Abstract
Aggressive tumor behavior poses a serious threat to the success of cancer therapy. Altered cancer metabolism is a hallmark feature of tumor initiation, progression, and metastases. During these processes, the tumor cells suffer bioenergetic and nutrient demand, which is met by metabolic reprogramming or preferential nutrient usage facilitated by the acquisition of driver oncogenic mutations and inactivation of tumor-suppressor genes. The metabolic heterogeneity and plasticity of tumor cells provide cellular fitness and survival advantage in the harsh tumor microenvironment (TME), resulting in aggressive tumor growth and resistance to chemotherapies. Besides, other cell types, including stroma, immune cells, and extracellular matrix in the TME, undergo metabolic switching that influences disease progression. Because aberrant glucose metabolism is central to tumor cell metabolic reprogram, various clinical trials targeting glucose uptake and its metabolites in combination with other molecular targets have been focused on reducing tumor progression by inhibiting the metabolic interplay. Here, we describe in detail how the metabolic plasticity of cancer cells and TME results in tumor progression and aggressiveness. In addition, we highlight the current approaches being explored for therapeutic intervention. This overview will help in understanding the intricated metabolic networks and open new avenues of cancer treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Glucose metabolism
- Heterogeneity
- Cancer stem cells
- Immune cells
- Hypoxia
- Tumor progression
- Lactic acid
- Chromatin
2.1 Introduction
Metabolic alterations are a characteristic hallmark feature of tumor cells, facilitating tumor cell proliferation, invasion, immune evasion, and metastases [1]. These aggressive features impose a serious therapeutic hurdle in cancer treatment [1] and are responsible for almost 90% of cancer-related mortality and morbidity [2]. Typically, cancer metastasis involves three main steps—invasion, intravasation, and extravasation. The initial step of metastasis involves detachment of tumor cells from the primary site and invasion of the local milieu directly via blood vessels (intravasation) or lymphatic system. The invasion or dissemination of tumor cells from the primary site to surrounding tissue/stroma occurs either as single cells or clusters [3, 4]. However, only a small subset of disseminated tumor cells survive the shear stress and protective immune cells attached to the endothelial linings of blood vessels and extravasate to facilitate successful metastasis [5].
The tumor mass also harbors a small population of “stemlike” cells known as cancer stem cells (CSCs) that influence various aspects of tumor biology. CSC was first identified in acute myeloid leukemia in 1994 and its potential role in tumor aggressiveness, therapy, relapse, and metastasis of hematological and solid tumor cells was subsequently recognized [6, 7]. These CSCs (0.05–1%) are characterized by the expression of distinct surface markers based on the origin of tumors [8]. Like pluripotent stem cells, CSCs show several salient features such as surviving for longer periods, quiescence, resistance to apoptosis, and ability to undergo self-renewal and differentiation [6, 7]. Such self-renewal property allows CSCs to initiate uncontrolled proliferation with diverse molecular, cellular, and metabolically active phenotypes, subsequently resulting in the significant increase in heterogeneity of primary and metastatic tumors [7, 9]. The acquisition of heterogeneous tumor phenotypes increases the survival advantage during treatment with chemotherapy causing therapy resistance and relapse in various cancer types [9, 10]. To fulfill their energy and biosynthetic demand, tumor cells and CSC increase their nutrient uptake (glucose and glutamine) from the environment [1, 11]. The marked increase in the glucose consumption by tumor cells compared to normal cells in the presence of oxygen (O2) was first discovered by Otto Warburg (1926) and is known as the Warburg effect [12]. The Warburg effect is well established in a variety of tumors [1] and has been exploited for tumor diagnosis and staging by positron-emission tomography (PET) using radiolabeled glucose analog 18F-fluorodeoxyglucose (18F-FDG) [13].
Altered cancer cell metabolism is associated with various stages of tumorigenesis. As altered metabolism enhances the cellular fitness of tumor cells by increasing the nutrient uptake, it is essential to understand how these nutrients are utilized, and what metabolic changes occur as a result of preferential nutrient uptake in the tumor microenvironment (TME) in order to promote the tumor progression [1].
This chapter describes in detail the role of altered glucose metabolism in tumor progression and metastasis, metabolic heterogeneity of CSCs, and its association with chemoresistance. In addition, we summarize how the metabolic plasticity of tumor cells influences the TME, leading to disease aggressiveness or therapeutic resistance. We also highlight the potential therapeutic approaches being used to target cancer metabolism.
2.2 Altered Glucose Metabolism in Tumor Cells
Human somatic cells cultured in petri dish undergo limited cell division and become senescent to die due to the “Hayflick limit” named after the first observation by Leonard Hayflick in 1961 [14]. However, tumor cells overcome this “limit” to facilitate limitless cell division, by accumulating oncogenic mutation, inactivating tumor-suppressor genes, and sustaining telomerase activity. This process is driven by the metabolic rewiring of tumor cells to improve their cellular fitness and selective survival advantage [1]. Typically, in normal cells, the influx of glucose is driven by extracellular signals rather than bioenergetic demand. For instance, mammary epithelial cells cultured in detached condition from extracellular matrix have suppressed glucose uptake despite high glucose present in the medium, resulting in decreased mitochondrial function and ATP production [1]. However, constitutive activation of AKT alone can stimulate glycolysis to restore the mitochondrial function and maintain ATP levels despite growth factor deprivation. In normal cells, glucose diffuses into the mitochondria, where it enters the tricarboxylic acid cycle (TCA) to oxidize glucose to carbon dioxide and generate NADH and FADH2 molecules with a little amount of lactate generation via oxidative phosphorylation (OXPHOS) pathway. NADH and FADH2 then enter the electron transport chain to generate net two ATP molecules per glucose consumed.
In 1926, Otto Warburg observed that cancer cells preferentially utilize glycolysis even in the presence of O2 to support their energy requirement (Warburg effect) [12]. The aerobic glycolysis generates building blocks for macromolecules (proteins, lipids, and nucleotides) required to maintain enhanced growth and proliferation of cancer cells [1]. However, aerobic glycolysis is highly inefficient as it generates only two ATP molecules per molecule of glucose metabolized compared to 36 ATP molecules generated via OXPHOS. This low energy production is compensated by PI3K/AKT signaling, a key master regulator of glucose uptake. During PI3K/AKT signaling, AKT drives the transcription of the glucose transporter GLUT1 and its translocation to the cell surface.
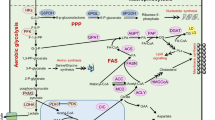
AKT also induces the hexokinase (HK) activity to phosphorylate glucose and prevents effluxing of glucose back to the extracellular space. In addition, AKT also activates the phosphofructokinase and thus promotes the irreversible function of glycolysis. Increased GLUT1 and HK activity increases the glucose uptake by 100-fold in tumor cells, leading to the generation of more ATP molecules during aerobic glycolysis than OXPHOS [12]. However, during aerobic glycolysis, the tumor cells generate high amounts of lactate as a by-product (Fig. 2.1). Inhibiting this pathway by inhibitors targeting PI3K or receptor tyrosine kinases can result in the blockade of glucose uptake by the tumor cells [15, 16]. Moreover, aberrant activation of the PI3K/AKT pathway is shown to induce growth factor-independent tumor progression [1].
