Abstract
This paper presents the synthesis, structural analysis, and DC conductivity of zinc ferrite (ZnFe2O4) and lanthanum-doped zinc nanoferrite synthesized via green synthesis route. The formation of ZnFe2O4 and lanthanum-doped ZnFe2O4 nanoferrites was confirmed by X-ray Diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FTIR) techniques. The Expo-2014 software was used for indexing and structural analysis of the nanoferrites. The Fityk software was used for the estimation of the crystallite size of the synthesized nanoferrites. The structural analysis revealed the normal spinel structure of the ZnFe2O4 and lanthanum-doped ZnFe2O4. The DC conductivity study was performed using the four-probe method. The resistivity measurement was carried out in the temperature range of 318–453 K. The DC conductivity study showed the semiconductor behavior of the ZnFe2O4 and lanthanum-doped ZnFe2O4. From the resistivity measurement using the four-probe method the activation energies of the ZnFe2O4 and lanthanum-doped ZnFe2O4were calculated.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Zinc ferrite belongs to a class of spinel ferrite. The general formula of spinel ferrite is MFe2O4 (where M is a divalent metal ion). In spinel ferrite, M2+ and Fe3+ occupy the tetrahedral (A) and octahedral (B) interstitial sites of the fcc lattice formed by O2− ions, respectively [1, 2]. The unit cell of a spinel ferrite consists of eight divalent metal ions, 16 trivalent ions, and 32 oxygen atoms [3]. The magnetic ferrites are useful in a variety of fields such as gas sensing [4, 5], diagnostic medicine [6, 7], data storage [8, 9], and transformer cores [10]. The ferrites are essential inductive components in electronic circuits such as filters, low-noise amplifiers, voltage-controlled oscillators, and impedance matching networks. In recent days, zinc ferrite (ZnFe2O4) and its composites are drawing greater attention due to their wide range of applications. The zinc ferrite is used in commercial applications such as magnetic resonance imaging (MRI), Li-ion batteries [11], and gas sensors [12]. The zinc ferrite (ZnFe2O4) seems to be an ideal candidate to use as a soft magnet and low loss material at high frequency [13]. The doping of rare-earth metal ions would change the texture of magnetic ferrites [14]. Due to the high electrical insulation property of rare-earth materials, the inclusion of rare-earth ions could alter the magnetic and electrical properties [15, 16]. Due to the excellent applications of zinc ferrite and its nanocomposites, zinc ferrite and lanthanum-doped zinc ferrite have been prepared by the green synthesis route.
In recent years there is a great interest in preparing nanoparticles using biomolecules as it is cost-effective, the input energy for synthesis is less and the precursors used are nontoxic. It is also known to reduce particle aggregation. In the present study, ZnFe2O4 and lanthanum-doped ZnFe2O4 nanoferrites were prepared using biomolecules extracted from Tulsi (Ocimum sanctum) leaf extract. To understand the electrical behavior of the synthesized ZnFe2O4 and lanthanum-doped ZnFe2O4 nanoferrites, the DC conductivity measurement was also carried out.
2 Experimental
2.1 Preparation of ZnFe2O4
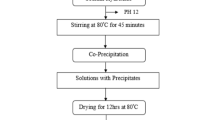
The chemicals used in the present study were analytical grade zinc nitrate hexahydrate [Zn (NO3)2.6H2O], iron nitrate [Fe(NO3)3.9H2O], and lanthanum nitrate [La(NO3)2.6H2O]. The Tulsi leaf extract was used as a reducing agent for the synthesis of ZnFe2O4 and lanthanum-doped ZnFe2O4 nanoferrites. The Tulsi (Ocimum sanctum) leaves were collected locally, first washed with tap water and then with distilled water. The leaves were dried in a cool place for seven days. The dried leaves were then ground to powder using a pestle and mortar. About 4 g of Tulsi leaf powder was weighted using an electronic balance and added to 100 mL of distilled water and the mixture was stirred well using a magnetic stirrer for 1 h at 50 °C. The leaf extract was filtered using filter paper and pure leaf extract was obtained. The pH of the obtained leaf extract was about 6.5. The pH plays an important role in controlling the morphology and yield of nanoparticle synthesis. About 1.070 g of zinc nitrate and 3.232 g of iron nitrate were added to the leaf extract. The solution was stirred using a magnetic stirrer and a few drops of ammonia solution were added to adjust the pH between 7 and7.5 and the stirring was continued at 90 °C until all the water evaporated completely. Finally, the product was heated at 250 °C in a laboratory oven to facilitate a self-propagating combustion reaction of redox mixture to form crystalline ZnFe2O4. The steps involved in the synthesis of ZnFe2O4 and lanthanum-doped ZnFe2O4 are shown in Fig. 1.
