Abstract
Alzheimer’s disease (AD) is recognized as complex neurological disease marked by gradual dementia and deterioration of cognitive function. As per World Alzheimer Report (2015), 46.8 million individuals have mental illness and the number will increase twofold every 20 years. Synthetic drugs used to treat cognitive loss in AD patients are tacrine, donepezil, rivastigmine, and memantine. They improve overall memory temporarily, but cause other side effects. Medicinal plants used in traditional medicinal systems have been used to treat progression of disorders of neurons like memory deficits, i.e., amnesia, dementia, and AD many years ago. Natural compounds isolated from plant sources, like phenolics, terpenoids, and alkaloids have been recommended to have the potential to cure AD because of their inflammation/oxidative stress reducing capacity and anti-amyloidogenic properties. In the present review, we discussed the mechanism of neuroprotective effect of medicinal plant extracts and non-phenolic compounds, i.e., Bacoside A (Bacopa monnieri), acetyl-11-keto-β-boswellic acid (Indian Frankincense), caffeine (Coffea arabica and C. canephora), Galantamine (Galanthus nivalis), ginkgolide B and bilobalide (Ginkgo biloba), Hederacolchiside E (Hedera colchica), Huperzine (Huperzia serrata), and Withanolide-A and Withaniferin-A (Withania somnifera).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
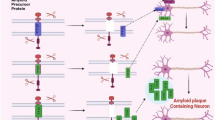
Neurological diseases are increasingly recognized as major causes of death and disability worldwide. The most prevalent neurological diseases are Parkinson’s (PD), multiple sclerosis (MS), AD, and Huntington’s disease (HD). The most important pathological symptoms of neuro degenerative diseases are mitochondrial dysfunction, synapse loss, elevated oxidative stress, protein abnormalities, decreased neuronal survival, and nitrosative stress (Winner et al. 2011). It is accepted that among neurodiseases, AD is most complex neurological disease characterized by gradual dementia and failure of cognitive function. The prevalence of AD accounts for about 60% of all cases (van Marum 2008). As per World Alzheimer Report (2015), 46.8 million individuals have mental illness and the number will increase twofold every 20 years. The disorder is typified by variations in different genes, such as the β amyloid precursor protein (APP) and persenilins (PS1, PS2), proteins such as BACE (Aβ cleaving enzyme), APOE, PS1/2, and APP, tau and secretases play a crucial role in the immunopathology of AD.
The approved medicine used to treat cognitive impairment in AD patients are tacrine, donepezil, rivastigmine, galantamine, and memantine. They improve overall memory temporarily, but cause gastrointestinal complexity, such as diarrhea, hepatotoxicity, nausea, weight loss, etc. (Kim et al. 2010). Herbal recipes used in traditional medicinal systems are fundamentally preventive, protective, nutritive, and curative. These traditional herbs have been used to treat progression of disorders of neurons like abstraction and AD since years ago. Because these traditional herbs and isolated chemical components may be less toxic than synthetic drugs. The herbal drugs used in medicinal systems of Indian tradition to cure AD are Centella asiatica (Apiaceae), Bacopa monnierii (Scrophulariaceae), Curcuma longa (Zingiberaceae), Withania somnifera (Solanaceae), Celastrus paniculatus (Celastraceae), Nardostachys jatamansi (Caprifoliaceae), Tinospora cordifolia (Menispermaceae), Morinda citrifolia (Rubiaceae), etc. Natural compounds such as alkaloids, terpenoids, and phenolic components of plant origin have been proved to have the potential therapeutic value to prevent AD because of their inflammation/oxidative stress reducing capacity and anti-amyloidogenic properties (Kim et al. 2010).
The present work is concentrated on the role of the potential traditional herbal extracts and their active principles in the prevention of AD with special reference to non-phenolic compounds of the herbal drugs to understand its possible mechanism.
1.1 Bacopa monnieri (L.) Wettst.
B. monnieri (L.) Wettst. (Family: Scrophulariaceae) is known as Brahmi (Telugu) or Aindri (Sanskrit). Medhya rasayana is a popular brain tonic prescribed by Ayurveda practitioners for intellectual, cognition rejuvenation and in memory loss conditions (Vishnupriya and Padma 2017). Studies on standardized extract of B. monnieri revealed that the extract significantly reversed scopolamine induced impaired the acquisition and recapture of memory producing both retrograde and anterograde amnesia in Morris water maze mice model (Saraf et al. 2011). Further, Holcomb et al. (2006) evaluated the efficacy of B. monnieri extract on expression studies of M146L presenilin-1 mutations and Swedish amyloid precursor protein in PSAPP mice. The results showed that B. monnieri extract has downregulated amyloid (Abeta) gene expression 60% and reversed behavioral changes in incessant locomotion and Y-maze performance in PSAPP mice. Bacosides (Fig. 16.1), isolated from B. monnieri, significantly reduced stress in adult male Sprague Dawley rats via modulating the expression levels of Hsp 70 and activities of superoxide dismutase and cytochrome P450 (Chaudhari et al. 2017).