Apart from PI3K/AKT signaling, oncogenic proteins such as Ras are known to increase the transcription of GLUT1 [17, 18]. In pancreatic cancer, Kras mutation is an early oncogenic insult that initiates pancreatic intraepithelial neoplasia development and later progresses to pancreatic ductal adenocarcinoma (PDAC) with additional genetic mutations, including Trp53. Increased glycolysis is a key feature of Kras-driven tumorigenesis [17, 19]. Abrogation of Kras signaling in the PDAC murine model has been shown to result in tumor regression along with severe reduction of Glut1 transcription and rate-limiting glycolytic enzymes [20]. Apart from elevated glycolysis, Kras also fuels the glycolytic intermediates to pentose phosphate and hexosamine biosynthesis [20]. At the molecular level, Kras-driven glycolysis is mediated by the activation of MAP kinase, which increases the cMyc-dependent transcription of glycolytic enzymes. During cellular stress, such as starvation, mutant Kras cooperates with other antioxidant enzymes such as paraoxonase 2 (Pon2) to increase glycolysis in PDAC [21]. In lung cancer, mutant Kras is responsible for metabolic heterogeneity and metabolizes the glucose differently based on the degree of lesion (low to high grade) in KrasG12D;Trp53−/− lung tumors [22]. In addition, lung cancer patients and NSCLC cell lines (49%) also gain homozygous mutation for Kras (G12D) [23, 24], which influences the glycolytic switch, maintenance of redox balance, channeling of glucose metabolites to the TCA cycle, and biosynthesis of glutathione [22, 25]. Increased glutathione in the homozygous mutant Kras in NSCLC protects the cells from reactive oxygen species (ROS)-mediated abnormalities, thereby increasing the selective growth of these cells during lung tumor progression [26].
2.3 Cancer Stem Cells Exhibit Heterogeneous Metabolic Characteristics
Stem cells are undifferentiated cells with a unique capacity for self-renewal and multiple differentiation in multicellular organisms [27, 28]. As somatic cells have limited cell division, replenishing of the damaged cells is achieved by stem cells and self-renewing its progenitors for maintaining the tissue homeostasis. At physiological condition, stem cells reside in the hypoxic microenvironment, which enables them to maintain their undifferentiated state, proliferate, and commit to cell fate [29]. Due to spatial residence, stem cells rely heavily on anaerobic glycolysis to support their energy requirement [30]. The reliance of stem cells on glycolysis is due to fewer or immature mitochondria, which protects the genome from ROS generated by OXPHOS and limits oxidation of proteins and lipids [31]. A key driver for glucose metabolism in a low-O2 environment is the activation of transcription factor hypoxia-inducible factor 1 α (HIF1α). During anaerobic glycolysis, HIF1α heterodimerizes with HIF1β to promote the transcription of glycolytic genes [32]. The hypoxic condition stabilizes the HIF1α protein by preventing hydroxylation and facilitates the expression of pyruvate dehydrogenase kinase (PDK2 and 4) to prevent pyruvate from entering into the TCA cycle, thus blocking mitochondrial respiration. However, depletion of HIF1α in stem cells results in the reversal of this phenotype, thereby allowing the cells to undergo mitochondrial respiration rather than glycolysis. The transition from glycolysis to mitochondrial respiration is responsible for the exhaustion of hematopoietic stem cells, and thus suggests the pivotal role of HIF1α in maintaining the hematopoietic stem cell function [33].
Like stem cells, CSCs have the ability to self-renew and maintain an undifferentiated state, remain quiescent, and activate DNA repair machinery. CSCs are associated with tumor initiation, relapse, therapy resistance, and metastatic dissemination [7, 10]. Several studies have identified and characterized CSCs in various malignancies for use as biomarkers or targeted therapies [34]. The stemness features are tightly regulated by several transcription factors (TF) such as OCT4, SOX2, KLF4, and Nanog. Shinya Yamanaka, in 2006, first demonstrated that four TFs (Oct4, cMyc, Sox2, and Klf4) could induce pluripotency in the mouse embryonic fibroblast suggesting the importance of TFs in stemness [35]. Like cancer cells, CSCs also undergo metabolic adaptation to the cellular environment, such as hypoxia versus normoxia and proliferative versus quiescence. Such changes in the cellular environment cause a shift in the metabolic states that gives rise to cellular heterogeneity in CSCs [11, 36]. The existence of heterogeneity in tumor cells and CSCs represents a major therapeutic hurdle in several cancers.
Though CSCs are metabolically very active, controversy regarding their energy metabolism (glycolytic or mitochondrial respiration) is still under scrutiny. In general, glycolytic activity is mainly responsible for maintaining the stemness traits of stem cells, embryonic stem cells, and induced pluripotent stem cells. For example, increased glycolysis in non-small cell lung cancer (NSCLC) leads to the elevation of ABCG2 transporter in the side population [37] via activation of the AKT pathway. Constitutive expression of active AKT also increases the glycolytic rate and aerobic glycolysis independently of the growth factor [37, 38]. Apart from glycolysis, CSCs also utilize OXPHOS for alternative energy generation in response to their physiological needs, suggesting its metabolic flexibility. Recent findings have shown that liver CSCs are highly OXHPOS dependent compared to the non-stem cells, which was evident from increased mitochondrial DNA copy number, mitochondrial content, and ROS. In addition, as a result of the treatment with 2-deoxy-D-glucose (2-DG), the high OXPHOS liver CSCs promote the expression of stemness surface markers CD133 and CD44 [39]. Overall, we now understand that CSCs can undergo metabolic reprogramming (glycolysis or OXPHOS) to support their stemness.
2.4 Metabolic Plasticity Drives Cancer Cell Metastasis
As tumor cells are highly active metabolically, there is a dramatic change in the TME with increased hypoxia, nutrient shortage, and lactic acid buildup. Most of the metabolic pathways are interconnected and flexible, allowing the tumor cells to reprogram their metabolic activity for glucose catabolism and maintain the redox balance during changing microenvironment. The metabolic plasticity ensures the survival of the tumor cells by increasing their cellular fitness during nutrient starvation. For example, in the case of chronic glucose starvation in serous ovarian cancer cells, tumor cells undergo metabolic reprogramming to generate cell types that are highly heterogenic. Such generation of heterogenic cell types is driven by the ZEB1-dependent transcription of NNMT (nicotinamide N-methyltransferase), which is highly expressed in the metastatic and recurrent tumors compared to matched primary carcinoma. In addition, ZEB1-dependent expression of NNMT also confers resistance to glucose dependence and increases the migration of ovarian cancer cells suggesting metabolic adaptation during glucose restriction [40].
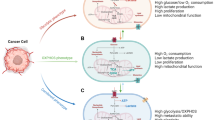
Tumor cells increase their metastatic potential by metabolic reprogramming by shifting from glycolysis to OXPHOS [41]. The metabolic shift to OXHPOS is coordinated by transcriptional coactivator PGC-1α (peroxisome proliferator-activated receptor-gamma coactivator-1α), a key regulator involved in mitochondrial biogenesis and metabolism [42]. Recent studies using the systems biology approach by utilizing AMPK and HIF1 signatures in The Cancer Genome Atlas indicated the presence of a hybrid phenotype that enables the cells to consume various types of nutrients [41, 43]. It also provides cellular advantages such as efficient energy production through multiple metabolism pathways, synthesizes biomass for rapid cell proliferation, and maintains ROS at a moderate level to favor ROS-mediated signaling [44]. Such phenotype was evident in circulating tumor cells isolated from highly metastatic mouse basal type breast cancer cell line (4T1) [45]. The hybrid phenotype is characterized by high levels of HIF1/pAMPK (AMP-activated kinase), which favors both glycolysis and OXPHOS. In contrast, another phenotype with high HIF1/low pAMPK expression and low HIF1/high pAMPK expression in triple-negative breast cancer exclusively favored glycolysis and OXPHOS, respectively [41]. Such metabolic plasticity creates a major clinical hurdle, considering that the current clinical strategies targeting metabolism have been largely ineffective. Thus, simultaneous targeting of both the pathways (glycolysis and OXPHOS) may be critical to eliminate these metabolically highly flexible tumor cells [41, 46].
2.5 Lactic Acid Secretion, Utilization, and Tumor Progression
As a result of increased metabolic rate in tumor cells, there is a significant accumulation of lactic acid and H+ in the cytosol. Almost 85% of the incoming glucose is converted to lactic acid, which needs to be eliminated from the tumor cells to prevent acidosis and support higher rates of glycolysis. This elimination of lactic acid and H+ from the cytosol to the microenvironment is assisted by the increased expression of monocarboxylate transporter isoforms (MCT1 and 4) and Na-driven proton release, respectively [47, 48]. Overexpression of MCT1 and 4 has been associated with poor prognosis and high mortality in several cancers [47]. The dependence on MCTs to expel lactate is based on the fact that lactic acid is a weak acid, which prevents them from diffusion across the membrane. However, studies have shown that the dissociation of lactate to H+ generation is not the primary cause for acidosis. Rather the coupling of ATP hydrolysis and glycolysis is the major source of H+ production which contributes to acidification (low pH) [49].
