Abstract
This work highlights that the CO2 footprint of cement can be reduced significantly by blending Portland cement clinker with thermally activated (calcined) clay (CC). Investigations on pure meta phases obtained via calcination of native kaolin, montmorillonite, illite and muscovite reveal that they noticeably increase the water demand and decrease workability of the cement. The effect depends on the fineness and internal porosity of the calcined clay and the chemical composition of the native clay. A comparison of three industrial calcined samples of mixed layer clays originating from natural deposits in Germany, India and China confirmed the increased water demand of composite cements holding up to 40 wt% of these calcined clays. The increase in water demand correlates with the amorphous part and the meta kaolin content. Also, the particle size and morphology of the calcined clay impact water demand. For one sample holding ~50% meta kaolin, an increase in superplasticizer dosage of ~400% as compared to neat OPC was recorded. Whereas, a high content of meta kaolin proved to be favorable with respect to rapid early strength development as a result of its high pozzolanic reactivity. It can be concluded that calcined clays offer the potential of significant CO2 reduction in cement manufacture, however higher superplasticizer dosages need to be used. Still, because of the low CO2 footprint of superplasticizers a substantial savings in CO2 emission can be realized, and the cement industry can progress into an era of more eco-friendly binders.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The production of Ordinary Portland Cement (OPC) comes with a significant environmental impact because per ton of OPC, no less than 850 kg of the greenhouse gas CO2 are emitted [1]. This means that at the current volume of global cement production of about 4.4 billion tons, over 3 billion tons of CO2 are released. This represents roughly 7% of total anthropogenic CO2 emission and even exceeds that from the global air traffic. Consequently, there is a dire need to reduce the environmental footprint of cement.
Recently, a new concept for the partial substitution of cement clinker by thermally activated (calcined) clays has been introduced, with the most prominent example being the so-called LC3 cement [2]. Its clinker content is reduced to 50%, with the remainder being calcined clay (30%), limestone powder (15%) and gypsum (5%) [3]. The main advantages of LC3 lie in the worldwide availability of huge clay deposits and the relatively low calcination temperature for the clay (~650 to 850 °C vs. 1450 °C for Portland cement).
In this paper, at first the characteristic properties of four different pure calcined clays—meta kaolin, meta montmorillonite, meta illite and meta muscovite—will be presented and their particle size, water demand and behavior towards a common industrial polycarboxylate (PCE) superplasticizer will be compared in order to understand the specifics of each pure calcined clay. Thereafter, three industrially produced calcined mixed layer clays obtained from different clay deposits were blended with a Portland cement CEM I 42.5 R at a clinker substitution rate of 30 wt% and their behavior in cement with respect to water demand, response to superplasticizer and strength development was analyzed. The overall goal of the study was to develop a more fundamental understanding of the behavior of thermally activated clays blended into cement and to assess their general usefulness in practical applications.
2 Materials and Methods
Cement. An ordinary Portland cement (OPC) CEM I 42.5 R (Schwenk Zement KG, Allmendingen plant, Germany) was used in the study. Its phase composition is shown in Table 1. The average particle size (d50 value) measured by laser granulometry was 19.8 µm. A density of 3.15 g/cm3 was determined by helium pycnometry and for the specific surface area (Blaine fineness) a value of 3,020 cm2/g was obtained.
Pure calcined clay samples. Meta kaolin was prepared industrially from >73 wt% pure kaolin via flash calcination between 550° and 650 °C. Meta montmorillonite was self-prepared via 2 h calcination in a lab-scale muffle furnace at 800 °C. In the same furnace, meta illite and meta muscovite were prepared via 1 h calcination at 770 °C and 800 °C, respectively.
Calcined mixed layer clay samples. Three mixed layer clay samples were subject to calcination at 750 °C (German CC) and 800 °C (Indian and Chinese CC). The mineralogical analysis of these three samples is presented in Table 2.
PCE superplasticizer. As superplasticizer, a commercial industrially produced acrylic acid-co-methallyl polyethylene glycol polycarboxylate (HPEG PCE) was used. This kind of PCE is most popular in the Asian market. Owed to its high anionic charge density, this type is mostly applied in precast concrete.
3 Results and Discussion
3.1 Pure Calcined Clay Samples
As a first, the pure calcined clay samples were characterized.