2.2 Preparation of lanthanum- Doped ZnFe2O4
About 4 g of Tulsi leaf powder was added to 200 mL of distilled water and was stirred using a magnetic stirrer for 1 h at 50 °C. The mixture was filtered using filter paper and to get pure leaf extract. About 1.070 g of zinc nitrate, 3.232 g of iron nitrate, and 0.057 g (5 wt% relative to zinc nitrate) lanthanum nitrate were added to the leaf extract and finally, ammonia solution was added to maintain the pH between 7 and 7.5. The stirring was continued at 90 °C until the water is evaporated completely. The final product was heated at 250 °C and crystallized lanthanum-doped ZnFe2O4 was obtained as the combustion product.
2.3 Characterization
The phase purity of the powders was checked using an X-ray diffractometer (XRD, Bruker D8 Advance) and Expo-2014 crystal structure solution. The full width at half maxima (β) was estimated using Fityk software [17]. The crystallite size was found from substituting the value of β in the Scherrer`s equation:
where D—Average crystallite size (nm); K—Scherrer constant. K varies from 0.68 to 2.08. K = 0.94 for spherical crystallites with cubic symmetry; λ-X-ray wavelength; β- FWHM (Full width half maximum) obtained from XRD peak.
The FTIR analysis of ZnFe2O4 and lanthanum-doped ZnFe2O4was carried out using a Bruker FTIR spectrometer. The DC conductivity study was carried out with the four-probe setup (Mars Edpal Pvt (Ltd), India) and constant current power supply (SES instruments, India). For the DC measurement, the nanoferrite powder was ground into a fine powder using mortar and pestle. The ground fine powder of nanoferrite was pelletized using a hydraulic press at a pressure of 2 tons. The nanoferrite pellet was cut into a square shape using a sharp razor. The square-shaped pellet was then polished smoothly for good electrical contact. The four-probe setup consists of four equally spaced spring-loaded pen-type probes with a spacing of 0.2 cm. The pellet under study was placed in between a thin mica sheet and the probes. The four-probe setup was manually adjusted with gentle pressure. The current and voltage were measured using digital multimeters. The DC measurement was carried out within the temperature range of 318–473 K while cooling.
3 Results and Discussion
3.1 X-Ray Diffraction Analysis
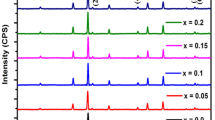
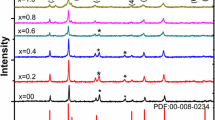
Figure 2 shows the XRD pattern of ZnFe2O4.The figure shows major XRD peaks at 2θ values of 30.26, 35.64, 37.28, 43.32, 53.75, 57.30, 62.93, 71.41, 74.47 and 75.48°. The XRD pattern matches well with the patterns reported in the literature [18, 19]. Figure 3 shows the XRD indexing for lanthanum-doped ZnFe2O4. The figure shows major XRD peaks at 2θ values of 30.17, 35.54, 37.18, 43.19, 47.29, 53.59, 57.13, 62.74, 71.18, 74.23 and 75.23°. The 2θ values, interplanar spacing (d), Miller indices, (h, k, l) and lattice constant (a) obtained for ZnFe2O4 on the basis of XRD data and Expo-2014 are summarized in Table 1 [20]. In Table 2, 2θ values, interplanar spacing (d), Miller indices, (h, k, l) and lattice constant (a) are listed for lanthanum-doped ZnFe2O4 on the basis of XRD data and Expo-2014. The values of (h, k, l), d-spacing, and lattice constant obtained by indexing the XRD data using the Expo-2014 reveal the formation of cubic spinel structure for both ZnFe2O4 and lanthanum-doped ZnFe2O4.
