Abstract
The formation of allotetraploids of red crucian carp (Carassius auratus red var., ♀) × common carp (Cyprinus carpio L., ♂) fills a gap for producing both fertile female and male allotetraploids in vertebrates, which is important for genetic breeding and evolutionary studies. In fish breeding, this lineage provides the valuable resources of diploid gametes for the production of allotriploids. In the early 1990s, using diploid gametes generated from the allotetraploids and haploid gametes generated from Japanese white crucian carp (Carassius cuvieri) or Xingguo red carp (Cyprinus carpio var. singuonensis), triploid Xiangyun crucian carp and triploid Xiangyun carp were produced, respectively. Triploid Xiangyun crucian carp and triploid Xiangyun carp are farmed and popular among consumers in 28 provinces and municipalities in China due to their growth superiority, high meat quality, strong resistance, infertility, and other advantageous traits. Farming of these triploids achieves significant economic, social, and ecological benefits. In addition, new types of allotriploid fish are produced by crossing the male allotetraploids with female goldfish or red crucian carp, which have advantageous traits in growth rate, shape, and meat quality. On the other hand, the diploid eggs and diploid sperm from allotetraploid fish are used to establish gynogenetic and androgenetic diploid hybrid lineages, respectively. Subsequently, the improved tetraploid hybrids are generated from the gynogenetic and androgenetic diploid hybrid lineages. By crossing the improved allotetraploid hybrids with improved red crucian carp, triploid Xiangyun crucian carp II is produced. This chapter mainly introduces the morphological traits, reproductive physiology, genetic characteristics, and applications of the allotriploid fish.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 The Formation, Appearance, and Karyotype of Triploid Xiangyun Crucian Carp and Triploid Xiangyun Carp
4.1.1 The Formation and Morphological Traits of Triploid Xiangyun Crucian Carp and Triploid Xiangyun Carp
By crossing between female Japanese white crucian carp (Carassius cuvieri, 2n = 100) and male allotetraploids of red crucian carp (Carassius auratus red var., ♀) × common carp (Cyprinus carpio L., ♂) hybridization, triploid Xiangyun crucian carp (3n = 150) was formed. By crossing between female Xingguo red carp (Cyprinus carpio var. singuonensis, 2n = 100) and male allotetraploids of red crucian carp (♀) × common carp (♂) hybridization, triploid Xiangyun carp (3n = 150) was generated.
Triploid Xiangyun crucian carp had a flat-sided body with a humped back and the abdomen was slightly pointed. The upper part of the body side was steel gray and the lower side was silver gray with a white belly. The snout was blunt. The terminal mouth was blunt and oblique with the thick lower lip. Either side of the lower jaw had only one pair of very short barbels. The appearance of triploid Xiangyun crucian carp was shown in Fig. 4.1.
Triploid Xiangyun carp was spindle-shaped with a high back and round belly. Generally, the back was gray-black, while the belly was silver-gray. Triploid Xiangyun carp had a terminal mouth. Two pairs of barbels were found at the corners of the mouth.
4.1.2 The Karyotype and DNA Content of Triploid Xiangyun Crucian Carp and Triploid Xiangyun Carp
The chromosome numbers of kidney cells in triploid Xiangyun crucian carp and triploid Xiangyun carp were mainly from 145 to 150 (Zhang et al. 2005; He 1999). The modes (3n = 150) of triploid Xiangyun crucian carp and triploid Xiangyun carp accounted for 78.0% and 78.6% of all the chromosome numbers in cells. These shreds of evidence confirmed that triploid Xiangyun crucian carp and triploid Xiangyun carp were both triploids with chromosome numbers as 3n = 150. The karyotypes were both 33 m + 51sm + 33st + 33 t. Atypia chromosomes and specific chromosomes with satellites and secondary constrictions were not found due to the different gonadal development appearances.
The DNA contents were analyzed using the blood of triploid Xiangyun crucian carp and triploid Xiangyun carp by flow cytometry, respectively. The DNA content in the blood sample was tested by flow cytometry. The analysis showed that the DNA content of triploid Xiangyun crucian carp was 1.53 times that of diploid common carp. The DNA content of triploid Xiangyun carp was 1.48 times that of diploid common carp. All these ratios had no significant difference to 1.5:1 (P > 0.05). The result was identical to the chromosome number (3n = 150) of triploid Xiangyun crucian carp and triploid Xiangyun carp which proved that triploid Xiangyun crucian carp and triploid Xiangyun carp were triploids (Liu et al. 1999).