Heterogeneous distribution of glucose in the intratumoral area, apart from activating HIF1α, also activates the oncogene cMYC to upregulate LDHA (lactate dehydrogenase A), leading to the generation of NAD+ which in turn activates glycolysis, thus maintaining the vicious cycle [50, 51]. Besides HIF1α and cMYC, lactate also regulates the transcription of RAS, PI3KCA, E2F1, tumor-suppressor genes (BRCA1 and BRCA2), and genes that mediate cell cycle and cell proliferation [52]. On the contrary, cMYC and tumor suppressor P53 also activate the transcription of MCT1 to favor lactate uptake [53, 54]. HIF1α activates the transcription of MCT4 to expel lactate from the cells [55]. Under physiological conditions, lactate concentration in the blood and normal tissues ranges between 1.5 and 3 mmol/L [56]. The levels can rise up to 40 mmol/L concentrations in tumors [57]. When lactate is not eliminated from the cells, it can lead to lactic acid acidosis, which is common in most highly mitotic tumors. Tumor-associated acidosis was first documented in acute leukemia patients in 1963 [58]. In general, lactic acid acidosis in cancer patients results from a failure in lactate clearance from the liver due to deficiencies in thiamine and/or riboflavin. Thiamine functions as a cofactor that facilitates the conversion of pyruvate to acetyl-CoA by pyruvate dehydrogenase. Due to thiamine deficiency, this conversion from pyruvate to acetyl-CoA prevents the entry of the latter into the TCA cycle [59]. Thus, balancing lactic acid production and expulsion by cancer cells is essential to prevent intracellular acidification and apoptosis.
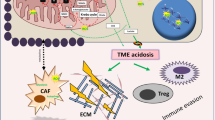
Though lactate was previously considered as a “metabolic waste” product of glycolysis, recent studies have demonstrated the role of lactate levels in driving tumor progression, immune escape, angiogenesis, cell migration, and drug resistance [51, 56]. TME is composed of stromal cells, endothelial cells, and immune cells. Immune cells primarily surveil the body to eliminate any pathogen, including tumor cells. However, tumor cells release anti-inflammatory cytokines and recruit immunosuppressive cell types in the TME to inhibit the immune response [60]. Lactate accumulation also dampens the antitumor activity of NK cells and NKT cells by inducing apoptosis [61, 62]. In several tumors, tumor-associated macrophages undergo polarization in response to lactate-induced transcription of vascular endothelial growth factor (VEGF) and arginase 1 [63]. Furthermore, lactate also assists the tumor cells in evading immune response by expressing its receptor G protein-coupled receptor 81 (GPCR81). In lung cancer cells, the activation of GPCR81 receptor results in the upregulation of programmed death-ligand 1 (PD-L1) in the membrane, which blocks the antitumor immune response. On the contrary, blocking the LDHA enzyme which converts pyruvate to lactate in the tumor cells increases the efficiency of programmed cell death 1 (PD1) therapy [64].
Higher lactate in the TME is associated with an increased metastasis in various cancers [48] and correlates with poor clinical outcome [56]. The mechanisms by which lactate promotes metastasis are multifactorial: (1) modifies several cell adhesion molecules, such as integrins, which assist in cell binding to the extracellular matrix, making them more migratory [65], and (2) induces the expression of proteases (MMP9, cathepsin B, and hyaluronidase) to degrade the surrounding tissues, thereby allowing tumor cells to metastasize [66, 67]. Apart from metastasis promotion, lactate buildup is also associated with the induction of therapy resistance. In NSCLC, prolonged treatment with tyrosine kinase inhibitors (EGFR and MET) results in a metabolic shift towards increased glycolysis and lactate production. This lactate, in turn, promotes the secretion of hepatocyte growth factor by cancer-associated fibroblast (CAF) in an NFkB-dependent manner to activate MET signaling to induce therapy resistance [68]. Thus, targeting lactate metabolism or uptake has proven to be an important strategy for cancer therapy.
2.6 Glucose Metabolism, Chromatin Structure, and Chemoresistance
Changes in the global chromatin structure are associated with gene expression, DNA repair, and tumor progression [69]. Typically opening and closing of chromatin structure is facilitated by the acetylation of histones (H3, H4, H2A, and H2B in nucleosome core) catalyzed by the balanced action of histone acetyl transferase (HAT) and histone deacetylase (HDACs). During harsh metabolic reactions, tumor cells meet the increasing demand for energy and precursors for biosynthesis by initiating the distinct transcription of metabolic genes via chromatin remodeling [70]. The metabolites generated during the metabolic reaction are taken up by the cells actively or passively through the plasma membrane or nuclear membrane to modify the chromatin structure or processed by the metabolic enzymes to function as a substrate or cofactor for the chromatin-remodeling enzymes. Acetyl-CoA is one such metabolic by-product that functions as a substrate for HAT activity. The canonical histone acetylation involves addition of acetyl group at lysine residue which is derived from the metabolite acetyl-CoA. Acetyl-CoA generated during glucose metabolism is funneled through mitochondrial metabolism via a citrate intermediate, which is exported and lysed in the cytosol by ATP-citrate lyase to generate acetyl-CoA. Therefore, nutrient availability is vital in regulating the chromatin structure and gene expression during metabolic reprogramming.
The study by Liu et al. (2015) has shown that inhibiting glycolysis with 2-DG or silencing two rate-limiting enzymes, hexokinase-1 (HK1) and pyruvate kinase (PKM), results in condensing of the chromatin structure and reduced tumor cell proliferation [71]. Besides, increased glycolysis results in higher accumulation of cellular acetyl-CoA, a substrate for acetyltransferases, which increases the histone acetylation, thereby enabling the cells to undergo efficient DNA repair and induce chemoresistance [71].
Another chromatin-associated protein, MORC2, a member of the Microrchidia family CW-type zinc finger (MORC) family of proteins, is upregulated in several cancers [72]. It also regulates transcription by modifying the chromatin structure [73, 74]. During tumorigenesis, MORC2-mediated transcription is catalyzed by the interaction with histone HDAC1, HDAC4, and EZH2 [75]. Likewise, during glucose metabolism, cMYC directly targets the expression of HK2, PFKM, ENO1, GLUT1, and LDHA [76], while MORC2 is known to regulate LDHA by cooperating with cMYC to promote the migration of breast cancer cells [75, 77, 78]. As numerous metabolic pathways converge onto cMYC regulation, attempts to block or restore altered pathways driven by cMYC can lead to novel strategies in cancer treatment.
2.7 Cross Talk Between Tumor Microenvironment and Metabolism in Disease Progression
As discussed earlier, metabolic plasticity allows tumor cells to adapt themselves to changing TME [1]. Aberrant tumor vasculature in the TME causes heterogeneous perfusion (O2 and nutrients) across the tumor vessels, which promotes a hypoxic environment [79]. The competitive metabolic milieu in the TME also results in the variable nutrient utilization among tumor cells, immune cells, and stromal population [80, 81]. Besides, tumor cells adapt to their metabolic needs in the hypoxic conditions of TME through HIF1, which activates enzymes of glycolytic flux. Overall, the intratumoral metabolic heterogeneity by the nonuniform distribution of nutrients is influenced by various factors, including the composition of TME, disease stage, and mutation load [82]. Here, we will discuss in detail how stroma, extracellular matrix (ECM), and immune cell metabolism are reprogrammed by tumor cells and influence the disease progression.