The Fityk analysis was made with the main XRD peak at 2θ = 35.42°. Figure 4 shows the Gaussian fit to the XRD peak at 2θ = 35.42° for ZnFe2O4 and Fig. 5 shows the Gaussian fit to the XRD peak at 2θ = 35.38° for lanthanum—doped ZnFe2O4. From the Fityk analysis, the full width half maximum (β) was estimated to be 0.409041 for ZnFe2O4 and 0.286329 for lanthanum-doped ZnFe2O4. The crystallite size of ZnFe2O4 was found to be 21.31 nm and for lanthanum-doped ZnFe2O4 it was found to be 30.43 nm.
3.2 FTIR Analysis
The FTIR absorption spectra of the synthesized ferrite samples are shown in Figs. 6 and 7. The characteristics absorption peak at 603 cm−1 for ZnFe2O4is attributed to Fe–O vibration of regular spinel sample and the characteristics absorption peak at 404 cm−1 for ZnFe2O4 is attributed to Zn–O vibration of spinel ferrite sample [21, 22]. The characteristics absorption peak at 557 cm−1 for lanthanum-doped ZnFe2O4 is attributed to Fe–O vibration of regular spinel sample. The characteristics absorption peak at 423 cm−1 for lanthanum-doped ZnFe2O4 is attributed to Zn–O vibration of spinel ferrite sample. The FTIR analysis results so obtained are in accordance with the results obtained from the XRD analysis.
3.3 DC Conductivity Study
Figure 8 depicts the variation of electrical conductivity with varying temperatures and Fig. 9 shows the plot of log10 (ρ) versus 1/T. It is evident from the figure that electrical conductivity increases with increasing temperature for both ZnFe2O4 and lanthanum-doped ZnFe2O4. The increase of electric conductivity of ferrites with temperature shows the semiconducting nature of the ferrites. This nature can be attributed to the thermal enhancement of carrier mobility [23, 24]. However, the conductivity of lanthanum-doped ZnFe2O4 was lower than that of ZnFe2O4. This may be due to the occupation of lanthanum ions in the octahedral sites. The occupation of La3+ ions in octahedral sites reduces electron hopping. The increase of conductivity with temperature indicates the semiconductor behavior of the ZnFe2O4 and lanthanum-doped ZnFe2O4. For both ZnFe2O4 and lanthanum-doped ZnFe2O4, the plot of log10ρ versus 1000/T is linear. By finding the slope of the log10 (ρ) versus 1000/T line, the activation energy Ea can be determined using the relation:
For ZnFe2O4, Ea was estimated as 0.352 eV. This value is in agreement with the reported value 0.331 eV [25]. For lanthanum-doped ZnFe2O4 the Ea was estimated as 0.364 eV. The activation energy of lanthanum-doped ZnFe2O4 is greater than that of ZnFe2O4. This reveals the fact that doping of rare-earth ions reduces the number of Fe3+ ions in octahedral site which also slow down the electron hopping between Fe3+ and Fe2+ [26].
4 Conclusions
Zinc ferrite (ZnFe2O4) and lanthanum-doped zinc nanoferrite were synthesized via a green synthesis route using Tulsi leaf extract. The XRD and FTIR analysis revealed the normal spinel structure for both ZnFe2O4 and lanthanum-doped ZnFe2O4. The XRD structural analysis shows higher lattice constant and crystallite size for lanthanum-doped ZnFe2O4 compared to ZnFe2O4.This is attributed to the accumulation of La3+ in the octahedral lattice sites of the ZnFe2O4. The DC conductivity study confirmed semiconducting behavior for both ZnFe2O4 and lanthanum-doped ZnFe2O4 nanoferrites. The conductivity of lanthanum-doped ZnFe2O4 decreased in comparison to ZnFe2O4 within the measured temperature range. The reason for decreased conductivity for lanthanum-doped ZnFe2O4 is the occupation of La3+ in the octahedral sites. From this study, it was observed that by doping of La3+ions in ZnFe2O4 the resistivity increases and thus it reveals that doping of lanthanum increases the electrical insulation of ZnFe2O4. Future studies are aimed at the cyclic voltammetric studies of the synthesized ferrites.