4.2 The Mechanism of Sterility and Rapid Growth Appearance in Allotriploid Fish
4.2.1 The Sterility of Triploid Xiangyun Crucian Carp and Triploid Xiangyun Carp
In terms of genetics, triploid Xiangyun crucian carp had three sets of chromosomes. Therefore, the chromosome pairing was abnormal during meiosis and could not form normal gametes. In terms of reproductive physiology, triploid Xiangyun crucian carp showed gonadal infertility. The gonads of triploid Xiangyun crucian carp could be classified into three types including testis, ovary, and fat-like type, and all of them could not generate normal gametes. More than 10 years of cultivation experiments had also proved that triploid Xiangyun crucian carp and triploid Xiangyun carp were sterile.
Triploid Xiangyun crucian carp had genetic characteristics with three sets of chromosomes, resulting in biological characteristic changes of the hypothalamus, pituitary, and gonadal axis (the reproductive axis) and ultimately leading to infertility. The related infertility characteristics and infertility mechanisms were shown as follows.
4.2.1.1 The Gonadal Development of Triploid Xiangyun Crucian Carp
During the breeding season, the gonadal structures of triploid Xiangyun crucian carp at the age of 1 and 2 were similar which could be divided into testis, ovary, and fat-like types (Liu et al. 2002). The results of histological sections were shown in Fig. 4.2a–c.
The gonadal development of triploid Xiangyun crucian carp. (a) Ovary type gonad in which there were a lot of cystic-arranged small cells with lower differentiation degree. Small number of primary oocytes could be seen in the small cells. Bar = 20 μm. (b) Testis type gonad. Spermatids were in seminiferous lobules. Some spermatids were vacuolated and degenerated. Bar = 50 μm. (c) Fat-like type gonad. Structures of adipocyte could be observed. Bar = 25 μm
Testis type: The testes of triploid Xiangyun crucian carp were white and brown observed with naked eyes. Bilaterally asymmetrical testes were common. Several individuals only had un-well developmental testis or even no testis could be found on one side, while well developmental testis could be observed on the other side. The shapes of the testes had uneven thickness. Under the light microscope, the testes were full of seminiferous lobules. Spermatids were found to be distributed in seminiferous lobules. Vacuoles could be found in several spermatids and showed degenerative forms with abnormal mature sperm. The observation results showed that intracellular substances were disintegrating and disappearing in degenerative spermatids by the transmission electron microscopy. At the same time, mature sperm could be found in testes of Japanese white crucian carp.
Ovary type: Bilaterally asymmetrical ovaries were common with uneven thickness. Under the light microscope, the cells in ovaries were cystically arranged with small volumes. Under the electron microscopy, these small cells were in a lower differentiated degree and no yolk was found. Since there were no obvious oocyte characteristics, it was difficult to identify which were spermatogonia or oogonia. These small cells were inlaid with a few primary oocytes at phase II and a few large degraded oocytes at phase III. In the degenerative oocytes, the yolk was dissolved. Irregular curve and inward shrinkage could be observed at the outside of oocytes. In the ovaries of Japanese white crucian carp, most oocytes were large with the abundant yolk. No cystic-arranged small cell structure could be found in ovaries from Japanese white crucian carp.
Fat-like type: Only two white adipose tissues on two sides of the dorsal mesentery in the gonads were observed under naked eyes. This type had neither testicular structures nor ovarian structures. Cells were full of fat particles. The cytoplasm and nucleus were pulled aside. When preparing histological sections, fat particles in the adipocyte were easily dissolved by organic solvent (such as xylene) and became blurred. Under the electron microscope, the fat particles in adipocyte could be observed. In these adipose tissues, no evidence of germ cells could be found.
The gonads of triploid Xiangyun carp also included the three types mentioned above (Liu et al. 2000).