Stroma: The contribution of stroma for tumor growth and progression is well established in different cancers, but how alterations in stromal composition support tumor growth are still unclear. The metabolic interplay between cancer cells and TME is a well-recognized hallmark of tumors. The accumulation of different metabolic intermediates and their by-products in the TME activates stromal cells through paracrine signaling and alters their phenotype [83]. Stroma modulation by growing tumor is synonymous to the regeneration of damaged tissue and involves (a) monocyte recruitment and activation to pro-inflammatory M1 phenotype for clearance of necrotic tissue and subsequent transition to M2 phenotype; (b) fibroblast recruitment, their differentiation to myofibroblasts, and secretion of ECM for surrounding cells survival; and (c) immunosuppressive milieu characterized by Tregs, M2 macrophages, and myeloid-derived suppressor cells [84]. This stromal regeneration by tumors is driven by alteration of metabolic consumption in the TME, which includes autophagy in stromal fibroblasts by glucose depletion and AMPK activation and secretion of nonessential amino acids, which leads to enhanced tumor growth [85, 86]. CAFs are the main component of tumor stroma and engage in tumor progression by promoting tumor cells to undergo EMT and enhancing the stem cell traits and metastatic dissemination [87, 88]. Accumulating evidence shows that CAFs undergo metabolic reprogramming during their activation, including utilization of aerobic glycolysis and increased autophagy for mobilization of the nutrients into the TCA cycle [89, 90]. Also, CAF-derived exosome is seen to mediate metabolic reprogramming [91]. While in PDAC, the oncogenic mutation is observed to regulate signaling in both the tumor cells and adjacent stromal cells. By cell-specific proteome labeling and multivariate phosphoproteomics, it is observed that tumor cell KRAS (KRASG12D) interacts with fibroblast to initiate reciprocal signaling in tumor cells. This reciprocal signaling results in distinct tumor cell phosphoproteome, which regulates tumor cell proliferation and apoptosis and increases mitochondrial capacity [92]. Tumor cells also interact with the CAFs and reprogram their cellular metabolism to adapt to the nutrient deprivation in the harsh TME. One such classic example is the reciprocal interplay between prostate cancer cells and CAFs which results in EMT and metabolic shift in the tumor cells. As prostate cancer cells come in contact with CAFs, it reprograms the metabolism of cancer cells towards aerobic metabolism, thereby decreasing the dependence on glucose and shift towards aerobic metabolism. This process is driven by reducing GLUT1 expression and increasing the lactate load by MCT1. Therefore, prostate cancer cells by inducing symbiosis with CAFs utilize their by-products, favoring them to grow in a low-glucose environment [88]. While MCT1 can induce lactate uptake or secretion in cancer cells, MCT4 promotes lactate efflux in CAFs through HIF1α induction under hypoxic conditions and results in tumor promotion [93]. In fact, triple-negative breast cancer patients with high stromal MCT4 expression show poor prognosis [94]. In addition, stroma-associated pancreatic stellate cells also secrete nonessential amino acids, decreasing the tumor cell dependence on glucose and serum-derived nutrients [85]. Likewise, CAFs in ovarian tumors utilize carbon to produce glutamine for cancer cells. This shows the existence of novel cross talk between tumor cells and CAFs in metabolic regulation of tumor cells [95]. Thus, targeting the glutamine pathway in both tumor and stroma resulted in a significant decrease in tumor growth [96]. However, the mechanistic link between CAFs and tumor nutrient demand is not clear. A detailed understanding of these pathways would help in dissecting the actionable targets, including targeting both tumor and TME simultaneously. This approach of simultaneous targeting is limited by the cell-dependent function of different actionable target proteins. These targets are present in both tumor epithelium and TME but possess opposite functions. For example, prostate tumor epithelium-mediated downregulation of p62 in stromal fibroblasts resulted in impaired metabolism through reduced mTOR activity and cMYC expression and release of ROS and IL-6, which in turn enhanced epithelial invasion and tumorigenesis [97]. Therefore, inhibiting their activity in tumor cells could be compensated by increased stromal reactivation.
ECM: Extracellular matrix (ECM) consists of an intricate network of secreted proteins that provide biochemical and mechanical support to different tissues and organs. Tumor cells interact with ECM via transmembrane integrin receptors to control cell migration, proliferation, and metabolism. Tumor relieves anchorage dependence and gets disengaged from the ECM for metastases and dissemination. However, ECM detachment results in impaired glucose uptake, reduced cellular ATP levels, and increased ROS production. Tumor cells endure this stressful environment by altering their nutrient utilization from glycolysis to glutamine-derived TCA metabolism mediated by AMPK-regulated NRF2 expression [98]. Glutamate production through AMPK-mediated glutamine metabolism helps to reduce oxidative stress following anchorage independence. ECM composition and organization are influenced by the presence of CAFs in TME [99]. Higher collagen content has been correlated with altered metabolism in breast cancer due to reduced oxygen and glucose consumption and increased glutamine consumption by tumor cells [100]. In head and neck squamous cell carcinoma (HNSCC), cancer cell-derived glutamate promotes ECM remodeling by maintaining the redox state in CAFs, and aspartate from CAFs sustains cancer cell proliferation [101]. These opposite results might be due to different tumor types and altered TME composition. ECM undergoes continuous remodeling by expressing a variety of matrix-degrading enzymes, resulting in altered nutrient uptake by the tumor cells. For instance, hyaluronan degradation in ECM enhances transporter GLUT1 mobilization to the plasma membrane and promotes glucose uptake and increased migration of cancer cells [102]. In a nutshell, the studies mentioned above fill a gap in understanding the varying metabolic requirements of cells in anchorage-dependent and -independent conditions. A better understanding of the underlying mechanisms of ECM remodeling and metabolic rewiring in tumors could encourage the development of novel therapeutic interventions.
Immune cells: The hallmarks of TME, including hypoxia, low pH, lactate accretion, waste accumulation, and very high demand for nutrients, create a competitive niche for different cells present in the TME [81, 103]. Multiple studies have demonstrated that this nutrient-competitive milieu favors tumor progression and dampens effector T-cell functions but not necessarily their proliferation [104,105,106]. Metabolic heterogeneity in the TME plays a key role in the differential intratumoral immune cell recruitment. Metabolic reprogramming by cell-intrinsic and -extrinsic nutrient availability in the TME results in the differential activity of immune cells [107, 108]. Also, tumor cells, by employing the Warburg pathway, limit the nutrient supply to immune cells, thereby inducing the immunosuppression [103]. Increased glycolysis is a hallmark of metabolic alterations of activated immune cells, including macrophages, NK cells, dendritic cells, B cells, and effector T cells [109]. Multiple studies have described T-cell activation by complex metabolic regulation [110, 111]. Earlier studies have shown the association between the differentiation state of T cells (naïve, effector, or memory) and their metabolic activity [112]. Naïve T cells have basal glucose requirements and depend mainly on fatty acid oxidation and glutaminolysis for their nutrient supply, while activated T cells undergo metabolic switching towards glucose metabolism. For T-cell activation, CD28 costimulation promotes glucose uptake via the PI3K-AKT pathway, and TCR activation induces glutaminolysis through ERK/MAPK pathway [113]. Additionally, enhanced mTOR activity results in the activation of CD8+ T cell and stabilization of HIF1α required for CD4+ T-cell proliferation and activation. Effector T-cell subsets, including TH17, TH1, TH2, and activated CD8+ T cells, have been shown to possess high glycolytic activity as seen by increased mTOR activation. Thus, metabolic reprogramming in activated T cells through PI3K-AKT, mTOR, AMPK, and HIF1α signaling pathways gives rise to similar metabolic profiles of both cancer cells and activated T cells [114,115,116]. This has been one of the major challenges posed by therapeutic interventions directed towards cancer cells.
