Abstract
Chronic viral hepatitis, caused by hepatitis B and hepatitis C viruses, is one of the most common causes of end-stage liver disease and liver cancer in the world, and accounts for almost 4000 deaths per day. Fundamental research in chronic viral hepatitis has led to dramatic changes in its diagnosis, prevention and management. Indeed, the scientists credited with the discovery of hepatitis B (Baruch Blumberg) and hepatitis C (Harvey J. Alter, Michael Houghton and Charles M. Rice) were honoured with the Nobel Prize in 1976 and 2020, respectively. The story of the unravelling of the mysteries behind “serum hepatitis” and the establishment of its viral aetiology is an incredible testament to the remarkable accomplishments of modern biomedical research. In this chapter, we embark on a journey into the discovery of hepatitis B and C viruses, discuss the work of the Nobel laureates and throw light on the work of the unsung heroes without whose contributions, the discoveries may never have seen the light of day.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Chronic viral hepatitis, caused by Hepatitis B and Hepatitis C viruses, is one of the most common causes of end-stage liver disease and liver cancer in the world, and accounts for almost 4000 deaths per day (Cooke et al. 2019). In fact, mortality from chronic viral hepatitis is comparable to that of tuberculosis, malaria and HIV. As per the Global Hepatitis Report of the World Health Organisation (WHO), the global burden of Hepatitis B and Hepatitis C is estimated to be 257 million and 71 million, respectively (WHO 2017). In India, approximately 50 million individuals are estimated to have chronic Hepatitis B. Higher prevalence of Hepatitis B has been noted in several tribal pockets in India (Tandon et al. 1996). The prevalence of Hepatitis C in India is estimated to be 0.5–1% with regions of higher prevalence in the North East and Punjab (Sood et al. 2018; Goel et al. 2019). The prevalence of both viruses is much higher in high-risk groups like intravenous drug users.
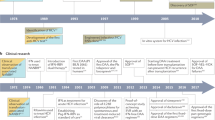
Fundamental research in chronic viral hepatitis has led to dramatic changes in its diagnosis, prevention and management. It is thus apt that the scientists credited with the discovery of both these viruses have been honoured with the Nobel Prize which was ordained by Sir Alfred Nobel for being awarded to those whose work “has conferred the greatest benefit to humankind”. In 1976, Baruch Blumberg received the Nobel Prize in Medicine for his discovery of Hepatitis B and in 2020, Harvey J. Alter, Michael Houghton and Charles M. Rice were awarded the same for their contributions in discovering Hepatitis C. The work of these men revolutionised fields of virology, immunology and hepatology and is a remarkable story of the triumph of passion, determination, and innovative, multifaceted research. In this chapter, we will embark on a journey into the discovery of Hepatitis B and C viruses. While discussing the work of the Nobel laureates, we will also be throwing light on the work of the unsung heroes without whose contributions, the discoveries may never have seen the light of day.
9.2 Viral Hepatitis: A Brief Primer
The human hepatitis virus includes five viruses (A-E). Hepatitis A and E are transmitted by the faeco-oral route and present acutely with jaundice. Unremarkable recovery is usually the rule although sometimes acute liver failure and even death can occur. Hepatitis B and C viruses are transmitted parenterally by contaminated injections or blood. While acute presentations can occur with Hepatitis B and rarely with Hepatitis C, the bigger concern with these two viruses is the establishment of chronic infection with propensity to progress to end-stage liver disease and hepatocellular cancer. Hepatitis D is only seen in the context of chronic Hepatitis B infection and is extremely rare in India.
9.3 Prelude
9.3.1 Early Human Understanding of Hepatitis
The earliest description of jaundice can be seen in the Babylonian Talmud (dating back to around 4500 BC) and in ancient Ayurvedic and Chinese texts. The Greek physician, Hippocrates (460–370 BC) described an outbreak of jaundice in Thassos (Wong et al. 2015; Khuroo and Sofi 2020; Trepo 2014). Medieval Europe was afflicted by repeated epidemic outbreaks of jaundice because of the poor standards of hygiene and overcrowding. The communicable nature of the disease was widely suspected and an early evidence of human intervention to stop the spread of the disease can be seen in a letter written by Pope Zacharis to the Archbishop of Mainz in 751 AD. The Pope requested the Archbishop to delay communion to people with jaundice till the healthy individuals have been attended. Intriguingly, he also asked for the horses to be buried.