XRD analysis revealed the mineralogical compositions. According to this data it is evident that meta kaolin contains the highest content of amorphous phase (93 wt%) while meta montmorillonite exhibited the lowest (10 wt%). Generally, the amorphous content presents the dehydroxylated phases which are responsible for the pozzolanic activity of a calcined clay [4]. Hence, the data suggest that meta kaolin exhibits the highest reactivity (thus producing a particularly high early strength) while meta montmorillonite and meta illite will hydrate slower in cement and meta muscovite can be expected to present the least reactive of the four clinker phases.
It is well established that the pozzolanic reactivity of thermally activated clays is also much impacted by the fineness of the CC sample. To elucidate further, a particle size analysis was performed for the four meta clay samples. It revealed that meta illite consisted of particularly fine particles whereas meta montmorillonite presented a relatively coarse material. Relative to their d50 values, the order as follows could be established: Mmo ≫ Mmu > Mka ≫ Mil.
In the next step, the specific water demand of each meta clay sample was determined. The “water demand” is defined as the amount of water which is required to achieve a specific spread flow (e.g. 18 ± 0.5 cm) from a suspension of the meta clay in synthetic cement pore solution. According to this experiment, different meta clays exhibited significantly different water demands, as is evident from Fig. 1. By far the highest water demand was recorded for meta muscovite (w/b ratio = 2.70) as a consequence of its high surface area and the internal porosity of the particles. As expected, the lowest water demand was observed for the relatively coarse meta montmorillonite, and medium water demand values could be assigned to meta kaolin and meta illite.
The data presented in Fig. 1 signify that all meta clay samples cause a higher water demand than the Portland cement sample. This allows to predict that when such calcined clay materials are blended with cement clinker, then the water demand of the resulting composite cement will increase as compared to the neat OPC. The increase will be less when the raw clay used in the calcination predominantly contains montmorillonite, but will be most pronounced when the calcined clay is rich in meta illite or meta muscovite. The consequence of this behavior is that cements blended with meta muscovite require particularly high dosages of superplasticizers to reduce the water content to practical values which typically lie around 0.5 to achieve acceptable strength values whereas meta kaolin and meta montmorillonite are more benign and require less superplasticizer to reach the required water-to-binder ratio of ~0.5.
In order to elaborate more on the dispersing behavior of the different meta clay phases when treated with the HPEG PCE superplasticizer, the spread flow of pastes prepared from the calcined clay samples in synthetic cement pore solution and admixed with the acrylic acid-co-methallyl polyethylene glycol polycarboxylate (HPEG PCE) superplasticizer was examined. There, the water-to-binder ratios exhibited in Fig. 1 which produce a spread flow value of 18 cm were used for each CC sample. The results are exhibited in Fig. 2.
The data clearly suggest that the suspension of meta muscovite is very easy to disperse, i.e. a low dosage (0.1% by weight of CC) of the HPEG PCE is sufficient to increase the spread diameter from 18 to 28 cm. In comparison, meta montmorillonite already requires a PCE addition of 0.4% to reach only 26 cm spread flow which presents the maximum achievable value. Furthermore, meta kaolin first is thickened by dispersant addition, but then at dosages >0.2% strongly responds to the polycarboxylate and reaches high fluidity (~28 cm at a dosage of 1.0%). Most surprising is the behavior of the meta illite paste which does not become fluid even at a dosage of 1.0% of PCE. It can be assumed that its pronounced fineness and the concomitant high surface area necessitate extremely high dispersant additions to cover these surfaces via physical adsorption.
3.2 Calcined Mixed-Layer Clay Samples
As mentioned before, actual natural clay and marl deposits always contain a mixture of different clays, and the variations in composition can be enormous. For this reason, three samples of calcined mixed layer clays from Germany, India and China were probed for their behavior in cement, and an attempt was made to correlate their properties with the content of individual clay components such as the portion of meta kaolin, meta illite etc.
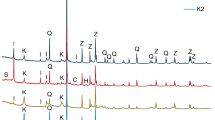
At first, the particle size distribution of the three samples was captured via laser granulometry. The results are displayed in Fig. 3.