References
Kooti M, Sedeh AN (2013) Synthesis and characterization of NiFe2O4 magnetic nanoparticles by combustion method. J Mater Sci Technol 29:34–38. https://doi.org/10.1016/j.jmst.2012.11.016
Sadri F, Ramazani A, Ahankar H, Taghavi Fardood S, Azimzadeh Asiabi P, Khoobi M, Joo SW, Dayyani N (2016) Aqueous- phase oxidation of alcohols with green oxidants (Oxone and hydrogen peroxide) in the presence of MgFe2O4 magnetic nanoparticles as an efficient and reusable catalyst. J Nanostruct 6:264–272
Prasad BBVSV, Ramesh KV, Srinivas A (2019) Structural and magnetic studies on Co-Zn nanoferrite synthesized via sol-gel and combustion methods. Mater Sci-Poland 37:39–54. https://doi.org/10.2478/msp-2019-0013
You J, Chen X, Zheng B, Geng X, Zhang C (2017) Suspension plasma-sprayed ZnFe2O4 nanostructured coatings for ppm-level acetone detection. J Therm Spray Technol 26:728–734. https://doi.org/10.1007/s11666-017-0536-7
Jeseentharani V, George M, Jeyaraj B, Dayalan A, Nagaraja K (2013) Synthesis of metal ferrite (MFe2O4, M = Co, Cu, Mg, Ni, Zn) nanoparticles as humidity sensor materials. J Exp Nanosci 8:358–370. https://doi.org/10.1080/17458080.2012.690893
Issa B, Obaidat I, Albiss B, Haik Y (2013) Magnetic nanoparticles: surface effects and properties related to biomedicine applications. Int J Mol Sci 14:21266–21305. https://doi.org/10.3390/ijms141121266
Niemirowicz K, Markiewicz KH, Wilczewska AZ, Car H (2012) Magnetic nanoparticles as new diagnostic tools in medicine. Adv Med Sci 57:196–207. https://doi.org/10.2478/v10039-012-0031-9
Galloway JM, Talbot JE, Critchley K, Miles J, Bramble J (2015) Developing biotemplated data storage: room temperature biomineralization of L10CoPt magnetic nanoparticles. Adv Funct Mater 25:4590–4600. https://doi.org/10.1002/adfm.201501090
S-ichi O, Namai A, Yoshikiyo M, Imoto K, Tamazaki K, Matsuno K, Inoue O, Ide T, Masada K, Goto M, Goto T, Yoshida T, Miyazaki T (2016) Multimetal-substituted epsilon-iron oxide ϵ-Ga0.31Ti0.05Co0.05Fe1.59O3 for next-generation magnetic recording tape in the big-data era. Angew Chem Int 55:11403–11406. https://doi.org/10.1002/anie.201604647
Ngoua Teu Magambo JS, Bakri R, Margueron X, Le Moigne P, Mahe A, Guguen S, Bensalah T (2017) Planar magnetic components in more electric aircraft: review of technology and key parameters for DC–DC power electronic converter. IEEE Trans Transp Electrification 3:831–842. https://doi.org/10.1109/tte.2017.2686327
Zhong X-B, Yang Z-Z, Wang H-Y, Lu L, Jin B, Zha M, Jiang Q-C (2016) A novel approach to facilely synthesize mesoporous ZnFe2O4 nanorods for lithium ion batteries. J Power Sourc 306:718–723. https://doi.org/10.1016/j.jpowsour.2015.12.102
Srivastava R, Yadav BC (2014) Nanostructured ZnFe2O4thick film as room temperature liquefied petroleum gas sensor. J Exp Nanosci 10:703–717. https://doi.org/10.1080/17458080.2013.880001
Sathiyamurthy K, Rajeevgandhi C, Bharanidharan S, Sugumar P, Subashchandrabose S (2020) Electrochemical and magnetic properties of zinc ferrite nanoparticles through chemical co-precipitation method. Chem Data Collect 28:100477. https://doi.org/10.1016/j.cdc.2020.100477
Kumar L, Kar M (2012) Effect of Ho3+ substitution on the cation distribution, crystal structure and magnetocrystalline anisotropy of nanocrystalline cobalt ferrite. J Exp Nanosci 9:362–374. https://doi.org/10.1080/17458080.2012.661474