Glycolysis is important in immune cell programming from TH17 to Treg type [117, 118]. The different metabolic requirement of various immune cells is dictated by their functional activity. This is consistent with the idea that CD28 signaling for T-cell activation is dependent on increased glucose uptake while M2 macrophages and Tregs can survive in low-glucose conditions as they utilize fatty acid oxidation for nutrient requirement [119, 120]. In fact, switching of Treg metabolic pathway to fatty acid oxidation may be due to suppression of mTOR by AMPK [81, 121]. Also hypoxia in TME induces high adenosine concentrations by tumor cells; it exerts an immunosuppressive effect through the binding of adenosine receptors in various immune cells [122]. Likewise, lactate accumulation by excessive glycolytic activity in the TME engenders metabolic reprogramming of both tumor and immune cells and angiogenesis through increased VEGF secretion [56, 88]. In one study, excessive lactate accumulation resulted in reduced T-cell effector function and polarization towards Treg phenotype [123, 124] (Fig. 2.2). In addition, reduced activation of infiltrated immune cells (T cell, B cell, and NK cell) and poor monocyte differentiation by excessive lactate concentrations in the TME endow tumor cells with the ability to proliferate at higher levels. While T cells rely solely on glycolysis for their nutrient requirement, hypoxia-induced mitochondrial function loss has also been linked to T-cell exhaustion through MYC-regulated pathway [125]. Nevertheless, there remains a gap in metabolic heterogeneity and its association with immune cell type due to limitations in traditional technologies that help determine the metabolic profile. Recent advancements in flow cytometry and mass spectrometry-based analysis have encouraged researchers to develop innovative approaches of profiling patient samples at a single-cell level. CyTOF-based multiplexing in flow cytometry has allowed single-cell metabolic profiling of human CD8+ T cells in colorectal carcinoma patients [126]. This study suggested that the metabolic heterogeneity in the peripheral and tumor-infiltrating CD8+ T-cell subsets causes differences in their functional attributes. Therefore, delineating the effect of metabolic reprogramming on tumor immune cell function and distribution will allow intervention with pharmacological inhibitors to remodel the immune response.
2.8 Therapeutic Targeting of Glucose Metabolism
Developing therapeutic strategies targeting the Warburg pathway in tumors has been a long-standing approach to eliminate or delay tumor progression. Several drugs targeting enzymes and intermediates of glycolytic pathways have been evaluated in clinical trials [127] with little success. It is now becoming clear that cancer cells exhibit hybrid metabolism (glycolysis and OXPHOS) under stress conditions induced by the oncogenic activation of Ras, MYC, and c-SRC or ROS generation [128, 129]. Such metabolic plasticity orchestrates the tumor cell proliferation and metastasis by maintaining ROS levels and efficient energy production [45]. In fact, there exist reports indicating the synergistic effect of a combination of glycolytic inhibitor 2-DG decreasing the glucose uptake and metformin inhibiting OXPHOS activity on the growth and metastatic potential of tumor cells [130]. Regardless of the impressive data with 2-DG in several preclinical studies, clinical data are not very satisfactory [131, 132]. A recent clinical trial in PC patients with 2-DG was stopped due to slow accrual. Likewise, clinical trials of other cancers with 2-DG were not satisfactory and unambiguous. Clinical trials combining 2-DG with other chemotherapeutic agents including cisplatin, docetaxel, or radiation are currently ongoing [127]. Data obtained from initial trials are quite encouraging and might open new avenues for cancer treatment. Several other anti-glycolytic agents target different enzymes and intermediates of the glycolytic pathway, including glucose uptake and phosphorylation, fructose phosphorylation, glucotriose metabolism, pyruvate formation, oxidation, lactate dehydrogenase, and tumor acidosis [127]. One of the most effective anti-glycolytic agents, 3-bromopyruvate (3-BrPA), a pyruvate analog, acts by targeting GAPDH and inhibiting both tumor glycolysis and mitochondrial OXPHOS. As a result, cancer cells undergo energy deficiency through ATP diminution and apoptosis and eventually die, leading to decreased tumor growth. In addition, studies have shown the anticancer effect of 3-BrPA through suppressing tumor invasion, angiogenesis, and metastasis. 3-BrPA has shown antitumor potential not only as a single agent but also acting synergistically in combination with cytotoxic agents and ABC transporters to restore drug sensitivity [133, 134]. As 3-BrPA is stable in the acidic TME, it has the potential for efficient tumor cell killing with reduced off-target toxicity. However, nonspecific alkylation by 3-BrPA can induce toxicity in the normal immune and stem cells. Therefore, several attempts are being made for local-regional delivery of 3-BrPA through catheters, microencapsulation, or intra-arterial routes to minimize the toxicity [135, 136]. Likewise, synergistic inhibition of glycolysis and OXPHOS by a combination of metformin with bromodomain and extra-terminal motif (BET) inhibitor, JQ-1, has been tested in pancreatic cancer [137]. These combinatorial targeting strategies could provide ways to overcome therapy resistance and achieve durable responses. The metabolic plasticity of cancer cells in the harsh TME is mediated by a cross talk between gene regulation and metabolic pathways [1]. A recent study devised a theoretical framework to couple gene signatures and metabolic interplay in the hybrid metabolism phenotype. This study indicated a direct correlation between AMPK and OXPHOS, and HIF1 and glycolysis, highlighting the significance of targeting abnormal metabolism in cancer by modulating both genes and metabolic pathways [41]. The multifaceted interactions between different signaling pathways regulate metabolic reprogramming in cancer cells, allowing them to proliferate and sustain therapeutic resistance. The inhibition of key metabolic regulators, including KRAS, MYC, P53, HIF1α, and PI3K/AKT/mTOR pathways, could be an effective approach towards tumor killing. For instance, targeting KRAS in PDAC patients showed promising results in preclinical studies; however, it had no positive influence on patient survival [17]. Similarly, preclinical studies targeting EGFR and CDK4/6 by afatinib and palbociclib have shown great promise in reducing tumor progression by reducing metabolic reprogramming in HNSCC [138]. Several ongoing preclinical and clinical studies targeting HIF1α, MYC, and P3K/mTOR pathways in various cancers are under progress. Nevertheless, the metabolic plasticity of cancer cells poses a serious therapeutic challenge in targeting a specific pathway as they can overcome the inhibitory effect by activating the alternative metabolic pathways. In addition, other cells of TME, including stroma, fibroblasts, and immune cells, also influence the metabolic milieu of tumor cells and help them survive in a stressful environment. Therefore, current approaches focus on combining anti-glycolytic agents that target different metabolic pathways or their combination with other chemotherapeutic agents to overcome the therapeutic resistance. Overall, the knowledge acquired from these studies will help develop an understanding on future therapeutic perspectives based on metabolic reprogramming.
2.9 Concluding Remarks
Metabolic reprogramming is employed by tumor cells/CSCs to survive and grow in the harsh TME to generate energy and precursors for the biosynthetic process and maintain their redox balance. This reprogramming is achieved by acquiring mutations in the oncogene and tumor-suppressor genes which activates the downstream signaling pathways associated with tumor progression, metastases, and therapy resistance. Apart from metabolic switching from glycolysis to OXPHOS, tumor cells also acquire a hybrid phenotype and utilize both metabolic pathways. While most studies are limited to investigating altered metabolism in tumor cells, a broader understanding of metabolic cooperativity between the tumor cells and stromal compartments may help delineate intricated metabolic pathways and exploit them for novel anticancer therapies.
