Abstract
Sexually transmitted diseases (STDs) pose a challenge in pregnant females due to their harmful effects on the mother and the fetus/neonate. Many of the STDs can lead to adverse pregnancy outcomes like ectopic pregnancy, abortion, premature rupture of membranes, and low birth weight. In this chapter, we describe the four common STDs seen in pregnancy- herpes simplex virus (HSV), human papilloma virus (HPV), chlamydia, and gonorrhea. HSV infection, besides causing painful genital lesions, can lead to severe morbidity and mortality if it is acquired in third trimester. The child usually gets infected intrapartum, but rarely may acquire infection transplacentally and may sometimes manifest severe illness. HPV-induced warts may become more florid during pregnancy, but usually regress postpartum. Intrapartum HPV infection can lead to anogenital warts in neonates, or rarely laryngeal papillomas. Chlamydia and gonorrhea are common bacterial STDs where the neonate may get infected intrapartum and can develop ophthalmia neonatorum or systemic illness. Adequate preventive and screening measures and appropriate treatment can prevent the maternal and fetal morbidity associated with these infections.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sexually transmitted diseases
- Herpes simplex virus
- Neonatal herpes
- Human papilloma virus
- Juvenile laryngeal papillomatosis
- Chlamydia
- Gonococcus
- Ophthalmia neonatorum
- HPV vaccine
1 Herpes, Gonorrhea, Chlamydia, and HPV in Pregnancy (Prevention, Screening, Treatment, and Outcomes)
1.1 Introduction
Sexually transmitted diseases (STD) are very common in young females. They present with wide range of clinical manifestations. They can be asymptomatic, may cause mild discomfort, or may cause significant complications like infertility or ectopic pregnancy. STDs are of special concern during pregnancy due to their potential to cause adverse obstetric outcomes and neonatal infections. Screening and treatment of STDs during pregnancy represent an overlooked opportunity and is often underutilized.
In this chapter, we will discuss the four common STDs that are encountered during pregnancy, namely Herpes simplex virus (HSV), Human Papilloma Virus (HPV), Chlamydia, and Gonorrhea.
1.2 Herpes
HSV infection is a common STD in the reproductive age group [1]. HSV is an enveloped, double-stranded DNA epitheliotropic virus related to the Alphaherpesvirinae, a subfamily of the Herpesviridae family [2].
As per the World Health Organization (WHO), globally, 3.7 billion people under the age of 50 years are infected with HSV-1 infection, and 491 million people aged 15–49 years are infected with HSV-2 infection [3]. The prevalence of HSV-2 in Asian countries varies from 10% to 30% [1]. Studies from different regions in India have shown HSV-2 seroprevalence from 5.8% to 18.9%. HSV-2 seroprevalence among pregnant women has been reported to be 8.7% in north India and approximately 11.3% in south India [4, 5].
1.2.1 Pathogenesis
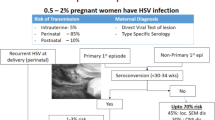
HSV is a double-stranded DNA virus and has two types HSV-1 and HSV-2. Primary herpes infection refers to a new infection acquired for the first time in a person lacking both pre-existing HSV-1 and HSV-2 antibodies. When a person with pre-existing HSV antibodies (against type 1 or 2) is infected initially with the other HSV type, it is called the first non-primary episode. Primary HSV infection with both types is acquired across mucous membranes and nonintact skin.
After the primary infection, the virus remains latent in nerve ganglia and can cause recurrent lesions. HSV-1 remains latent in trigeminal ganglia and usually causes orolabial lesions. HSV-2 commonly infects lumbosacral ganglia and causes recurrent genital lesions. However, both types of viruses can infect orofacial and anogenital regions, and their management remains the same. Recurrent infections are associated with antibodies against the same HSV type [1, 2]. Both primary and recurrent infections with HSV can be asymptomatic.
1.2.2 Clinical Features
1.2.2.1 Maternal Manifestations
Incubation period of primary genital herpes is 2–20 days, and symptoms can last up to 21 days. Women usually present with painful blisters and ulcerative lesions over external genitals, cervix, inner thigh, buttocks, perineum, and perianal area. She may have dysuria, vulval pain, vaginal soreness and discharge, with or without inguinal lymphadenopathy. Prodromal symptoms can range from fever, headache, and myalgia to severe manifestations including meningitis and autonomic neuropathy.
Severe manifestations of HSV infection are more common in pregnancy. The pregnant woman is at higher risk for severe and frequent symptomatic episodes of orolabial and genital herpes and also of asymptomatic viral shedding at term [6]. Disseminated HSV infection during pregnancy is a rare but serious complication of primary HSV infection in the third trimester. It can manifest as disseminated skin lesions, encephalitis, hepatitis, thrombocytopenia, leucopenia, coagulopathy, with a maternal case fatality rate of up to 50% [1]. There is also a high rate of transmission of infection to the fetus.
During pregnancy, recurrent genital herpes is much more frequent than primary HSV infection. Most episodes of recurrent genital herpes during pregnancy are asymptomatic or present with mild symptoms and few localized lesions. Recurrent genital herpes is also associated with a much lower transmission risk to the fetus than primary genital HSV infection. The fetal infection rate with recurrent genital HSV is <1%, compared with 30–50% fetal transmission rates with primary genital HSV infection during pregnancy [1, 2]. Viral shedding is higher with HSV-2 infection as compared to HSV-1 and can occur even in the absence of symptoms and evident lesions. Type-specific antibodies to HSV are usually formed within the first 12 weeks after maternal infection and persist indefinitely. These maternal antibodies may reduce fetal infection by transplacental passage and by decreasing viral shedding in the maternal genital tract.
Maternal to neonatal HSV transmission mainly occurs during antenatal (5–8%) and perinatal (85–90%) period; postnatal transmission is rare. As mentioned above, in-utero HSV transmission is higher with primary HSV infections and HSV-2 infections, and also with maternal infection during the first 20 weeks of gestation. Fetal HSV infection can cause spontaneous abortions, congenital anomalies, fetal growth retardation, preterm labor, and still birth [2]. Rates of perinatal transmission of HSV infection are increased with primary or non-primary third trimester maternal infections, absence of maternal neutralizing antibodies, prolonged rupture of membranes, vaginal mode of delivery, and with invasive fetal monitoring [6].
1.2.2.2 Neonatal Manifestations
The infected newborn may present with congenital infection or with neonatal herpes. Congenital herpes is characterized by skin vesicles (appearing within 48 h of birth) or scarring, eye lesions (chorioretinitis, microphthalmia, and cataract), neurologic damage (intracranial calcifications, microcephaly, seizures, and encephalomalacia), growth retardation, or impairment in psychomotor development.
Neonatal herpes infection is usually acquired at the time of delivery or occasionally in the early postnatal period. It is classified into three groups:
-
1.
Localized to skin, eyes, or mouth, representing 30% of neonatal herpes and has the best prognosis. Neurological and ocular morbidity after antiviral treatment is <2%.
-
2.
HSV encephalitis, presenting between 10 days to 4 weeks of age. Mortality is around 6%, while neurological morbidity is 70%, even after antiviral treatment.
-
3.
Disseminated infection with multiple organ involvement, occurring mostly in preterm infants of mothers with primary herpes infection. It carries the worst prognosis, with mortality rates of around 30% and neurological morbidity of around 17% even after treatment [6].
1.2.3 Diagnosis
Clinical diagnosis of genital herpes can be made when characteristic multiple vesicular lesions on an erythematous base are present. However, diagnosis may be difficult when herpetic lesions resemble other skin conditions or when the patient has non-specific symptoms. Laboratory tests are required to confirm the diagnosis, which can be done by virus isolation or by detecting an antibody response against it [7].
-
1.