B. monnieri extract significantly decreased glutamate- and β-amyloid protein (25–35) challenged toxicity in cortical neurons by reducing intracellular oxidative stress and by inhibiting lipid peroxidation in neuronal cells (Limpeanchob et al. 2008). Amyloid-beta (Aβ42) polypeptide plays remarkable role in AD development due to neuronal dysfunction. Bacoside A, saponin isolated from B. monnieri significantly reduced toxicity of cells and suppressed fibril formation both in membrane vesicles and in buffer solution (Malishev et al. 2017). Methanolic extract of B. monnieri significantly inhibited S-nitroso-N-acetyl-penicillamine (SNAP) induced generation of free radical and damage of DNA in concentration-dependent manner in isolated rat astrocytes (Russo et al. 2003). In a double-blind placebo and randomized experiment, BacoMind, a standardized phytochemical enriched B. monnieri extract, improved cognitive functions such as attentiveness and remembrance in patients (Barbhaiya et al. 2008), in AD patients (Goswami et al. 2011) and in medical students (Kumar et al. 2016).
Further, Usha et al. (2008) conducted a clinical experiment on efficiency of BacoMind on cognitive enhancement in children. The results showed that BacoMind at 225 mg/day for a span of 4 months, significantly increased working memory and short-term verbal memory in 28 children volunteers. Rai et al. (2015) evaluated protective effect of CDRI-08, a special extract of B. monnieri in scopolamine-induced neurotoxicity amnesic mice. In results, pretreatment of CDRI-08 to amnesic mice treated with scopolamine bring back the spatial memory. The mechanism of action the drug found to be enhancing the expression levels of GluN2B subunit and decreased levels of acetylcholinesterase activity prefrontal cortex and hippocampus, similar to the normal mice. Oral administration of Medhya Rasayana, an Ayurvedic drug prepared from B. monnieri significantly reduced anticholinesterase activity and secured the cholinergic neurons than the standard drugs rivastigmine, donepezil, and galantamine. It further reduced the deposition levels of β-amyloid proteins in hippocampal and stress-induced damages in hippocampal regions of rat models in in vivo study (Chowdhuri et al. 2002). Peth-Nui et al. (2012) investigated the effect of standardized B. monnieri extract on brain function and remembrance in 23 males and 37 female healthy elders for a period of 12 weeks and concluded that the B. monnieri extract improved attention, cognitive processing, and working memory partly by inhibiting the activity of acetyl cholinesterase (AChE) activity.
1.2 Boswellia serrata Roxb.
B. serrata Roxb. (Family: Burseraceae) is known as olibanum or Indian Frankincense. Its oleo gum resin is traditionally used in Indian traditional systems (Ayurveda or Unani or ethnomedicine) to treat several types of human ailments like pain and cardiac debility, inflammatory health ailments, blood disorders, rheumatism, urinary disorders, and corneal ulcer (Paranjpe 2001; Khan 1885). Nrf2, a transcription factor, plays a main role in regulation of antioxidant genes/proteins such as heme oxygenase-1 (HO-1). Dysregulation of HO-1 is connected to pathogenesis of variety of neurodegenerative diseases including AD (Habtemariam 2019). Medication with acetyl-11-keto-β- boswellic acid (AKBA, Fig. 16.2) resulted noteworthy reduction in the volume of infarcts and number of apoptotic cells and upregulated expression amounts of HO-1 and Nrf2 in brain tissues of tested animals.
Further, AKBA increased the expression of HO-1 and Nrf2 expression in oxygen and glucose deprivation induced oxidative stress in primary cultured neurons (Ding et al. 2014). In another experiment, B. serrata at 50 mg/kg significantly improved memory retrieval and step-through latency in streptozotocin-induced Alzheimer disease in rat models (Zaker et al. 2015). Medication with water extract of B. serrata gum resin for 42 days of experimental schedule, significantly decreased streptozotocin-induced Alzheimer disease symptoms, i.e., decreased step-through latency, increased the decimal of step-through into the dark compartment and increased the spending time in the dark compartment (Beheshti and Aghaie 2016).
1.3 Coffea arabica L.
Methylxanthines isolated from the coffee (Coffea arabica L. and C. canephora L.) reported to have antagonists on adenosine-receptors. Caffeine (Fig. 16.3) a indifferent, competitive inhibitor of A2A and A1 receptors of adenosine, diffused in CNS. General depressant reaction is due to binding of adenosine to specific targets, lowering blood pressure and slowing rate of hart beat (Gu et al. 1992). As per epidemiological reports, caffein and A2A receptor inhibitor contribute a crucial role in delaying and avoidance of the emergence of AD. Maia and de Mendonca (2002) case study results demonstrated that people consumed two cups of coffee per day for 20 years have low risk for onset of AD than the people consumed less coffee. Systematic review on case control and prospective studies on efficiency of coffee on AD, concluded that coffee has therapeutic effects (Barranco Quintana et al. 2007). These consequences were in line with previous reports in which consumption of coffee was regularly protective against PD for women and men without estrogen therapy (Ascherio et al. 2001, 2004; Ascherio and Chen 2003).
