Abstract
The mechanism reversing bone resorption to formation during the remodeling cycle has remained a black box for many years—but starts now to be understood. First, the use of specific cell activity markers in histological sections oriented along the axis of the remodeling cycles, allowed identifying the successive cell activities linking the onset of resorption and the onset of formation. A dense osteoclast population initiating resorption is followed by an alternation of osteoclasts and osteoprogenitors (i.e., reversal cells) lining the eroded surface. This cell arrangement thus reveals the existence of a mixed “reversal-resorption” phase allowing several waves of resorption and multiple interactions between osteoclasts and osteoprogenitors. Importantly, the density of osteoprogenitors continuously grows along the eroded surface until reaching a threshold density permissive for initiation of bone formation. Next, the analysis of proliferation and differentiation markers indicated that the cell layer forming a canopy over the remodeling site is the local source of osteoprogenitors for recruitment onto the eroded surface. Functional support for this view was provided by the fact that decreased canopy coverage is associated with less recruitment of osteoprogenitors on the eroded surface and lower activation frequency of bone formation, throughout a diversity of pathophysiological conditions.
The present invited review was completed and submitted to the publisher on 08-Nov-19.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Coupling
- Bone loss
- Bone remodeling cycle
- Reversal phase
- Osteoprogenitors
- Bone formation
- Osteoclasts
- Canopy
- Bone marrow envelope
- Osteoblastogenesis
1 Understanding Bone Remodeling Requires Attention for the Reversal Phase
Bone remodeling replaces the existing bone matrix with a new bone matrix. It is achieved by local cell teams of osteoclasts resorbing bone and osteoblasts forming new bone—which are called basic multicellular units (BMUs). Bone loss is commonly ascribed to an imbalance of these two activities within a BMU [1, 2]. Bone resorption and formation are therefore a major research focus, whether in drug design, development of clinical markers, bone morphometry, or investigation of pathophysiological mechanisms.
However, common sense tells us that the conversion of a “resorption surface” into a “formation surface” cannot just be fortuitous and must be driven by a specific mechanism that also deserves attention. This conversion means that the bone surface “calls in” osteoblast lineage cells after having “called in” osteoclasts. It is actually the event that enrolls the osteoblasts in the BMU team—and more specifically those that will initiate bone formation, thereby coupling/reversing resorption to formation. It is the expected site of action of the molecular factors involved in coupling resorption and formation [3]. This central role is well demonstrated by the observations presented in Chap. 7 “Significance of Reversal Resorption Phase in Bone Loss” indicating that coupling/reversal may actually fail, with the consequence that initiation of bone formation does not occur, resulting in bone loss [4, 5]. Of note, this origin of bone loss (absence of osteoblasts) is mechanistically different from bone loss due to BMU imbalance (malfunction of the osteoclast-osteoblast team), and may demand different diagnostic and therapeutic considerations. There is thus a need for attention for the reversal mechanism—in addition to the current attention for bone resorption and formation.
2 Early Observations on Reversal and the Need for Appropriate Approaches to Understand the Reversal Mechanism
The reversal phase of the bone remodeling cycle was first defined by Baron in the 1970s as an intermediate step occurring between osteoclast resorption and the onset of bone matrix deposition [6]. Reversal was histologically characterized by the elongated profiles of mono-nucleated cells lining the eroded surface [6, 7]. These cells received the name of reversal cells, but their nature and role remained unknown for many years as their characterization was mostly morphological (see below). Reversal surfaces were found to represent on average as much as 80% of the eroded surface in human bone [4, 8]. However, this value was shown to vary depending on the pathophysiological condition [4, 8]. An increase in this value was suggested to reflect delayed (or absent) onset of bone formation and thus delayed (or absent) coupling. Situations suggesting uncoupling were repeatedly reported, but their pathophysiological relevance did not receive the attention one would have expected [3]. Only a few attempts to systematically investigate reversal were done during its early period of discovery: a rat model where a wave of remodeling was induced in alveolar bone, showed that the prevalence of bone surfaces undergoing reversal peaked later than those undergoing resorption and before those undergoing formation [9]. Also, the probability of detecting reversal cells at different excavation depths in human bone was the highest at depths intermediate between those where osteoclasts and osteoblasts were the most abundant [10]. These data contributed to the well-known representations of a remodeling unit with the reversal phase positioned as a single continuous event occurring between a period of pure resorption and onset of formation [11]. However, one should be aware that these drawings are based on averages of measurements obtained over whole bone surfaces and then aligned in a “logical” order, but they are not in the “real” order of the events within a remodeling unit (see below).
