promoting agent, also helps increase crop yield, which has been demonstrated by the application of T. harzianum (Th3) in irrigated and dry areas of Kota and Jaipur districts Rajasthan, which is also ecologically competent (Sharma et al. 2012). Seed treatment with T. harzianum, A. sativum, and A. indica on par with the foliar spray of mancozeb showed results against Alternaria blight disease of mustard caused by A. brassicae and A. brassicicola and increasing the yield (Jagana et al. 2013). Seed treatment with Trichoderma species inhibited the growth of oilseed-borne fungi like Aspergillus flavus, Alternaria alternata, Curvularia lunata, Fusarium moniliforme, F. oxysporum, Rhizopus nigricans, Penicillium notatum, and Penicillium chrysogenum, which affects oilseed crops like soybean, sesame, and sunflower (Jat and Agalave 2013). More examples are listed below in Table 22.1.
2.1.8 Seed Biopriming
Seed priming is when the seeds are hydrated to allow the metabolic process of germination to take place but not sprouting. Priming enhances the biocontrol of Trichoderma by regulating water levels in the seed. It allows effective colonization of seed surface before planting, given the right pH and matrix material (Harman and Bjorkmann 1998). Seed priming of three rhizosphere isolates of T. harzianum enhanced growth and induced resistance in sunflower against downy mildew caused by Plasmopara halstedii (Nagaraju et al. 2012).
Treating seeds with biocontrol agents and then incubating under warm and moist conditions until before the emergence of radical is referred to as biopriming. This technique has potential advantages over the simple coating of seeds as it results in rapid and uniform seedling emergence. Trichoderma conidia germinate on the seed surface and form a layer around bioprimed seeds. Such seeds tolerate adverse various soil conditions better. Biopriming could also reduce the amount of biocontrol agents that are applied to the seed. Seed biopriming is successfully used in tomato, brinjal, soybean, and chickpea in the Tarai region of Uttaranchal (Mishra et al. 2001). Three rhizosphere competent microbial strains, viz., P. fluorescens OKC, Trichoderma asperellum T42, and Rhizobium sp. RH4, individually and in combination in bioprimed seeds of chickpea and rajma in pots and fields, showed higher germination percentage and better plant growth in both the crops than non-bioprimed control plants. It was also observed that the combined application of the microbes enhanced seed germination and plant growth better than their application. Among the combinations, all combinations comprising Trichoderma showed better results than the others. The triple microbial combination demonstrated the best results in seed germination and seedling growth in both chickpea and rajma (Yadav et al. 2013).
2.1.9 Liquid Coating
Liquid coating is a seed coating system that involves the application of Trichoderma to the seed with an aqueous adhesive or binder (pelgel or polyox-N-10) and a particulate material (Agro-lig or muck soil) to optimize pH level, including a bulking agent (Taylor et al. 1991). The Agro-lig is reported to have physical and chemical characteristics which favor the growth of the fungus.
2.1.10 Soil Application
Soil is the home for both beneficial and pathogenic microbes. Delivering Trichoderma spp. to the soil will increase the population dynamics of augmented fungal antagonists and thereby suppress the establishment of pathogenic microbes onto the infection court. There are several reports on applying biocontrol agents to the soil either before or at the time of planting for control of a wide range of soilborne fungal pathogens (Baby and Manibhushanrao 1996; Kumar et al. 2009; Kumar 2010). Soil application of T. viride either alone or in combination with other treatments significantly reduced red rot caused by C. falcatum (Reddy et al. 2009). Srivastava et al. (2010) suggested that the soil application of T. viride was best in controlling seedling blight, color rot, stem rot, and root rot disease of Jute. Soil application of organic preparation of Trichoderma was effective in managing seed-borne pathogenic fungi F. oxysporum, F. moniliforme, F. solani, B. theobromae, A. alternata, and R. solani and in the seedling establishment of Dalbergia sissoo Roxb (Mustafa et al. 2009). Trichoderma can colonize farmyard manure (FYM), and therefore the application of colonized FYM to the soil is more appropriate and beneficial. This is the most effective method of application of Trichoderma, particularly for the management of soilborne diseases. Soil application of T. asperellum strain GDFS1009 granules produces beneficial effects on maize growth and resistance to stalk rot caused by F. graminearum (He et al. 2019).
2.1.11 Root Treatment
Seedling roots can be treated with spore or cell suspension of antagonists either by drenching the Trichoderma in nursery beds or by dipping roots in Trichoderma suspension before transplanting. Root dipping in antagonist’s suspension not only reduces disease severity but also enhances seedling growth in rice, tomato, brinjal, chili, and capsicum (Singh and Zaidi 2002). There are also reports on the reduction of sheath blight disease of rice by root dip of seedlings before transplantation (Vasudevan et al. 2002).
2.1.12 Foliar Spraying/Wound Dressing
The efficacy of biocontrol agents for foliar diseases is greatly affected by the fluctuation of microclimate. Phyllosphere is subjected to diurnal and nocturnal, cyclic and non-cyclic variation in temperature, relative humidity, dew, rain, wind, and radiation. Hence, the water potential of phylloplane microbes will constantly be varying. It will also vary between leaves or the periphery of the canopy and on sheltered leaves. Higher relative humidity could be observed in the shaded, dense region of the plant than that of peripheral leaves. The dew formation is greater in the center and periphery. The concentration of nutrients like amino acids, organic acids, and sugars exuded through stomata, lenticels, hydathodes, and wounds varies highly. It affects the efficacy and survival of antagonists in phylloplane (Andrews 1992). The liquid suspension of Trichoderma has been successfully applied to the aerial plant parts for the biocontrol of Alternaria leaf spot of Vicia faba (Kumar et al. 2002). Khan and Sinha (2005, 2007) emphasized the usefulness of T. harzianum and T. virens in foliar sprays and talc-based formulations to reduce disease incidence of sheath blight of rice. Sharma et al. (2012) carried out field trials in Rajasthan on the groundnut root rot disease caused by multiple pathogen complex mainly A. niger, A. flavus, S. rolfsii, Thielaviopsis basicola, R. solani, and P. aphanidermatum by the application of T. harzianum in the form of powder and liquid bioformulation found effective in controlling disease in the field. Singh et al. (2000) managed citrus scab caused by Elsinoe fawcettii. They found that T. harzianum and E. purpurascens reduced the disease incidence in the field on spraying by 17.8 and 10%, respectively. Though the foliar application of Trichoderma reduces the severity of diseases under field conditions, it is not technically feasible due to increased dosage and economy realized from the crop. Hence, dosage and frequency of application have to be standardized based on the crop value, which could be a reliable and practical approach.
2.1.13 Types of Formulations
Significant research on biocontrol is centered on the use of spores of Trichoderma directly to seed. Technologies become viable only when the research findings are transferred from the lab to the field. Though Trichoderma has excellent potential in managing diseases, it could not be used as spore suspension under field conditions. Thus, the culture of Trichoderma should be immobilized in certain carriers and should be prepared as formulations for easy application, storage, commercialization, and field use.
For the successful development of a formulation of biocontrol agents, it is important not only to provide a substrate that will promote the synthesis of the desired enzymes, which help in its biocontrol mechanisms but also to provide sufficient substrate so as not to limit the synthesis of the enzymes at the time they are required. These include the utilization of a large number of agro-wastes as a substrate for the mass production of Trichoderma, the use of a wide variety of solid substrates with less expenditure, and higher reproducibility (Kulkarni and Shalini 2007). Two types of formulation used widely are either liquid or solid formulations (Table 22.2).