1.4 Galanthus nivalis L.
G. nivalis L. (Family: Amaryllidaceae) known as snowdrop, is widely grown in gardens and naturalized particularly in northern Europe. The active principle isolated from this plant is an alkaloid, galantamine. Galantamine is commercially accepted as AChEi drug to treat AD patients at different stages in many countries. Castillo (2017) assessed the efficiency of galantamine on beta-amyloid peptide-challenged damage of DNA and cell viability in SH-SY5Y neuroblastoma cell lines of human in vitro studies. The results revealed that treatment with galantamine exhibited noteworthy recovery of cell longevity, reduced the death of necrotic cells and exerted anticancer effects. Lopes et al. (2013) assessed the neuroprotective activity of galantamine alone or in mixed with memantine, an N-methyl-d-aspartate (NMDA) receptor inhibitor, in rat primary cultures of cortical neurons. Galantamine exhibited its neuroprotective effect via activation of α7 and α4b2 nicotinic acetylcholine receptor at a concentration of 5 μ mol/L.
Prins et al. (2014) conducted a clinical, placebo-controlled, randomized, double-blind trial experiment to investigate the neuroprotective effect of galantamine in 364 mild cognitive impairment patients for a time schedule of 1 year at 16–24 mg flexible-dose every day. They concluded that patients received galantamine showed a lower rate of whole brain atrophy than patients received placebo. In another experiment, galantamine significantly attenuated β-amyloid1–40 and thagapsigarin challenged cell toxicity of SH-SY5Y cell lines and bovine chromaffin cell. The mechanism related to neuroprotective effect of galantamine was by activation of levels of α7 nicotinic receptors and enhancing the expression levels of Bcl-2, the antiapoptotic protein (Arias et al. 2004). In in vitro study, galantamine significantly modulated regulation of signal transmission via intracellular cascade implicated in rat hippocampal slices of brain ischemia-reperfusion model put through glucose and oxygen deprivation (OGD) subsequently reoxygenation. Galantamine expressed its neuroprotective mechanism by reducing the induction of iNOS and secretion of NO caused by OGD via Jak2. It also decreased ROS generation by NADPH oxidase (NOX) activation (Egea et al. 2012). Kihara et al. (2004) investigated the efficiency of galantamine on β-amyloid-increased glutamate toxicity using cortical neurons of primary rat cultures. The defensive effect of galantamine was expressed by partial inhibition of α7 nAChR antagonists.
1.5 Ginkgo biloba L.
G. biloba L. (Family: Ginkgoaceae), known as the maidenhair tree or Ginkgo, belongs to Gymnosperms. The usage of Ginkgo biloba L. to treat neuro diseases was started by German physicians in 1965. EGb 761, a patented and commercialized extract of G. biloba leaves was developed in the early 1970s (Wagner 1999). The standardized products of Ginkgo extensively prescribed in Europe and the USA to treat various types of neurodegenerative diseases. EGb 761 extracts rich in flavonoids and terpenic lactones may be responsible for its therapeutic action. The terpenoid fraction consists mainly diterpenic lactones, sesquiterpene trilactone, the bilobalide, and the ginkgolides A, B, C, J and M (Birks and Grimley 2009).
G. biloba extract and active ingredient ginkgolide B (GB), a terpene trilactone solution reduced the endothelial permeability coefficients and upregulated ZO-1 and occludin expression, the tight junction proteins in endothelial cells (Fang et al. 2010). Administration of ginkgolide B demonstrated that GB reduced the neurological deficits core and enhanced the amount of nestin, mRNA expression levels of neurotrophic factor derived from the brain and growth factor of the epidermis. Further, it enhanced brain-derived neurotrophic factors expression levels in animal models with middle cerebral artery occlusion (MCAO) (Zheng et al. 2018). Further, ginkgolide B derivative, 10-O-(N, N, dimethyl aminoethyl)-ginkgolide B methane sulfonate, (XQ-1H) significantly reduced high lipid profile, cerebral infarct size and improved Blood–Brain Barrier (BBB) permeability in hyperlipidemic rats (Fang et al. 2015). Pretreatment with ginkgolide K (GK) reduced mitochondrial fission, modulated mitochondrial dysfunction and inhibited mitochondrial permeability transition pore opening through a GSK-3β-dependent mechanism of action in both MCAO mice and OGD/R neuroblastoma cells (Zhou et al. 2017).
EGb 761 and its component, ginkgolide A, modulated Aβ-induced disease symptoms such as reduced chemotaxis behavior, 5-HT hypersensitivity and paralysis in a transgenic Caenorhabditis elegans (Wu et al. 2006). In in vitro study, ginkgolide B attenuated pro-oxidant Aβ25–35 peptide induced oxidative stress symptoms such as higher amount of ROS/RNS and decreased concentration of mitochondrial Apurinic/apyrimidinic endonuclease 1 (APE1) in human neuroblastoma SH-SY5Y and IMR-32 cells (Kaur et al. 2015). Bilobalide, diterpene constituent of G. biloba, showed neuroprotection in ischemia-induced neurotoxicity in mice model. The mechanism involves drug reversed ischemia-induced brain damage, mitochondrial dysfunction, leading to reduction in the release of glutamate amount (Schwarzkopf et al. 2013).