In the absence of knowledge about the functional mechanism of reversal and the absence of appropriate methods to gain this knowledge, the place of reversal in pathophysiology could not be appreciated and was mostly not considered. More recently, however, the combination of different approaches allowed a breakthrough. These approaches included (1) the use of markers revealing relevant features and specific cell activities in histological sections [4, 12]; (2) the generation of histological sections oriented along the operational axis of the remodeling events, making it possible to capture in a continuum the successive events occurring between the initial resorption episode up to the initiation of bone formation [13, 14]; (3) attention for tissue areas that are usually not taken into consideration, including the bone marrow neighboring the bone surfaces [12, 15,16,17,18,19]; (4) taking advantage of intracortical pores to measure strictly intracortical bone loss and relate this loss to the specific biological events of this pore [5]; (v) comparing critical histological features in diverse pathophysiological situations where bone remodeling is differently affected (hyperparathyroidism, osteoporosis induced by age, menopause, glucocorticoids, multiple myeloma) or challenged with drugs [3]. These approaches reveal that the critical activity underlying reversal is osteoprogenitor recruitment. It occurs during a period where resorption still occurs [14] and is supported by integrated activities of osteoclasts, osteoblast lineage cells, capillaries, and maybe other cell types [3]. Reversal appears at the heart of the decision of whether resorbed bone should be further resorbed, left unreconstructed, or replaced by new bone. It can now be understood how reversal impacts pathophysiology—which leads one to revisit the classical views on bone remodeling and bone loss [13, 14]. The present chapter focuses on the functional mechanism of reversal, thereby providing the mechanistic background necessary to explain the relevance of reversal to bone loss (Chap. 7 “Significance of Reversal Resorption Phase in Bone Loss”).
3 Reversal Cells Are Osteoprogenitors
The nature of the reversal cells has been a matter of debate, and especially the nature of the reversal cells colonizing the eroded surface right after the osteoclast has moved away, as reviewed earlier [3, 20]. These cells were proposed to be macrophage-like because they were observed to take up resorption debris left by the osteoclasts in the resorption lacuna. It was also proposed they might be pre-osteoclasts based on their TRAcP immunoreactivity, a typical osteoclast marker. It is only when cell markers could systematically be analyzed in histological bone sections, that the osteoblastic nature of reversal cells was revealed: they show Runx2, alkaline phosphatase, type 3 collagen, and other markers [4, 14, 20]. Markers also revealed the “maturing” nature of this osteoblast lineage cell population, thereby explaining the reported phenotypic diversity of reversal cells [10]. (1) Those next to osteoclasts most often correspond to early osteoprogenitors (high prevalence of smooth muscle actin), are more elongated, and show catabolic characteristics (phagocytosing resorption debris and rich in MMP13), thereby supporting osteoclastic resorption [4, 20, 21]. (2) Those next to osteoid-covered surfaces most often appear more differentiated towards mature bone-forming osteoblasts (high prevalence of osterix) and are more plump [4, 20]. It is expected that the shift from catabolic to anabolic characteristics of the reversal cells is a key in the control of bone remodeling and deserves attention.
4 Osteoprogenitors Are Intermixed with Osteoclasts on Eroded Surfaces: A Newly Recognized “Mixed Reversal-Resorption” Phase
The current remodeling cycle models show reversal as one period of pure reversal following a period of pure resorption, and preceding formation [11]. As mentioned above, such models are only drawings and not pictures captured from a real BMU, as conventional morphometry is based on randomly oriented sections and thus very rarely oriented along the operational axis of the remodeling unit. Thus the real order of the events occurring on the eroded surface remained uninvestigated for a long time [13]. A recent study took advantage of the fact that the orientation of intracortical BMUs is mostly parallel to the long axis of the diaphysis of long bones, and disclosed in real time the successive events connecting the initial resorption episode to the onset of bone formation as a functional continuum [14]. These events occur on the walls of the so-called cutting cone, which had remained poorly known in the absence of the use of markers.