A deep tank fermentation system is employed in a liquid formulation, making it a preferred approach for biomass production in Europe and North America (Churchill 1982). Inexpensive growth media such as molasses and brewer’s yeast are used for production in liquid formulation (Papavizas et al. 1984). The advantage of this formulation is the optimization of biomass production and quality which allows control of nutrients, pH, temperature, and other environmental factors, thus reducing contamination (Whipps 1997).
2.1.13.1 Solid Formulation
Solid formulation or fermentation is the alternative method for inoculum production. Agricultural waste materials such as wheat and rice straw, sugarcane bagasse, ground corn cobs, sawdust, rice bran are used as food base or substrate alone or in combination for the growth of Trichoderma. Cumagun and Lapis (1993) used rice bran as a food base for Trichoderma spp. with tapioca flour as a binding agent to produce pellets. Provision of food base in the formulation should, in most cases, favor the antagonist (Papavizas et al. 1984) or a food base that can only be utilized by the antagonist in which the pathogen can be inhibited (Nelson et al. 1988). This method of formulation requires only minimal cost, especially in small-scale production. However, it is bulky as it requires considerable space for production, inoculation, and storage, including drying and milling. Solid and liquid formulations require drying to obtain stable products with prolonged shelf life (Jin et al. 1992). Spray drying is preferred among the different drying techniques for large-scale production of microorganisms containing dried powders due to its low cost (Morgan et al. 2006). Various solid formulations methods are listed in Table 22.3.
2.1.13.1.1 Talc-Based Formulation
In India, talc-based formulations of T. viride were developed at Tamil Nadu Agricultural University, Coimbatore, for seed treatment of pulse crops and rice (Jeyarajan et al. 1994). Trichoderma is grown in the liquid medium, mixed with talc powder in the ratio of 1:2 and dried to 8% moisture under shade. The talc formulations of Trichoderma have a shelf life of 3–4 months. It has become quite popular in India to manage several soilborne diseases of various crops through seed treatment at 4–5 g/kg seed. Several private industries produce large quantities of talc formulations in India for supply to the farmers. The annual requirement of Trichoderma has been estimated as 5000 tons to cover 50% area in India (Jeyarajan 2006).
2.1.13.1.2 Vermiculite-Wheat Bran-Based Formulation
Trichoderma is multiplied in a molasses-yeast medium for 10 days. 100 g vermiculite and 33 g wheat bran are sterilized in an oven at 70 °C for 3 days. Then, 20 g of fermented biomass, 0.05 N medium, and concentrated or entire biomass with HCl are added, mixed well, and dried in the shade (Lewis 1991).
2.1.13.1.3 Pesta Granules-Based Formulation
Fermenter biomass (52 ml) is added to wheat flour (100 g) and mixed by gloved hands to form a cohesive dough. The dough is kneaded, pressed, and folded by hand several times. Then one mm thick sheets (pesta) are prepared and air-dried till it breaks crisply. After drying, the dough sheet was ground and passed through a mesh and granules were collected (Connick et al. 1991).
2.1.13.1.4 Alginate Prills Based Formulation
Sodium alginate is dissolved in one portion, and distilled water (25 g/750 ml) and food base are suspended in another portion (50 g/250 ml). These preparations are autoclaved and, when cool, is blended with biomass. The mixture is added dropwise into CaCl2 solution to form spherical beads, which are air-dried and stored at 5°C (Fravel et al. 1999).
2.1.13.1.5 Press Mud-Based Formulation
Press mud is available as a byproduct of the sugar factory, and this can be used as a substrate for mass multiplication of Trichoderma. The method involved uniformly mixing of 9 days old culture of T. viride prepared in potato dextrose broth into 120 kg press mud. Water was sprinkled intermittently to keep it moist. Gunny bags covered this to permit air movement and trap moisture under shade. Within 25 days, nucleus culture for further multiplication becomes ready. The same was added to 8 tons of press mud, mixed thoroughly, and incubated for eight days under shade conditions before being applied in the field. By this, we added 8000 times more inoculums in the soil than the recommended doses of biopesticides, which rapidly get established, showing rapid and visible effect. Similarly, other substances could also be effectively used for the multiplication of different bioagents at the mass level (Sabalpara 2014).
2.1.13.1.6 Coffee Husk-Based Formulation
In Karnataka, India, Sawant and Sawant (1996) developed a Trichoderma formulation based on the coffee husk, waste from the coffee curing industry. This product effectively managed Phytophthora foot rot of black pepper and is widely used in Karnataka and Kerala.
2.1.13.1.7 Banana Waste-Based Formulations
The mass multiplication protocol of Trichoderma sp. in banana waste was proposed by Balasubramanian et al. (2008). For the same banana waste, urea, rock phosphate, culture of Bacillus polymyxa, P. sajor-caju, and T. viride are used. A pit of other banana waste, viz. sheath pseudostem and core, is chopped in the length of 5–8 cm. A pit is prepared, and different ingredients are placed in five different layers. Each layer contains one tone banana waste, 5 kg urea, 125 kg rock phosphate, and one-liter broth culture of B. polymixa, P. sajor-caju, and T. viride. Five different layers are prepared similarly and mixed thoroughly in Banana. Banana waste is decomposed within 45 days, and enriched culture is mass available for field application.
2.1.13.2 Liquid Formulation
2.1.13.2.1 Oil-Based Formulations
They were prepared by mixing the conidia harvested from the solid-state/liquid state fermentation with vegetable/mineral oils in a stable emulsion formulation. In such formulations, microbial agents are suspended in a water-immiscible solvent such as a petroleum fraction (diesel, mineral oils) and vegetable oils (groundnut, etc.) with the aid of a surface-active agent. This can be dispersed in water to form a stable emulsion. Emulsifiable concentrates require a high concentration of an oil-soluble emulsifying agent to rapidly form a homogenous emulsion on dilution in water. The oils used should not have toxicity to the fungal spores, plants, humans, and animals. Such formulations of Trichoderma are now being used as foliar sprays. Oil-based formulations are suitable for foliar sprays under dry weather and have a prolonged shelf life. The spores can survive longer on the plant surface, even during the dry weather, as the spores are covered by oil that protects them 5°C from drying. Batta (2005) developed an emulsion formulation of T. harzianum to control post-harvest decay of apple caused by Botrytis cinerea. Invert-emulsion formulation of T. harzianum with a shelf life of 8 months has been developed using indigenous constituents at the erstwhile Project Directorate of Biological Control (PDBC) in India. This formulation has been found to be effective against soilborne diseases of groundnut.
2.1.13.2.2 Adjuvants, Spreaders, and Stickers
Performance of Trichoderma in the formulations can be increased by incorporating water-soluble adjuvants, oils, stickers, and emulsions. It increases the efficacy of biocontrol agents by supplying nutrients and protecting the microbes from desiccation and death (Connick et al. 1991; Bateman et al. 1993; Barnes and Moore 1997; Green et al. 1998; Ibrahim et al. 1999). The incorporation of carboxymethyl cellulose (CMC) in formulations serves as stickers in the uniform seed coating of microbes. Though adjuvants and stickers increase the efficacy of bio-products, it has its demerits. Adjuvants/stickers in the formulations will be diluted when exposed to rain or heavy dew. It would alter the efficacy of formulations by reducing the establishment or colonization of Trichoderma onto the infection court. Sometimes spray application of emulsions or oil-based formulations may be toxic to plants.