9.3.2 Jaundice and the Military
Epidemics and outbreaks of jaundice were a frequent scourge in military camps and during Wars in the seventeenth–nineteenth century (Wong et al. 2015). This “campaign jaundice” was known as “Soldatengelbschut” in German and “jauniesse des camps” by the French. In the late eighteenth century, the ravishing army of Napoleon suffered serious setbacks in Egypt with hundreds and thousands falling ill. More than 70,000 cases of jaundice were reported during the American Civil War. The Franco-Prussian War and Boer War similarly witnessed outbreaks of jaundice which significantly dampened troop motivation (Schmidt 1999).
9.3.3 Early Scientific Work on the Etiology of Jaundice
Precious little was known about the origin and causes of jaundice till the nineteenth century. Various reasons were attributed including bad air, foul mood, bad water, dietary changes and small “germs”. Virchow, the Father of Pathology, proposed the “cattarhal” mechanism in which jaundice resulted from blockage of bile ducts by mucus (Gruber and Virchow 1865). His theory was later disproved by Murchison in 1874 who demonstrated the absence of biliary obstruction in a series of autopsies among patients who had expired during outbreaks of jaundice in Essen, London and Rotterdam (Murchison et al. 1886). In hindsight, most of the outbreaks that we have previously described were probably caused by the faeco-orally transmitted Hepatitis A and E viruses.
9.3.4 The Vaccination Era
With the dawn of the era of vaccines, transfusion hepatitis or serum hepatitis came into attention. The link was first identified by the keen observations of Lurmen while investigating an outbreak of jaundice in a Bremen dock in 1883–1884. He found that almost 15% of the 1289 individuals who were vaccinated using a particular batch of small pox vaccine developed jaundice within 6 months while none of the 500 individuals who were vaccinated using a separate batch. Lurmen concluded that vaccination was the “etiological source of the icterus epidemic” (Lürman 1885). Later, Stokes reported that the incidence of jaundice was 10 times higher in patients who received intravenous arsphenamin for syphilis at the Mayo Clinic, USA, from 1917 to 1920. After excluding arsphenamine and syphilis, he suggested infection spread by hematogenous means as the possible etiology (Stokes et al. 1920). Similar observations and conclusions were drawn by McCallum in 1943 in venereal disease centres across the USA (MacCallum 1943).
9.3.5 World War II: Key Observations About Changing Patterns of Hepatitis
In the meantime, the association between jaundice and wars was once again witnessed. In World War II, around 2 lakh cases of jaundice were reported in the US troops. More than 50 lakh cases were reported amongst the Germans including the military and civilian population (Wong et al. 2015). Several outbreaks were reported in the Mediterranean coast, North Africa and West Asia. On the flip side, this had a hidden benefit. The Allied powers, particularly the USA and Great Britain, invested significant amounts of money and infrastructure in research in hepatitis in order to gain a strategic advantage over the Germans.
During World War II, a new epidemiological pattern began to emerge. Many of the cases were related to vaccination, sharing of syringes and needles, and transfusion of blood or plasma. The US War Secretary declared that between 1st January and 4th July, 1942, approximately 28,000 cases of jaundice developed following vaccination against yellow fever with a mortality of 0.22% (Dooley 2005). The Health Ministry of Great Britain published a document titled “Homologous Serum Jaundice” in 1943, which reported on outbreaks of jaundice following transfusion of plasma and blood. They reviewed the available literature and made the very important observation that the incidence of hepatitis in institutions that used washed syringes and shared needles ranged from 30 to 60% compared to practically zero in institutions that used sterilized or boiled syringes. They correctly surmised that the hepatitis agent was transmitted during “venipuncture and intravenous injections” (MacCallum 1947).