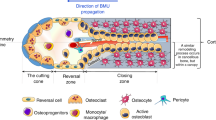
The analysis of such cutting cones indicates that the remodeling cycle starts with densely packed osteoclasts at the “tip” of the cutting cone, that osteoprogenitors colonize the eroded surface as soon as the osteoclast moves away, and that sparsely distributed osteoclasts occur all along the “wall” of the cutting cone—until formation starts [14]. Very important information provided by this study is that the reversal cells and osteoclasts are intermixed on the eroded surface reflecting a “mixed reversal-resorption period” (Fig. 1), which allows multiple loops of crosstalk between osteoclasts and osteoprogenitors [13, 14]. This is in contrast with the two single consecutive periods of resorption and reversal described in the textbooks—which were leaving only little room for osteoclast-osteoprogenitor interactions [11]. Interestingly also, the length of the reversal-resorption phase was shown to vary a lot among different cutting cones (fivefold, 250–1250 μm in [14]), thus meaning very different durations of osteoclast-osteoprogenitor interactions in different cutting cones as discussed in [14].
Key features of the mechanism reversing bone resorption to formation. This cartoon stresses (i) the existence of a mixed reversal-resorption phase allowing multiple osteoclast-osteoprogenitor interactions; (ii) that recruitment of osteoprogenitors on the eroded surface is a MUST to initiate bone formation and stop resorption; (iii) that a critical local source of osteoprogenitors is the bone marrow envelope that forms a canopy above the bone remodeling site; (iv) that capillaries in close contact with canopies are likely to contribute to the recruitment process
5 The Reversal-Resorption Events: Bone Resorption, Osteoprogenitor Recruitment, and Control of Resorption by Osteoprogenitor Recruitment
In classical literature, osteoclasts at the tip of the cutting cones receive the most attention. They appear responsible for initiating resorption and canal elongation. Important, however, osteoclasts occurring on the walls of the cutting cone during the reversal-resorption phase are responsible for widening the cutting cone and the longer the reversal-resorption phase, the wider it becomes, in accordance with the longer duration of exposure to osteoclasts [14]. Of note, the radial resorption of the reversal-resorption phase was found to account on average for 83% of the overall resorption and is thus the main contributor to overall resorption [14].
Sections along the operational axis of BMUs made it possible to systematically analyze the progressive changes occurring in the osteoprogenitor cell population during the reversal-resorption phase. The most striking change proved to be cell density [14]: cell densities of osteoprogenitors gradually increased during the reversal-resorption phase and bone formation was never initiated below a given cell density. This indicates the need for a threshold to allow the onset of bone formation (Fig. 1). This demonstration is strengthened by the fact that this threshold is independent of the length of the reversal-resorption zone—which furthermore reflects a widespread variation in osteoprogenitor recruitment rates among different canals [14]. This is a central observation, because it demonstrates that osteoprogenitor recruitment on eroded surfaces is a permissive event for bone formation and that its rate determines how soon formation is initiated.
As the rate of osteoprogenitor recruitment determines the waiting time for initiation of bone formation and because resorption lasts during this whole waiting time, the rate of osteoprogenitor recruitment also determines the extent of resorption (Fig. 1) [14]. This remarkable property stresses that the extent of resorption is subordinated to osteoprogenitor recruitment: so, if recruitment does not reach the threshold density, resorption may continue leading to pathological bone loss. This explains why malfunction of reversal leads to bone loss and provides a mechanistic basis for the observations presented in Chap. 7 “Significance of Reversal Resorption Phase in Bone Loss”. The data supporting this mechanism are obtained in intracortical bone—which allows stringent analyses—but as discussed elsewhere [14], they are very likely to hold true in cancellous bone.