Serological tests—Patients develop antibodies to HSV within few weeks of acquiring infection, and these antibodies persist indefinitely. Serological assays can detect antibodies that are common for HSV-1 and 2 and also type-specific antibodies. Serological tests can be used to differentiate between primary or recurrent infection, identify asymptomatic carriers, and diagnose symptomatic patients with negative viral yield. Type-specific serological tests are based on the HSV-specific glycoprotein G2 (HSV-2) and glycoprotein G1 (HSV-1).
-
2.
Culture—HSV can be grown in wide variety of cell lines. Sensitivity rates of HSV culture are approximately 75% and 50% for primary and recurrent infection, respectively, with 100% specificity rates [7]. Cell-cultures can differentiate between HSV-1 and HSV-2 infections. Samples for viral culture should be collected within 3 days of the appearance of lesions, as isolation rates are significantly reduced once lesions start crusting. Due to suboptimal sensitivity and difficulty in the transportation of specimens, viral culture is not the initial preferred diagnostic test.
-
3.
Amplification techniques—Polymerase chain reaction (PCR) is a very sensitive test and can detect viral DNA even in low concentrations, and can also differentiate between the HSV types. It is the test of choice for diagnosing the central nervous system and systemic infections and neonatal herpes.
-
4.
Cytology—Direct microscopic examination of material from base of lesion (Tzanck test) can be done. Smears taken from the lesion are stained by Geimsa, hematoxylin-eosin, or Papanicolaou stains; giant cells or intranuclear inclusions (Cowdry bodies type A) are characteristic. Although cytology results are rapid, the sensitivity is low and it cannot be used to differentiate between the two HSV types. Cytology is now seldom used to diagnose genital HSV infection in clinical practice. A direct immunofluorescence assay using fluorescein-tagged antibodies (commercially available as IMAGEN™) is commercially available to detect HSV antigens from genital lesions.
1.2.4 Screening
Universal screening for HSV in asymptomatic pregnant women is not recommended by most of the societies like the Centers for Disease Control and Prevention (CDC) and American College of Obstetricians and Gynecologists (ACOG). However, type-specific serologic tests might be useful to council at-risk pregnant women to prevent them from acquiring genital herpes during pregnancy. Serologic screening may also be used to identify women who have a past history of HSV infection so they can be offered suppressive antiviral therapy. In the absence of lesions, cultures for HSV are not indicated for women in the third trimester, even if they have a history of recurrent genital herpes.
1.2.5 Management
1.2.5.1 Management of Primary Genital Herpes in Pregnancy and Labor
Whenever a pregnant patient presents with symptoms of genital herpes, a history of any such previous infection should be inquired, and genital swabs should be taken to confirm the diagnosis by culture or PCR. The antiviral drugs, acyclovir, valacyclovir, and famciclovir, are all category B drugs and approved for use for HSV infection in pregnancy. Acyclovir is widely used and well tolerated in pregnancy without any dose adjustment. Acyclovir 400 mg orally three times a day for 7–10 days (intravenous for disseminated HSV) reduces the duration and severity of symptoms, and the duration of viral shedding. Fetal harm has not been reported with valacyclovir and famciclovir, but due to limited safety data, they are not used as first-line drugs during pregnancy. The treatment recommendations as given by ACOG are given in Table 19.1 [8]. Paracetamol and topical lidocaine 2% gel are used for symptomatic pain relief. If possible, delivery should be delayed by at least 6 weeks to ensure the transplacental passage of protective maternal antibodies to the fetus [1, 2, 6]. Patients who acquire infection in the first or second trimester may additionally be given suppressive dose of acyclovir 400 mg three times a day, daily from 36 weeks of gestation till delivery. This helps in reducing the maternal genital herpes lesions at term and need of cesarean section. Patients who acquire primary infection after 28 weeks of pregnancy need continuous treatment with daily suppressive dose of acyclovir 400 mg three times a day until delivery.
Cesarean section is recommended in cases of primary and non-primary genital herpes, especially if infection has been acquired within 6 weeks of delivery. However, cesarean delivery does not have any protective effect if membranes have ruptured for more than 4 h. If the woman presents with herpetic lesions at onset of labor, swabs should be taken from the lesions and type-specific HSV antibody testing (IgG to HSV-1 or HSV-2) should be done, as it is difficult to distinguish between primary and recurrent lesions clinically. The presence of antibodies for the same type of HSV as isolated in the genital swab would confirm the recurrent episode, and an elective cesarean section may be avoided. If the patient presents in advanced stage of labor or chooses vaginal delivery, then intravenous acyclovir 5 mg/kg every 8 hours to mother and intravenous 20 mg/kg, 8 hourly subsequently to neonate after delivery should be started. However, definitive efficacy data for this approach is lacking. Any invasive procedure like fetal scalp electrodes application, fetal blood sampling, artificial rupture of membranes and instrumental deliveries should be avoided if possible [6].
1.2.5.2 Management of Recurrent Genital Herpes in Pregnancy and Labor
Majority of recurrent genital herpes episodes during pregnancy are mild and short lasting. Recurrent herpes lesions usually resolve within 7–10 days without any antiviral treatment. Symptomatic management with paracetamol is sufficient, along with saline bathing. Risk of neonatal herpes is low (0–3%) due to transplacental maternal protective antibodies. Hence, cesarean delivery is indicated only for obstetric indications. However, these transplacental maternal antibodies do not protect the fetus against the neuro-ophthalmic complications. Daily suppressive dose of acyclovir 400 mg three times a day from 36 weeks of gestation until the onset of labor is recommended to reduce viral shedding and recurrence of genital lesions. Sequential PCR cultures are not indicated in this setting [1, 2, 6].
1.2.5.3 Special Circumstances
-
1.
Management of genital herpes in preterm premature rupture of membrane (PPROM)—A woman with primary genital herpes presenting with PPROM (before 37 weeks of pregnancy), should be managed by a multidisciplinary team. If delivery is imminent, cesarean section is preferred over vaginal delivery to reduce the risk of neonatal herpes. If conservative management is planned, intravenous acyclovir 5 mg/kg, 8 hourly should be given to the mother, along with prophylactic corticosteroids to reduce the complications of prematurity in neonates. In a woman with recurrent genital herpes presenting with PPROM, risk of neonatal transmission is low. Management of these patients should be done according to standard PPROM guidelines, along with the addition of oral acyclovir 400 mg three times a day [6].
-
2.
Management of genital herpes in HIV positive mother—HIV positive females are at an increased risk of more severe and recurrent episodes of genital herpes during pregnancy, and increased perinatal transmission of both HSV and HIV. Hence, suppressive acyclovir treatment should be started from 32 weeks (instead of 36 weeks) in a pregnant HIV seropositive woman with HSV infection. Suppressive treatment of HSV is not recommended in HIV seropositive pregnant patients without any history of genital herpes [6].
1.2.5.4 Management of Neonates of Mothers with Genital Herpes
Risk of vertical transmission is low in babies born by cesarean section to a woman with primary genital herpes. They are managed conservatively. If a baby is born by vaginal delivery within 6 weeks of primary herpes infection, risk of vertical transmission is very high. Swabs from skin, conjunctiva, oropharynx, and rectum of the neonate should be taken and sent for HSV PCR, even in the absence of any clinical manifestations. Lumbar puncture is not required in the absence of skin lesions. These neonates should receive intravenous acyclovir 20 mg/kg, 8 hourly as an empirical treatment [6].
Neonates born to mothers with recurrent herpes are managed conservatively, as risk of neonatal herpes is very low in these children. In the absence of herpetic lesions around the nipples, breast feeding can proceed as normal. Parents should be educated about hand hygiene to reduce risk of postnatal infection. They should report immediately if the baby develops any lesions over skin, eye, and mucous membrane, has lethargy, poor feeding or irritability.