Xavier S, Thankachan S, Jacob BP, Mohammed EM (2013) Effect of samarium substitution on the structural and magnetic properties of nanocrystalline cobalt ferrite. J Nanosci 2013:1–7. https://doi.org/10.1155/2013/524380
Zhou B, Zhang Y-W, Liao C-S, Yan C-H, Chen L-Y, Wang S-Y (2004) Rare-earth-mediated magnetism and magneto-optical Kerr effects in nanocrystalline CoFeMn0.9RE0.1O4 thin films. J Magn Magn Mater 280:327–333. https://doi.org/10.1016/j.jmmm.2004.03.031
Wojdyr M (2010) Fityk: a general-purpose peak fitting program. J Appl Crystallogr 43:1126–1128. https://doi.org/10.1107/s0021889810030499
Abbas SI, John HT, Fraih AJ (2017) Preparation of nano crystalline zinc—ferrite as material for micro waves absorption by sol-gel methods. Indian J Sci Technol 10:1–6. https://doi.org/10.17485/ijst/2017/v10i21/113197
Kanagesan S, Hashim M, AB Aziz S, Ismail I, Tamilselvan S, Alitheen N, Swamy M, Purna Chandra Rao B (2016) Evaluation of antioxidant and cytotoxicity activities of copper ferrite (CuFe2O4) and zinc ferrite (ZnFe2O4) Nanoparticles synthesized by sol-gel self-combustion method. Appl Sci 6:184. https://doi.org/10.3390/app6090184
Altomare A, Cuocci C, Giacovazzo C, Moliterni A, Rizzi R, Corriero N, Falcicchio A (2013) EXPO2013: a kit of tools for phasing crystal structures from powder data. J Appl Crystallogr 46:1231–1235. https://doi.org/10.1107/s0021889813013113
Din IU, Tasleem S, Naeem A, Shaharun MS, Al GMJ, Kaisy, (2013) Zinc ferrite nanoparticle synthesis and characterization; effects of annealing temperature on the size of nanoparticles. Aust J Basic Appl Sci 7:154–162
Vinosha PA, Mely LA, Jeronsia JE, Monica FH, Raja K, Das SJ (2017) Study of structural, optical, dielectric and magnetic properties of zinc ferrite synthesized by co-precipitation. Nano Hybrids Compos 17:1–9. https://doi.org/10.4028/www.scientific.net/nhc.17.1
Hankare P, Patil N, Patil R, Patil D, Delekar S (2013) Structural, electrical and magnetic properties of copper substituted Zn–Mn ferrites. J Mater Sci Mater Electron 24:4028–4032
Yadav SP, Shinde SS, Kadam AA, Rajpure KY (2013) Structural, morphological, dielectrical and magnetic properties of Mn substituted cobalt ferrite. J Semicond 34 093002. https://doi.org/10.1088/1674-4926/34/9/093002
Sekulic DL, Lazarevic ZZ, Sataric MV, Jovalekic CD, Romcevic NZ (2015) Temperature-dependent complex impedance, electrical conductivity and dielectric studies of MFe2O4 (M = Mn, Ni, Zn) ferrites prepared by sintering of mechanochemical synthesized nanopowders. J Mater Sci Mater Electron 26:1291–1303. https://doi.org/10.1007/s10854-014-2491-0
Pervaiz E, Gul IH (2013) Influence of rare earth (Gd3+) on structural, gigahertz dielectric and magnetic studies of cobalt ferrite. J Phys Conf Ser 439:012015. https://doi.org/10.1088/1742-6596/439/1/012015
Acknowledgements
The authors are thankful to DST-SAIF, KOCHI for XRD measurements.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Udagani, C., Mahalakshmi, H.R., Kumar Govind, N., Keerthiveni, J. (2022). Green Synthesis and Characterization of Zinc Ferrite and Lanthanum- Doped Zinc Ferrite. In: Mudali, U.K., Aruna, S.T., Nagaswarupa, H.P., Rangappa, D. (eds) Recent Trends in Electrochemical Science and Technology. Springer Proceedings in Materials, vol 15. Springer, Singapore. https://doi.org/10.1007/978-981-16-7554-6_14
Download citation
DOI: https://doi.org/10.1007/978-981-16-7554-6_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7553-9
Online ISBN: 978-981-16-7554-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)