Consequently, comprehensive knowledge on the usage of adjuvants, stickers is crucial for increasing the efficacy of formulations. Some commercial products of Trichoderma spp. available in India are listed in Table 22.1.
2.1.14 Characteristics of Trichoderma for Formulation Development
To develop a successful Trichoderma formulation, Trichoderma spp. should possess (Jeyarajan and Nakkeeran 2000)
-
1.
High rhizosphere competence
-
2.
Highly competitive saprophytic ability
-
3.
Enhanced plant growth
-
4.
Ease for mass multiplication
-
5.
Broad-spectrum of action
-
6.
Excellent and reliable control
-
7.
Safe to environment
-
8.
Compatible with other bioagents
-
9.
Should tolerate desiccation, heat, oxidizing agents, and UV radiations.
2.2 Paecilomyces spp.: Formulations, Mechanism with Some Success Stories
The fungus Paecilomyces lilacinus (Thom) Samson, a nematode egg parasite, is currently used as a biological control agent against various plant–parasitic nematodes. The genus Paecilomyces was first described (Bainier 1907) as closely related to Penicillium and comprising only one species, P. variotii Bainier. The genus Paecilomyces has many species, both pathogenic and saprophytic, and can be found in a wide range of habitats, including soil (Samson 1974).
2.2.1 Mechanisms
Paecilomyces, microbial mechanisms involved in disease suppression have been direct, such as parasitism, competition or antibiosis, and indirect, which involve plant protection through induced systemic resistance (ISR) mechanisms (Di Francesco et al. 2016; Lugtenberg et al. 2017; Obrien 2017; Latz et al. 2018).
2.2.2 Parasitism
Chitinase production by P. javanicus leads to mycelia inhibition of Aspergillus nidulans, Colletotrichum gloeosporioides, Rhizoctonia solani, and Sclerotium rolfsii (Chen et al. 2007). Various studies refer to the nematicidal activity of Paecilomyces. Species of this genus, namely P. lilacinus, can penetrate both the eggshells and structural components of juvenile and adult stages of different species of nematodes through spore germination and subsequent hyphal branching and appressoria formation (Khan et al. 2006; Dong et al. 2007). Regarding the production of lytic enzymes causing a nematicidal effect, the synthesis of amylases, lipases, proteases, and chitinases associated with this species has been described (Morton et al. 2004; Park et al. 2004; Khan et al. 2006; Gortari et al. 2008; Gine and Sorribas 2017). Overexpression of genes regulating the synthesis of these enzymes increases P. lilacinus virulence and parasitic ability against Meloidogyne incognita, Panagrellus redivivus, and Caenorhabditis elegans (Wang et al. 2010; Yang et al. 2011).
2.2.3 Competition
In vitro synthesis of hydroxamate and carboxylate siderophores such as ferrirubin trihydroxamate has been described mainly in P. lilacinus and P. variotii (Vala et al. 2000; Renshaw et al. 2002; Moreno-Gavíra et al. 2020). While this mechanism directly impacts control, competition is often accompanied by other mechanisms (Latz et al. 2018). The rapid growth of Paecilomyces species prevents the development of specific pathogens (Adebola and Amadi 2010; Arora et al. 2017). For instance, spraying sunflower seeds with P. variotii spores prevents penetration and infection by the pathogen Macrophomina phaseolina (Anis et al. 2010). However, this competition can sometimes harm the rest of the beneficial microbiota (Yu et al. 2015).
2.2.4 Antibiosis
The production of secondary metabolites with antimicrobial effect by Paecilomyces species has been widely described. Among them, we can highlight the synthesis of alkaloids, phenolic compounds, volatile organic compounds, steroids, flavonoids, peptides, polyketides, quinones, and terpenoids (Mousa and Raizada 2013; Lugtenberg et al. 2016). Li et al. (2020) recently described a total of 148 active metabolites produced by different Paecilomyces species that can be used for drug or agrochemical development. In the following sections, we will show the importance of these metabolites in the biological control of pests and diseases.
2.2.5 Management of Plant Diseases by Paecilomyces
The effectiveness of Paecilomyces against different species of plant pathogenic bacteria is reported. Paecilomyces variotii isolated from municipal solid waste compost showed a reduction in 27% of diseases caused by X. campestris in melon and a decrease in the pathogen population (Suarez-Estrella et al. 2013). Sornakili et al. (2020) reported the inhibition of Erwinia carotovora, Xanthomonas, oryzae PV. oryzae, and Ralstonia solanacearum with in vitro inhibition between 13 and 45% using P. tenuis, an endophyte isolated from rice leaves.
Paecilomyces species have shown their antagonistic effect against plant pathogenic fungi causing root and aerial plant diseases through various mechanisms. Plasmolysis in spore germ tubes or hyphal melanization in Pyrenophora tritici-repentis (Larran et al. 2016), hyphal lysis in Moniliophthora roreri was caused by Paecilomyces sp. (Suarez-Estrella et al. 2013), mycoparasitism of F. oxysporum caused by P. variotii and P. lilacinus (Jacobs et al. 2003), or antibiosis against R. solani (Horn et al. 1992) were reported.
Yang et al. (2015) observed inhibited S. sclerotiorum mycelial growth and sclerotia germination and reduced disease severity after using P. lilacinus on a rapeseed crop. In tomatoes, spraying P. variotii spores on the leaves significantly reduces damage caused by Alternaria solani (Varma et al. 2008). Conversely, the increase in polyphenols and antioxidant activity due to P. lilacinus on okra roots improves plant development and control of various phytopathogenic fungi causing root rot (Shafique et al. 2015).
As a hematophagous fungus, Paecilomyces has been widely studied and can be found in various biological formulations for agricultural use (Dong et al. 2007). There are many examples where Paecilomyces spp. act as nematicidal agents, especially against Meloidogyne spp. and against other genera such as Globodera (Lima-Rivera et al. 2016), Rotylenchulus, Heterodera, Xiphinema, or Pratylenchus (Favre-Bonvin et al. 1991). One example is the use of P. lilacinus and P. fumosoroseus against M. incognita or M. javanica, which drastically reduces their populations (Favre-Bonvin et al. 1991; Walters and Barker 1994; Siddiqui and Akhtar 2009; Nesha and Siddiqui 2017) in in vitro (Khan et al. 2004; Perveen and Shahzad 2013) and field tests (Brand et al. 2004; Saha et al. 2016). The spores of these species must germinate on the host to penetrate and colonize its surface to modify its physiology (Favre-Bonvin et al. 1991). Paecilomyces act according to the fungal and nematode species it parasitizes.
Paecilomyces spp. can act at different nematode developmental stages by infecting eggs, young or adult nematodes. Nematode eggshell is the main barrier against parasite agents and resistance to chemical nematicides and biological compounds. Paecilomyces species can secrete enzymes to degrade this barrier and deploying mechanisms involved in nematode parasitism (Roumpos 2005; Sexton and Howlett 2006). Thus, observations have shown that Meloidogyne incognita eggs at early stages of development are more vulnerable than eggs containing fully developed juveniles, although the latter is also affected (Jatala et al. 1980; Dunn et al. 1982; Eapen et al. 2005). Williams et al. (1999) confirm that eggs are parasitized by P. lilacinus at all stages, including unhatched juveniles. Egg infection occurs when hyphae lie flat on the egg surface, and appressoria are formed. Then, the fungus spreads, and conidiophores are formed. Studies carried out by Khan et al. (2006) concluded that said juveniles show various degrees of deformities and developmental abnormalities, such as reduced mobility inside the eggs. Different studies show the significant role of proteases and chitinases in the penetration of the fungus through eggshells. Thus, M. arenaria eggshells showed vitelline membrane disaggregation and chitin and lipid layer destruction after using P. lilacinus (Morgan-Jones et al. 1984).