If a neonate born to a mother with HSV infection presents with signs of sepsis or poor feeding, surface swabs and blood culture should be taken for herpes simplex culture and PCR, respectively. Intravenous acyclovir 20 mg/kg every 8 h should be started empirically. Further management should be done by neonatologist according to the clinical condition of the baby and culture report [6].
1.3 Human Papilloma Virus
HPV infection is the most common sexually transmitted viral infection in world, and is the main causative factor for carcinoma cervix and other anogenital neoplasms. It is also responsible for certain non-genital head and neck cancers (oral cavity, pharynx, and larynx).
HPV infection is common in reproductive age. Global prevalence of anogenital HPV in females with normal cytology of cervix is around 11.7% [9]. In India, the prevalence of HPV type 16 or 18 in women is 5% with normal cytology, 28.2% with low-grade intraepithelial lesion (LSIL), 62.8% with high-grade intraepithelial lesion (HSIL), and 83.2% with cervical cancer. Around 14.6% to 64.2% of patients infected with HPV type 6 or 11 have visible genital warts. HPV prevalence in pregnant women ranges from 9.6% to 46.7%. Approximately 5% of pregnant females with HPV infection have abnormal cervical cytology [10, 11].
1.3.1 Pathogenesis
HPV belongs to Papovaviridae family of viruses. It has an icosahedral protein capsid and a tightly coiled circular double-stranded DNA of about 8000 base pair length. HPV genome is organized into three major functional regions, including an upstream regulatory region that regulates transcription from the early and late regions of the viral genome. The early region has genes encoding for proteins (E1, E2, E4, E5, E6, and E7) involved in viral replication, transcription control and cellular transformation. The late region includes genes that encode for structural capsid proteins L1 and L2.
Out of 184 types of HPV identified, 40 infect anogenital tract of males and females and are transmitted through sexual contact. HPVs are divided into three groups according to their neoplastic potential:
-
1.
Low-risk types: Types 6, 11, 40, 42, 43, 44, 54, 61, 72, 73, and 81, are associated with genital warts, condyloma, and low-grade dysplastic lesions.
-
2.
High-risk types: Types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 59, 68, and 82. Types 16 and 18 are the most common among this group and are responsible for precancerous lesions and cancers of cervix and other anogenital cancers.
-
3.
Probable high-risk types; Types 26, 53, and 66 [12].
Young women of age 20–35 years are more susceptible to HPV infection. HPV is an epitheliotropic virus and its replication cycle is linked to epithelial cell differentiation. The infection is initiated when virus gains access to basal epithelial cells through micro-abrasions during sexual intercourse, and its circular DNA remains episomal inside the human cell nuclei. Upon entry into the dividing cells, there is initial replication of the viral genome, in concert with the differentiation of infected basal cells. These cells carry the viral genome with them as they move through the upper epithelial layers and aid in the transmission of infection.
Majority of HPV infections are cleared by host innate immunity. However, in certain cases, when HPV persists, and viral genome integrates with the host genome, over-expression of E6 and E7 genes takes place. Depending on the virus type and host factors, low-grade or high-grade lesions may be produced. There is increased production of E6 and E7 oncoproteins which bind and interfere with functions of tumor suppressor genes p53 and retinoblastoma protein (pRb), respectively, leading to abnormal and uncontrolled cellular proliferation and carcinogenesis [13].
1.3.2 Clinical Manifestations
A meta-analysis of 28 studies showed significantly increased risk of HPV infection in pregnant females as compared to non-pregnant females (16.8% vs. 12.3%) [14]. Pregnancy is a state of mild immunosuppression and the hormonal and immunological changes during pregnancy may be responsible for increased risk of HPV infection in pregnancy. The upstream regulatory region of HPV 16 contains a steroid hormone receptor binding element, which may contribute to increased HPV replication during pregnancy [15]. Restitution of the immune function in postpartum period can cause spontaneous regression of cervical intraepithelial lesions (CIN) in 37–74% cases [9, 13].
A meta-analysis of 45 studies that analyzed 14,470 pregnant females found higher prevalence of HPV infection in cases of spontaneous abortions and preterm deliveries. Various studies have detected HPV DNA in amniotic fluid, placenta, fetal membranes, and umbilical cord blood [16]. HPV can infect trophoblastic cells of the placenta, and maternal HPV infection has been associated with spontaneous abortions and preterm delivery. However, the exact mechanism of adverse pregnancy outcomes is not yet clear.
The possible mode of vertical transmission of HPV infection from mother to fetus/neonate is still not clear. The most likely mode of transmission is passage through infected birth canal. Neonates born to mothers with HPV infection are at a higher risk of developing infantile genital and anal condyloma acuminatum and juvenile laryngeal papillomatosis (JLP). JLP is a rare infection with an incidence of 1.7–4.3 per 100,000 neonates. Risk factors associated with JLP are being a firstborn child, vaginal delivery with prolonged labor, and age of mother less than 20 years. JLP is commonly due to HPV type 6 and 11 [17].
For better understanding of HPV perinatal transmission and natural history of HPV infection, a large prospective cohort study- Human Papillomavirus perinatal transmission and risk of HPV persistence among children (HERITAGE) study is being done in Montreal, Canada. The preliminary data showed a high prevalence of HPV in pregnant women in the first trimester (45%), and of these, 80% remained positive in the third trimester. The overall prevalence of HPV infection was 14%. HPV positivity rates among children born of infected mothers was 11% from birth to 3 months of age. HPV was detected in children in multiple sites, including the conjunctiva [18].
1.3.3 Diagnosis
HPV infection may be diagnosed by characteristic clinical lesions or by molecular tests done on cervicovaginal specimens. As this virus cannot be grown in tissue culture, nucleic acid tests are employed to confirm the HPV infection.
1. Clinical examination—HPV associated condylomas and warts are visible with naked eye. (Fig. 19.1) Visual inspection with acetic acid (VIA), cytology, and colposcopy with biopsy give indirect evidence of HPV infection. (Fig. 19.2).
2. Non-amplified hybridization techniques—These include Southern blot for DNA, Northern blot for RNA, dot blot, and in-situ hybridization assays. They are not used commonly as these tests require large amount of purified nucleic acids, are poorly reproducible, and have low sensitivities.
3. Signal amplification assays—These are based on the amplification of DNA/RNA hybrids. Hybrid capture 2 (HC2) gives a semi-quantitative value using chemiluminescence. The available probes can detect the presence of the 13 high-risk types (types 16/18/31/33/35/39/45/51/52/56/58/59/68) but cannot identify the individual genotype. Similarly, a probe for low-risk types (types 6/11/42/43/44) can detect the presence of any of the five low-risk viruses but cannot specify the genotype.
4. Target amplification assays—These are PCR-based tests that amplify the target HPV DNA and also identify the specific HPV genotype. E6/E7 mRNA assay test is also based on target amplification of E6/E7 mRNA of high-risk HPV types. The most commonly used commercial target amplification test is the APTIMA™ HPV assay, which is FDA approved.
1.3.4 Screening
CDC, ACOG, and Federation of Obstetric and Gynecological Society of India (FOGSI) recommend cervical cancer screening by cytology every 3 years or by co-testing (HPV+ cytology) every 5 years for women aged 21–65 years. However, there are no specific recommendations and guidelines for HPV and cervical cancer screening during pregnancy.
Incidence of cervical cancer is low during pregnancy, around 3.3 to 26 cases per 100,000 births [19]. Still, cervical cancer screening should be a part of routine antenatal care in developing countries, where it may be the only opportunity for some women to be screened for cervical cancer. Broom brush, instead of endocervical brush should be used for cytology acquisition during pregnancy.
Recommendation for pregnancy with abnormal cervical screening: Pregnant women with positive high-risk oncogenic HPV (not type 16 or 18) and normal cytology or LSIL in cervical smear should have repeat HPV DNA testing after 12 months. Pregnant woman positive for high-risk oncogenic HPV (not type 16 or 18) along with HSIL or abnormal glandular cervical smear, or positive for oncogenic HPV (type 16 or 18) regardless of cytology results, need early colposcopic evaluation [19].