1.6 Hedera colchica (K. Koch) K. Koch
H. colchica (K. Koch) K. Koch (Family: Araliaceae) commonly called ivy/Persian ivy, is an evergreen woody vine, native to Turkey. Hederacolchiside E, saponin, active principle isolated from H. colchica reported to have antioxidant activity (Gülçin et al. 2004). Neuroprotective efficiency of hederacolchiside E, isolated by bioactivity-guided fractionation from root extract of Pulsatilla koreana, was studied in in vivo and in vitro methods (Han et al. 2007). Hederacolchiside E reversed scopolamine-challenged cognitive impairment in rats and reduced amyloid-beta peptide (1–42) induced cell toxicity in human SK-N-SH cells. Hederacolchiside E and its 11 derivative compounds were assessed for neuroprotective effects against H2O2- and Aβ1–42-induced neurotoxicity using cell-based assays (Li et al. 2018). Compound 1 and 7 exhibited noteworthy reduction in LDH release levels, amount of intracellular ROS, and extent of malondialdehyde (MDA) enhancement in Aβ1–42-induced cells.
1.7 Huperzia serrata (Thunb. ex Murray) Trevis.
H. serrata (Family: Lycopodiaceae) known as the club moss, has been used by tribes of China to treat strain, swellings, contusion, and schizophrenia. Liu et al. (1986) isolated active chemical constituent, the alkaloid (−)-huperzine A, reported to possess AChE inhibition, which was associated with the traditional uses of this plant.
Huperzine A significantly modulated the D-galactose (D-gal) induced neurodegenerative disease symptoms such as increased cellular senescence and auditory brainstem response (ABR) threshold and reduced neurofilament in the cochlear tissues in Sprague Dawley rats. Further it also suppressed expression levels of NF-kB in Schwann cells and notably blocked D-gal-stimulated expression amounts of pro-inflammatory markers such as IL-1b, IL-6 and TNF-α (Li and Shi 2019). Huperzine A attenuated beta-amyloid protein, H2O2, ischemia, glutamate, and staurosporine-induced cell toxicity and programmed cell death in animal models. The protective mechanism of Huperzine A involved in restoring the oxidative stress controls the expression levels of apoptotic markers i.e., Bcl-2, caspase-3, P53, and Bax, mitochondrial protection and interferes with APP metabolism (Wang and Tang 2005).
In in vivo study, Huperzine A exhibited noteworthy reduction in pathological symptoms, i.e., memory deficiency and neurodegeneration, induced by Aβ1–40 in both hippocampus and cortex of animal models, and inhibited amyloid deposits formation in cortex (Wang et al. 2001). Additionally, huperzine A also reported that it can directly process the amyloid precursor protein (Peng et al. 2006). With regard to cell culture studies, treatment with (-)-huperzine increased the secretion levels of α-APPs, and inhibited Aβ in embryonic kidney 293 cells of human, transfected with the Swedish mutation (Peng et al. 2006, 2007). Acetylcholinesterase (AChE) inhibitory potential and attenuation of Aβ-induced toxicity in NG108-15 and PC cells were studied (Zhang et al. 2002).
Receptors of glutamate play crucial role in the alteration of synaptic plasticity and play noteworthy roles in learning and memory (Contractor and Heinemann 2002). Cells treated with huperzine-A (100 nM) significantly modulated the glutamate (100 μM) induced morphological changes, such as reduction of axon-like processes and diffusion of cell aggregates and cell viability of cell cultures of rat cerebellum (Ved et al. 1997). Huperzine A at 10 μM concentration attenuated N-methyl-d-aspartate (NMDA) challenged cell viability in neuronal cultures in concentration-dependent manner. The binding capacity of huperzine A to proximal to the phencyclidine binding sites and MK-801 is the diagnostic feature in the process of NMDA receptor modulating activity (Gordon et al. 2001). Neuroprotective effect of huperzine A and enantiomers of huperzine A demonstrated by inhibiting the MK-801 binding capacity in the cerebral cortex of animal models was studied (Zhang et al. 2000). Huperzine A was identified as an efficient drug in pre- and post-treatment experiments, against N-methyl-d-aspartate induced seizures and epilepsy (Coleman et al. 2008).