Evidence shows that various hydrolytic proteins, such as proteases (mainly serine proteases), collagenases, and chitinases, are involved in nematode cuticle penetration and subsequent cell degradation (Huang et al. 2004; Morton et al. 2004; Ahman et al. 2002; Yang et al. 2011; Pau et al. 2012). Likewise, different secondary metabolites produced by Paecilomyces also play a significant role in nematode control (Yan et al. 2011). Nematode control effectiveness using Paecilomyces depends on the crop itself, affecting fungal activity in many cases (Al-Hazmi et al. 2017). Thus, using an antagonist in combination with organic substances increases parasitism by Paecilomyces in both eggs and larvae of nematodes (Siddiqui and Futai 2009).
3 Conclusions
The growth of agricultural production has led to several new challenges, making further growth possible only if these challenges are met appropriately and timely due to excessive use of chemical fertilizers and pesticides becoming a matter of concern. So, biological control can be an alternate system, which may play an essential role in achieving the goal of agriculture agents. Different methods have been tried to test the efficiency of these biocontrol agents, which involves mutation and protoplasm fusion via chemical agents. There is a need for producing these biological agents with better environmental factors for the growth of biocontrol agents. The main challenge is the cost associated with the formulation and safety assessment during the production of commercial biocontrol agents. The biocontrol approach emerged as the promising alternative approach, which provides and ensures a sustainable management system.
The success of biopesticides to suppress pests and diseases depends on the availability of microbes as a product or formulation, which facilitate the technology to transfer from lab to land. The constraints to biopesticides development and utilization mirror some of those factors that limit the development worldwide. Some constraints include lack of the right screening protocol for the selection of promising candidates of Trichoderma, inconsistent performance, and poor shelf life, awareness, training, and education shortfalls.
References
Adebola MO, Amadi JE (2010) Antagonistic activities of Paecilomyces and Rhizopus species against the cocoa black pod pathogen (Phytophthora palmivora). Afr Sci 11:235–239
Ahman J, Johanson T, Olsson M, Punt PJ, Van den Hondel CAMJJ, Tunlid AS (2002) Improving the pathogenicity of a nematode trapping fungus by genetic engineering of a subtilisin with nematotoxic activity. Appl Environ Microbiol 689:3408–3415
Al-Hazmi AS, Dawabah AAM, Al-Nadhari SN, Al-Yahya FA (2017) Comparative efficacy of different approaches to managing Meloidogyne incognita on green bean. Saudi J Biol Sci 24:149–154
Andrews JH (1992) Biological control in the phyllosphere. Annu Rev Phytopathol 30:603–635. https://doi.org/10.1146/annurev.py.30.090192.003131
Anis M, Abbasi MW, Zaki MJ (2010) Bioefficacy of microbial antagonists against Macrophomina phaseolina on sunflower. Pak J Bot 42:2935–2940
Arora K, Sharma S, Krishna SB, Adam JK, Kumar A (2017) Non-edible oil cakes as a novel substrate for DPA production and augmenting biocontrol activity of Paecilomyces variotii. Front Microbiol 8:753
Baby UI, Manibhushanrao K (1996) Fungal antagonists and VA mycorrhizal fungi for biocontrol of Rhizoctonia solani, the rice sheath blight pathogen. In: Manibhushanrao K, Mahadevan A (eds) Recent developments in biocontrol of plant pathogens. Today and Tomorrow’s Printers and Publishers, Allahabad
Bainier G (1907) Mycothe ‘que de l’e’cole de Pharmacie. XI Paecilomyces, genre nouveau de Muce’dine’es. Bull Soc Mycol 23:26–27
Baker KF, Cook RJ (1974) Biological control of plant pathogens. W. H. Freeman and Co, San Francisco, p 433
Balasubramanian C, Udaysoorian P, Prabhu C, Kumar GS (2008) Enriched compost for yield and quality enhancement in sugarcane. J Ecobiol 22:173–176
Barnes S, Moore D (1997) The effect of fatty, organic or phenolic acids on the germination of conidia of Metarhizium flavoviride. Mycol Res 10:662–666
Bateman R, Carey M, Morre D, Prior C (1993) The enhanced infectivity of Metarhizium flaviviridae in oil formulations to desert locust at low humidities. Ann Appl Biol 122:145–152
Batta YA (2005) Postharvest biological control of apple gray mould by Trichoderma harzianum formulated in an invert emulsion. Crop Prot 23(1):19–26
Bhagat S, Pan S (2010) Biological management of root and collar rot (Rhizoctonia solani) of French bean (Phaseolus vulgaris). Indian J Agric Sci 80(1):42–50
Bhagat D, Koche M, Ingle RW, Mohod YN (2010) Evaluate the suitability of locally available substrates for mass multiplication of cellulolytic fungi and bacteria. J Plant Dis Sci 5:27–29
Brand D, Roussos S, Pandey A, Zilioli PC, Pohl J, Soccol CR (2004) Development of a bionematicide with Paecilomyces lilacinus to control Meloidogyne incognita. Appl Biochem Biotechnol 118:81–88
Brunner K, Montero M, Mach RL, Peterbauer CK, Kubicek CP (2003) Expression of the ech42 (endochitinase) gene of Trichoderma atroviride under carbon starvation is antagonized via a BrlA-like cis-acting element. FEMS Microbiol Lett 218:259–264
Carsolio C, Benhamou N, Haran S, Cortes C, Gutierrez A, Chet I, Herrera-Estrella A (1999) Role of the Trichoderma harzianum endochitinase gene ech42 in mycoparasitism. Appl Environ Microbiol 65:929–935
Chaverri P, Branco-Rocha F, Jaklitsch W, Gazis R, DegenkoSL T, Samuels GJ (2015) Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 107(3):558–590
Chen CC, Kumar HA, Kumar S, Tzean SS, Yeh KW (2007) Molecular cloning, characterization, and expression of a chitinase from the entomopathogenic fungus Paecilomyces javanicus. Curr Microbiol 55:8–13
Chen J, Sun S, Miao C, Wu K, Chen Y, Xu L, Guan H, Zao L (2016) Endophytic Trichoderma gamsii YIM PH30019: a promising biocontrol agent with hyperosmolar, mycoparasitism and antagonistic activities of induced volatile organic compounds on root rot pathogenic fungi of Panax notoginseng. J Ginseng Res 40:315–324
Chet I, Inbar J (1994) Biological control of fungal pathogens. Appl Biochem Biotechnol 48:37–43
Chet IJ, Inbar Y, Hadar (1997) Fungal antagonists and mycoparasites. In: Wicklow DT, Soderstrom B (eds) The mycota, environmental and microbial relationships, vol 4. Springer, Berlin, pp 165–184
Chet I, Benhamou N, Haran S (1998) Mycoparasitism and lytic enzymes. In: Harman GE, Kubicek CP (eds) Trichoderma and gliocladium, Enzymes, biological control and commercial applications, vol 2. Taylor and Francis, London, pp 153–171
Churchill BW (1982) Mass production of microorganisms for biological control