The gonad weight and gonadosomatic index of triploid Xiangyun crucian carp and Japanese white crucian carp in the same period were measured. The gonadosomatic index of the ovary in triploid Xiangyun crucian carp was lower than that in diploid Japanese white crucian carp. Similarly, gonadosomatic index of testis in triploid Xiangyun crucian carp was lower than that in diploid Japanese white crucian carp. The “gonadosomatic index” (fat-like gonad weight/body weight) of fat-like gonads in triploid Xiangyun crucian carp was lower than the average value of gonadosomatic index in ovary and testis of diploid Japanese white crucian carp. In the same reproductive stage, the gonadosomatic index of ovary and testis in diploid Japanese white crucian carp was 2.85 and 1.94 times of that in triploid Xiangyun crucian carp. The average gonadosomatic index in ovary and testis of diploid Japanese white crucian carp was 5.60 times of “gonadosomatic index” of fat-like gonads in triploid Xiangyun crucian carp (Liu et al. 2002). Combined with the results of the gonadal histological section, it could be concluded that triploids had no normal gonadal development and could not produce mature sperm and eggs. In addition, more than 10 years of cultivation had also proved the infertility of triploid Xiangyun crucian carp.
In addition, the ovary development of triploid Xiangyun crucian carp undertook more suppression than testis, which was consistent with the observation from histological sections. Ovaries of triploid Xiangyun crucian carp were mainly occupied with small cells with a lower degree of differentiation, while only a few bigger oocytes with yolk were observed. Therefore, the ovarian gonadosomatic index was low. In the testis of triploid Xiangyun crucian carp, the testes were mainly occupied with spermatids. Though there were not a large number of mature sperm, the testicular structures were similar to the testis of diploid fish. Thus, the difference of testicular gonadosomatic index between triploid and diploid fish was smaller compared to that of ovarian gonadosomatic index. No germ cell existed in the fat-like gonad of triploid Xiangyun crucian carp. The “gonadosomatic index” had significant differences from the average gonadosomatic index of ovary and testis in diploid Japanese white crucian carp (Liu et al. 2002).
4.2.1.2 The Chromosome Behavior of Triploid Xiangyun Crucian Carp in Meiosis
Triploid Xiangyun crucian carp and triploid Xiangyun carp were allotriploids with genome 3n = 150. Since the gonads were sterile, they could not produce mature sperm and eggs. Thus, these triploid fishes also could not reproduce itself. The results from chromosome pairing observation of triploid Xiangyun crucian carp spermatocytes in the first meiotic metaphase indicated that most chromosomes formed paired bivalent and unpaired univalent (Zhang et al. 2005). The bivalents were thick and deeply stained. Several ring-shaped bivalents were found. Univalents were slender and lightly stained without pairing. During the meiosis of triploid Xiangyun crucian carp, the numbers of the bivalent and univalent formation were consistent with the result of somatic cell detection that was 3n = 150 (Zhang et al. 2005).
A similar result was reported. For example, in artificial autotriploid transparent-colored crucian carp, the underdeveloped ovaries had a large number of bivalents and univalent but a few trivalents and ployvalents. It had been regarded that the disorder of chromosome pairing affected the development and growth of germ cells, leading to the block of gonadal development (Gui et al. 1990). In autotriploid, the three chromosome sets had high homology and it was easy to form trivalents. However, the chromosome sets in the allotriploids shared low homology and it had less possibility to form trivalents.
During meiosis, the coexistence of bivalents and univalents in triploid Xiangyun crucian carp would easily lead to aneuploid spermatid which contributed to abortion and abnormal development of mature sperm. In terms of the chromosome, the three sets of chromosomes were disordered in the pairing and separation stages during meiosis, which can cause aneuploid gametes. The suppressed development of germ cells led to nondevelopment or abortion which gave a better explanation of why both testis and ovary types in triploid Xiangyun crucian carp were sterile (Zhang et al. 2008). In the testis of triploid Xiangyun crucian carp, some primary spermatocytes could develop to spermatids and then aborted. In a few cases, some triploid fish could produce a small amount of sperm, but they could not form survived offspring due to the chromosomal aneuploidy of the sperm. Oocytes with yolk were in the ovary from triploid Xiangyun crucian carp but the overall development was abnormal and degenerated. Ultimately, normal mature eggs could not be generated.