9.3.6 Existence of Two Types of Hepatitis: A Vital Piece of the Puzzle
These observations led to the recognition that there were two distinct types of hepatitis based on clinical and epidemiologic characteristics. The first type (“infectious hepatitis”) was characterised by a brief incubation period, faeco-oral transmission, and was often clustered in outbreaks and epidemics. The second type (“serum hepatitis”) had a longer incubation period and was transmitted by injections or transfusion of blood and blood products. Further, patients with prior infectious hepatitis were immune to new onset “infectious hepatitis” while patients with “serum hepatitis” had no immunity against “infectious hepatitis”. The conclusions drawn were remarkably accurate. Indeed, as early as 1947, McCallum suggested the nomenclature of Hepatitis A and B, well before the discovery of the respective viruses (Tandon et al. 1996; MacCallum 1947).
9.3.7 The Dark Phase of Human Experimentation
The history of the discovery of hepatitis viruses would not be complete without alluding to the questionable ethics of some of the studies that were carried out during this time, particularly during World War II and the immediate post-war period. One of the vexing problems facing researchers at this time was that humans appeared to be the only hosts for the infective agents of hepatitis and all attempts to grow the agent in cellular cultures and animals were futile. We have alluded to the remarkable conclusions about the distinct forms of hepatitis that were made in the 1940s. On a darker note, many of these conclusions were drawn from human experimentation. Various researchers, including McCallum, Stokes, Havens and Neefe, carried out research that involved inoculating healthy human volunteers with faeces, serum or duodenal fluids from patients with hepatitis (Tandon et al. 1996; Khuroo and Sofi 2020; Trepo 2014). Human experimentation in hepatitis continued through the next two decades. A wealth of information on the epidemiology and natural history of hepatitis was obtained from a series of human experiments that were carried out on mentally challenged children at Willowbrook State School, a government-funded hostel cum in New York from 1956 to 1971 (Krugman 1986). Krugman was both the protagonist and villain who was credited and later blamed for these trials. While the studies had no methodological flaws and there is no reason to doubt the honest and scientific intentions of Krugman, they generated a substantial amount of debate on ethics in medical research particularly with respect to informed consent and mental competency to give the same (Goldby 1971). However, many leading scientists of that time had spoken out in favour of Krugman.
While these appalling practices are indefensible, it should be noted that human sensibilities and ethics evolve with time. With advancements in tissue engineering and the development of organoids, it is not difficult to foresee that several decades into the future, new drugs would be tested on fully functional 3-dimensional tissue-organ systems instead of human volunteers. The current practice of controlled human trials may well be deemed as unethical by the future generations of researchers.
9.4 Serendipitous Discovery of Hepatitis B
Baruch Blumberg was a geneticist who was primarily interested in studying if differences in serum lipoproteins were linked to the genetic susceptibility to disease. While working at the National Institute of Health (NIH), Bethesda, USA, he studied blood samples obtained from indigenous populations in remote corners of the world to see if serum protein polymorphisms were related to disease susceptibility. He also collected blood samples of haemophilia patients who had received multiple blood transfusions. He believed that these patients who had received transfusions from unrelated donors would have antibodies against the pleomorphic serum proteins that could be detected by immunodiffusion in agar-gel. In 1965, his team published the seminal paper on the discovery of the Australia antigen in an Australian aborigine which showed immunologic reaction with the sera of the patients with haemophilia (Krugman 1986). The Australia antigen is now recognised to be the surface antigen of the Hepatitis B virus (HBsAg). Blumberg looked for antibodies against this new antigen in the sera of 107 patients with a history of multiple blood transfusions and detected it in 10.3% patients. They then tested the sera of 1704 healthy individuals and 659 patients to determine the prevalence of antibodies against the “Australia antigen”. While none were detected among Americans, antibodies were present in 3.8% of foreigners and 11.4% patients with leukaemia (Krugman 1986). Blumberg speculated that the Australia antigen may increase the susceptibility of leukaemia or may be related to a leukaemia-causing virus and even suggested that it may be useful in the early diagnosis of leukaemia. He could not have been further away from the truth.