6 Where Do the “Osteoprogenitors Colonizing the Eroded Surface” Come from? Bone Lining Cells, Bone Marrow Envelope/Canopy Cells, Capillaries
Bone lining cells (BLCs) defined as the cells in strict contact with the quiescent bone matrix, are very likely contributors to the generation of the reversal cell population, since BLCs pre-exist on the bone surface and were identified as osteoprogenitors in several cell tracing models [22,23,24]. They are believed to retract upon passage of the resorbing osteoclast [25,26,27] and to spread on the eroded surface after its passage (attracted by chemotactic- and haptotactic factors generated by the osteoclast: see below). However, these cells are too few to allow initiation of bone formation, and the proliferation index assessed on reversal surfaces is rather low, so that “importation” is mandatory [18]. As reviewed elsewhere, insight into the importation route was gained by comparing cell proliferation, cell densities, and differentiation markers in the immediate environment of reversal surfaces of human cancellous bone [3]. The bone marrow envelope (BME) appears to play an important role in this importation [3, 18, 28, 29]. The BME is defined as a layer of CD271+ cells surrounding the bone marrow [30, 31]. At the level of the quiescent surface, it is interposed between the BLCs and the bone marrow. This envelope is easily overlooked because its thinness does not allow detection at the light microscope level, and at the electron microscopy level, BME cells appear very similar to BLCs, with long cell extensions aligned parallel to the bone surface [18]. On the other hand, however, BME cells have their own specific characteristics suggesting that they may represent a distinct cell category [3, 18]. (1) Whereas BLCs are believed to be post-differentiated osteoblasts, the BME cells might correspond to a subpopulation of reticular adventitial cells that colonized the periphery of the primitive bone marrow cavity during development [32]. (2) Whereas BLCs are in direct contact with the bone matrix, the BME cells are not, and dissociate from BLCs when taking a bone marrow plug [28] or when lifted by osteoclasts at the onset of the bone remodeling cycle [18]. Accordingly, at remodeling sites, the BME appears as a canopy (Fig. 1) that covers all the bone surface cells involved in remodeling, thereby delineating a so-called bone remodeling compartment [3, 12, 18]. (3) The transition from quiescent to remodeling surfaces induces distinct molecular characteristics in the canopy layer compared to the bone surface [30]. First, canopy cells show a 2–3 times higher proliferation index compared with the cells sitting on the eroded surface [18, 30]. Of note, this greater proliferation does not lead to increased cell densities at the canopy level, while instead the cell densities on the reversal surface are increased despite a low proliferation [18, 30]. This opposite behavior of cell densities and proliferation at the canopy and reversal surface-level points to the canopy as the main contributor to the increased number of osteoprogenitors, which then translocate to the bone surface. Second, the canopy shows a high prevalence of early osteoblast differentiation markers like smooth muscle actin and tenascin, whereas the opposite is true for the later osteoblast lineage marker osterix. Runx2, however, is present in the majority of canopy and reversal cells [18, 30].
The critical role of the canopy in osteoprogenitor recruitment on eroded surfaces is also convincingly shown by pathophysiological situations where canopy coverage of eroded surfaces is deficient (including aging, postmenopausal and glucocorticoid osteoporosis, myeloma), as canopy deficiency repeatedly coincides with deficient osteoprogenitor recruitment and deficient initiation of bone formation [12, 15,16,17, 33]. This repetitive coincidence in several diverse situations taken together with the close proximity of the canopy to the eroded surface and the assessments of cell markers, proliferation index and cell densities, strongly suggests a critical contribution of canopy cells to the recruitment of osteoprogenitors on eroded surfaces (Fig. 1) [3]. The triggers of these molecular changes were discussed elsewhere and were related to the changes in cell–cell and cell–matrix interaction at the onset of bone remodeling [3]. Of special importance is the generation of numerous contacts between canopy cells and both osteoclasts and capillaries (Fig. 1)—because the latter two are sources of coupling/osteogenic/proliferation factors [3, 12, 18, 19].
In relation to this dense network of canopy-associated capillaries above eroded surfaces, one should also mention the existence of circulating osteoblast-lineage cells [34], as well as the suggested role of osteoprogenitor-bearing transition vessels in osteoblast recruitment to bone formation sites in mouse [35, 36]. Thus, in the same way, eroded surfaces may recruit osteoprogenitors from capillaries, and this may occur through translocation across the canopy as suggested by electron microscopy observations [19]. Recruitment from capillaries may also be relevant to intracortical remodeling as it appears the only possible importation route.