In a placebo-controlled, double-blind, parallel, multi-centered clinical experiment, it showed noteworthy developments in behavior, cognition and daily living activities than patients given with placebo was studied in China (Xu et al. 1995, 1999; Zhang et al. 2002a). Similarly, the efficiency of huperzine-A on learning and memory enhancement clinical experiment in school students with 34 pairs was studied in double-blind method in China (Sun et al. 1999). They conclude that huperzine A-treated students group showed better performance in Chinese-language lesson quizzes than the placebo-treated group. A multi-centered, randomized, prospective, double-blind, three-arm, dose-escalation clinical experiment was conducted to assess the neuroprotective effect of huperzine A in 210 patients with AD in the USA. Patients treated with 400 μg of huperzine A demonstrated a 2.27-point enhancement in the Alzheimer’s Disease Assessment Scale-Cognitive Subscale, while 0.29- point decline was observed in the after 11 weeks treatment period (Rafii et al. 2011).
1.8 Withania somnifera Dunal.
W. somnifera Dunal. (Family: Solanaceae) is locally called as Aswagandha (Sankskrit). In Ayurveda, the root of Ashwagandha has been used as a Rasayana and it is used as stimulant, diuretic, tonic, aphrodisiac, narcotic, astringent, and thermogenic (Singh et al. 2011). W. somnifera root extract showed a noteworthy improvement in the gripping ability, motor movement patterns and restored tyrosine hydroxylase and antioxidant enzyme activity in paraquat (PQ) and maneb challenged nigrostriatal dopaminergic neurodegeneration in mice models (Prakash et al. 2013). Withanolide-A (Fig. 16.4), a major chemical constituent of W. somnifera root showed neuroprotective ability by modulating brain damage, morphology of brain tissue, levels of neurotransmitter in cerebral and brain tissue morphology in mice (Mukherjee et al. 2020). Banu et al. (2019) investigated the neuroprotective efficiency of withaniferin-A, steroidal lactone characterized from roots of W. somnifera on aging induced oxidative stress in substantia nigra (SN) and striatum (ST) of aged rat brain. In conclusion, withaniferin-A significantly modulated the aging induced oxidative stress biochemical changes such as reduced motor activity, increased free radical concentration, reduced antioxidant enzyme levels, increased active caspase-3 activity, enhanced apoptotic nuclear morphology in striatum and substantia nigra of aged rat. Further, Rabhi et al. (2019) studied the neuroprotective activity of CR-777, a glutathione derivative of withaferin A (Fig. 16.5), neurotoxicity caused by 6-hydroxydopamine (6-OHDA), α-synuclein (α-Syn) and 1-methyl-4-phenylpyridinium (MPP+), in rat dopaminergic neuron cells. In 6-OHDA-treated neurons, CR-777 at nanomolar concentrations enhanced neurite network and cell survival and reduced the expression levels of α-Syn in rat dopaminergic neurons.
2 Perspectives
Medicinal plants used in traditional medicinal systems have been used in the prevention of neurological diseases since long time. Natural compounds isolated from plant sources such as terpenoids, alkaloids, and phenolic components have been reported to have the potential therapeutic value to prevent AD because of their anti-amyloid genic, reduction capacity of oxidative stress and inflammation properties. Some of the potential medicinal plant extracts and its chemical constituents scientifically proved its intellectual, cognition rejuvenation and memory enhancing power in AD patients, such as Bacopa monnieri extract and its active principle bacoside A, ginkgolides from Ginkgo biloba, withanolides and withaniferin from Withania somnifera, huperzine from Huperzia serrata, etc. Extensive research studies are to be conducted in order to establish the long-term usage and efficacy of using phytochemicals as potential therapeutics for neurological diseases and sustainable utilization of drug yielding plants by using biotechnological interventions.
References
Arias E, Alés E, Gabilan NH, Cano-Abad MF, Villarroya M, García AG, López MG (2004) Galantamine prevents apoptosis induced by β-amyloid and thapsigargin: involvement of nicotinic acetylcholine receptors. Neuropharmacology 46:103–114
Ascherio A, Chen H (2003) Caffeinated clues from epidemiology of Parkinson’s disease. Neurology 61:S51–S54
Ascherio A, Zhang SM, Hernan MA (2001) Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol 50:56–63