It was the team of Alfred Prince at the New York Blood Centre who unravelled the significance of Blumberg’s mysterious discovery. He identified a novel antigen (designated as SH) in the serum of a haemophiliac with multiple transfusions who had developed “serum hepatitis” following a surgery for bleeding peptic ulcer which had required multiple transfusions (Goldby 1971; Blumberg et al. 1965). The patient’s serum had been collected in the intervening incubation period prior to the development of hepatitis. Prince subsequently demonstrated that the SH antigen was present in almost 80% patients with serum hepatitis and only 0.1% of healthy individuals. Further, it was found that the Australia antigen and SH antigen were similar and while this antigen was associated with “serum hepatitis”, there was no association with “infectious hepatitis” (Prince 1968).
The serendipitous nature of Blumberg’s discovery can be easily understood from the words of Harvey Alter, who was the second author in the landmark paper on Australia antigen. He wrote, “the chronological events surrounding the Australia antigen stand out as a monument to non-directed medical research and as a tribute to investigative perseverance. This tale of serendipity began in the mid-1960s when the Australia antigen was first reported by a geneticist who had been seeking new inherited polymorphisms among serum proteins, by a blood banker looking for non-cellular causes of febrile, non-haemolytic transfusion reactions and by a technologist destined to become a commercial airline pilot. A research interest in viral hepatitis was conspicuously absent in this investigative team. The significance of the Australia antigen, found when the serum of an Australian aborigine formed a precipitin line with the serum of a multiply transfused haemophiliac, was, at that time, unknown” (Alter 1981).
In 1976, Blumberg was honoured with the Nobel Prize in Medicine for the discovery of the Hepatitis B virus. Many authorities feel that the exclusion of Prince from this prestigious award was a travesty. Nonetheless, the contributions of Blumberg in Hepatitis B research were humongous.
He continued his work at the Institute of Cancer Research in Philadelphia and found that the Australia antigen was present in the sera of 20% patients presenting with acute hepatitis. He also showed a high prevalence of the Australia antigen in patients with Downs syndrome who had abnormal liver function tests on blood biochemistry with evidence of hepatitis on liver biopsy, and in patients with post-transfusion hepatitis. He thus surmised that this antigen was probably derived from a virus that caused hepatitis (London et al. 1969). He also noted that the Australia antigen had a diameter of 20 μm (London et al. 1969). The complete 42 μm viral particle was identified by Dane et al. in 1970 (Dane et al. 1970). By treating the Dane particles with detergent, Almeida et al. subsequently demonstrated that the “Australia antigen” was the surface antigen of the Hepatitis B virus (HBs Ag) and also found another antigen known as the core antigen (Almeida et al. 1971). HBs Ag was found to be non-infective but immunogenic and an excellent surrogate marker for the presence of Hepatitis B infection. Blumberg along with Millman also received the first patent for a prototype Hepatitis B vaccine in 1969 using HBs Ag isolated from the blood of infected patients (Blumberg 2002). Using the principles demonstrated by Blumberg, Maurice Hilleman, a researcher working at Merck developed Heptavax B, which was the first Hepatitis B virus vaccine to be approved by the FDA in 1983 (Szmuness et al. 1981).
9.5 Discovery of Hepatitis C: A Tale of Innovation and Collaboration
9.5.1 Non-A Non-B Hepatitis
The possibility of a second parenterally transmitted hepatitis virus was suggested by Gocke et al. in 1970 when they reported that many of the cases of post-transfusion hepatitis occur in patients who have been received blood from donors who were negative for Australia antigen (Gocke et al. 1970). At that time, a definitive conclusion could not be drawn due to constraints in the accuracy of the agar gel diffusion technique described by Blumberg. A sentinel moment in research in viral hepatitis was the development of a radioimmunoassay technique for detecting even small amounts of HBs Ag (Australia antigen) and its antibody. This test was easier to perform and was more accurate than the agar gel diffusion technique (Walsh et al. 1970).