1.3.5 Management
There is no specific treatment for the virus itself. Postpartum regression of lesions has been documented in various studies, and expectant management is preferred for all low-grade lesions and high-grade lesions with no invasion. However, patients should be kept under follow-up, and undergo repeat colposcopy with biopsy 6 weeks postpartum. When indicated, colposcopy and directed biopsies are safe in pregnancy. Cervical biopsy is required when cytology is suspicious for malignancy or invasive carcinoma is suspected during colposcopy.
1.3.5.1 Management of Pregnant Woman with Genital Warts and Condylomas
In majority of cases, genital warts may increase during pregnancy and then regress spontaneously during puerperium. Definitive treatment is therefore usually delayed till delivery. Small lesions do not require any treatment, and larger lesions can be treated by keratolytic agents (80–90% trichloroacetic acid solution) or by cryotherapy. Occasionally, condylomas can show rapid growth during pregnancy and may become necrotic and macerated. These lesions may need surgical excision, which can be done in the second or third trimesters. 5-fluorouracil, podophyllin, and interferon should not be used during pregnancy.
Risk of perinatal transmission of HPV to oropharyngeal mucosa of newborn is low, and the presence of genital warts does not affect the mode of delivery, unless they are large and necrotic, or obscure the vaginal canal. In these cases, cesarean section is recommended [17].
1.3.5.2 Management of Pregnant Women with Invasive Disease
Pregnant women with suspected or confirmed cervical cancer should be referred to a gynecological oncologist. A multidisciplinary team should review and manage the patient based on the duration of pregnancy, stage of disease, and patient preference.
1.3.5.3 Management of Neonates
Anogenital warts and laryngeal papillomas are not seen commonly in neonates. Affected infants may present with weak cry, episodes of choking, or stridor. Treatment is directed towards removing papillomas, decreasing the spread of disease, and maintaining airway. The disease may resolve on its own or may require repeated surgical excision of the papillomas.
1.4 Chlamydia
Chlamydia trachomatis (CT) is the most common bacterial STD worldwide. In USA it accounted for 1,758,668 cases in 2018 [20]. Based on 2018 global STD surveillance from WHO, global estimation of new CT cases in 2016 was 127 million [21]. As CT infections are asymptomatic in majority of men, and women, the true burden of disease may be underestimated [22]. Detection of infection among women is twice as high as compared to men, probably due to increased screening in women. In India, the prevalence of CT infection is 0.9–25.5% among asymptomatic population, 10–50% among symptomatic women, and 0.1–2.5% among pregnant women [23].
1.4.1 Pathogenesis
Chlamydiae are gram-negative anaerobic, obligate intracellular bacteria. Chlamydia genus contains three species that infect humans: Chlamydia trachomatis (CT), Chlamydia psittaci, and Chlamydia pneumoniae.
Based on differences in their cell wall and outer membrane proteins, CT is divided into different serologically variant strains (serovars) and genotypes, which cause different illnesses. Serovars A, B, Ba, and C cause trachoma, which is the leading cause of preventable infectious blindness in the world. Serovars D-K cause genital tract infections, pelvic inflammatory disease, and neonatal infections. Serovars L1-L3 cause lymphogranuloma venereum (LGV) and genital ulcers.
Serovar identification is not important for therapeutic purpose except in cases of LGV, where longer treatment duration is indicated. Serovars can be identified by serologic typing or by molecular gene typing. Chlamydia possesses both DNA and RNA, has a cell wall and ribosomes. It lacks metabolic and synthetic pathways and depends on the host cell for intermediates, like ATP. Chlamydia has a complex reproductive cycle. It exists in two stages—the extracellular elementary bodies, which are the infectious form, and the intra-cytoplasmic reproductive forms called the reticulate bodies (RB). Elementary bodies are the metabolically inactive forms, and once taken up by the host cell, they differentiate into metabolically active RB within 6–8 h. RB undergoes binary fission within the host cells and re-organizes to form elementary body inclusions within 2–3 days. These inclusions rupture to infect other cells. The trachoma serovars mainly target squamocolumnar epithelial cells, whereas LGV serovars involve lymphoid cells.
The pathogenesis of host injury in CT disease involves direct cytotoxicity because of its intracellular replicative cycle, immune-mediated injury, and immune cross-reactivity between chlamydial and host cell antigens. The chlamydial heat-shock protein, which shares antigenic epitopes with similar proteins of other bacteria and with human heat-shock protein, may sensitize the host. CT causes chronic asymptomatic infections in the majority. However, persistent or recurrent CT infections can lead to scarring of mucous membranes and fibrosis.
CT transmission can occur through sexual contact (horizontal transmission), direct contact with the infected tissue, and by vertical transmission to the fetus during vaginal delivery. Genito-ocular autoinoculation can also occur. The incubation period of sexually transmitted chlamydial infection is around 1–3 weeks.
1.4.2 Clinical Manifestations
In majority of pregnant women, chlamydial infections are asymptomatic. However, during pregnancy, cervical ectopy due to increased estrogen levels may aggravate the shedding of the bacteria. Symptoms of CT during pregnancy are similar to those seen in the non-pregnant state, including mucopurulent cervicitis with vaginal discharge, lower abdominal pain, post-coital bleeding, increased urinary frequency, and dysuria. Urine analysis of women with urethritis will show sterile pyuria. Extra-genital presentations of CT can also occur during pregnancy, and include conjunctivitis, perihepatitis with right upper quadrant pain (Fitz-Hugh-Curtis syndrome), pharyngitis, and reactive arthritis. Ascending genital infections, either spontaneously or secondary to induced abortion, can lead to endometritis, chorioamnionitis, salpingitis and pelvic inflammatory disease (PID). Tubal scarring due to PID can lead to tubal infertility and ectopic pregnancy. CT infections are now considered a major risk factor for ectopic pregnancy.
Chlamydial infection during pregnancy can lead to adverse pregnancy outcomes like spontaneous or recurrent abortion, PROM, preterm labor, stillbirth, or low birth weight. Infections during early gestation (less than 24 weeks), in particular, increase the risk of preterm birth by two- to threefold. The exact mechanism is not well understood, but chlamydial DNA is frequently isolated from placenta of women with preterm deliveries before 32 weeks of gestation [24].
CT infection in pregnant women may lead to late postpartum endometritis, which develops between 2 days to 6 weeks after delivery. These women may present with secondary postpartum hemorrhage, with or without fever, lower abdominal pain, and vaginal discharge.
1.4.2.1 Chlamydial Infection in the Newborn
Newborn infants can acquire chlamydial infection during passage through the infected birth canal. Infection risk after cesarean delivery is considered to be lower than after vaginal delivery. The overall risk for infants born to women with untreated chlamydial infections is approximately 50–75% [25].
Chlamydia can cause ophthalmia neonatorum (neonatal conjunctivitis) in 20–50% of infected newborns [26]. Symptoms may present from first week until 3 months of age and include mild conjunctival redness with scant watery to severe mucopurulent discharge. Loss of vision is very rare, and majority of infections will resolve spontaneously; however, if left untreated conjunctival scarring may result. Nasopharynx is another common site of infection in neonates; these infections are usually self-limited and asymptomatic. Chlamydial pneumonia occurs in 5–20% of infants, typically between 1 and 3 months of age [25]. The pneumonia tends to be subacute; infants are usually afebrile with mild tachypnea and distinctive pertussis like non-productive cough (staccato cough). Chest radiograph reveals hyperinflation and bilateral diffuse infiltrates. Peripheral eosinophilia is frequent. Although mortality is rare, pneumonia can be more severe in premature infants and may require hospitalization. Untreated newborns may have apneic spells, feeding difficulties and may need ventilatory support. An association has been suggested between neonatal chlamydial infection and asthma and chronic lung disease later in life.