Ascherio A, Weisskopf MG, O'Reilly EJ (2004) Coffee consumption, gender, and Parkinson’s disease mortality in the cancer prevention study II cohort: the modifying effects of estrogen. Am J Epidemiol 160:977–984
Banu MR, Ibrahim M, Prabu K, Rajasankar S (2019) Neuroprotective efficacy of withaferin A on aging mediated oxidative stress in striatum and Substantia nigra of wistar albino rat. Drug Invent Today 12:425–431
Barbhaiya HC, Desai RP, Saxena VS, Wasim PP, Geetharani P, Allan JJ, Venkateswarlu K, Amit A (2008) Efficacy and tolerability of BacoMind on memory improvement in elderly participants—a double blind placebo controlled study. J Pharmacol Toxicol 3:425–434
Barranco Quintana JL, Allam MF, Serrano Del Castillo AR, Fernandez-Crehuet Navajas R (2007) Alzheimer’s disease and coffee: a quantitative review. Neurol Res 29:91–95
Beheshti S, Aghaie R (2016) Therapeutic effect of frankincense in a rat model of Alzheimer’s disease. Avicenna J Phytomed 6:468–475
Birks J, Grimley EJ (2009) Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev CD003120
Castillo WO (2017) Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease. Neural Regen Res 12:916–917
Chaudhari KS, Tiwari NR, Tiwari RR, Sharma RS (2017) Neurocognitive effect of nootropic drug Brahmi (Bacopa monnieri) in Alzheimer’s disease. Ann Neurosci 24:111–122
Chowdhuri DK, Parmar D, Kakkar P, Shukla R, Seth P, Srimal R (2002) Antistress effects of bacosides of Bacopa monnieri: modulation of Hsp70 expression, superoxide dismutase and cytochrome P450 activity in rat brain. Photother Res 16:639–645
Coleman BR, Ratcliffe HR, Oguntayo SA, Shi X, Doctor BP, Gordon RK, Nambiar MP (2008) [+]-Huperzine A treatment protects against N-methyl-d-aspartate-induced seizure/status epilepticus in rats. Chem Biol Interact 175:387–395
Contractor A, Heinemann SF (2002) Glutamate receptor trafficking in synaptic plasticity. Sci STKE 156:Re 14
Ding Y, Chen M, Wang M, Wang M, Zhang T, Park J (2014) Neuroprotection by acetyl-11-keto-[bgr]-boswellic acid, in ischemic brain injury involves the Nrf2/HO-1 defense pathway. Sci Rep 4
Egea J, Martín-de-Saavedra MD, Parada E, Romero A, del Barrio LAO, Rosa AO, García AG, López MG (2012) Galantamine elicits neuroprotection by inhibiting iNOS, NADPH oxidase and ROS in hippocampal slices stressed with anoxia/reoxygenation. Neuropharmacology 62:1082–1090
Fang W, Deng Y, Li Y, Shang E, Fang F, Lv P, Bai L, Qi Y, Yan F, Mao L (2010) Blood brain barrier permeability and therapeutic time window of Ginkgolide B in ischemia-reperfusion injury. Eur J Pharm Sci 39:8–14
Fang W, Sha L, Kodithuwakku ND, Wei J, Zhang R, Han D, Mao L, Li Y (2015) Attenuated blood-brain barrier dysfunction by XQ-1H following ischemic stroke in hyperlipidemic rats. Mol Neurobiol 52:162–175
Gordon RK, Nigam SV, Weitz JA, Dave JR, Doctor BP, Ved HS (2001) The NMDA receptor ion channel: a site for binding of huperzine A. J Appl Toxicol 21:S47–S51
Goswami S, Saoji A, Kumar N, Thawani V, Tiwari M, Thawani M (2011) Effect of Bacopamonnieri on cognitive functions in Alzheimer’s disease patients. Int J Collab Res Internal Med Public Health 3:285–293
Gu L, Gonzalez FJ, Kalow W, Tang BK (1992) Biotransformation of caffeine, paraxanthine, theobromine and theophylline by cDNA-expressed human CYP1A2 and CYP2E1. Pharmacogenet Genomics 2:73–77
Gülçin I, Mshvildadze V, Gepdiremen A, Elias R (2004) Antioxidant activity of saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta Med 70:561–563
Habtemariam S (2019) The Nrf2/HO-1 axis as targets for flavanones: neuroprotection by pinocembrin, naringenin, and eriodictyol. Oxidative Med Cell Longev:1–15
Han CK, Choi WR, Oh KB (2007) Cognition-enhancing and neuroprotective effects of hederacolchiside-E from Pulsatilla koreana. Planta Med 73:665–669
Holcomb LA, Dhanasekaran M, Hitt AR, Young KA, Riggs M, Manyam BV (2006) Bacopa monniera extract reduces amyloid levels in PSAPP mice. J Alzheimers Dis 9:243–251
Kaur N, Dhiman M, Perez-Polo JR, Mantha AK (2015) Ginkgolide B revamps neuroprotective role of apurinic/apyrimidinic endonuclease 1 and mitochondrial oxidative phosphorylation against Aβ25-35-induced neurotoxicity in human neuroblastoma cells. J Neurosci Res 93:938–947
Khan A (1885) Muheet-e-Azam. vol IV, Part-II, Darmataba Nizami, Kanpur (1314 H.), pp 129–132
Kihara T, Sawada H, Nakamizo T, Kanki R, Yamashita H, Maelicke A, Shimohama S (2004) Galantamine modulates nicotinic receptor and blocks Aβ-enhanced glutamate toxicity. Biochem Biophys Res Commun 325:976–982
