Abstract
Biological disease-modifying antirheumatic drugs (bDMARDs) have changed the physicians’ therapeutic approach to Spondyloarthritis (SpA) since their advent. They are the single most effective treatment option by a distance for axial SpA (axSpA), where the science of rheumatology had found itself limited until a couple of decades back. Apart from their numero uno position in the management of Ankylosing Spondylitis (AS), they are also very effective for treating peripheral SpA (pSpA), as well as non-radiographic axSpA (nr-axSpA). The following text shall provide an overview of the various bDMARDs and their applications in SpA.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
3.1 Introduction
Biological disease-modifying antirheumatic drugs (bDMARDs) have changed the physicians’ therapeutic approach to Spondyloarthritis (SpA) since their advent. They are the single most effective treatment option by a distance for axial SpA (axSpA), where the science of rheumatology had found itself limited until a couple of decades back. Apart from their numero uno position in the management of Ankylosing Spondylitis (AS), they are also very effective for treating peripheral SpA (pSpA), as well as non-radiographic axSpA (nr-axSpA). The following text shall provide an overview of the various bDMARDs and their applications in SpA.
3.2 Overarching Principles for Use of Biologicals in Spondyloarthritis
-
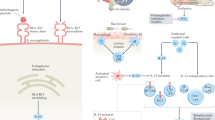
A chronic triggering of inflammation in genetically predisposed individuals takes place in SpA. Studies have shown that control of inflammation at the very earliest provides the best chance to prevent the ensuing bone formation and the resultant ankylosis. It gives the patients the best chance to maintain function and prevent deformity [1].
-
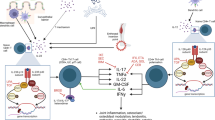
Research over the years had already implicated the dysregulation of cytokine production as the key process driving the inflammation in SpA. With further studies, the cytokines began to be identified. Then came the evidence of involvement of the Interleukin (IL)-23/IL-17 axis. With the above knowledge at hand, tumor necrosis factor-alpha inhibitors (TNFi) and IL-17inhibitors (IL-17i) and IL-12/23i were developed and were found to be effective in blocking the cascade of inflammation in SpA.
-
The guidelines for prescribing bDMARDs in SpA in different countries have minor variations, but they are generally recommended in moderate to severe disease not responding to the conventional drugs.
-
In axSpA, bDMARDs are recommended where a trial of nonsteroidal anti-inflammatory drugs (NSAIDs) fails to produce a response or these agents are not tolerated. In psoriatic and non-psoriatic peripheral SpA, bDMARDs are recommended where conventional DMARDs have failed or are not tolerated.
-
Apart from high axial or peripheral skeletal disease activity, another indication for the institution of bDMARD therapy is severe eye, skin, or intestinal inflammation, which might be organ threatening.
-
Although the primary consideration is to provide the best care possible, the cost is of concern when using biologicals. It is for this reason, that EULAR recommends that the choice of bDMARD may be driven by cost where similar outcomes can be expected with the agents under consideration [2].
-
Studies have shown that both NSAIDs and TNFi are more effective in patients with shorter disease duration, thus it would be prudent to conclude that early treatment with bDMARDs is likely to result in better outcomes.
3.3 Biological Agents Approved for Spondyloarthritis
The US Food and Drug Administration (USFDA) approved TNFi for the treatment of AS include infliximab (IFX), etanercept (ETN), adalimumab (ADA), certolizumab pegol (CZP), golimumab (GOL), Secukinumab (SEC), and Ixekizumab are IL-17i approved by the agency for AS [3].
3.3.1 Infliximab in Spondyloarthritis
-
IFX is a chimeric mouse–human monoclonal antibody. It binds soluble as well as transmembrane TNF alpha.
-
Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy (ASSERT) was the landmark trial that established the efficacy of IFX in AS. The drug was found to be effective as early as within 2 weeks of initiation and continued to be effective during the 24 weeks of the study [4].