1.4.3 Diagnosis
Laboratory tests for chlamydia have evolved over the years. Nucleic acid amplification tests (NAAT) are the mainstay of the diagnosis currently and have largely replaced the initial gold standard test of cell culture. The various methods available for diagnosis are as listed below:
-
1.
Microscopy: Direct microscopic examination of tissue scrapings with Giemsa or iodine staining or immunofluorescence is simple and cost-effective. The presence of characteristic inclusion bodies is pathognomic of CT infection. The test has high sensitivity for diagnosing neonatal conjunctivitis, but relatively less sensitive for diagnosing adult conjunctivitis and genital tract infections.
-
2.
Cell Culture: Chlamydia cannot be cultured on artificial medium and need tissue media like McCoy, HeLa 229, or Buffalo Green Monkey Kidney cells for their growth. The characteristic intra-cytoplasmic inclusions can be detected after 48–72 h of culture inoculation. Use of fluorescence labeled monoclonal antibodies specific for chlamydial lipopolysaccharide (LPS) and major outer membrane proteins (MOMP) increase detection rates. Culture methods are dependent on viable organisms and have variable sensitivity from 60% to 80%. Their use is restricted to research laboratories [27].
-
3.
Antigen Detection: Two types of antigen detection tests are available.
-
(a)
Direct immunofluorescent antibody test (DFA)—DFA assays are based on monoclonal antibodies directed against MOMP of chlamydial elementary bodies. Detection of 10 or more elementary bodies is considered a positive result. DFA assays have sensitivity of 80–90%, and specificity of 98–99% compared to culture methods, but have lower sensitivity as compared with NAATs. DFA is used as confirmatory test for positive results with other non-culture tests like enzyme immunoassays [28].
-
(b)
Enzyme Immunoassays (EIA)—EIAs use monoclonal or polyclonal antibodies for the detection of chlamydial LPS antigens in clinical specimens. EIAs have a sensitivity of 65–75% compared to NAATs, but have high false positive rates due to cross-reactivity with LPS of other gram-negative bacteria and chlamydial species [29]. Positive results need to be confirmed by repeating the test with more specific monoclonal antibodies to chlamydial LPS or by doing DFA test.
-
(a)
-
4.
Nucleic Acid Amplification Tests (NAATs): NAATs include polymerase chain reaction, transcription-based amplification, and strand amplification assays. These tests do not require viable organisms or any specific storage or transport medium and have a high sensitivity of 90–96% compared to culture and non-culture methods [30, 31]. NAATs can be performed on either endocervical or vaginal swabs or first void urine samples. Vaginal swabs have the highest sensitivity and can be self-collected. NAATs done on vaginal swabs are now considered the “gold standard” for diagnosis of CT infections.
Rapid NAAT based tests have been developed, which include:
-
(a)
XPert C.trachomatis/N.gonorrhoeae (CT/NG) assay—This test is approved for use on endocervical or vaginal swabs and urine samples and can provide results within 90 min. The Xpert test uses a modular cartridge base for testing specimens by nucleic acid amplification. The use of cartridges minimizes processing steps and so results can be provided earlier as compared to other NAATs, which may take 1–2 days to process.
-
(b)
CT/NG NAAT assay (Binx io)—It is FDA approved for use on vaginal swabs and can provide results as early as 30 min. Rapid tests have a sensitivity of 96% and specificity of 99% [32].
-
(a)
-
5.
Serology Tests: Testing for antibodies is not recommended for screening of genital chlamydial infections because a positive test cannot distinguish a current from past infection. However, serological tests can be used for diagnosing infections in the neonate, with the micro-immunofluorescence test (MIF) being the method of choice for serodiagnosis. An IgM titre of 1:32 or greater is considered diagnostic of neonatal CT pneumonia [27]. IgG antibodies are not useful as they may also represent passively transferred maternal antibodies.
1.4.4 Screening for Chlamydial Infection
NAATs done on endocervical swabs or self-taken vulvovaginal swabs and even on the first void urine sample are the screening tests of choice. All pregnant women should be screened for chlamydia to prevent maternal postnatal complications and chlamydial infection in infants. Antenatal screening, even in asymptomatic pregnant females, can be used as window of opportunity for the treatment of infected individuals. The US Preventive Services Task Force (USPSTF) recommends screening for chlamydia in all pregnant women who are under 25 years of age, and pregnant women 25 years or older with risk factors for chlamydial infection [33]. ACOG also recommends screening of all pregnant women with increased risk. The screening recommendations of CDC, American Medical Association, and American Academy of Pediatrics are similar to the USPSTF. They recommend testing all pregnant women at their first antenatal visit and retesting in the third trimester in women with continued risk factors and in those who test positive at their first prenatal visit. Pregnant women at higher risk for chlamydial infection include those with HIV infection and those with a new sex partner, more than one sex partner, a sex partner with concurrent partners, or a sex partner who has a sexually transmitted infection.
1.4.5 Management
Treatment of chlamydia during pregnancy is indicated to prevent adverse pregnancy outcomes and reduce the risk of perinatal transmission. Even women with untreated chlamydia infection who present in labor should be treated immediately, even though treatment at this stage does not reduce the risk of neonatal transmission.
Doxycycline and quinolones are avoided in pregnancy. Azithromycin 1gram orally given as a single dose is the recommended regimen for the treatment of chlamydial infection in pregnant women. Other effective and safe treatment options for the pregnant female include erythromycin, amoxycillin, and clindamycin, for a duration of 7 days. (Table 19.2) [34] Neisseria gonorrhoeae infection can coexist with chlamydia in a significant percentage of patients and requires additional treatment if detected.
As chlamydial infection does not provide long-term immunity and there are high chances of reinfection, these patients need to be followed up. Follow-up strategies include test of cure and retesting. Test of cure is done by NAAT 3–4 weeks after completion of therapy to document treatment success. Test of cure is not performed before 3 weeks as NAAT may be positive even in the presence of non-viable organisms. Retesting by NAAT 3 months after treatment of chlamydial infection is recommended to rule out recurrent or repeat infections. The first-line treatment with azithromycin remains effective for reinfection.
1.4.5.1 Treatment of Sex Partner
Treatment of sexual partners is important to prevent reinfection of the patient. Treatment of sexual partners within the preceding 60 days from the onset of patient’s symptoms or chlamydial diagnosis is particularly important. A pragmatic approach to partner treatment is by “Expedited partner therapy” (EPT), where the patient takes medication (single dose of 1gram azithromycin) or prescription to her partner without the healthcare provider first examining him. The patient and her partner are advised to maintain abstinence for at least 7 days after treatment or till they are free of symptoms.
Treatment of infants—Antenatal screening and treatment of infected women remain the best methods to prevent transmission of infection to the newborn. Neonatal ocular prophylaxis is not indicated, and treatment of neonatal conjunctivitis is initiated only after a positive diagnostic test. Treatment for chlamydial pneumonia is based on clinical and radiological findings. Erythromycin in a dose of 50 mg/kg/day orally in four divided doses for 14 days is the treatment of choice for both conjunctivitis and neonatal pneumonia. Topical therapy for the treatment of neonatal conjunctivitis is not as effective as systemic therapy. The efficacy of erythromycin treatment for neonatal conjunctivitis is approximately 90% and for pneumonia is 80%, and a second course of therapy might be required in infants with unresolved infections [35]. An alternative treatment regimen is azithromycin suspension for 3 days. An association between erythromycin, azithromycin and the development of pyloric stenosis in infants less than 6 weeks of age has been observed.