Using this improved armamentarium of reliable serological tests for detecting both Hepatitis B and Hepatitis A, Harvey J. Alter and his colleagues working at the Department of Blood Transfusion in the Bethesda centre of NIH, USA, found that a considerable number of cases of post-transfusion hepatitis was attributable to neither Hepatitis A nor Hepatitis B. This entity was popularly referred to as non-A non-B hepatitis. Using injectable extracts prepared from the serum of patients with non-A non-B hepatitis, Alter successfully transmitted the disease in five chimpanzees in 1974. He demonstrated that all chimpanzees developed deranged liver function parameters and features of hepatitis on liver biopsy after an average incubation period of 13.4 weeks (Alter et al. 1972, 1978; Feinstone et al. 1975). His findings were confirmed by Tabor et al. who transmitted non-A non-B hepatitis to four chimpanzees who subsequently developed hepatitis after a prolonged incubation period (Tabor et al. 1978). The scientific community quickly inferred that the transmissibility of non-A non-B hepatitis and other findings implicated an infective agent probably a virus.
9.5.2 Search for the Virus: A Decade of Failures
The search began in earnest to identify this novel entity. However, all efforts to detect the virus over the next 15 years were in vain. The virus eluded detection by all the traditional methods of microscopy, culture and serology. At least 19 claims to have found the virus were made during this time but all of them failed to show immunological reactivity with a well characterised serum pool prepared by Alter from patients with non-A non-B hepatitis (Tandon et al. 1996).
9.5.3 A Game-Changing Innovation Backed Up by Dogged Perseverance
In the early 1980s, the application of molecular techniques was in a fledgling state. The implementation of this approach by Michael Houghton and his team at Chiron Corp., California, USA ultimately led to the identification of the virus. Houghton and his colleagues, Qui-Lim Choo and George Kuo, collaborated with Daniel Bradley of the Centre of Disease Control and Prevention (CDC), Atlanta, USA and developed a library of cDNA using hepatic and pancreatic tissue samples obtained from chimpanzees infected with non-A non-B hepatitis. These were then transferred to bacteria using lambda bacteriophages and cloned. Serum of patients with non-A non-B hepatitis was used as a likely source of antibodies. The hypothesis was that some of the proteins encoded by these cDNA would immunologically react with these antibodies in the serum of patients with non-A non-B hepatitis.
In 1989, after 6 years of painstaking research, the first such antigen epitope (corresponding to the viral envelope) was identified from a clone called 5-1-1. This clone hybridised with a single-stranded RNA fragment of 10,000 nucleotides obtained from infected chimpanzees but no such phenomenon was observed with genetic material obtained from non-infected chimpanzees. This finding suggested that the genetic material of the clone was present only in infected chimpanzees and possibly originated from a RNA virus. Further, they demonstrated that the antigen expressed by the clone 5-1-1 showed immunological reaction with antibodies present in the serum of chimpanzees infected with non-A non-B hepatitis and also with the isolate that had been prepared and characterised by Alter (Choo et al. 1989). The expressed antigen did not cross-react with antibodies against Hepatitis A and Hepatitis B. Moreover, patients with non-A non-B hepatitis were found to be seropositive for antibodies against 5-1-1 (Kuo et al. 1989). The etiologic agent of non-A non-B hepatitis was finally thus identified. This positive-stranded RNA virus was named Hepatitis C and was subsequently classified as a Flavivirus.
The novel methods adopted by Houghton drastically changed the field of virology and heralded a molecular revolution. Innovations and refinements in molecular techniques like PCR allowed the sequencing of the whole genome of the SARS COVID-19 within weeks of it first being reported in China. It is also prudent to reflect on the numerous failures in the period between Alter’s work in chimpanzees in 1974 and Houghton’s discoveries in 1989. Houghton’s team themselves went through 6 years of exasperating failures. Perseverance is key in research. Failure in research and indeed any aspect of life is not a cause for concern but rather a lesson in “what and how not to do”.