References
Pavlova NN, Thompson CB (2016) The emerging hallmarks of cancer metabolism. Cell Metab 23:27–47
Seyfried TN, Huysentruyt LC (2013) On the origin of cancer metastasis. Crit Rev Oncog 18:43–73
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147:992–1009
Lintz M, Muñoz A, Reinhart-King CA (2017) The mechanics of single cell and collective migration of tumor cells. J Biomech Eng 139:0210051–0210059
Strilic B, Offermanns S (2017) Intravascular survival and extravasation of tumor cells. Cancer Cell 32:282–293
Yu Z, Pestell TG, Lisanti MP, Pestell RG (2012) Cancer stem cells. Int J Biochem Cell Biol 44:2144–2151
Visvader JE, Lindeman GJ (2012) Cancer stem cells: current status and evolving complexities. Cell Stem Cell 10:717–728
Bao B, Ahmad A, Azmi AS, Ali S, Sarkar FH (2013) Overview of cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr Protocol Pharmacol, Chapter 14, Unit-14.25
Rich JN (2016) Cancer stem cells: understanding tumor hierarchy and heterogeneity. Medicine 95:S2–S7
Ayob AZ, Ramasamy TS (2018) Cancer stem cells as key drivers of tumour progression. J Biomed Sci 25:20
Yadav UP, Singh T, Kumar P, Sharma P, Kaur H, Sharma S, Singh S, Kumar S, Mehta K (2020) Metabolic adaptations in cancer stem cells. Front Oncol 10:1010
Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41:211–218
Almuhaideb A, Papathanasiou N, Bomanji J (2011) 18F-FDG PET/CT imaging in oncology. Ann Saudi Med 31:3–13
Shay JW, Wright WE (2000) Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol 1:72–76
Benz MR, Herrmann K, Walter F, Garon EB, Reckamp KL, Figlin R, Phelps ME, Weber WA, Czernin J, Allen-Auerbach MS (2011) (18)F-FDG PET/CT for monitoring treatment responses to the epidermal growth factor receptor inhibitor erlotinib. J Nucl Med 52:1684–1689
Lheureux S, Lecerf C, Briand M, Louis MH, Dutoit S, Jebahi A, Giffard F, Fournier CB, Batalla A, Poulain L, Aide N (2013) (18)F-FDG is a surrogate marker of therapy response and tumor recovery after drug withdrawal during treatment with a dual PI3K/mTOR inhibitor in a preclinical model of cisplatin-resistant ovarian cancer. Transl Oncol 6:586–595
Pupo E, Avanzato D, Middonti E, Bussolino F, Lanzetti L (2019) KRAS-driven metabolic rewiring reveals novel actionable targets in cancer. Front Oncol 9:848
Murakami T, Nishiyama T, Shirotani T, Shinohara Y, Kan M, Ishii K, Kanai F, Nakazuru S, Ebina Y (1992) Identification of two enhancer elements in the gene encoding the type 1 glucose transporter from the mouse which are responsive to serum, growth factor, and oncogenes. J Biol Chem 267:9300–9306
Xie H, Hanai J, Ren JG, Kats L, Burgess K, Bhargava P, Signoretti S, Billiard J, Duffy KJ, Grant A, Wang X, Lorkiewicz PK, Schatzman S, Bousamra M 2nd, Lane AN, Higashi RM, Fan TW, Pandolfi PP, Sukhatme VP, Seth P (2014) Targeting lactate dehydrogenase—a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab 19:795–809
Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik J-H, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, DePinho RA (2012) Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149:656–670
Nagarajan A, Dogra SK, Sun L, Gandotra N, Ho T, Cai G, Cline G, Kumar P, Cowles RA, Wajapeyee N (2017) Paraoxonase 2 facilitates pancreatic cancer growth and metastasis by stimulating GLUT1-mediated glucose transport. Mol Cell 67:685–701.e686
Kerr EM, Gaude E, Turrell FK, Frezza C, Martins CP (2016) Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities. Nature 531:110–113
Junttila MR, Karnezis AN, Garcia D, Madriles F, Kortlever RM, Rostker F, Brown Swigart L, Pham DM, Seo Y, Evan GI, Martins CP (2010) Selective activation of p53-mediated tumour suppression in high-grade tumours. Nature 468:567–571
Feldser DM, Kostova KK, Winslow MM, Taylor SE, Cashman C, Whittaker CA, Sanchez-Rivera FJ, Resnick R, Bronson R, Hemann MT, Jacks T (2010) Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature 468:572–575
Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC (2009) Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal 11:2685–2700
Kerr EM, Martins CP (2018) Metabolic rewiring in mutant Kras lung cancer. FEBS J 285:28–41
Weissman IL, Anderson DJ, Gage F (2001) Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 17:387–403
Seita J, Weissman IL (2010) Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med 2:640–653
Ito K, Suda T (2014) Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 15:243–256
Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA (2010) The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7:380–390
Perales-Clemente E, Folmes CD, Terzic A (2014) Metabolic regulation of redox status in stem cells. Antioxid Redox Signal 21:1648–1659
Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92:5510–5514
Roy IM, Biswas A, Verfaillie C, Khurana S (2018) Energy producing metabolic pathways in functional regulation of the hematopoietic stem cells. IUBMB Life 70:612–624
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H (2020) Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther 5:8
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Tanabe A, Sahara H (2020) The metabolic heterogeneity and flexibility of cancer stem cells. Cancers 12:2780
Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL, Hu Y, Wang P, Ju HQ, Xu RH, Huang P (2014) Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ 21:124–135
Hoxhaj G, Manning BD (2020) The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer 20:74–88
Liu G, Luo Q, Li H, Liu Q, Ju Y, Song G (2020) Increased oxidative phosphorylation is required for stemness maintenance in liver cancer stem cells from hepatocellular carcinoma cell line HCCLM3 cells. Int J Mol Sci 21:5276
Kanska J, Aspuria P-JP, Taylor-Harding B, Spurka L, Funari V, Orsulic S, Karlan BY, Wiedemeyer WR (2017) Glucose deprivation elicits phenotypic plasticity via ZEB1-mediated expression of NNMT. Oncotarget 8:26200–26220
Jia D, Lu M, Jung KH, Park JH, Yu L, Onuchic JN, Kaipparettu BA, Levine H (2019) Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc Natl Acad Sci U S A 116:3909–3918
Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370
Yu L, Lu M, Jia D, Ma J, Ben-Jacob E, Levine H, Kaipparettu BA, Onuchic JN (2017) Modeling the genetic regulation of cancer metabolism: interplay between glycolysis and oxidative phosphorylation. Cancer Res 77:1564–1574
Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J (2008) ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320:661–664
LeBleu VS, O’Connell JT, Herrera KNG, Wikman H, Pantel K, Haigis MC, De Carvalho FM, Damascena A, Chinen LTD, Rocha RM (2014) PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16:992–1003
Paudel BB, Quaranta V (2019) Metabolic plasticity meets gene regulation. Proc Natl Acad Sci U S A 116:3370–3372
Payen VL, Mina E, Van Hée VF, Porporato PE, Sonveaux P (2020) Monocarboxylate transporters in cancer, molecular. Metabolism 33:48–66
Pérez-Tomás R, Pérez-Guillén I (2020) Lactate in the tumor microenvironment: an essential molecule in cancer progression and treatment. Cancers (Basel) 12:3244
Robergs RA, Ghiasvand F, Parker D (2004) Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 287:R502–R516
Semenza GL (2013) HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123:3664–3671
de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J (2019) Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol 9:1143–1143
San-Millán I, Julian CG, Matarazzo C, Martinez J, Brooks GA (2019) Is lactate an oncometabolite? Evidence supporting a role for lactate in the regulation of transcriptional activity of cancer-related genes in MCF7 breast cancer cells. Front Oncol 9:1536
Doherty JR, Yang C, Scott KEN, Cameron MD, Fallahi M, Li W, Hall MA, Amelio AL, Mishra JK, Li F, Tortosa M, Genau HM, Rounbehler RJ, Lu Y, Dang CV, Kumar KG, Butler AA, Bannister TD, Hooper AT, Unsal-Kacmaz K, Roush WR, Cleveland JL (2014) Blocking lactate export by inhibiting the Myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res 74:908–920