1.5 Gonorrhea
There has been a global increase in the incidence of Neisseria gonorrhoeae in the past few years. The estimated annual incidence of gonococcal infection globally is 86.9 million adults [36]. The highest disease burden is seen among women 20–24 years of age. The true global burden is difficult to establish due to under-reporting and asymptomatic infections.
1.5.1 Pathogenesis
N.gonorrhoeae are aerobic, encapsulated, non-spore forming, gram-negative diplococci. There are nine species of Neisseriae that infect humans, but only N. gonorrhoeae and N. meningitis are pathogenic. The important outer membrane proteins of gonococcus include pili, opacity-associated protein (Opa), and porins (previously designated protein I). Pili are important virulence factors mediating bacterial adherence and host mucosal penetration. Opa proteins bind to receptors on immune cells and mediate immune escape. Porins exhibit antigenic variations and form the basis for gonococcal serotyping. Two main serotypes have been identified. The PorB.1A strains are often associated with disseminated gonococcal infection (DGI), and PorB.1B strains usually cause local genital infections. Gonococcal lipo-oligosaccharide (LOS) is an endotoxin that provokes an immune response.
N. gonorrhoeae infects the columnar mucosal epithelium of urogenital tracts, rectum, pharynx, or conjunctiva. Women have higher chances of contracting the infection per exposure (60–90%), as compared to men [37]. Concurrent STDs like trichomonas vaginalis and chlamydial infection may be seen in 40–50% of cases [38]. Neonates can acquire infection during passage through the birth canal. Gonococcus has also been isolated from infants delivered by cesarean section of infected mothers, with prolonged rupture of membranes.
1.5.2 Clinical Manifestations
The incubation period of urogenital gonorrhea ranges from 2 to 8 days. Patients can be completely asymptomatic or have dysuria, vaginal discharge, pelvic pain, or fever (Fig. 19.3). Salpingitis and infertility may be the sequelae of untreated gonorrhea infection.
Acute gonococcal infection in pregnancy, is usually limited to vulvovaginal area. However, increased rate of pharyngeal infections is being reported in pregnancy, likely due to altered sexual practices. Gonococcal pharyngitis may present as mild sore throat but is often asymptomatic. Rectal infections usually have coexisting genital infections and present with symptoms of proctitis with mucopurulent discharge.
Disseminated gonococcal infection (DGI) is a rare condition in pregnancy with an incidence of 0.04–0.09% [39]. Patients in second and third trimester of pregnancy are more susceptible to develop DGI. Disseminated infection occurs when gonococci invade bloodstream following initial pharyngeal, genital tract, or rectal mucosal infection. Patients usually present with fever, malaise, and anorexia. Small erythematous macules can be seen on skin of arms and legs, which can evolve into pustular lesions, finally becoming hemorrhagic necrotic lesions. The face and trunk are spared. Migratory polyarthralgia, tenosynovitis of the hands and feet, arthritis of large joints like knees can occur. Other less common consequences include meningitis, endocarditis, pharyngitis, hepatitis, pericarditis, pneumonia, and osteomyelitis. Often the diagnosis is based on high clinical suspicion, and appropriate cultures should be obtained.
Gonorrhea in pregnancy can have serious consequences. Gonococcal salpingitis and PID are more common in first trimester and are often associated with fetal loss. Premature rupture of membranes, chorioamnionitis, and sepsis in infant are common complications of maternal gonococcal infection in the third trimester. Preterm delivery rates as high as 12–40% have been reported, most likely due to maternal cytokine release and increased fetal corticotropin-releasing hormone in response to the gonococcal infection. Women with gonococcal infection who undergo medical or surgical termination of pregnancy are at increased risk of post-abortion endometritis [40].
1.5.3 Neonatal Manifestations
Neonates have 30–35% chance of acquiring infection during passage through the birth canal [41]. Ophthalmia neonatorum is the most common manifestation of N. gonorrhoeae in neonates. Signs of conjunctival infection usually develop 2–5 days after birth but can appear as early as few hours after delivery. Purulent conjunctivitis, eyelid edema, and in severe cases corneal ulcerations with permanent scarring, corneal perforations, and blindness can result. Conjunctival infection can be prevented by applying 1% topical silver nitrite (Crede’s method). Early antibiotic treatment causes prompt healing and prevents systemic spread. Though rare, ophthalmia neonatorum can be associated with gonococcal meningitis.
In addition to conjunctivitis, other localized infections in neonates can involve pharynx, vagina, urethra, anus, or scalp. Disseminated gonococcal infection in the infant may present as sepsis, meningitis, or arthritis. Septicemia can develop after prolonged rupture of membranes. Gonococcal arthritis typically presents in 1–4 weeks after delivery. Symptoms are usually non-specific, including fever and feeding difficulties followed by erythematous swelling of the affected joints. Unlike adult arthritis, skin lesions are seldom seen, and usually multiple joints are involved.
1.5.4 Diagnosis
Pregnant women presenting with mucopurulent vaginal discharge, intrapartum and postpartum fever, and also mothers of infants with ophthalmia neonatorum should undergo testing for N. gonorrhoeae.
-
1.
Nucleic Acid Amplification Test (NAAT)—These tests are US Food and Drug Administration approved for use on urine, endocervical, and vaginal swabs, but not cleared for use in rectal, oropharyngeal, or conjunctival specimens. Major disadvantage of NAATs is their inability to provide information on anti-microbial resistance, so in cases of treatment failure, relevant specimen should be obtained for culture.
-
2.
Culture—Culture methods have sensitivity as high as 95–100% if specimens are collected and transported properly. CDC recommends selective (modified Thayer-Martin, Martin-Lewis, or modified New York City) and nonselective (e.g., chocolate agar) mediums for the growth and isolation of Neisseriae. Additional biochemical tests, NAATs or mass spectrometry must be performed to confirm the diagnosis of isolate as N. gonorrhoeae. Cultures have additional advantage of providing antibiotic sensitivity panel and genomic analysis if required.
-
3.
Microscopy—The presence of gram-negative, intracellular diplococci on microscopic examination of smears from endocervical secretions can be used to make a diagnosis of N. gonorrhoeae infection. However, because of lower sensitivity and specificity and requirement of technical expertise CDC does not recommend gram staining for detection of N. gonorrhoeae infection in endocervical, rectal, or pharyngeal specimens.
1.5.5 Screening for Gonorrhea Infection
USPSTF recommends screening of all pregnant women under 25 years of age and older women if at increased risk. CDC, ACOG, and FOGSI also recommend screening for gonorrhea in all sexually active women (including pregnant women) who are at increased risk for infection [42].
Risk factors for gonococcal infection include those with a new sex partner, having more than one sex partners, sex partner with concurrent partners or a sex partner who has an STD. Additional risk factors include inconsistent condom use among persons who are not in mutually monogamous relationships; previous or coexisting sexually transmitted infections; and exchanging sex for money or drugs.
Pregnant women should be offered a screening test at their first antenatal visit. Women who are at continued risk factor for gonorrhea should be retested in the third trimester.
1.5.6 Management
In view of increasing anti-microbial resistance, CDC has recently increased the recommended dose of ceftriaxone for the treatment of N. gonorrhoeae. The current recommendation is a single weight-based intramuscular injection (500 mg for all patients <150 kg weight) of ceftriaxone for treatment of uncomplicated gonococcal infection. A single oral dose of 800 mg cefixime is an alternative to parenteral ceftriaxone, albeit with lower efficacy. In cases where concurrent chlamydia infection is suspected, additionally azithromycin 1gm single oral dose is given. A single intramuscular injection of gentamycin (240 mg) plus a single oral dose of 2 g azithromycin is an alternative treatment option for penicillin-allergic patients [43].
Test of cure by either culture or NAAT is not necessary for uncomplicated gonococcal infections except for pharyngeal infections. Following treatment, CDC recommends retesting after 3 months due to increased risk of reinfection in these patients, regardless of whether their partners were treated or not. Patients should be retested in the third trimester if risk factors for reinfection persist. In cases of suspected treatment failure, clinical specimens for culture and sensitivity should be tested.