7 Site-Specific Targeting of Osteoprogenitors and Other Features Possibly Associated to Reversal
Reversal does not only demand proliferation and differentiation of osteoprogenitors as reviewed above, but also precise targeting of osteoprogenitors to the site where resorbed bone has to be reconstructed. Chemotaxis exerted by factors generated during osteoclast resorption has received much attention. A series of reports concern soluble factors able to attract osteoprogenitors from the deep bone marrow to the bone surfaces. Examples are TGF-β [37], IGF [37], PDGF [38,39,40], S1P [41]. Other studies stress the strength of immobilized factors for inducing osteogenesis in an even more “site-restricted” way. Examples are factors bound to the eroded matrix, such as TRAcP [43] and demineralized collagen remnants [21, 44] and fibronectin [44] or EphrinB2 bound to the osteoclast membrane [42]. Obviously, these immobilized factors are relevant for haptotaxis of the osteoprogenitors already positioned right next to the eroded surface, such as BLCs and canopy cells.
Another intriguing question is whether osteocytes play a role specifically during reversal. There is no clear answer, but osteoclastic factors like LIF [45] and cardiotrophin [46] were reported to make osteocytes produce less sclerostin—which should thus favor bone formation. Osteocytes are also speculated to respond to the changes in a mechanical strain that are generated by the presence of resorption lacunae [47].
A well-known hallmark of the reversal surface is the cement line [48]. It consists of basophilic material deposited on eroded surfaces and is rich in mucopolysaccharides and osteopontin. It is mostly ascribed a role in cell adhesion and in connecting new bone matrix packages to pre-existing ones. However, it remains unknown whether the cement line plays a role in the activation of osteogenesis.
8 Consequences of the New Knowledge About Reversal for Treatment of Bone Loss and Routine Morphometry
This new knowledge of the reversal mechanism highlights that the reversal phase must be taken into account to appropriately address bone remodeling. First, it is now clear that reversal may fail, resulting in uncoupling of resorption from formation, although standard morphometry presupposes coupling as a rule. This uncoupling represents a mechanism of bone loss with a distinct etiology, i.e., absence of osteoblasts (thus abortion of the remodeling cycle and no BMU generation) instead of malfunction of the osteoclast-osteoblast team within the BMUs as commonly stated. Obviously, this origin of bone loss should be taken into account when searching for treatment strategies to prevent it. Second, routine morphometry should therefore include assessment of reversal. This may be challenging, but some of the features that have been used to unravel the reversal mechanism are worthwhile to consider: i.e., thorough identification of all eroded surfaces (broken lamellae), their quantification discriminating osteoclast and reversal surface, and characterization of the reversal surface with respect to cell density and canopy coverage. Furthermore, standard morphometric indices should be interpreted cautiously: for example, the so-called activation frequency of bone remodeling can be interpreted as such only in coupled remodeling, as this parameter is actually the activation frequency of bone formation (and would be more safely called this way). Third, during the mixed reversal-resorption phase, osteoclasts appear to make the osteoprogenitor activity shifting from catabolic (promoting resorption) to anabolic (osteoblastogenesis). This remarkable property should guide further research to understand how bone mass and structure are maintained throughout adult life, despite continuous remodeling.
References
Compston JE, Mcclung MR, Leslie WD. Seminar osteoporosis. Lancet. 2019;393:364–76.
Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20(2):177–84.
Delaisse J-M. The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2016;5:1–8.
Andersen TL, Abdelgawad ME, Kristensen HB, Hauge EM, Rolighed L, Bollerslev J, et al. Understanding coupling between bone resorption and formation: are reversal cells the missing link? Am J Pathol. 2013;183(1):235–46.
Andreasen CM, Delaisse J-M, van der Eerden BCJ, van Leeuwen JPTM, Ding M, Andersen TL. Understanding age-induced cortical porosity in women: the accumulation and coalescence of eroded cavities upon existing intracortical canals is the main contributor. J Bone Miner Res. 2018;33(4):606–20.
Baron R. Importance of the intermediate phases between resorption and formation in the measurement and understanding of the bone remodeling sequence. In: Meunier PJ, editor. Bone histomorphometry: second international workshop. Paris: Armour Montagu; 1977. p. 179–83.