Connick W, Daigle D, Quimby P (1991) An improved invert emulsion with high water retention for mycoherbicide delivery. Weed Technol 5:442–444
Cook RJ, Baker KF (1983) The nature and practice of biological control of plant pathogens. American Phytopathological Society Press, St. Paul, p 539
Cumagun CJR (2014) Advances in formulation of trichoderma for biocontrol. In: Biotechnology and biology of trichoderma. Elsevier, Amsterdam, pp 527–531
Cumagun CJR, Lapis DB (1993) Note: practical approach in mass production of Trichoderma spp. as a means of biological control against sheath blight of rice. Philipp Agric 76:251–257
Das BC, Hazarika DK (2000) Biological management of sheath blight of rice. Indian Phytopathol 53(4):433–435
De La Cruz J, Hidalgo-Gallego A, Lora JM, Benitez T, Pintor-Toro JA, Llobell A (1992) Isolation and characterization of three chitinases from Trichoderma harzianum. Eur J Biochem 206:859–867
Degenkolb T, Grafenhan T, Berg A, Nirenberg HI, Gams W, Brückner H (2006) Peptaibiomics: screening for polypeptide antibiotic (peptaibiotics) from plant protective Trichoderma species. Chem Biodivers 3:593–610
Delgado-Jarana J, Moreno-Mateos MA, Benitez T (2003) Glucose uptake in Trichoderma harzianum: role of gtt l. Eukaryot Cell 2:708–717
Di Francesco A, Martini C, Mari M (2016) Biological control of postharvest diseases by microbial antagonists: How many mechanisms of action Eur. J Plant Pathol 145:711–717
Djonovic S, Pozo MJ, Dangott LJ, Howell CR, Kenerley CM (2006) Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol Plant-Microbe Interact 19:838–853
Do Nascimento Silva R, Sousha Rocha JR, Oliveira NT (1998) In vitro antagonistic potential of Trichoderma spp. against Colletotrichum gloeosporioides agent of anthracnose in passion fruit (Passiflora). Boletin-Micologico 13(1):103–110
Dong LQ, Yang JK, Zhang KQ (2007) Cloning and phylogenetic analysis of the chitinase gene from the facultative pathogen Paecilomyces lilacinus. J Appl Microbiol 103:2476–2488
Druzhinina I, Kubicek CP (2005) Species concepts and biodiversity in Trichoderma and Hypocrea: from aggregate species to species clusters. J Zhejiang Univ Sci 6(2):100–112
Dubey SC, Bhavani R, Singh B (2011) Integration of soil application and seed treatment formulations of Trichoderma species for management of wet root rot of mungbean caused by Rhizoctonia solani. Pest Manag Sci 67:1163–1168
Dunn MT, Sayre RM, Carrell A, Wergin WP (1982) Colonization of nematode eggs by Paecilomyces lilacinus(Thom) Samson as observed with scanning electron microscope. Scan Electron Microsc 3:1351–1357
Eapen SJ, Beena B, Ramana K (2005) Tropical soil microflora of spice-based cropping systems as potential antagonists of root-knot nematodes. J Invertebr Pathol 88:218–225
Eisendle M, Oberegger H, Buttinger R, Illmer P, Haas H (2004) Biosynthesis and uptake of Siderophores is controlled by the PacC-mediated ambient-pH regulatory system in Aspergillus nidulans. Eukaryot Cell 3(2):561–563
Favre-Bonvin J, Ponchet M, Djian C, Arpin N, Pijarowski L (1991) Acetic acid: A selective nematicidal metabolite from culture filtrates of Paecilomyces lilacinus (Thom) Samson and Trichoderma longibrachiatum Rifai. Nematologica 37:101–112
Franken P, Khun G, Gianinazzi-Pearson V (2002) Development and molecular biology of arbuscular mycorrhizal fungi. In: Osiewacz HD (ed) Molecular biology of fungal development. Marcel Dekker, New York, pp 325–348
Fravel DR, Rhodes DJ, Larkin RP (1999) Production and commercialization of biocontrol products. In: Albajes R, Lodovica Gullino M, Van Lenteren JC, Elad Y (eds) Integrated pest and disease management in greenhouse crops. Kluwer Academic Publishers, Boston, pp 365–376
Geremia RA, Goldman GH, Jacobs D, Ardrtes W, Vila SB, Van Montagu M, Herrera-Estrella A (1993) Molecular characterization of the proteinase-encoding gene, prb1 related to mycoparasitism by Trichoderm harzianum. Mol Microbiol 8:603–613
Gine A, Sorribas FJ (2017) Effect of plant resistance and BioAct WG (Purpureocillium lilacinum strain 251) on Meloidogyne incognita in a tomato–cucumber rotation in a greenhouse. Pest Manag Sci 73:880–887
Gortari MC, Galarza BC, Cazau MC, Hours RA (2008) Comparison of the biological properties of two strains of Paecilomyces lilacinus (Thom) Samson associated to their antagonistic effect onto Toxocara canis eggs. Malays J Microbiol 4:35–41
Green S, Wade-Stewart S, Boland G, Teshler M, Liu S (1998) Formulating microorganisms for biological control of weeds. In: Boland G, Kuykendall L (eds) Plant-microbe interactions and biological control. Marcel Dekker, Inc., New York, pp 249–281
Haran S, Schickler H, Chet I (1996) Molecular mechanisms of lytic enzymes involved in the biocontrol activity of Trichoderma harzianum. Microbiology 142(9):2321–2331
Harman GE, Bjorkmann T (1998) Potential and existing uses of Trichoderma and Gliocladium for plant disease control and plant growth enhancement. In: Harman GE, Kubicek CP (eds) Trichoderma and Gliocladium, vol 2. Enzymes, biological control and commercial applications. Taylor and Francis, London, pp 229–265
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species – opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
He A, Jia L, Xin-hua W, Quan-guo Z, Wei S, Jie C (2019) Soil application of Trichoderma asperellum GDFS1009 granules promotes growth and resistance to Fusarium graminearum in maize. J Integr Agric 18(3):599–606
Hjeljord L, Tronsmo A (1988) Trichoderma and Gliocladium in biological control: an overview. In: Harman GE, Kubicek CP (eds) Trichoderma and Gliocladium, vol 2. Enzymes, biological control and commercial applications. Taylor and Francis, London, pp 129–155
Horn WS, Smith JL, Bills GF, Raghoobar SL, Helms GL, Kurtz MB (1992) Sphingofungins E and F: novel serinepalmitoyl transferase inhibitors from Paecilomyces variotii. J Antibiot 45:1692–1696
Howell CR, Puckhaber LS (2005) A study of the characteristics of P and Q strains of Trichoderma virens to account for differences in biological control efficacy against cotton seedling diseases. Biol Control 33:217–222
Huang XW, Zhao NH, Zhang KQ (2004) Extracellular enzymes serving as virulence factors in nematophagous fungi involved in infection of the host. Res Microbiol 115:811–816
Ibrahim L, Butt T, Beckett A, Clark S (1999) The germination of oil formulated conidia of the insect pathogen Metarhizium anisolpliae. Mycol Res 103:901–907
Jacobs H, Gray SN, Crump DH (2003) Interactions between nematophagous fungi and consequences for their potential as biological agents for the control of potato cyst nematodes. Mycol Res 107:47–56
Jagana M, Zacharia S, Basayya A (2013) Management of Alternaria blight in Mustard. Ann Plant Prot Sci 21(2):441–442