San-Millán I, Brooks GA (2017) Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg effect. Carcinogenesis 38:119–133
Singh D, Arora R, Kaur P, Singh B, Mannan R, Arora S (2017) Overexpression of hypoxia-inducible factor and metabolic pathways: possible targets of cancer. Cell Biosci 7:62
Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, Mueller-Klieser W (2000) High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 60:916–921
Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, Mueller-Klieser W (2001) Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys 51:349–353
Roth GJ, Porte D Jr (1970) Chronic lactic acidosis and acute leukemia. Arch Intern Med 125:317–321
Masood U, Sharma A, Nijjar S, Sitaraman K (2017) B-cell lymphoma, thiamine deficiency, and lactic acidosis. Proc (Bayl Univ Med Cent) 30:69–70
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA (2014) Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014:149185
Harmon C, Robinson MW, Hand F, Almuaili D, Mentor K, Houlihan DD, Hoti E, Lynch L, Geoghegan J, O’Farrelly C (2019) Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol Res 7:335–346
Kumar A, Pyaram K, Yarosz EL, Hong H, Lyssiotis CA, Giri S, Chang CH (2019) Enhanced oxidative phosphorylation in NKT cells is essential for their survival and function. Proc Natl Acad Sci U S A 116:7439–7448
Bronte V (2014) Tumor cells hijack macrophages via lactic acid. Immunol Cell Biol 92:647–649
Brown TP, Bhattacharjee P, Ramachandran S, Sivaprakasam S, Ristic B, Sikder MOF, Ganapathy V (2020) The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene 39:3292–3304
Busco G, Cardone RA, Greco MR, Bellizzi A, Colella M, Antelmi E, Mancini MT, Dell’Aquila ME, Casavola V, Paradiso A, Reshkin SJ (2010) NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J 24:3903–3915
Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, Baba Y (2013) Acidic extracellular microenvironment and cancer. Cancer Cell Int 13:89
Cardone RA, Casavola V, Reshkin SJ (2005) The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer 5:786–795
Apicella M, Giannoni E, Fiore S, Ferrari KJ, Fernández-Pérez D, Isella C, Granchi C, Minutolo F, Sottile A, Comoglio PM, Medico E, Pietrantonio F, Volante M, Pasini D, Chiarugi P, Giordano S, Corso S (2018) Increased lactate secretion by cancer cells sustains non-cell-autonomous adaptive resistance to MET and EGFR targeted therapies. Cell Metab 28:848–865.e846
Brock MV, Herman JG, Baylin SB (2007) Cancer as a manifestation of aberrant chromatin structure. Cancer J 13:3–8
Dai Z, Ramesh V, Locasale JW (2020) The evolving metabolic landscape of chromatin biology and epigenetics. Nat Rev Genet 21:737–753
Liu XS, Little JB, Yuan ZM (2015) Glycolytic metabolism influences global chromatin structure. Oncotarget 6:4214–4225
Ding QS, Zhang L, Wang BC, Zeng Z, Zou XQ, Cao PB, Zhou GM, Tang M, Wu L, Wu LL, Yu HG, Guo Y, Zhou FX (2018) Aberrant high expression level of MORC2 is a common character in multiple cancers. Hum Pathol 76:58–67
Li DQ, Nair SS, Ohshiro K, Kumar A, Nair VS, Pakala SB, Reddy SD, Gajula RP, Eswaran J, Aravind L, Kumar R (2012) MORC2 signaling integrates phosphorylation-dependent, ATPase-coupled chromatin remodeling during the DNA damage response. Cell Rep 2:1657–1669
Xie H-Y, Zhang T-M, Hu S-Y, Shao Z-M, Li D-Q (2019) Dimerization of MORC2 through its C-terminal coiled-coil domain enhances chromatin dynamics and promotes DNA repair. Cell Commun Signal 17:160
Guddeti RK, Thomas L, Kannan A, Karyala P, Pakala SB (2021) The chromatin modifier MORC2 affects glucose metabolism by regulating the expression of lactate dehydrogenase A through a feed forward loop with c-Myc. FEBS Lett 595(9):1289–1302
Miller DM, Thomas SD, Islam A, Muench D, Sedoris K (2012) c-Myc and cancer metabolism. Clin Cancer Res 18:5546–5553
Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV (2000) Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem 275:21797–21800
Dang CV, Le A, Gao P (2009) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15:6479–6483
Guillaumond F, Leca J, Olivares O, Lavaut MN, Vidal N, Berthezene P, Dusetti NJ, Loncle C, Calvo E, Turrini O, Iovanna JL, Tomasini R, Vasseur S (2013) Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A 110:3919–3924
Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP (2009) The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8:3984–4001
Reina-Campos M, Moscat J, Diaz-Meco M (2017) Metabolism shapes the tumor microenvironment. Curr Opin Cell Biol 48:47–53
Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, Wodzak M, Klimko C, McMillan E, Butt Y, Ni M, Oliver D, Torrealba J, Malloy CR, Kernstine K, Lenkinski RE, DeBerardinis RJ (2016) Metabolic heterogeneity in human lung tumors. Cell 164:681–694
Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB (2009) ATP-citrate Lyase links cellular metabolism to histone acetylation. Science 324:1076
Schwörer S, Vardhana SA, Thompson CB (2019) Cancer metabolism drives a stromal regenerative response. Cell Metab 29:576–591
Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, Kimmelman AC (2016) Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536:479–483
Katheder NS, Khezri R, O’Farrell F, Schultz SW, Jain A, Rahman MM, Schink KO, Theodossiou TA, Johansen T, Juhász G, Bilder D, Brech A, Stenmark H, Rusten TE (2017) Microenvironmental autophagy promotes tumour growth. Nature 541:417–420
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16:582–598
Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, Lanciotti M, Serni S, Cirri P, Chiarugi P (2012) Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res 72:5130
Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21:297–308
Robertson-Tessi M, Gillies RJ, Gatenby RA, Anderson AR (2015) Impact of metabolic heterogeneity on tumor growth, invasion, and treatment outcomes. Cancer Res 75:1567–1579
Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D (2016) Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife 5:e10250
Tape CJ, Ling S, Dimitriadi M, McMahon KM, Worboys JD, Leong HS, Norrie IC, Miller CJ, Poulogiannis G, Lauffenburger DA, Jorgensen C (2016) Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell 165:1818
Knudsen ES, Balaji U, Freinkman E, McCue P, Witkiewicz AK (2016) Unique metabolic features of pancreatic cancer stroma: relevance to the tumor compartment, prognosis, and invasive potential. Oncotarget 7:78396–78411
Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R, Sneddon S, Martinez-Outschoorn UE, Sotgia F, Lisanti MP (2012) Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell cycle 11:1108–1117
Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P (2014) Metabolism of stromal and immune cells in health and disease. Nature 511:167–176
Yang L, Achreja A, Yeung TL, Mangala LS, Jiang D, Han C, Baddour J, Marini JC, Ni J, Nakahara R, Wahlig S, Chiba L, Kim SH, Morse J, Pradeep S, Nagaraja AS, Haemmerle M, Kyunghee N, Derichsweiler M, Plackemeier T, Mercado-Uribe I, Lopez-Berestein G, Moss T, Ram PT, Liu J, Lu X, Mok SC, Sood AK, Nagrath D (2016) Targeting stromal glutamine synthetase in tumors disrupts tumor microenvironment-regulated cancer cell growth. Cell Metab 24:685–700
Valencia T, Kim JY, Abu-Baker S, Moscat-Pardos J, Ahn CS, Reina-Campos M, Duran A, Castilla EA, Metallo CM, Diaz-Meco MT, Moscat J (2014) Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell 26:121–135
Endo H, Owada S, Inagaki Y, Shida Y, Tatemichi M (2020) Metabolic reprogramming sustains cancer cell survival following extracellular matrix detachment. Redox Biol 36:101643
Nazemi M, Rainero E (2020) Cross-talk between the tumor microenvironment, extracellular matrix, and cell metabolism in cancer. Front Oncol 10:239
Morris BA, Burkel B, Ponik SM, Fan J, Condeelis JS, Aguirre-Ghiso JA, Castracane J, Denu JM, Keely PJ (2016) Collagen matrix density drives the metabolic shift in breast cancer cells. EBioMedicine 13:146–156