Dempster DW, Compston JE, Drezner MK, Kanis FHG, John AM, Ott S, et al. Histomorphometry nomenclature: a 2012 update of the report of the ASBMR histomorphometry nomenclature David. J Bone Miner Res. 2014;28(1):2–17.
Baron R, Vignery A, Lang R. Reversal phase and osteopenia: Defective coupling of resorption to formation in the pathogenesis of osteoporosis. In: Deluca HF, Frost HM, Jee WSS, Johnston CC, Parfitt AM, editors. Osteoporosis: recent advances in pathogenesis and treatment. University Park Press, Baltimore, MD; 1980. p. 311–20.
Van Tran PT, Vignery A, Baron R. Cellular kinetics of the bone remodeling sequence in the rat. Anat Rec. 1982;202(4):445–51.
Eriksen EF, Melsen F, Mosekilde L. Reconstruction of the resorptive site in iliac trabecular bone: a kinetic model for bone resorption in 20 normal individuals. Metab Bone Dis Relat Res. 1984;5(5):235–42.
Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev. 1986;7(4):379–408.
Andersen TL, Sondergaard TE, Skorzynska KE, Dagnaes-Hansen F, Plesner TL, Hauge EM, et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol. 2009;174(1):239–47.
Dempster DW. Tethering formation to resorption: reversal revisited. J Bone Miner Res. 2017;32(7):1389–90.
Lassen NE, Andersen TL, Pløen GG, Søe K, Hauge EM, Harving S, et al. Coupling of bone resorption and formation in real time: new knowledge gained from human Haversian BMUs. J Bone Miner Res. 2017;32(7):1395–405.
Andersen TL, Hauge EM, Rolighed L, Bollerslev J, Kjærsgaard-Andersen P, Delaisse J-M. Correlation between absence of bone remodeling compartment canopies, reversal phase arrest, and deficient bone formation in post-menopausal osteoporosis. Am J Pathol. 2014;184(4):1142–51.
Jensen PR, Andersen TL, Hauge E-M, Bollerslev J, Delaissé J-M. A joined role of canopy and reversal cells in bone remodeling – lessons from glucocorticoid-induced osteoporosis. Bone. 2014;73:16–23.
Jensen PR, Andersen TL, Søe K, Hauge EM, Bollerslev J, Amling M, et al. Premature loss of bone remodeling compartment canopies is associated with deficient bone formation: a study of healthy individuals and patients with Cushing’s syndrome. J Bone Miner Res. 2012;27(4):770–80.
Kristensen HB, Andersen TL, Marcussen N, Rolighed L, Delaisse J-M. Osteoblast recruitment routes in human cancellous bone remodeling. Am J Pathol. 2014;184(3):778–89.
Kristensen HB, Andersen TL, Marcussen N, Rolighed L, Delaisse J-M. Increased presence of capillaries next to remodeling sites in adult human cancellous bone. J Bone Miner Res. 2013;28(3):574–85.
Abdelgawad ME, Delaisse J-M, Hinge M, Jensen PR, Alnaimi RW, Rolighed L, et al. Early reversal cells in adult human bone remodeling: osteoblastic nature, catabolic functions and interactions with osteoclasts. Histochem Cell Biol. 2016;145(6):603–15.
Everts V, Delaissié JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, et al. The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J Bone Miner Res. 2002;17(1):77–90.
Kim SW, Lu Y, Williams EA, Lai F, Lee JY, Enishi T, Balani DH, Ominsky MS, Ke HZ, Kronenberg HMWM. Sclerostin antibody administration converts bone lining cells into active osteoblasts. J Bone Miner Res. 2017;32(5):892–901.
Kim SW, Pajevic PD, Selig M, Barry KJ, Yang JY, Shin CS, et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res. 2012;27(10):2075–84.
Matic I, Matthews BG, Wang X, Dyment NA, Worthley DL, Rowe DW, et al. Quiescent bone lining cells are a major source of osteoblasts during adulthood. Stem Cells. 2016;34(12):2930–42.
Ferrier J, Xia SL, Lagan E, Aubin JE, Heersche JNM. Displacement and translocation of osteoblast-like cells by osteoclasts. J Bone Min Res. 1994;9(9):1397–405.