1.5.7 Treatment of Partners
Sexual partners of the infected patients should be evaluated and treated particularly with a preceding history of contact within last 60 days of onset of symptoms or diagnosis. Alternatively, expedited partner therapy can be done with a single 800 mg oral dose of cefixime. If chlamydial infection has not been excluded, then cefixime 800 mg single oral dose along with doxycycline 100 mg twice daily is given for 7 days. In case of resistant gonococcal infection, the sex partners should be treated with the same regimens as selected for the patient.
1.5.8 Treatment of Neonates
The best method to prevent gonococcal infection among infants is antenatal screening and treatment of pregnant women, and neonatal ophthalmic prophylaxis, with erythromycin (0.5%), 1% silver nitrate, or 1% tetracycline ointment. Neonatal gonococcal conjunctivitis and systemic gonococcal infections are treated with ceftriaxone.
1.6 Prevention of STDs
Prevention can be done at primary, secondary, and tertiary levels. Primary prevention involves health education and lifestyle modifications so as to prevent the infection. Young adults should be educated regarding safe sexual practices and hygiene. Pregnant females should use barrier methods like condoms, avoid contact with an infected partner, and be in long term mutually monogamous relationship to prevent sexually transmitted infections. Secondary prevention involves early detection and treatment so as to prevent the complications associated with the infection. It is very important to take detailed history on the first antenatal visit regarding any genital lesions, both in the patient and her partner. Screening, especially in high-risk cases can detect infection even in asymptomatic females. Tertiary prevention involves appropriate treatment of acute and chronic infection.
Many prophylactic and therapeutic vaccines have been explored for chlamydia, gonorrhea, herpes, and HPV. However, till date, only prophylactic vaccines against HPV have been successful.
There are three prophylactic vaccines available against HPV, which are recombinant vaccines and contain virus-like particle without core DNA.
-
Bivalent vaccine (Cervarix)—This vaccine is against types 16 and 18, with ASO4 as an adjunct.
-
Quadrivalent vaccine (Gardasil)—It is against types 6, 11, 16, and 18, with an aluminum containing adjunct.
-
Nonavalent vaccine (Gardasil 9)—This vaccine is against types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
Vaccination of adolescents and young girls by either bivalent or quadrivalent or nonavalent vaccines, 2 doses in 9–15 years of age, and 3 doses in 15–45 years of age in 6 months duration is 88% effective against HPV infection and its endpoint complications including pre-malignant and malignant lesions.
1.6.1 HPV Vaccination During Pregnancy
HPV vaccines trigger a more robust immune response than the natural infection. HPV vaccines are category B drugs and are not recommended during pregnancy. However, accidental vaccination of a pregnant woman does not need any intervention, and no congenital abnormalities have been reported in these cases. A pregnancy test is not routinely advised before vaccination.
1.7 Conclusion
Sexually transmitted infections pose a challenge in pregnant females due to their harmful effects on the mother and her fetus/neonate. Many of the infections can lead to adverse pregnancy outcomes like ectopic pregnancy, abortion, PROM, and low birth weight. Besides causing painful lesions, herpes simplex infection can lead to severe morbidity and mortality, if it is acquired in third trimester. The child usually gets infected intrapartum, but rarely may acquire infection transplacentally, and may have mild to severe illness. HPV-induced warts may become more florid during pregnancy, but usually regress postpartum. Intrapartum HPV infection can lead to anogenital warts in neonates or rarely laryngeal papillomas. Chlamydia and gonorrhea are common bacterial STDs where the neonate may get infected intrapartum. Adequate preventive and screening measures and appropriate treatment can prevent the maternal and fetal morbidity associated with these infections.
Key Points
-
1.
Human simplex virus has two types, of which type-2 is responsible for causing maximum genital infections. Herpes infection may be asymptomatic or may present with painful vesicular lesions. Primary infection in pregnancy is the main cause of neonatal herpes.
-
2.
Diagnosis of HSV infection should be confirmed by virus isolation or by detecting an antibody response against it.
-
3.
Suppressive dose of acyclovir 400 mg three times a day, daily from 36 weeks of gestation till delivery, helps in reducing the herpes lesions at term, and need of cesarean section.
-
4.
Cesarean section is recommended in cases of primary and non-primary genital herpes especially if infection has been acquired within 6 weeks of delivery.
-
5.
Human papilloma virus is responsible for causing many pre-malignant and malignant lesions of anogenital area. HPV infection in pregnancy may lead to spontaneous abortion, preeclampsia, preterm delivery, premature rupture of membranes, and low birth weight.
-
6.
Juvenile laryngeal papillomatosis is a rare infection in neonates who are exposed to vaginal secretions of mothers with active HPV lesions for more than 10 hours.
-
7.
HPV does not require active treatment in pregnancy, except treatment of large warts by excision.
-
8.
Chlamydia trachomatis infection in pregnancy can be asymptomatic or may present with symptoms like mucopurulent vaginal discharge, lower abdominal pain, increased urinary frequency, or dysuria.
-
9.
Chlamydial infections in pregnancy may lead to adverse pregnancy outcomes like spontaneous or recurrent abortions, premature rupture of membranes, preterm labor, stillbirth, or low birth weight. Neonates may acquire infection during passage through infected birth canal. Most common manifestation of neonatal chlamydia infection is ophthalmia neonatorum.
-
10.
Chlamydial infection can be diagnosed by examination of tissue scrapings for intra-cytoplasmic inclusion bodies, isolation of organism by cell culture, or by antigen detection. Nucleic acid amplification tests are the gold standard for diagnosing chlamydia infections.
-
11.
Single oral dose of 1 g azithromycin is recommended for the treatment of chlamydia infection in pregnancy.
-
12.
Neisseria gonorrhea infection in pregnancy usually presents with vulvovaginal infection. It may present as pharyngitis, proctitis, or rarely as disseminated gonococcal infection, where mortality is high.
-
13.
Maternal gonococcal infection can lead to premature rupture of membranes, chorioamnionitis, and preterm delivery. Neonates can acquire infection during passage through the birth canal, and can have neonatal conjunctivitis, or disseminated infection.
-
14.
Nucleic acid amplification tests are considered to be gold standard for diagnosing gonococcal infection.
-
15.
Due to high prevalence of anti-microbial resistance in gonococci, high dose ceftriaxone is recommended for treatment. Following treatment, retesting is advised after 3 months due to increased risk of reinfection.
-
16.
There are no specific recommendations for screening of HSV and HPV during pregnancy. However, screening is advised for chlamydia, and gonorrhea infections in all pregnant women less than 25 years and others at risk.
-
17.
Of all the sexually transmitted infections, prophylactic vaccine is available only for human papilloma virus.
References
Anzivino E, Fioriti D, Mischitelli M, Bellizzi A, Barucca V, Chiarini F, et al. Herpes simplex virus infection in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention. Virol J. 2009;6:40.
Straface G, Selmin A, Zanardo V, De Santis M, Ercoli A, Scambia G. Herpes simplex virus infection in pregnancy. Infect Dis Obstet Gynecol. 2012;2012:385697.
https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus. Accessed on 24 January 2021.
Biswas D, Borkakoty B, Mahanta J, Walia K, Saikia L, Akoijam BS, et al. Seroprevalence and risk factors of herpes simplex virus type-2 infection among pregnant women in Northeast India. BMC Infect Dis. 2011;11:325.
Hochberg CH, Schneider JA, Dandona R, Lakshmi V, Kumar GA, Sudha T, et al. Population and dyadic-based seroincidence of herpes simplex virus-2 and syphilis in southern India. Sex Transm Infect. 2015;91(5):375–82.
https://www.rcog.org.uk/globalassets/documents/guidelines/management-genital-herpes.pdf. Accessed on 24 January 2021.