9.5.4 The Final Piece of the Puzzle
By the beginning of the 1990s decade, scientists had discovered the modes of transmission of HCV, established its infectivity in chimpanzees and unravelled its nuclear structure. However, whether infection with the virus alone was sufficient to cause clinically significant disease was yet unknown. Charles M. Rice and his team at Washington University, USA and Kunitada Shimotohno who was leading a Japanese research team characterised a highly conserved region at the 3′ non-translated end of the Hepatitis C viral genome (Kolykhalov et al. 1996; Tanaka et al. 1996). Rice correctly surmised that the highly conserved nature suggested that it was important in viral replication. Rice and Alexander Kolykhalovthen constructed a library of cDNA clones using an isolate previously characterised by Alter. A consensus sequence was developed by Sanger’s sequencing of many cDNA sequences followed by reconstruction using restriction enzymes. A complete genomic clone was ultimately genetically engineered using the previously described 3′ untranslated region of the Hepatitis c viral genome and the consensus sequence. RNA transcribed from these cDNA clones was infective to chimpanzees and resulted in hepatitis (Kolykhalov et al. 1997). Thus, the link between Hepatitis C virus and liver disease was firmly established.
9.6 The Landscape of Hepatitis B and C in 2020: Reaping the Benefits
Bar-Gal et al. recently isolated the complete genome of Hepatitis B from hepatic extracts of a Korean mummy (Bar-Gal et al. 2012). Dating estimates of the most recent common ancestor places the origins of this ancient Hepatitis B sequence to be 3000–100,000 years old. Yet, within six decades of the discovery of the viruses implicated in chronic hepatitis, we have developed ways to diagnose, prevent and treat them. Indeed, the pace of advancement of our knowledge and technical prowess is exponential once the initial spark has been lit by stalwarts.
Highly accurate, readily available and cheap serological tests have facilitated the easy screening of Hepatitis B and C infections. Routine screening of all blood donors for antibodies against these viruses is now the norm all over the world. Recognition of the modes of viral transmission led to the adoption of universal safety precautions which has greatly reduced occupational risk in health care workers. Hepatitis B vaccination is now a part of the universal immunisation schedule. Importantly, as Hepatitis B is a leading cause of liver cancer, this vaccine is also the first anti-cancer vaccine.
The non-specific antiviral agent interferon (discovered in 1957) was the first drug that was approved for the treatment of Hepatitis B in 1991 (Trepo 2014). In the same year, lamivudine, a nucleoside analogue, was shown to prevent the replication of Hepatitis B by inhibiting RNA-dependent DNA polymerase (an enzyme involved in reverse transcription). In 1998, Lamivudine was the first of several oral drugs belonging to a class known as “nucleoside inhibitors” that was approved for the treatment of Hepatitis B (Trepo 2014). Although highly effective, lamivudine and many other “nucleoside inhibitors” are constrained by the development of resistance due to genetic mutations. Currently, “nucleoside inhibitors” with a high barrier to resistance like tenofovir are used for treating Hepatitis B. Pegylated Interferon α also continues to be a viable treatment option although it is uncommonly used in India and most parts of the world because of adverse effects, tolerability issues and need for injections. The main problem with currently available Hepatitis B therapies is that while they are excellent in supressing the viral load, they cannot eliminate the infection completely. This is because some of the Hepatitis B DNA exists as covalently closed circular DNA (cccDNA) in a plasmid-like form inside the host nucleus and acts as a template for progeny production. cccDNA cannot be effectively targeted by currently available means. Elimination of cccDNA is the holy grail of research in Hepatitis B management. Epigenetic modulation and gene editing techniques like the CRISPR-CAS system hold potential in targeting cccDNA with the promise of “complete cure” in the future (Lok et al. 2017).