Perez-Amodio S, Beertsen W, Everts V. (pre-)osteoclasts induce retraction of osteoblasts before their fusion to osteoclasts. J Bone Miner Res. 2004;19(10):1722–31.
Karsdal MA, Fjording MS, Foged NT, Delaissé J-M, Lochter A. Transforming growth factor-β-induced osteoblast elongation regulates osteoclastic bone resorption through a p38 mitogen-activated protein kinase- and matrix metalloproteinase-dependent pathway. J Biol Chem. 2001;276(42):39350–8.
Bi LX, Mainous EG, Yngve DA, Buford WL. Cellular isolation, culture and characterization of the marrow sac cells in human tubular bone. J Musculoskelet Neuronal Interact. 2008;8(1):43–9.
Simmons D. The in vivo role of bone marrow fibroblast-like stromal cells. Calcif Tissue Int. 1996;58(3):129–32.
Jensen PR, Andersen TL, Sikjær TT, Rejnmark L, Ejersted CDJ. Molecular changes triggered in local osteoprogenitors at the onset of bone remodeling. J Bone Miner Res. 2017;32(Suppl 1):S332.
Tormin A, Li O, Brune JC, Walsh S, Schütz B, Ehinger M, et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117(19):5067–77.
Bianco P, Riminucci M. The bone marrow stroma in vivo: ontogeny, structure, cellular composition and changes in disease. In: Beresford JN, Owen ME, editors. Marrow stromal cell culture [internet]. Cambridge: Cambridge University Press; 1998. p. 11–24.
Andersen TL, Søe K, Sondergaard TE, Plesner T, Delaisse J-M. Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells: research paper. Br J Haematol. 2010;148(4):551–61.
Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352(19):1959–66.
Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–8.
Caire R, Roche B, Picot T, Aanei CM, He Z, Campos L, et al. Parathyroid hormone remodels bone transitional vessels and the leptin receptor-positive Pericyte network in mice. J Bone Miner Res. 2019;00:1–15.
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, et al. TGF-Β1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15(7):757–65.
Sanchez-Fernandez MA, Gallois A, Riedl T, Jurdic P, Hoflack B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PLoS One. 2008;3(10).
Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20(11):1270–8.
Tokunaga A, Oya T, Ishii Y, Motomura H, Nakamura C, Ishizawa S, et al. PDGF receptor β is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008;23(9):1519–28.
Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA. 2008;105(52):20764–9.
Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4(2):111–21.
Sheu TJ, Schwarz EM, O’Keefe RJ, Rosier RN, Puzas JE. Use of a phage display technique to identify potential osteoblast binding sites within osteoclast lacunae. J Bone Miner Res. 2002;17(5):915–22.
Abdelgawad ME, Søe K, Andersen TL, Merrild DMH, Christiansen P, Kjærsgaard-Andersen P, et al. Does collagen trigger the recruitment of osteoblasts into vacated bone resorption lacunae during bone remodeling? Bone. 2014;67:181–8.
Walker EC, McGregor NE, Poulton IJ, Solano M, Pompolo S, Fernandes TJ, et al. Oncostatin M promotes bone formation independently of resorption when signaling through leukemia inhibitory factor receptor in mice. J Clin Invest. 2010;120(2):582–92.
Walker EC, McGregor NE, Poulton IJ, Pompolo S, Allan EH, Quinn JMW, et al. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for normal bone remodeling. J Bone Miner Res. 2008;23(12):2025–32.
Erben RG. Hypothesis: coupling between resorption and formation in cancellous bone remodeling is a mechanically controlled event. Front Endocrinol (Lausanne). 2015;6:1–5.
McKee MD, Nanci A. Osteopontin: an interfacial extracellular matrix protein in mineralized tissues. Connect Tissue Res. 1996;35(1–4):197–205.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Delaisse, JM., Andersen, T.L., Kristensen, H.B., Jensen, P.R. (2022). Mechanism Reversing Bone Resorption to Formation During Bone Remodeling. In: Takahashi, H.E., Burr, D.B., Yamamoto, N. (eds) Osteoporotic Fracture and Systemic Skeletal Disorders. Springer, Singapore. https://doi.org/10.1007/978-981-16-5613-2_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-5613-2_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5612-5
Online ISBN: 978-981-16-5613-2
eBook Packages: MedicineMedicine (R0)