Singh A, Preiksaitis J, Ferenczy A, Romanowski B. The laboratory diagnosis of herpes simplex virus infections. Can J Infect Dis Med Microbiol. 2005;16(2):92–8.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Management of genital herpes in pregnancy: ACOG practice bulletin, number 220. Obstet Gynecol. 2020;135(5):e193–202.
Pradhan SR, Mahata S, Ghosh D, Sahoo PK, Sarkar S, Pal R, et al. Human papillomavirus infections in pregnant women and its impact on pregnancy outcomes: possible mechanism of self-clearance, human papillomavirus, Rajamanickam Rajkumar. Intech Open. Available at: https://www.intechopen.com/books/human-papillomavirus/human-papillomavirus-infections-in-pregnant-women-and-its-impact-on-pregnancy-outcomes-possible-mech
Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human papillomavirus and related diseases in India. Summary Report 17 June 2019.
Gilson R, Nugent D, Werner RN, Ballesteros J, Ross J. 2019 IUSTI-Europe guideline for the management of anogenital warts. J Eur Acad Dermatol Venereol. 2020;34(8):1644–53.
Pandey D, Solleti V, Jain G, Das A, Shama Prasada K, Acharya S, et al. Human papillomavirus (HPV) infection in early pregnancy: prevalence and implications. Infect Dis Obstet Gynecol. 2019;2019:4376902.
Sun L, Herkanaidu PK, Mohur P, Ramudoo J. Effect of pregnancy on HPV infection and on its mode of management. Med J Obstet Gynecol. 2017;5(2):1099.
Liu P, Xu L, Sun Y, Wang Z. The prevalence and risk of human papillomavirus infection in pregnant women. Epidemiol Infect. 2014;142(8):1567–78.
Gloss B, Bernard HU, Seedorf K, Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987;6(12):3735–43.
Ambühl LM, Baandrup U, Dybkær K, Blaakær J, Uldbjerg N, Sørensen S. Human papillomavirus infection as a possible cause of spontaneous abortion and spontaneous preterm delivery. Infect Dis Obstet Gynecol. 2016;2016:3086036.
Rekhawasin T, Chayachinda C, Thamkhantho M, Thuwasee T, Munn A. Perinatal outcomes of pregnancy with anogenital warts at the time of delivery. J Med Assoc Thail. 2020;103:270–5.
Trottier H, Mayrand MH, Coutlée F, Monnier P, Laporte L, Niyibizi J, et al. Human papillomavirus (HPV) perinatal transmission and risk of HPV persistence among children: design, methods and preliminary results of the HERITAGE study. Papillomavirus Res. 2016;2:145–52.
Cancer Council Australia Cervical Cancer Screening Guidelines Working Party. National Cervical Screening Program: Guidelines for the management of screen-detected abnormalities, screening in specific populations and investigation of abnormal vaginal bleeding. Sydney: Cancer Council Australia. Available at: https://wiki.cancer.org.au/australia/Guidelines:Cervical_cancer/Screening.
Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2018. Atlanta: US Department of Health and Human Services; 2019.
World Health Organization. Report on global sexually transmitted infection surveillance 2018. Available at: https://apps.who.int/iris/bitstream/handle/10665/277258/9789241565691-eng.pdf?sequence=5&isAllowed=y. Accessed on 24 January 2021.
Huai P, Li F, Chu T, Liu D, Liu J, Zhang F. Prevalence of genital Chlamydia trachomatis infection in the general population: a meta-analysis. BMC Infect Dis. 2020;20(1):589.
Thomas P, Spaargaren J, Kant R, Lawrence R, Dayal A, Lal JA, et al. Burden of Chlamydia trachomatis in India: a systematic literature review. Pathog Dis. 2017;75(5):ftx055.
Adachi K, Nielsen-Saines K, Klausner JD. Chlamydia trachomatis Infection in pregnancy: the global challenge of preventing adverse pregnancy and infant outcomes in Sub-Saharan Africa and Asia. Biomed Res Int. 2016;2016:9315757.
Rours GI, Duijts L, Moll HA, Arends LR, de Groot R, Jaddoe VW, et al. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. Eur J Epidemiol. 2011;26(6):493–502.
Hammerschlag MR. Chlamydial infections. J Pediatr. 1989;114(5):727–34.
Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016;4(3):25.
Black CM. Current methods of laboratory diagnosis of Chlamydia trachomatis infections. Clin Microbiol Rev. 1997;10(1):160–84.
Chernesky MA, Mahony JB, Castriciano S, Mores M, Stewart IO, Landis SJ, et al. Detection of Chlamydia trachomatis antigens by enzyme immunoassay and immunofluorescence in genital specimens from symptomatic and asymptomatic men and women. J Infect Dis. 1986;154(1):141–8.
Tabrizi SN, Unemo M, Limnios AE, Hogan TR, Hjelmevoll SO, Garland SM, et al. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol. 2011;49(10):3610–5.
Johnson RE, Newhall WJ, Papp JR, Knapp JS, Black CM, Gift TL, et al. Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections--2002. MMWR Recomm Rep. 2002;51(RR-15):1–38.
Gaydos CA, Van Der Pol B, Jett-Goheen M, Barnes M, Quinn N, Clark C, et al. 3rd; CT/NG Study Group. Performance of the Cepheid CT/NG Xpert rapid PCR test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2013;51(6):1666–72.
LeFevre ML, U.S. Preventive Services Task Force. Screening for Chlamydia and gonorrhoea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(12):902–10.
Romero R, Nygaard I. CDC updates guidelines for treating sexually transmitted diseases. Am J Obstet Gynecol. 2015;213(2):117–8.
Hammerschlag MR, Chandler JW, Alexander ER, English M, Koutsky L. Longitudinal studies on chlamydial infections in the first year of life. Pediatr Infect Dis. 1982;1(6):395–401.
Unemo M, Seifert HS, Hook EW III, Hawkes S, Ndowa F, Dillon JR. Gonorrhea. Nat Rev Dis Primers. 2019;5(1):79.
Philpott A, Knerr W, Maher D. Promoting protection and pleasure: amplifying the effectiveness of barriers against sexually transmitted infections and pregnancy. Lancet. 2006;368(9551):2028–31.
Christmas JT, Wendel GD, Bawdon RE, Farris R, Cartwright G, Little BB. Concomitant infection with Neisseria gonorrhoeae and Chlamydia trachomatis in pregnancy. Obstet Gynecol. 1989;74(3 Pt 1):295–8.
Phupong V, Sittisomwong T, Wisawasukmongchol W. Disseminated gonococcal infection during pregnancy. Arch Gynecol Obstet. 2005;273(3):185–6.
Burkman RT, Tonascia JA, Atienza MF, King TM. Untreated endocervical gonorrhea and endometritis following elective abortion. Am J Obstet Gynecol. 1976;126(6):648–51.
Laga M, Meheus A, Piot P. Epidemiology and control of gonococcal ophthalmia neonatorum. Bull World Health Organ. 1989;67(5):471–7. Erratum in: Bull World Health Organ 1990; 68(5):690
Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. Erratum in: MMWR Recomm Rep. 2015 28; 64(33):924.
St Cyr S, Barbee L, Workowski KA, Bachmann LH, Pham C, Schlanger K, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1911–6.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bhatia, S., Gupta, R., Yadav, S. (2022). Herpes, Gonorrhea, Chlamydia, and HPV Infection. In: Mehta, S., Grover, A. (eds) Infections and Pregnancy. Springer, Singapore. https://doi.org/10.1007/978-981-16-7865-3_19
Download citation
DOI: https://doi.org/10.1007/978-981-16-7865-3_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7864-6
Online ISBN: 978-981-16-7865-3
eBook Packages: MedicineMedicine (R0)