-
The drug has a half-life of 9 days and is recommended to be given at a dose of 5 mg per kg at 0, 2, and 6 weeks, and then every 6 weeks thereafter.
3.3.2 Etanercept in Spondyloarthritis
-
ETN is a fusion protein that binds to soluble TNF and lymphotoxin alpha. Studies have shown that the molecule has very low immunogenicity.
-
A randomized controlled trial in 2003 found ETN highly effective in AS with improvements in the patient-reported measures, acute phase reactants as well as function. The safety was comparable to the drug’s trials in rheumatoid arthritis (RA) and psoriatic arthritis (PsA) [5].
-
The drug has a half-life of 4 days and is recommended to be given at a dose of 25 mg twice a week. Studies have also shown 50 mg once a week to be effective as well.
3.3.3 Adalimumab in Spondyloarthritis
-
ADA is a human monoclonal antibody that binds soluble as well as transmembrane TNF alpha.
-
The ABILITY-1 trial showed that ADA was effective in the treatment of nr-axSpA. The ABILITY-2 trial showed the molecule’s efficacy in non-psoriatic peripheral SpA [6, 7].
-
ADA has a half-life of 14 days and is recommended to be given at a dose of 40 mg every other week.
3.3.4 Certolizumab Pegol in Spondyloarthritis
-
CZP is a Fab fragment of a humanized anti-TNF antibody fused to polyethylene glycol, that binds TNF alpha.
-
The RAPID-axSpA and RAPID-PsA studies found CZP efficacious in axSpA and PsA. The patients showed improvement in the extraarticular domains of PsA as well, including skin disease, nail disease, enthesitis, and dactylitis [8, 9].
-
The drug has a half-life of 14 days and is recommended to be given at a dose of 400 mg at 1, 2, and 4 weeks, and thereafter at 200 mg fortnightly or 400 mg every 4 weeks.
3.3.5 Golimumab in Spondyloarthritis
-
GOL is a human monoclonal antibody that binds soluble as well as transmembrane TNF alpha.
-
The results of the GO-RAISE study published in 2008 showed the efficacy and safety of GOL in the treatment of AS in 2008 [10].
-
GOL has a half-life of 14 days and is recommended to be given at a dose of 50 or 100 mg every month.
3.3.6 Secukinumab and Ixekizumabin Spondyloarthritis
-
SEC is a human monoclonal antibody against interleukin-17A.
-
The MEASURE1 and MEASURE2 trials showed the efficacy of SEC in patients with active AS. The studies showed the efficacy of a 150-mg dose with a loading dose in the first 4 weeks [11].
-
SEC has a half-life of 27 days and is recommended to be given at a dose of 150 mg at 0, 1, 2, 3, and 4 weeks and then every 4 weeks for SpA.
-
Ixekizumab is another IL-17i approved for use in AS. It has a half-life of 13 days and it is used at a dose of 160 mg at dose 0 and then 80 mg every 4 weeks.
3.3.7 Ustekinumab in Spondyloarthritis
-
Ustekinumab is a human IL-12 and IL-23 antagonist approved for use in psoriasis, psoriatic arthritis, and Crohn’s disease.
-
It has a half-life of 20–39 days and is recommended to be given at a dose of 45 mg at 0 and 4 weeks and then every 12 weeks for PsA.
3.4 Biologics in Various Spondyloarthritides
While there is a unifying concept to the SpA family, the differences that exist in various diseases and manifestations of the family translate into a need for customizing the bDMARD therapy. A summary of where different biological agents are effective is given in Table 3.1.
3.4.1 Axial Spondyloarthritis
-
AxSpA includes radiographic (r-axSpA) and non-radiographic (nr-axSpA) diseases. The initial approvals for use of bDMARDs in axSpA came only for r-axSpA. The efficacy was also found higher in this subgroup. It was, however, later recognized to be due to a higher degree of certainty of diagnosis in r-axSpA compared to nr-axSpA.
