Abstract
Water-borne coatings are environmentally friendly compared solvent born coatings where polymer particles (the binder) and other formulation ingredients are dispersed in water. However, most water-borne coatings contain 5–10% of organic molecules like plasticizers and coalescing agents. These organic molecules evaporate from the drying coating posing an environmental burden. Many approaches have been investigated, but there is still no good working alternative for the plasticizers. The coalescing agents temporarily lower the Tg of the binder to facilitate particle deformation, after evaporation the glass transition temperature is restored to the original Tg again. A versatile alternative is to use lower Tg binders and create a “jump” in Tg during film formation. Our wok uses this nano-confinement effect, which creates a jump in Tg when the polymer chains are coming in close contact with inorganic nanoparticles surfaces, get immobilized. This close contact happens in the particle deformation stage, automatically leading to the correct timing of the two processes, particle deformation and increase of Tg. Another advantage of this approach is that no new chemistries need to be introduced. However it turns out that this approach only properly functions in a narrow window of nanoparticle loading.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Water-borne coatings
- NoVOC coatings
- Nanoparticles
- Inorganic surface-polymer interactions
- Glass transition temperature change
1 Introduction
Confinement effects due to the presence of hard nanoparticles in a polymer matrix have a profound effect on polymer \({T}_{g}\)[1]. So far the nano-confinement effect has been studied from casting films out of a polymer solution in the presence of inorganic nanoparticles [1]. The interactions between the polymer chain segments and/or the end groups of the chain play an important role, leading to either an increase (for interaction) or a decrease in (repulsive forces) Tg. We demonstrate the effect of end groups in determining the Tg via a simple equation to work out the concentration of nanoparticles for maximum \({T}_{g}\) in a composite for a given polymer. Using polystyrene-silica system with various end groups and polyvinyl alcohol-silica nanocomposite with interacting groups present throughout the polymer chain, we demonstrate that loading of nanoparticles for maximum \({T}_{g}\) is governed mostly by interactions of chain ends with the nanoparticle surface.
2 Methods
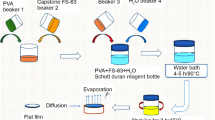
For the polyvinyl alcohol system with silica we used aqueous solution of PVA of different molecular weights and cast films by drying For the polystyrene-silica combinations we used solutions in THF to cast the film [2]. We also studied whether similar Tg jumps can also be observed in latex systems. For the latex systems we used composite of latex and silica and analyzed the Tg using Dynamic Mechanical Analysis and Differential Scanning Calorimetry. Finally, a recipe for water-borne coatings that introduces nano-silica particles in the formulation according to the experimental composition that gave maximum Tg was prepared. The corresponding coatings films were tested for gloss, scratch resistance and other typical paint film tests.
3 Results and Discussion
We observed the Tg jump phenomena due to the nano-confinement effect in PVA-silica, polystyrene-silica and latex-silica systems. We studied water based PVA system in depth under controlled conditions to establish the relationship between nanoparticle size and polymer molecular weight. A typical result when used silica nanoparticle (Fig. 1).
For the first time the nano-confinement effect was also shown to occur in a latex system by studying the composites formed by latex and varying amount of silica nanoparticles A typical example of Tg effect in latex-silica composite system is shown in Fig. 2.
The Tg increase in the latex system would mean that during the film formation process, at the stage where the particle deformation and polymer diffusions happens, the polymer chains are starting to interact with the surface of the inorganic particles through hydrogen bonding between hydroxyl groups on the surface and acid groups on the latex. This effect was evident in a non-tacky film obtained when we used a low Tg latex (15°C) in the final formulation.
Surprisingly all the Tg effects showed an optimum at a certain loading of the nanoparticles (Figs. 1 and 2). This optimum loading corresponding to the highest Tg depends on the particle size of the nanoparticles as well as the molecular weight of the polymers and their end groups.
We were able to derive an equation that predicts the location of this optimal loading.
For the latex systems, the resulting coatings performed like their regular counterparts, with the difference that no coalescing agents/plasticizers were added and instead a lower Tg latex was utilized (with the regular co-stabilizing acidic comonomers) and a low loading of commercial inorganic nanoparticles. The crucial factor is to select the optimum loading of these inorganic nanoparticles which can be predicted via our model but would otherwise be relatively difficult to establish experimentally.
The question might be asked why this effect does not play a role in regular coating formulations with an abundance of inorganic (pigment and filler) particles. The answer lies in the size of these particles, representing a much smaller surface area than the typical nanoparticles used in these studies, being in the 10–50 nm range whereas the pigment particles are approximately 10 times bigger and the fillers are at least 100 times bigger. To test whether this Tg effect finally translates to the preparation of SOC free water-based paint formulations we prepared coating formulations with low Tg latex and bench marked its performance with commercial formulations. We did not see any notable difference in performance in terms of color, gloss, tackiness or visual defects and the coating performed well in a one-year outdoor testing.
4 Conclusion
The nanoconfinement effect can be observed in films cast from solutions. We have shown that there is a strong influence on the loading of nanoparticles where this Tg jump is highest. The location depends on the combination of nanoparticles and polymer and the molecular weight as well as the end groups of the polymers. For the first time we were also able to show that the same effect can be utilized in water-borne coatings, leading to the elimination of coalescing agents/plasticizers and NoVOC water-borne coatings.
References
Rittigstein, P., Priestley, R.D., Broadbelt, L.J., Torkelson, J.M.: Model polymer nanocomposites provide an understanding of confinement effects in real nanocomposites. Nat. Mater. 2007(6), 278–282 (2007)
Panigrahi, R., et al.: Elucidating the role of interfacial hydrogen bonds on glass transition temperature change in a Poly (Vinyl Alcohol)/SiO2 polymer-nanocomposite by noncovalent interaction characterization and atomistic molecular dynamics simulation. Macromol. Rapid Commun. 41(21), 2000240 (2020). https://doi.org/10.1002/marc.202000240
Acknowledgement
The authors gratefully acknowledge the National Supercomputing Centre (NSCC), Singapore, for providing computational facilities and the financial support from the Agency for Science, Technology and Research (A*STAR), Singapore, Environmentally Friendly Specialty Products Programme, SERC152800043.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Thoniyot, P., Panigrahi, R., van Herk, A. (2022). NoVOC Water-Borne Coatings Through Utilization of the Nano-confinement Effect. In: Wei, Y., Chng, S. (eds) Proceedings of the 2nd International Conference on Advanced Surface Enhancement (INCASE 2021). INCASE 2021. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-16-5763-4_43
Download citation
DOI: https://doi.org/10.1007/978-981-16-5763-4_43
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5762-7
Online ISBN: 978-981-16-5763-4
eBook Packages: EngineeringEngineering (R0)