Kim J, Lee HJ, Lee KW (2010) Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J Neurochem 112:1415–1430
Kumar N, Abichandani L, Thawani V, Gharpure K, Naidu M, Venkat RG (2016) Efficacy of standardized extract of Bacopa monnieri (Bacognize®) on cognitive functions of medical students: a six-week, randomized placebo-controlled trial. Evid Based Complement Alternat Med 2016:4103423
Li CMM, Shi SMD (2019) Neuroprotective effect of huperzine A on D-galactose-induced hearing dysfunction. Ear Nose Throat J 100(3_suppl):269S–276S
Li HN, Liu Y, Zhang ZP, Wang Zp, Hao JZ, Li FR, Fan ZF, Zou LB, Cheng MS (2018) Synthesis, biological evaluation and structure-activity relationship studies of hederacolchiside E and its derivatives as potential anti-Alzheimer agents. Eur J Med Chem 143:376–389
Limpeanchob N, Jaipan S, Rattanakaruna S, Phrompittayarat W, Ingkaninan K (2008) Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol 120:112–117
Liu JS, Zhu YL, Yu CM, Zhou YZ, Han YY, Wu FW, Qi BF (1986) The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can J Chem 64:837–839
Lopes JP, Tarozzo G, Reggiani A, Piomelli D, Cavalli A (2013) Galantamine potentiates the neuroprotective effect of memantine against NMDA-induced excitotoxicity. Brain Behav 3:67–74
Maia L, de Mendonca A (2002) Does caffeine intake protect from Alzheimer’s disease? Eur J Neurol 9:377–382
Malishev R, Shaham-Niv S, Nandi S, Kolusheva S, Gazit E, Jelinek R (2017) Bacoside-A, an Indian traditional-medicine substance, inhibits β-amyloid cytotoxicity, fibrillation, and membrane interactions. ACS Chem Neurosci 8:884–891
Mukherjee S, Kumar G, Patnaik R (2020) Withanolide A penetrates brain via intra-nasal administration and exerts neuroprotection in cerebral ischemia reperfusion injury in mice. Xenobiotica 50:957–966
Paranjpe P (2001) Sallak.– Boswellia serrata. In: Indian medicinal plants—forgotten healers: a guide to ayurvedic herbal medicine. Chaukhamba Sanskrit. Pratishthan Publishers, Delhi, pp 233–234
Peng Y, Jiang L, Lee DY, Schachter SC, Ma Z, Lemere CA (2006) Effects of huperzine A on amyloid precursor protein processing and beta-amyloid generation in human embryonic kidney 293 APP Swedish mutant cells. J Neurosci Res 84:903–911
Peng Y, Lee DY, Jiang L, Ma Z, Schachter SC, Lemere CA (2007) Huperzine A regulates amyloid precursor protein processing via protein kinase C and mitogen-activated protein kinase pathways in neuroblastoma SK-N-SH cells over-expressing wild type human amyloid precursor protein 695. Neuroscience 150:386–395
Peth-Nui T, Wattanathorn J, Muchimapura S, Tong-Un T, Piyavhatkul N, Rangseekajee P, Ingkaninan K, Vittaya-areekul S (2012) Effects of 12-week Bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evid Based Complement Alternat Med 2012:606424
Prakash J, Yadav SK, Chouhan S, Singh SP (2013) Neuroprotective role of Withania somnifera root extract in maneb-paraquat induced mouse model of parkinsonism. Neurochem Res 38:972–980
Prins ND, van der Flier WA, Knol DL, Fox NC, Brashear HR, Nye JS, Barkhof F, Scheltens P (2014) The effect of galantamine on brain atrophy rate in subjects with mild cognitive impairment is modified by apolipoprotein E genotype: post-hoc analysis of data from a randomized controlled trial. Alzheimers Res Ther 6:47
Rabhi C, Arcile G, Le Goff G, Da Costa NC, Ouazzani J (2019) Neuroprotective effect of CR-777, a glutathione derivative of withaferin A, obtained through the bioconversion of Withania somnifera (L.) dunal extract by the fungus Beauveria bassiana. Molecules 24:4599
Rafii MS, Walsh S, Little JT, Behan K, Reynolds B, Ward C, Jin S, Thomas R, Aisen PS (2011) A phase II trial of huperzine A in mild to moderate Alzheimer disease. Alzheimer’s Disease Cooperative Study. Neurology 76:1389–1394
Rai R, Singh HK, Prasad S (2015) A special extract of Bacopa monnieri (CDRI-08) restores learning and memory by upregulating expression of the NMDA receptor subunit GluN2B in the brain of scopolamine-induced amnesic mice. Evid Based Complement Alternat Med 2015:254303
Russo A, Borrelli F, Campisi A, Acquaviva R, Raciti G, Vanella A (2003) Nitric oxide related toxicity in cultured astrocytes: effect of Bacopa monniera. Life Sci 73:1517–1526
Saraf MK, Prabhakar S, Khanduja KL, Anand A (2011) Bacopa monniera attenuates scopolamine-induced impairment of spatial memory in mice. Evid Based Complement Alternat Med 2011:236186
Schwarzkopf TM, Hagl S, Eckert GP, Klein J (2013) Neuroprotection by bilobalide in ischemia: improvement of mitochondrial function. Pharmazie 68:584–589