Bertero T, Oldham WM, Grasset EM, Bourget I, Boulter E, Pisano S, Hofman P, Bellvert F, Meneguzzi G, Bulavin DV, Estrach S, Feral CC, Chan SY, Bozec A, Gaggioli C (2019) Tumor-stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metab 29:124–140.e110
Sullivan WJ, Mullen PJ, Schmid EW, Flores A, Momcilovic M, Sharpley MS, Jelinek D, Whiteley AE, Maxwell MB, Wilde BR, Banerjee U, Coller HA, Shackelford DB, Braas D, Ayer DE, de Aguiar Vallim TQ, Lowry WE, Christofk HR (2018) Extracellular matrix remodeling regulates glucose metabolism through TXNIP destabilization. Cell 175:117–132.e121
Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, Tonc E, Schreiber RD, Pearce EJ, Pearce EL (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162:1229–1241
Chang CH, Curtis JD, Maggi LB Jr, Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL (2013) Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153:1239–1251
Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara H, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS (2015) Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol 35(Suppl):S185–S198
Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sánchez-García FJ (2016) Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol 7:52
Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D'Acquisto F, Bland EJ, Bombardieri M, Pitzalis C, Perretti M, Marelli-Berg FM, Mauro C (2015) Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol 13:e1002202
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513:559–563
Ganeshan K, Chawla A (2014) Metabolic regulation of immune responses. Annu Rev Immunol 32:609–634
MacIver NJ, Michalek RD, Rathmell JC (2013) Metabolic regulation of T lymphocytes. Annu Rev Immunol 31:259–283
Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, Anderson SM, Abel ED, Chen BJ, Hale LP, Rathmell JC (2014) The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab 20:61–72
Michalek RD, Rathmell JC (2010) The metabolic life and times of a T-cell. Immunol Rev 236:190–202
Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16:769–777
Shyer JA, Flavell RA, Bailis W (2020) Metabolic signaling in T cells. Cell Res 30:649–659
Sugiura A, Rathmell JC (2018) Metabolic barriers to T cell function in tumors. J Immunol 200:400–407
Andrejeva G, Rathmell JC (2017) Similarities and distinctions of cancer and immune metabolism in inflammation and tumors. Cell Metab 26:49–70
Sun L, Fu J, Zhou Y (2017) Metabolism controls the balance of Th17/T-regulatory cells. Front Immunol 8:1632
Cluxton D, Petrasca A, Moran B, Fletcher JM (2019) Differential regulation of human Treg and Th17 cells by fatty acid synthesis and glycolysis. Front Immunol 10:115
Bailis W, Shyer JA, Chiorazzi M, Flavell RA (2017) No oxygen? No glucose? No problem: fatty acid catabolism enhances effector CD8(+) TILs. Cancer Cell 32:280–281
Le Bourgeois T, Strauss L, Aksoylar H-I, Daneshmandi S, Seth P, Patsoukis N, Boussiotis VA (2018) Targeting T cell metabolism for improvement of cancer immunotherapy. Front Oncol 8:237–237
Chapman NM, Chi H (2014) mTOR signaling, Tregs and immune modulation. Immunotherapy 6:1295–1311
Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MKK, Huang X, Caldwell S, Liu K, Smith P, Chen J-F, Jackson EK, Apasov S, Abrams S, Sitkovsky M (2006) A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 103:13132
Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, Kastenberger M, Bogdan C, Schleicher U, Mackensen A, Ullrich E, Fichtner-Feigl S, Kesselring R, Mack M, Ritter U, Schmid M, Blank C, Dettmer K, Oefner PJ, Hoffmann P, Walenta S, Geissler EK, Pouyssegur J, Villunger A, Steven A, Seliger B, Schreml S, Haferkamp S, Kohl E, Karrer S, Berneburg M, Herr W, Mueller-Klieser W, Renner K, Kreutz M (2016) LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab 24:657–671
Allison KE, Coomber BL, Bridle BW (2017) Metabolic reprogramming in the tumour microenvironment: a hallmark shared by cancer cells and T lymphocytes. Immunology 152:175–184
Liu YN, Yang JF, Huang DJ, Ni HH, Zhang CX, Zhang L, He J, Gu JM, Chen HX, Mai HQ, Chen QY, Zhang XS, Gao S, Li J (2020) Hypoxia induces mitochondrial defect that promotes T cell exhaustion in tumor microenvironment through MYC-regulated pathways. Front Immunol 11:1906
Hartmann FJ, Mrdjen D, McCaffrey E, Glass DR, Greenwald NF, Bharadwaj A, Khair Z, Verberk SGS, Baranski A, Baskar R, Graf W, Van Valen D, Van den Bossche J, Angelo M, Bendall SC (2021) Single-cell metabolic profiling of human cytotoxic T cells. Nat Biotechnol 39:186–197
Abdel-Wahab AF, Mahmoud W, Al-Harizy RM (2019) Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol Res 150:104511
Dang CV (2010) Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res 70:859
Xie J, Wu H, Dai C, Pan Q, Ding Z, Hu D, Ji B, Luo Y, Hu X (2014) Beyond Warburg effect—dual metabolic nature of cancer cells. Sci Rep 4:4927
Cheong J-H, Park ES, Liang J, Dennison JB, Tsavachidou D, Nguyen-Charles C, Wa Cheng K, Hall H, Zhang D, Lu Y, Ravoori M, Kundra V, Ajani J, Lee J-S, Ki Hong W, Mills GB (2011) Dual inhibition of tumor energy pathway by 2-deoxyglucose and metformin is effective against a broad spectrum of preclinical cancer models. Mol Cancer Ther 10:2350
Maher JC, Krishan A, Lampidis TJ (2004) Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol 53:116–122
Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR (2007) 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res 67:3364
Ihrlund LS, Hernlund E, Khan O, Shoshan MC (2008) 3-Bromopyruvate as inhibitor of tumour cell energy metabolism and chemopotentiator of platinum drugs. Mol Oncol 2:94–101
Nakano A, Tsuji D, Miki H, Cui Q, El Sayed SM, Ikegame A, Oda A, Amou H, Nakamura S, Harada T, Fujii S, Kagawa K, Takeuchi K, Sakai A, Ozaki S, Okano K, Nakamura T, Itoh K, Matsumoto T, Abe M (2011) Glycolysis inhibition inactivates ABC transporters to restore drug sensitivity in malignant cells. PLoS One 6:e27222
Chapiro J, Sur S, Savic LJ, Ganapathy-Kanniappan S, Reyes J, Duran R, Thiruganasambandam SC, Moats CR, Lin M, Luo W, Tran PT, Herman JM, Semenza GL, Ewald AJ, Vogelstein B, Geschwind J (2014) Systemic delivery of microencapsulated 3-Bromopyruvate for the therapy of pancreatic cancer. Clin Cancer Res 20:6406–6417
Buijs M, Wijlemans JW, Kwak BK, Ota S, Geschwind JH (2013) Antiglycolytic therapy combined with an image-guided minimally invasive delivery strategy for the treatment of breast cancer. J Vasc Interv Radiol 24:737–743
Sancho P, Burgos-Ramos E, Tavera A, Bou Kheir T, Jagust P, Schoenhals M, Barneda D, Sellers K, Campos-Olivas R, Graña O, Viera CR, Yuneva M, Sainz B Jr, Heeschen C (2015) MYC/PGC-1α balance determines the metabolic phenotype and plasticity of pancreatic cancer stem cells. Cell Metab 22:590–605
Chaudhary S, Pothuraju R, Rachagani S, Siddiqui JA, Atri P, Mallya K, Nasser MW, Sayed Z, Lyden ER, Smith L, Gupta SD, Ralhan R, Lakshmanan I, Jones DT, Ganti AK, Macha MA, Batra SK (2021) Dual blockade of EGFR and CDK4/6 delays head and neck squamous cell carcinoma progression by inducing metabolic rewiring. Cancer Lett 510:79–92
Acknowledgments
The authors/work in the manuscript are supported, in part, by the grants from the National Institutes of Health (P01 CA217798, R01 CA195586, R01 CA 210637, R01 CA247471, R01 CA 206444, R01CA218545, and R01CA241752) and Veteran Affairs (I01 BX004676).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chaudhary, S., Shah, A., Pothuraju, R., Lakshmanan, I., Ganti, A.K., Batra, S.K. (2022). Cancer Metabolism and Aggressive Tumor Behavior. In: Macha, M.A., Bhat, A.A., Wani, N.A. (eds) Immuno-Oncology Crosstalk and Metabolism. Springer, Singapore. https://doi.org/10.1007/978-981-16-6226-3_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-6226-3_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-6225-6
Online ISBN: 978-981-16-6226-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)