-
Biological DMARDs are the cornerstone of therapy for active, moderate to severe axSpA. Younger age, shorter duration of disease, elevated acute phase reactants, HLA-B27 positivity, and inflammation on MRI are predictors of good response to bDMARDs [12].
-
Studies have failed to show any significant differences between various biological agents used for the treatment of axSpA. The choice of agent is hence driven by extraarticular manifestations (EAMs), comorbidities, cost, and local availability.
-
The newer studies have shown that TNFi and IL-17i reduce radiographic progression. But since the structural damage in axSpA is heterogeneous and is appreciable only over a longer term, it is not a useful tool to guide optimization of bDMARD therapy or switching from one agent to another.
-
The recommendations are to start patients with axSpA on NSAIDs and evaluate after 2–4 weeks. Then bDMARD therapy is initiated in those patients who do not respond or are intolerant to NSAIDs. TNFi are the first choice bDMARDs in axSpA and the current recommendations prefer IL17i over targeted synthetic DMARDs (tsDMARDs) as next line agents [2, 13].
3.4.2 Non-psoriatic Peripheral Spondyloarthritis
-
In non-psoriatic peripheral SpA (pSpA), conventional DMARDs like methotrexate, sulfasalazine, and leflunomide are used where NSAIDs or local glucocorticoid injections do not work. In patients who fail to respond or are intolerant to these agents, bDMARDs are initiated [14].
-
The CRESPA trial with golimumab showed the efficacy of this TNFi in pSpA and demonstrated that a drug-free remission was achievable in nearly 50 percent of these patients.
-
The ABILITY-2 trial and the study by Paramarta et al. established the efficacy of Adalimumab in pSpA. These studies were the first to demonstrate the concept of early treatment in pSpA [7, 15].
3.4.3 Psoriatic Arthritis
-
The choice of therapy in PsA is guided by the extents of axial involvement, peripheral joint involvement, skin disease, and other EAMs like enthesitis and dactylitis.
-
In predominantly axial disease not responding to NSAIDs, bDMARDs are started with the usual practice being to start with a TNFi [16].
-
NSAIDs or glucocorticoids are first used in mono or oligoarthritis and enthesitis, and then bDMARDs are instituted in non-responders.
-
In PsA patients with predominant polyarthritis, the usual practice is to start with conventional DMARDs and then move to bDMARDs if needed.
-
However, recent recommendations do provide the option to use upfront bDMARDs in those with severe arthritis and/or severe skin disease [17].
-
Rapid progression, erosions, elevated APRs, and active PsA at multiple sites are considered hallmarks of severe arthritis.
-
A PASI score of over 12, involvement of more than 5–10% body surface area (BSA) or physical or mental impairment (even in absence of high PASI or more than 5–10% BSA involvement) are considered to indicate severe psoriasis.
-
TNFi, IL-17i, and IL-12/23i have all been found to be effective in PsA. IL-12/23i agents are not used in axial disease.
-
TNFi are effective in skin and nail diseases but they paradoxically increase the lesions in some patients. IL-17i and Il12/23i are the preferred agents in patients with severe psoriasis.
-
When a patient with PsA does not respond to one bDMARD, it is recommended to switch to another agent of the same class or an agent of another class.
3.5 Extraarticular Manifestations and Choice of Biologicals in SpA
-
Even in absence of high skeletal disease activity in a patient with SpA, early bDMARD therapy may be warranted because of active inflammatory bowel disease (IBD). Among the TNFi, monoclonal antibody TNFi, e.g., adalimumab and infliximab are preferred over agents like etanercept in patients with IBD [18]. IL-17i are not preferred in patients with IBD. IL-12/23i on the other hand are preferred agents, particularly for Crohn’s disease [18].
-
In patients with recurrent uveitis, monoclonal antibody TNFi are preferred over other TNFi and non-TNFibDMARDs.