Jat JG, Agalave HR (2013) Antagonistic properties of Trichoderma species against oilseed-borne fungi. Sci Res Reporter 3(2):171–174
Jatala P, Kaltenback R, Bocangel M, Devaus AJ, Campos R (1980) Field application of Paecilomyces lilacinus for controlling Meloidogyne incognita on potatoes. J Nematol 12:226–227
Jeyarajan R (2006) Prospects of indigenous mass production and formulation of Trichroderma. In: Rabindra RJ, Ramanujam B (eds) Current status of biological control of plant diseases using antagonistic organisms in India. Project Directorate of Biological Control, Bangalore, pp 74–80
Jeyarajan R, Nakkeeran S (2000) Exploitation of microorganisms and viruses as biocontrol agents for crop disease management. In: Biocontrol potential and their exploitation in sustainable agriculture. Kluwer Academic/Plenum Publishers, Boston, pp 95–116
Jeyarajan R, Ramakrishnan G, Dinakaran D, Sridar R (1994) Development of products of Trichoderma viride and Bacillus subtilis for biocontrol of root rot diseases. In: Dwivedi BK (ed) Biotechnology in India. Bioved Research Society, Allahabad, pp 25–36
Jin X, Hayes KC, Harman GE (1992) Principles in the development of biological control systems employing Trichoderma species against soil-borne plant pathogenic fungi. In: Lantham GF (ed) Frontiers in industrial mycology. Chapman & Hall Inc., New York, pp 174–195
Jyoti, Singh DP (2016) Fungi as biocontrol agents in sustainable agriculture. In: Microbes and environmental management. Springer, New York, pp 172–192
Kamala T, Indira Devi S, Sharma KC, Kennedy K (2015) Phylogeny and taxonomical investigation of Trichoderma spp. from Indian region of Indo-Burma biodiversity hot spot region with special reference to Manipur. Bio Med Res Int 2015:285261
Khan A, Williams KL, Nevalainen HK (2004) Effects of Paecilomyces lilacinus protease and chitinase on the eggshell structures and hatching of Meloidogyne javanica juveniles. Biol Control 31:346–352
Khan A, Williams KL, Nevalainen HK (2006) Infection of plant-parasitic nematodes by Paecilomyces lilacinus and Monacrosporium lysipagum. Biol Control 51:659–678
Khan AA, Sinha AP (2005) Influence of different factors on the effectivity of fungal bioagents to manage rice sheath blight in nursery. Indian Phytopathol 58(3):289–293
Khan AA, Sinha AP (2007) Screening of Trichoderma spp. against Rhizoctonia solani the causal agent of rice sheath blight. Indian Phytopathol 60(4):450–456
Kopchinskiy A, Komon M, Kubicek CP, Druzhinina IS (2005) TrichoBLAST: a multilocus database for Trichoderma and Hypocrea identifications. Mycol Res 109:658–660
Kulkarni S, Shalini DS (2007) Trichoderma–a potential biofungicide of the millennium, technical bulletin – 5. University of Agricultural Sciences, Dharwad
Kumar S, Thakur M, Rani A (2014) Trichoderma: mass production, formulation, quality control, delivery and its scope in commercialization in India for the management of plant diseases. Afr J Agric Res 9(53):3838–3852
Kumar S (2013) Trichoderma: a biological weapon for managing plant diseases and promoting sustainability. Int J Agric Sci Med Vet 1:106–121
Kumar S (2010) Integrated management of maydis leaf blight of maize. Ann Plant Prot Sci 18(2):536–537
Kumar S, Upadhyay JP, Rani A (2009) Evaluation of Trichoderma species against Fusarium udum Butler causing wilt of Pigeon pea. J Biol Control 23(3):329–332
Kumar S, Upadhyay JP, Kumar S (2002) Biocontrol of Alternaria leaf spot of Vicia faba using antagonistic fungi. J Biol Control 20(2):247–251
Larran S, Simon MR, Moreno MV, Siurana MS, Perello A (2016) Endophytes from wheat as biocontrol agents against tan spot disease. Biol Control 92:17–23
Latorre BA, Lillo C, Rioja ME (2001) Eficacia de los tratamientos fungicidas para el control de Botrytis cinerea de la vid en function de la epoca de aplicacion. Cien Invest Agraria 8:61–66
Latz MA, Jensen B, Collinge DB, Jorgensen HJ (2018) Endophytic fungi as biocontrol agents: elucidating mechanisms in disease suppression. Plant Ecol Div 11:555–567
Lewis JA (1991) Formulation and delivery system of biocontrol agents with emphasis on fungi Beltsville symposia. Agric Res 14:279–287
Li XQ, Xu K, Liu XM, Zhang PA (2020) Systematic review on secondary metabolites of Paecilomyces species: chemical diversity and biological activity. Planta Med 86:805–821
Lima-Rivera DL, Lopez-Lima D, Desgarennes D, Velazquez-Rodriguez AS, Carrion G (2016) Phosphate solubilisation by fungi with nematicidal potential. J Soil Sci Plant Nutr 16:507–524
Lorito M, Mach RL, Sposato P, Strauss J, Peterbauer CK, Kubicek CP (1996) Mycoparasitic interaction relieves binding of the Cre1 carbon catabolite repressor protein to promoter sequences of the ech42 (endochitinase-encoding) gene in Trichoderma harzianum. Proc Natl Acad Sci U S A 93:14868–14872
Lugtenberg B, Rozen DE, Kamilova F (2017) Wars between microbes on roots and fruits. F1000 Res 6:343
Lugtenberg BJ, Caradus JR, Johnson LJ (2016) Fungal endophytes for sustainable crop production. FEMS Microbiol Ecol 92:194
Mach RL, Peterbauer CK, Payer K, Jaksits S, Woo SL, Zeilinger S, Kullnig CM, Lorito M, Kubicek CP (1999) Expression of two major chitinase genes of Trichoderma atroviride (T. harzianum P1) is triggered by different regulatory signals. Am Soc Microbiol Appl Envirol Microbiol 65(5):1858–1863
Mach RL, Zeilinger S (2003) Regulation of gene expression in industrial fungi: Trichoderma. Appl Microbiol Biotechnol 60:515–522
Manibhusanrao K, Sreenivasaprasad S, Baby UF, Joe Y (1989) Susceptibility of rice sheath pathogen to mycoparasites. Curr Sci 58(9):515–518
Mathre DE, Cook RJ, Callan NW (1999) From discovery to use: traversing the world of commercializing biocontrol agents for plant disease control. Plant Dis 83:972–983
Mcintyre M, Nielsen J, Arnau J, Vander Brink H, Hansen K, Madrid S (2004) Proceedings of the 7th European conference on fungal genetics, Copenhagen, Denmark
Mishra DS, Singh US, Dwivedi TS (2001) Comparative efficacy of normal seed treatment and seed bio priming with commercial formulations of Trichoderma sp. In: 53rd Annual meeting of Indian Phytopathological Society and National symposium on Eco-friendly approaches for plant disease management, Chennai, India, pp 21–23
Moreno-Gavíra A, Dianez F, Sanchez-Montesinos B, Santos M (2020) Paecilomyces variotii as a plant-growth promoter in horticulture. Agronomy 10:597
Morgan CA, Herman N, White PA, Vesey G (2006) Preservation of microorganisms by drying: a review. J Microbiol Methods 66:183–193
Morgan-Jones G, White JF, Rodriguez-Kabana R (1984) Phyto-nematode pathology: ultrastructural studies II. Parasitism of Meloidogyne arenaria eggs and larvae by Paecilomyces lilacinus. Nematropica 14:57–71