4.2.1.3 The Pituitary Structure of Triploid Xiangyun Crucian Carp
The microstructure, ultrastructure, and histochemical characteristics of pituitaries in diploid red crucian carp (2n = 100), triploid Xiangyun crucian carp (3n = 150), and allotetraploids of red crucian carp (♀) × common carp (♂) (4n = 200) were analyzed and compared in the breeding season and after breading season. The results showed that pituitaries of the three fishes all had six different types of secretory cells. The sizes of pituitaries were significantly different among various ploidy fishes. The volumes of pituitary secretory cells and nuclei in allotetraploids of red crucian carp (♀) × common carp (♂) were significantly larger than those in diploids and triploids. The volumes of pituitary secretory cells and nuclei in triploid fish were slightly larger than those in diploid fish (Long et al. 2006). In addition, the studies on blood and blood cells of tetraploid, triploid and diploid fishes showed that erythrocytes and their nucleus, neutrophils and monocytes also presented a proportional relationship of 4:3:2. With the increase of ploidy, the ratios of erythrocyte minor axis to major axis were decreased (Liu et al. 2004a). Allotetraploids of red crucian carp (♀) × common carp (♂) contained four sets of chromosomes (4n = 200). Meanwhile, the number of chromosomes or DNA content was two times that of their original diploids parents (common carp and red crucian carp, 2n = 100). Similarly, the volumes of triploid pituitary cells and nuclei were larger than those of diploid fish. As for germ cells, the volume of diploid sperm head generated from allotetraploids of red crucian carp (♀) × common carp (♂) was two times that of haploid sperm volume of common carp. The diameters of ovulated diploid mature eggs [(0.17 ± 0.01) cm] were also significantly larger than the diameters of haploid eggs generated from diploid red crucian carp [(0.13 ± 0.01) cm] (Liu et al. 2001).
During the breeding season, somatotropic hormone cells (STH cells) in diploids pituitary accounted for 25% of meso-adenohypophysis. STH cells accounted for 38% of meso-adenohypophysis in triploids, while STH cells accounted for 20% of meso-adenohypophysis in tetraploids. Compared with the diploids, STH cell number of triploids was 1.5 times that of the diploids (Long et al. 2006). Previous experiments proved that triploids grew faster with a high survival rate. Its growth rate was 21.78% and 70.83% faster than that of Japanese white crucian carp and Pengze crucian carp, respectively. However, allotetraploids of red crucian carp (♀) × common carp (♂) grew slowly compared with diploids and triploids. Therefore, the proportion of STH in pituitary may relate to the growth rate. The higher proportion of STH cells may lead to a fast growth rate. Comparative analysis of gonadotropic hormone cells (GTH cells) in different ploidy fishes showed that GTH cells of tetraploid fishes had the largest proportion of cells. This may be because the sexual maturity age of allotetraploids of red crucian carp (♀) × common carp (♂) was earlier than diploid crucian carp and diploid common carp.
During the breeding season, GTH cells in meso-adenohypophysis in tetraploids were similar to diploids. The GTH cells were full of a large amount of closely arranged secretory granules and globules. Since GTH had a close association with gonadal development, allotetraploid fish had normal development of testis and ovary and produced mature sperm and eggs which was similar to diploids. Both allotetraploid females and males were fertile. However, in GTH cells of triploid meso-adenohypophysis, the number of secretory granules and globules was far less compared to diploids and tetraploids which were distributed in the endoplasmic reticulum. It was speculated that the degeneration of secretory granules and globules in GTH cells or the increase of some inhibited factors that suppressed secretory granules led to the great difference between triploid fishes and other fishes including diploids and tetraploids. Besides, the structures of GTH cell indifferent ploidy fishes were significantly different after the breeding season. In diploid and tetraploid GTH cells, a large amount of vacuole-shaped structures could be observed. This was because the appearance, structure, and number of GTH cells would be changed with their reproduction activities, which meant vacuole structures were found due to a release of a large number of granules after egg ovulation. However, only a few vacuoles could be found in triploids and most of the secretory granules and globules were not released out. Therefore, it could be inferred that some factors suppressed the release of secretory globules and granules in triploid GTH cells.
4.2.1.3.1 The Comparison of the Pituitary Histochemistry Structure
Histological observation was conducted to the pituitaries of red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂). There was no significant difference in appearance. Their pituitaries were white in color, and round or chicken heart-shaped, which were located at the ventral of interbrain, connecting with the hypothalamus through pituitary stem. They were composed of two parts: neurohypophysis and adenohypophysis. Nerve fibers, capillaries, and glial cells constituted neurohypophysis. The extremely fine branches of nerve fibers were distributed in every part of the pituitary gland (adenohypophysis), gliocytes, and capillary network linked with each other closely. The pituitary gland consisted of pro-adenohypophysis, meso-adenohypophysis, and meta-adenohypophysis.