There, it is observed that the German CC exhibits a larger particle size (d50 value = 13.2 μm) than the Chinese CC (d50 value = 10.4 μm). The Indian CC differs significantly from them in that it contains at the same time relatively high fractions of fine and of coarse particles. All three CC samples exhibit higher fineness than the OPC sample which is used to formulate the composite cements. Thus, it becomes evident that in such CC blended cements, the water demand will increase.
Furthermore, the phase composition of the calcined mixed layer clays was assessed via XRD. It was found that the amorphous content increased from 60.8% for the German CC to 62.2% for the Chinese CC and to 78.9% for the Indian CC (see Table 1). Moreover, analysis of the raw clays utilized in the manufacture of these CCs produced kaolinite contents of 25% (German clay), 45% (Indian clay) and 51% (Chinese clay). This signifies that the Indian and the Chinese calcined clays are particularly rich in meta kaolin.
The dispersing effectiveness of the HPEG PCE sample on the composite cements holding 20, 30 or 40 wt% of the mixed layer CCs was probed next. Figure 4 shows the results. It was found that the binder holding the German CC required the lowest dosages to achieve the target spread flow value of 26 cm. The Indian mixed layer CC demands significantly higher dosages than the German CC and behaves more similar to the cement holding the Chinese CC which prompts exceptionally high PCE dosages. To summarize, for the composite cement holding 40 wt. % of the German CC, the PCE dosage increases by 60% whereas it rises by 280% for the Indian CC and by a staggering 420% for the Chinese CC.
4 Conclusion
This study first elaborates on the behavior of pure calcined kaolin, montmorillonite, illite and muscovite. It is shown that in comparison to an ordinary Portland cement, all calcined clays prompt a higher water demand and thus higher superplasticizer dosages to achieve the same workability as in neat Portland cement. This behavior is explained by their higher fineness (resp. surface area) in comparison to OPC. Moreover, it was found that the presence of meta muscovite and meta illite in composite cements (70:30) increases the dosage of HPEG PCE by as much as 14 times (Mmu) or 10 times (Mil) while meta kaolin prompts only a slight increase (5 times) and meta montmorillonite no increase at all. The results signify that when the calcined clay samples contain certain meta clays, then the selection of a high performance PCE presents the key for its applicability in cement.
Second, composite cements were prepared, and their water demand, response to superplasticizer addition and early strength were investigated. It was confirmed that the content of specific meta clay phases controls their behavior. For example, a high content of meta kaolin provides high early strength, but is very unfavorable with respect to workability and necessitates high addition rates of superplasticizer. As such, depending on the requirements for workability or strength development, either clay deposits which are rich in kaolin content or those which are low on this mineral should be selected.
The study also reveals that while calcined clays significantly reduce the CO2 footprint of cement and offer the potential of a more eco-friendly binder, they partially compromise this advantage because of higher superplasticizer dosages required. However, considering the very minor amount of CO2 associated with PCE production, still a significant savings can be realized from the use of calcined clay blended cements. This way, the cement industry can move into an era of more eco-friendly production.
References
The Cement Sustainable Initiative (CSI). 2016. Getting the Numbers Right, Project Emissions Report 2014.
Scrivener K, Martirena F, Bishnoi S, Maity S. 2018. Calcined clay limestone cements (LC3). In: Cement and Concrete Research. Volume 114. p. 49–56.
Scrivener K, Avet F, Maraghechi H, Zunino F, Ston J, Hanpongpun W, Favier A. 2019. Impacting factors and properties of limestone calcined clay cements (LC3). In: Green Mater. Volume 77. p. 3–14.
Hollanders S, Adriaens R, Skibsted J, Cizer Ö, Elsen J. 2016. Pozzolanic reactivity of pure calcined clays. In: Applied Clay Science. Volume 132-133. p. 552–560.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Li, R., Schmid, M., Sui, T., Plank, J. (2022). Influence of Calcined Clays on Workability of Low Carbon Composite Cements. In: Ha-Minh, C., Tang, A.M., Bui, T.Q., Vu, X.H., Huynh, D.V.K. (eds) CIGOS 2021, Emerging Technologies and Applications for Green Infrastructure. Lecture Notes in Civil Engineering, vol 203. Springer, Singapore. https://doi.org/10.1007/978-981-16-7160-9_68
Download citation
DOI: https://doi.org/10.1007/978-981-16-7160-9_68
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7159-3
Online ISBN: 978-981-16-7160-9
eBook Packages: EngineeringEngineering (R0)