Singh N, Bhalla M, de Jager P, Gilca M (2011) An overview on Ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Alternat Med 8:208–213
Sun QQ, Xu SS, Pan JL, Guo HM, Cao WQ (1999) Huperzine-A capsules enhance memory and learning performance in 34 pairs of matched adolescent students. Zhongguo Yao Li Xue Bao 20:601–603
Usha PD, Wasim P, Joshua JA, Geetharani P, Murali B, Mayachari AS, Venkateswarlu K, Saxena VS, Deepak M, Amit A (2008) BacoMind®: a cognitive enhancer in children requiring individual education programme. J Pharmacol Toxicol 3:302–310
van Marum RJ (2008) Current and future therapy in Alzheimer’s disease. Fundam Clin Pharmacol 22:265–274
Ved HS, Koenig ML, Dave JR, Doctor BP (1997) Huperzine A, a potential therapeutic agent for dementia, reduces neuronal cell death caused by glutamate. Neuroreport 8:963–967
Vishnupriya P, Padma VV (2017) A review on the antioxidant and therapeutic potential of Bacopa monnieri. Reactive Oxygen Species 23:111–120
Wagner H (1999) Phytomedicine research in Germany. Environ Health Perspect 107:779–781
Wang R, Tang XC (2005) Neuroprotective effects of huperzine A. A natural cholinesterase inhibitor for the treatment of Alzheimer’s disease. Neurosignals 14:71–82
Wang R, Zhang HY, Tang XC (2001) Huperzine A attenuates cognitive dysfunction and neuronal degeneration caused by β-amyloid protein-(1–40) in rat. Eur J Pharmacol 421:149–156
Winner B, Kohl Z, Gage FH (2011) Neurodegenerative disease and adult neurogenesis. Eur J Neurosci 33:1139–1151
World Alzheimer Report (2015) The global impact of dementia. Prevalence, incidence, cost and trends. Alzheimer’s Disease International (ADI), London
Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, Link CD, Luo Y (2006) Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci Off J Soc Neurosci 26:13102–13113
Xu SS, Gao ZX, Weng Z, Du ZM, Xu WA, Yang JS, Zhang ML, Tong ZH, Fang YS, Chai XS (1995) Efficacy of tablet huperzine-A on memory, cognition, and behavior in Alzheimer’s disease. Zhongguo Yao Li Xue Bao 16:391–395
Xu SS, Cai ZY, Qu ZW, Yang RM, Cai YL, Wang GQ, Su XQ, Zhong XS, Cheng RY, Xu WA, Li JX, Feng B (1999) Huperzine-A in capsules and tablets for treating patients with Alzhiemer disease. Zhongguo Yao Li Xue Bao 20:486–490
Zaker S, Beheshti S, Aghaie R, Noorbakhshnia M (2015) Effect of olibanum on a rat model of Alzheimer’s disease induced by intracerebroventricular injection of streptozotocin. Physiol Pharmacol 18:477–489
Zhang YH, Chen XQ, Yang HH, Jin GY, Bai DL, Hu GY (2000) Similar potency of the enantiomers of huperzine A in inhibition of [(3)H] dizocilpine (MK-801) binding in rat cerebral cortex. Neurosci Lett 295:116–118
Zhang HY, Liang YQ, Tang XC, He XC, Bai DL (2002) Stereoselectivities of enantiomers of huperzine A in protection against β-amyloid (25–35)-induced injury in PC12 and NG108–15 cells and cholinesterase inhibition in mice. Neurosci Lett 317:143–146
Zhang Z, Wang X, Chen Q, Shu L, Wang J, Shan G (2002a) Clinical efficacy and safety of huperzine alpha in treatment of mild to moderate Alzheimer disease, a placebo-controlled, double-blind, randomized trial. Zhonghua Yi Xue Za Zhi 82:941–944
Zheng PD, Mungur R, Zhou HJ, Hassan M, Jiang SN, Zheng JS (2018) Ginkgolide B promotes the proliferation and differentiation of neural stem cells following cerebral ischemia/reperfusion injury, both in vivo and in vitro. Neural Regen Res 13:1204–1211
Zhou X, Wang HY, Wu B, Cheng CY, Xiao W, Wang ZZ, Yang YY, Li P, Yang H (2017) Ginkgolide K attenuates neuronal injury after ischemic stroke by inhibiting mitochondrial fission and GSK-3β-dependent increases in mitochondrial membrane permeability. Oncotarget 8:44682–44693
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive licence to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Venkata Ratnam, K., Md. Bhakshu, L., Venkata Raju, R.R. (2022). Herbal Drugs: Its Mechanism to Prevent Alzheimer’s Disease with Special Reference to Non-phenolic Secondary Metabolites. In: Rajagopal, S., Ramachandran, S., Sundararaman, G., Gadde Venkata, S. (eds) Role of Nutrients in Neurological Disorders. Nutritional Neurosciences. Springer, Singapore. https://doi.org/10.1007/978-981-16-8158-5_16
Download citation
DOI: https://doi.org/10.1007/978-981-16-8158-5_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8157-8
Online ISBN: 978-981-16-8158-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)