By histochemical staining, six types of endocrine cell in the pituitaries of the three kinds of fishes were with litter difference (Long et al. 2006).
4.2.1.3.2 The Volume Comparisons of Pituitary Cells and Nuclei
The same type of cells in pituitaries of three ploidy level fishes had obvious difference in cell size. The diameters of GTH and STH increased with their ploidy levels increasing (Long et al. 2006). Apart from STH and GTH, there were other four secretory cells and their nuclei diameters increased with the ploidy level being higher.
Generally, the size of the nucleus and the number of chromosomes increased proportionately. And in order to maintain a constant karyoplasmic ratio, cells would enlarge proportionately with the nuclei enlarging. Therefore, polyploid cells and nuclei were usually bigger than those of diploids. But organs and bodies of polyploids were not necessarily larger than diploids. It was not to say tetraploids were twice the size of the diploids of the same age. It was because the number of cells reduced while cells were enlarging, which maintained the sizes of the bodies or organs more or less. Allotetraploids of red crucian carp (♀) × common carp (♂) contained four sets of chromosomes (4n = 200), and their chromosome numbers or DNA contents were twice that of their original diploid parents (red crucian carp and common carp, 2n = 100). Thus, it might be due to the tetraploids having completed four sets of chromosomes with high genetic materials that the volumes of the pituitary secretion cells and nuclei were significantly larger than those of diploids. This might also explain why triploid pituitary cells and nuclei were larger than those of diploids.
4.2.1.3.3 The Analysis on Proportion of Acidophile and Basophil Cells
Acidophile and basophil cells took different proportions in meso-adenohypophysis of different ploidy fishes. During the breeding season, basophils (e.g., GTH cells) were obviously more than eosinophils (e.g., STH cells) in meso-adenohypophysis of three kinds of fishes. This was because STH cells and GTH cells took different proportions with the change of reproductive season. In breeding season, GTH cells increased and enlarged, coupled with their increased secretory granules and decreased STH cell. Diploid STH cells accounted for 25.0% of the meso-adenohypophysis and triploid STH cells for 38.0%, but the tetraploid STH cells accounted for 20.0%. Compared with the diploids, the amount of triploid STH cells was 1.5 times. Feeding experiments have shown that triploids grew fast and had a high rate of survival. However, allotetraploids grew more slowly than diploids and triploids. Therefore, the authors supposed that the growth rate might be affected by the proportion of STH cells in pituitary. In addition, through a comparative analysis on GTH cells of different ploidy fishes, we found GTH cells accounted for the largest proportion in allotetraploid fish. The age of sexual maturity for allotetraploids of red crucian carp (♀) × common carp (♂) was earlier than the diploid crucian carp and diploid common carp which might be related to the larger proportion of GTH cells and strong secretive ability in allotetraploid fish.
4.2.1.4 The Ultrastructure Comparison of Pituitary Glands
GTH cells and STH cells in different ploidy fishes had not much difference in appearance. The ultrastructural features of STH cells and GTH cells in meso-adenohypophysis of three fishes were shown in Table 4.1.
As observed from the GTH cell ultrastructure of allotetraploids of red crucian carp (♀) × common carp (♂) meso-adenohypophysis, we found it developed well in breeding season like diploid red crucian carp. A large number of secretory granules and globules were full of the cells and arranged tightly. Because GTH cells had a close relationship with gonadal development, allotetraploids of red crucian carp (♀) × common carp (♂) could produce mature spermatozoa and eggs normally just like diploid red crucian carp. Previous studies showed that the morphology, structure, and number of GTH cells changed coupled with the change of reproductive activities. For example, vacuoles appeared in degenerate GTH cells after spawning because a large number of granules were released from cells (Liu 1993). We also found that, after breeding season, a large amount of vacuoles occurred in GTH cells of diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂), which indicated that they can conduct normal internal secretion activities and promote the gonadal development.