Long an unmet meet, the treatment of Hepatitis C was revolutionised in the past decade. Treatment failures and adverse effects were frequent in the interferon era. Research into directed therapies against Hepatitis C was greatly hindered in the 1990s by the absence of a method to maintain and propagate the virus in-vitro. The development of replicons by Bartenschlager and Lohmann in 1999, was the next momentous breakthrough in Hepatitis C research (Lohmann et al. 1999). These subgenomicrepicons were bicistronic RNA constructs that could replicate autonomously in human hepatoma-derived Huh-7 cells. Replicons facilitated the granular understanding of the Hepatitis C genome and its structural and non-structural proteins, precise characterisation of the viral replication complex, and permitted the screening and testing of directly acting antivirals (DAAs) in vitro. In the current generation, oral DAAs have efficacy rates of >95–99% with minimal adverse effects (Baumert et al. 2019). They represent a paradigm shift in the management of Hepatitis C and are offering the hope of cure to millions of patients. An effective vaccine against Hepatitis C is still lacking. Despite the availability of highly effective treatment, a vaccine is still deemed necessary to attain the ultimate goal of elimination of the scourge of Hepatitis C.
9.7 Conclusion
The story of the unravelling of the mysteries behind “serum hepatitis” is an incredible testament to the remarkable accomplishments of modern biomedical research. Acknowledgement of the work of these “masters of science” and the remarkably long way that we have come in our understanding of Hepatitis B and C provides much-needed encouragement to researchers as they try to fight the hitherto unknown threat of COVID-19.
References
Almeida JD, Rubenstein D, Stott EJ (1971) New antigen-antibody system in Australia-antigen-positive hepatitis. Lancet 2:1225–1226
Alter HJ (1981) Hepatitis B: a tribute to nondirected medical research. Semin Liver Dis 1:1–6
Alter HJ, Holland PV, Purcell RH, Lander JJ, Feinstone SM, Morrow AG et al (1972) Posttransfusion hepatitis after exclusion of commercial and hepatitis-B antigen-positive donors. Ann Intern Med 77:691–699
Alter HJ, Purcell RH, Holland PV, Popper H (1978) Transmissible agent in non-A, non-B hepatitis. Lancet 1:459–463
Bar-Gal GK, Kim MJ, Klein A, Shin DH, Oh CS, Kim JW et al (2012) Tracing hepatitis B virus to the 16th century in a Korean mummy. Hepatology 56:1671–1680
Baumert TF, Berg T, Lim JK, Nelson DR (2019) Status of direct-acting antiviral therapy for hepatitis C virus infection and remaining challenges. Gastroenterology 156:431–445
Blumberg BS (2002) Hepatitis B: the hunt for a killer virus. Princeton University Press, Princeton and Oxford, pp 72–146
Blumberg BS, Alter HJ, Visnich S (1965) A “new” antigen in leukemia sera. JAMA 191:541–546
Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M (1989) Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359–362
Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H et al (2019) Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 4:135–184
Dane DS, Cameron CH, Briggs M (1970) Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet 1:695–698
Dooley DP (2005) History of U.S. military contributions to the study of viral hepatitis. Mil Med 170:71–76
Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV (1975) Transfusion associated hepatitis not due to viral hepatitis type A or B. N Engl J Med 292:767–770
Gocke DJ, Greenberg HB, Kavey NB (1970) Correlation of Australia antigen with post-transfusion hepatitis. JAMA 212:877–879
Goel A, Seguy N, Aggarwal R (2019) Burden of hepatitis C virus infection in India: a systematic review and meta-analysis. J Gastroenterol Hepatol (Australia) 34:321–329. https://doi.org/10.1111/jgh.14466
Goldby S (1971) Experiments at the Willowbrook State School. Lancet 1:749