Morton O, Hirsch P, Kerry B (2004) Infection of plant-parasitic nematodes by nematophagous fungi–a review of the application of molecular biology to understand infection processes and to improve biological control. Nematology 6:161–170
Mousa WK, Raizada MN (2013) The diversity of anti-microbial secondary metabolites produced by fungal endophytes: an interdisciplinary perspective. Front Microbiol 4:65
Mukherjee PK, Latha J, Hadar R, Horwitz A (2004) Role of two G-protein alpha subunits, TgaA and TgaB in the antagonism of Trichoderma virens against plant pathogens. Appl Environ Microbiol 70:542–549
Mukherjee PK, Mukhopadhyay AN (1995) In situ mycoparasitism of Gliocladium virens on Rhizoctonia solani. Indian Phytopathol 48(1):101–102
Mukhopadhyay AN, Mukherjee PK (1996) Fungi as fungicides. Int J Trop Plant Dis 14:1–17
Mukhopadhyay AN, Shrestha SM, Mukherjee PK (1992) Biological seed treatment for control of soil borne plant pathogens FAO. Plant Prot Bull 40:21–30
Mustafa A, Khan MA, Inam-ul-Haq M, Khan SH, Pervez MA (2009) Mass multiplication of Trichoderma spp. on organic substrate and their effect in management of seed borne fungi. Pak J Phytopathol 21(2):108–114
Nagaraju A, Sudisha J, Murthy SM, Ito S (2012) Seed priming with Trichoderma harzianum isolates enhances plant growth and induces resistance against Plasmopara halstedii, an incitant of sunflower downy mildew disease. Australas Plant Pathol 41:609–620
Nelson EB, Harman GE, Nash GT (1988) Enhancement of Trichoderma induced biological control of Pythium seed rot and pre emergence damping-off of peas. Soil Biol Biochem 20:145–150
Nesha R, Siddiqui ZA (2017) Effects of Paecilomyces lilacinus and Aspergillus niger alone and in combination on the growth, chlorophyll contents and soft rot disease complex of carrot. Sci Hortic 218:258–264
Obrien PA (2017) Biological control of plant diseases. Australas Plant Pathol 46:293–304
Olson HA, Benson DM (2007) Induced systemic resistance and the role of binucleate Rhizoctonia and Trichoderma hamatum 382 in biocontrol of Botrytis blight in geranium. Biol Control 42:233–241
Pandya JR (2012) Isolation, mass multiplication and characterization of Trichoderma spp. under south Gujarat conditions. Ph.D. thesis submitted to N.A.U., Navsari, pp 35–120
Papavizas GC, Dunn MT, Lewis JA, Beagle-Ristaino J (1984) Liquid fermentation technology for experimental production of biocontrol fungi. Phytopathology 74:1171–1175
Parab PB, Diwakar MP, Sawant UK, Kadam JJ (2008) Studies on mass multiplication, different methods of application of bioagent Trichoderma harzianum and their survival in rhizosphere and soil. J Plant Dis Sci 3:215–218
Park JO, Hargreaves JR, McConville EJ, Stirling GR, Ghisalberti EL, Sivasithamparam K (2004) Production of leucinostatins and nematicidal activity of Australian isolates of Paecilomyces lilacinus (Thom) Samson. Lett Appl Microbiol 38:271–276
Pau CG, Leong S, Teck C, Wong SK, Eng L, Jiwan M (2012) Isolation of indigenous strains of Paecilomyces lilacinus with antagonistic activity against Meloidogyne incognita. Int J Agric Biol 14:197–203
Persoon CH (1794) Disposita methodica fungorum. Romer’s Neues Mag Bot 1:81–128
Perveen Z, Shahzad SA (2013) Comparative study of the efficacy of Paecilomyces species against root-knot nematode Meloidogyne incognita. Pak J Nematol 31:125–131
Prasad RD, Rangeshwaran R, Anuroop CP, Phanikumar PR (2002) Bioefficacy and shelf life of conidial and chlamydospore formulations of Trichoderma harzianum Rifai. J Biol Cont 16:145–148
Ragab MMM, Abada KA, Abd-El-Moneim ML, Abo-Shosha YZ (2015) Effect of different mixtures of some bioagents and Rhizobium phaseoli on bean damping-off under field condition. Int J Sci Eng Res 6(7):1009–1106
Reddy K, Krishnamma, Narayana P (2009) Efficacy of Trichoderma viride against Colletotrichum falcatum in Sugarcane. Indian J Plant Prot 37:111–115
Reithner B, Brunner K, Schumacher R, Stoppacher N, Pucher M, Brunner K, Zeilinger S (2007) Signaling via the Trichoderma atroviride mitogen-activated protein kinase Tmk1 differentially affects mycoparasitism and plant protection. Fungal Genet Biol 44:1123–1133
Renshaw JC, Robson GD, Trinci AP, Wiebe MG, Livens FR, Collison D, Taylor RJ (2002) Fungal siderophores: structures, functions and applications. Mycol Res 106:1123–1142
Roumpos C (2005) Ecological studies on Paecilomyces lilacinus strain 251 and their importance for biocontrol of plant-parasitic nematodes and environmental risk assessment. Cuvillier Verlag, Gottingen
Sabalpara AN (2014) Mass multiplication of biopesticides at farm level. J Mycol Plant Pathol 44(1):1–5
Saha M, Sarkar S, Sarkar B, Sharma BQ, Bhattacharjee S, Tribedi P (2016) Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res 23:3984–3999
Samson RA (1974) Paecilomyces and some allied hyphomycetes. Stud Mycol 6:1–119
Sawant IS, Sawant SD (1996) A simple method for achieving high cfu of Trichoderma harzianum on organic wastes for field applications. Indian Phytopathol 9:185–187
Schuster A, Schmoll M (2010) Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol 87:787–799
Sehrawat A, Sindhu SS (2019) Potential of biocontrol agents in plant disease control for improving food safety def. Lifesci J 4:220–225
Seidl V, Marchetti M, Schandl R, Allmaier G, Kubicek CP (2006) Epl1, the major secreted protein of Hypocrea atroviridis on glucose, is a member of a strongly conserved protein family comprising plant defense response elicitors. FEBS J 273:4346–4359
Sexton AC, Howlett BJ (2006) Parallels in fungal pathogenesis on plant and animal hosts. Eukaryot Cell 5:1941–1949
Shafique HA, Sultana V, Ara J, Ehteshamul-Haque S, Athar M (2015) Role of antagonistic microorganisms and organic amendment in stimulating the defense system of okra against root rotting fungi. Pol J Microbiol 64(2):157–162
Sharma P, Patel AN, Saini MK, Deep S (2012) Field demonstration of Trichoderma harzianum as a plant growth promoter in wheat (Triticum aestivum L). J Agric Sci 4(8):65–73
Sharma KK, Singh US, Sharma P, Kumar A, Sharma L (2015) Seed treatments for sustainable agriculture-A review. J Appl Nat Sci 7(1):521–539
Shoresh M, Gal-On A, Leibman D, Chet I (2006) Characterization of a mitogen-activated protein kinase gene from cucumber required for Trichoderma conferred plant resistance. Plant Physiol 142:1169–1179
Siddiqui ZA, Akhtar MS (2009) Effects of antagonistic fungi and plant growth promoting rhizobacteria on growth of tomato and reproduction of the root-knot nematode, Meloidogyne incognita. Australas Plant Pathol 38:22–28
Siddiqui ZA, Futai K (2009) Biocontrol of Meloidogyne incognita on tomato using antagonistic fungi, plant growth promoting rhizobacteria and cattle manure. Pest Manag Sci 65:943–948
Singh D, Kapur SP, Singh K (2000) Management of citrus scab caused by Elsinoe fawcettii. Indian Phytopathol 53(4):461–467