-
All the available bDMARDs can be used in patients with enthesitis and dactylitis refractory to NSAIDs and/or local glucocorticoid injections. Tofacitinib and Apremilast are other options in enthesitis refractory to the first-line therapy [16].
-
TNFi are effective in AA amyloidosis and also reduce the risk of cardiovascular manifestations and atherosclerotic cardiovascular disease associated with SpA. However, they are best avoided in patients with pre-existing heart failure [19, 20].
3.6 Summary
-
Biological disease-modifying anti-rheumatic drugs are effective options for the management of spondyloarthritis.
-
Choosing the best-suited agent based on the disease manifestations and other coexisting or comorbid conditions results in better outcomes.
-
Early initiation of biological agents in spondyloarthritis has the potential to minimize damage and halt structural progression.
References
Furst DE, Louie JS. Targeting inflammatory pathways in axial spondyloarthritis. Arthritis Res Ther. 2019;21:135.
van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van den Bosch F, Sepriano A, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–91.
Spondyloarthritis. https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Spondyloarthritis. Accessed 14 Jun 2021.
van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 2005;52:582–91.
Davis JC, Heijde DVD, Braun J, Dougados M, Cush J, Clegg DO, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48:3230–6.
Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis. 2013;72:815–22.
Mease P, Sieper J, Van den Bosch F, Rahman P, Karunaratne PM, Pangan AL. Randomized controlled trial of adalimumab in patients with nonpsoriatic peripheral spondyloarthritis. Arthritis Rheumatol. 2015;67:914–23.
Landewé R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis. 2014;73:39–47.
Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2014;73:48–55.
Inman RD, Davis JC, Heijde DVD, Diekman L, Sieper J, Kim SI, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58:3402–12.
Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373(26):2534–48. https://doi.org/10.1056/NEJMoa1505066.
Fragoulis GE, Siebert S. Treatment strategies in axial spondyloarthritis: what, when and how? Rheumatology. 2020;59:iv79–89.
Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Care Res. 2019;71:1285–99.
Carron P, Varkas G, Cypers H, Van Praet L, Elewaut D, Van den Bosch F, et al. Anti-TNF-induced remission in very early peripheral spondyloarthritis: the CRESPA study. Ann Rheum Dis. 2017;76:1389–95.
Paramarta JE, De Rycke L, Heijda TF, Ambarus CA, Vos K, Dinant HJ, et al. Efficacy and safety of adalimumab for the treatment of peripheral arthritis in spondyloarthritis patients without ankylosing spondylitis or psoriatic arthritis. Ann Rheum Dis. 2013;72:1793–9.
Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79:700–12.
Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res. 2019;71:2–29.
Bruner V, Atteno M, Spanò A, Scarpa R, Peluso R. Biological therapies for spondyloarthritis. Ther Adv Musculoskelet Dis. 2014;6:92–101.
Kobak S, Oksel F, Kabasakal Y, Doganavsargil E. Ankylosing spondylitis-related secondary amyloidosis responded well to etanercept: a report of three patients. Clin Rheumatol. 2007;26:2191–4.
Di Minno MND, Iervolino S, Peluso R, Scarpa R, Di Minno G. CaRRDs study group. Carotid intima-media thickness in psoriatic arthritis: differences between tumor necrosis factor-α blockers and traditional disease-modifying antirheumatic drugs. Arterioscler Thromb Vasc Biol. 2011;31:705–12.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pandey, B.D. (2022). Biologics in Spondyloarthritis. In: Jain, N., Duggal, L. (eds) Handbook of Biologics for Rheumatological Disorders. Springer, Singapore. https://doi.org/10.1007/978-981-16-7200-2_3
Download citation
DOI: https://doi.org/10.1007/978-981-16-7200-2_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7199-9
Online ISBN: 978-981-16-7200-2
eBook Packages: MedicineMedicine (R0)