Gruber W, Virchow R (1865) Ueber das Vorkommen und den Nachweis des hepatogenen, insbesondere des katarrhalischen Icterus. Arch Patholog Anat Und Physiol Und Für Klin Med 32:117–125
Khuroo MS, Sofi AA (2020) The discovery of hepatitis viruses: agents and disease. J Clin Exp Hepatol 10:391–401. https://doi.org/10.1016/j.jceh.2020.04.006
Kolykhalov AA, Feinstone SM, Rice CM (1996) Identification of a highly conserved sequence element at the 3′ terminus of hepatitis C virus genome RNA. J Virol 70:3363–3371
Kolykhalov AA, Agapov EV, Blight KJ, Mihalik K, Feinstone SM, Rice CM (1997) Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 277:570–574
Krugman S (1986) The Willowbrook hepatitis studies revisited: ethical aspects. Rev Infect Dis 8:157–162
Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH et al (1989) An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 244:362–364
Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R (1999) Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110–113
Lok AS, Zoulim F, Dusheiko G, Ghany MG (2017) Hepatitis B cure: from discovery to regulatory approval. J Hepatol 67(4):847–861
London WT, Sutnick AI, Blumberg B (1969) Australia antigen and acute viral hepatitis. Ann Intern Med 70:55–59
Lürman A (1885) Eine Icterus Epidemic. Berlin Klin Wochenschr 22:207
MacCallum FO (1943) Jaundice in syphilitics. Br J Vener Dis 19:63
MacCallum FO (1947) Homologous serum jaundice. Lancet 2:691–692
Murchison C, ByIhird LB, Fayrer J (1886) Clinical lectures on diseases of the liver, jaundice, and abdominal dropsy. Biomed J Digit Project 31
Prince AM (1968) An antigen detected in the blood during the incubation period of serum hepatitis. Proc Natl Acad Sci U S A 60:814–821
Schmidt PJ (1999) Blood: an epic history of medicine and commerce. Transfusion 39:793–793. https://doi.org/10.1046/j.1537-2995.1999.39070793.x
Sood A, Suryaprasad A, Trickey A, Kanchi S, Midha V, Foster MA et al (2018) The burden of hepatitis C virus infection in Punjab, India: a population-based serosurvey. PLoS One 13:1–18. https://doi.org/10.1371/journal.pone.0200461
Stokes JH, Ruedemann R Jr, Lemon WS (1920) Epidemic infectious jaundice and its relation to the therapy of syphilis. Arch Int Med 26:521–543
Szmuness W, Stevens CE, Zang EA, Harley EJ, Kellner A (1981) A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology 1(5):377–384
Tabor E, Gerety RJ, Drucker JA et al (1978) Transmission of non-A, non-B hepatitis from man to chimpanzee. Lancet 1:463–466
Tanaka T, Kato N, Cho MJ, Sugiyama K, Shimotohno K (1996) Structure of the 3′ terminus of the hepatitis C virus genome. J Virol 70:3307–3312
Tandon BN, Acharya SK, Tandon A (1996) Epidemiology of hepatitis B virus infection in India. Gut 38(Suppl 2):S56–S59
Trepo C (2014) A brief history of hepatitis milestones. Liver Int 34:29–37. https://doi.org/10.1111/liv.12409
Walsh JH, Yalow RS, Berson SA (1970) Radioimmunoassay of Australia antigen. Vox Sang 19(3):217–224
WHO (2017) Global hepatitis report. World Health Organization, Geneva. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf
Wong DT, Martin CM, Boyer JL, Jain D (2015) Historical path of discovery of viral hepatitis. HMSR 3:18–36
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Ethics declarations
All authors have no financial disclosures and declare no conflict of interest.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
De, A., Chawla, Y.K. (2022). Discovery of Hepatitis Viruses and Two Nobel Prizes: A Tale of Keen Observations, Serendipity, Collaborative Research, Astute Interpretations and Game-Changing Innovations. In: Sobti, R., Ganju, A.K. (eds) Biomedical Translational Research. Springer, Singapore. https://doi.org/10.1007/978-981-16-8845-4_9
Download citation
DOI: https://doi.org/10.1007/978-981-16-8845-4_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8844-7
Online ISBN: 978-981-16-8845-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)