Singh D, Maheshwari VK (2001) Biological seed treatment for the control of loose smut of wheat. Indian Phytopathol 54(4):457–460
Singh US, Zaidi NW (2002) Current status of formulation and delivery of fungal and bacterial antagonists for disease management in India. In: Rabindra RJ, Hussaini SS, Ramanujam B (eds) Microbial biopesticide formulations and application. Project Directorate of Biological Control, Bangalore, pp 168–179
Sornakili A, Thankappan S, Sridharan AP, Nithya P, Uthandi S (2020) Antagonistic fungal endophytes and their metabolite-mediated interactions against phytopathogens in rice. Physiol Mol Plant Pathol 112:101525
Srinivasan U, Highley TL, Bruce A (1995) The role of siderophore production in the biological control of wood decay fungi by Trichoderma spp. In: Biodeterioration-and-biodegradation proceedings of the 9th international biodeterioration and bio degradation symposium, Leeds, UK 5–10 September 1993, pp 226–231
Sriram S, Raguchander T, Babu S, Nandakumar R, Shanmugam V, Vidhysekaran P, Balasubramanian P, Samiyappan R (2000) Inactivation of phytotoxin produced by the rice sheath blight pathogen Rhizoctonia solani. Can J Microbiol 46:520–524
Srivastava SRK, Kumar RKN, Singh S (2010) Management of Macrophomina disease complex in Jute (Corchorus olitorius) by Trichoderma viride. J Biol Control 24(1):77–79
Suarez-Estrella F, Arcos-Nievas MA, López MJ, Vargas-García MC, Moreno J (2013) Biological control of plant pathogens by microorganisms isolated from agro-industrial composts. Biol Control 67:509–515
Taylor AG, Min T, Harman GE, Jin X (1991) Liquid coating formulation for the application of biological seed treatments of Trichoderma harzianum. Biol Control 1:16–22
Tiwari AK (1996) Biological control of chickpea wilt complex using different formulations of Gliocladium virens through seed treatment. Ph.D. Thesis, GB Pant University of Agriculture and Technology, Pantnagar India, p 167
Tjamos EC, Papavizas GC, Cook RJ (1992) Biological control of plant diseases: progress and challenges for the future. Plenum Press, New York, pp 255–265
Tseng SC, Liu SY, Yang HH, Lo CT, Peng KC (2008) Proteomic study of biocontrol mechanisms of Trichoderma harzianum EST 323 in response to Rhizoctonia solani. J Agric Food Chem 56:6914–6922
Tulasne L, Tulasne R (1860) De quelques spheries fungicoles, apropos d’un memoire de M. Antoine de Bary sur les Nyctalis. Ann Sci Nat Bot 13:5–19
Vala AK, Vaidya SY, Dube HC (2000) Siderophore production by facultative marine fungi. Indian J Mar Sci 29:339–340
Varma PK, Gandhi SK, Surender S (2008) Biological control of Alternaria solani the causal agent of early blight of tomato. J Biol Control 22:67–72
Vasudevan P, Kavitha S, Priyadarisini VB, Babujee L, Gnanamanickamm SS (2002) Biological control of rice diseases. In: Gnanamanickam SS (ed) Biological control of crop diseases. Marcel Decker, New York, pp 11–32
Viterbo A, Chet I (2006) TasHyd1, a new hydrophobin gene from the biocontrol agent Trichoderma asperellum is involved in plant root colonization. Mol Plant Pathol 7:249–258
Walters SA, Barker KR (1994) Efficacy of Paecilomyces lilacinus in suppressing Rotylenchulus reniformis on tomato. J Nematol 26:600
Wang J, Liu F, Pan C (2010) Enhancing the virulence of Paecilomyces lilacinus against Meloidogyne incognita eggs by overexpression of a serine protease. Biotechnol Lett 32:1159–1166
Weindling R (1934) Studies on lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctonia solani and other soil fungi. Phytopathology 24:1153–1179
Whipps JM (1997) Developments in the biological control of soilborne plant pathogens. In: Callow JA (ed) Advances in botanical research incorporating advances in plant pathology, vol 26. Springer, New York, pp 1–123
Williams K, Khan A, Holland R (1999) Infection of Meloidogyne javanica by Paecilomyces lilacinus. Nematology 1:131–139
Woo SL, Donzelli B, Scala F, Mach R, Harman GE, Kubicek CP, Del Sorbo G, Lorito M (1999) Disruption of the ech42 (endochitinase-encoding) gene affects biocontrol activity in Trichoderma harzianum. Mol Plant-Microbe Interact 12:419–429
Yadav SK, Dave A, Sarkar A, Singh HB, Sharma BK (2013) Co-inoculated biopriming with Trichoderma, Pseudomonas and Rhizobium improves crop growth in Cicer arietinum and Phaseolus vulgaris. Int J Agric Biol 6(2):255–259
Yan XN, Sikora RA, Zheng JW (2011) Potential use of cucumber (Cucumis sativus L.) endophytic fungi as seed treatment agents against root-knot nematode Meloidogyne incognita. J Zhejiang Univ Sci B 12:219–225
Yang F, Abdelnabby H, Xiao Y (2015) A mutant of the nematophagous fungus Paecilomyces lilacinus (Thom) is a novel biocontrol agent for Sclerotinia sclerotiorum. Microb Pathog 89:169–176
Yang J, Zhao X, Liang L, Xia Z, Lei L, Niu X (2011) Overexpression of a cuticle-degrading proteaseVer112 increases the nematicidal activity of Paecilomyces lilacinus. Appl Microbiol Biotechnol 89:1895–1903
Yang HH, Yang SL, Peng KC, Lo CT, Liu SY (2009) Induced proteome of Trichoderma harzianum by Botrytis cinerea. Mycol Res 113:924–932
Yedidia I, Shoresh M, Kerem Z, Benhamou N, Kapulnik Y, Chet I (2003) Concomitant induction of systemic resistance to Pseudomonas syringae pv lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl Environ Microbiol 69:7343–7353
Yedidia I, Benhamou N, Kapulnik Y, Chet I (2000) Induction and accumulation of PR proteins activity during early stages of root colonization by the mycoparasite Trichoderma harzianum strain T-203. Plant Physiol Biochem 38:863–873
Yu Z, Zhang Y, Luo W, Wang Y (2015) Root colonization and effect of biocontrol fungus Paecilomyces lilacinus on composition of ammonia-oxidizing bacteria, ammonia-oxidizing archaea and fungal populations of tomato rhizosphere. Biol Fertil Soils 51:343–351
Zaher EA, Abada KA, Zyton MA (2013) Effect of combination between bioagents and solarization on management of crown and stem rot of Egyptian clover. Am J Plant Sci 1(3):43–50
Zeilinger S, Reithner B, Scala V, Piessl I, Lorito M, Mach R (2005) Signal transduction by Tga3, a novel G protein alpha subunit of Trichoderma atroviride. Appl Environ Microbiol 71:1591–1597
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Huilgol, S.N., Nandeesha, K.L., Banu, H. (2022). Fungal Biocontrol Agents: An Eco-friendly Option for the Management of Plant Diseases to Attain Sustainable Agriculture in India. In: Rajpal, V.R., Singh, I., Navi, S.S. (eds) Fungal diversity, ecology and control management. Fungal Biology. Springer, Singapore. https://doi.org/10.1007/978-981-16-8877-5_22
Download citation
DOI: https://doi.org/10.1007/978-981-16-8877-5_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8876-8
Online ISBN: 978-981-16-8877-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)