During breeding season, secretory granules and globules disseminated in the GTH cell endoplasmic reticulum of triploid Xiangyun crucian carp were far less than in diploids and allotetraploids of red crucian carp (♀) × common carp (♂) of the same period. Presumably, this might be associated with the stunted gonadal development of triploid Xiangyun crucian carp. Degeneration of secretory granules and globules in GTH cells or the inhibition of the increase of secretory granules may result in the difference among triploids, diploids, and allotetraploids of red crucian carp (♀) × common carp (♂). In addition, after the breeding season, only a small number of vacuoles could be observed in the triploid Xiangyun crucian carp GTH cells. Since something inhibited the release of secretory granules and globules, most of them would not discharge. Considering the correlation of the development of GTH cells and gonad, we could conclude that discharge inhibition of secretory granules and globules in or after the breeding season was related with the stunted gonad of sterile triploids (Long et al. 2006).
4.2.1.5 The Comparative Analysis of HPG Axis Related Genes
4.2.1.5.1 Research on GtHβ
We analyzed the localization of Lhβ and Fshβ in pituitaries of different ploidy fish during the breeding season by using in situ hybridization (Long et al. 2009a). The locations of the two genes in triploid Xiangyun crucian carp were similar to those in tetraploids. Fshβ was found in the center of pituitary, namely, meso-adenohypophysis, but not in the pro-adenohypophysis and meta-adenohypophysis. Lhβ gene mainly existed in the meso-adenohypophysis.
4.2.1.5.2 The Comparative Analysis of Gonadotropin Genes
Differential gene expression analysis of GtHβ genes was carried out in red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂). The results of real-time PCR showed that both Fshβ and Lhβ genes were only expressed in the pituitaries but not in other tissues (Long et al. 2009a). By in situ hybridization, we found that Fshβ and Lhβ genes were all expressed in the meso-adenohypophysis (the center of pituitary) among different ploidy fishes. This was consistent with the fact that GTH cells produced gonadotropin and GTH cells of different ploidy fishes were mainly located at the meso-adenohypophysis. The above results indicated that these two genes were all able to promote the maturation of gametes and gonads.
Real-time PCR analysis was conducted in pituitaries of different ploidy fishes. We found that Fshβ and Lhβ genes showed great differential expression before and after the breeding season. In nonbreeding season, the expressions of Fshβ genes in allotetraploids of red crucian carp (♀) × common carp (♂) were slightly lower than in the diploids. It was contrary to this during breeding season. But the Fshβ gene was highly expressed in the pituitary of the triploid Xiangyun crucian carp before and after the breeding season. The Lhβ gene had similar expression patterns in these three fishes (Fig. 4.3a–d).
The results of real-time PCR showed the expression of Fshβ and Lhβ in the pituitaries of different ploidy fishes (Long et al. 2009a) (partially quoted). (a) In nonbreeding season, expressions of Fshβ genes in the pituitaries of different ploidy fishes. (b) In breeding season, expressions of Fshβ genes in the pituitaries of different ploidy fishes. (c) In nonbreeding season, expressions of Lhβ genes in the pituitaries of different ploidy fishes. (d) In breeding season, expressions of Lhβ genes in the pituitaries of different ploidy fishes. 2nP, 3nP, and 4nP represented pituitaries of diploid red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂) in nonbreeding season, respectively. 2nP-s, 3nP-s, and 4nP-s represented pituitaries of diploid red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂) in breeding season, respectively. Note: On basis of diploid pituitaries, the original content of Fshβ and Lhβ mRNA in diploid pituitaries was 1.
In diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂), with the gonadal development its Fshβ and Lhβ mRNA levels increased and decreased when gonads were degenerated. This was quite unlike salmonids which were of continuity. Such a phenomenon also happened in goldfish and common carp. It was supposed that this maybe due to the complete synchronism of ovary maturation of salmon fishes. They only produced one egg in the spawning season, while common carps were not completely synchronized, the ovaries of which contained a number of oocytes at different stages.
It was noteworthy that regardless of before or after the breeding season, the expressions of Fshβ and Lhβ genes in triploid Xiangyun crucian carp were higher than in diploid and tetraploid fishes. Combined with the previous ultrastructure of pituitaries, we could consume that after the breeding season the secretory granules and globules in GTH cells of triploid Xiangyun crucian carp could not be discharged normally like diploids and tetraploids. And gonadotropins of Fshβ and Lhβ mRNA at the molecular level after breeding season could not decrease normally which resulted in a higher expression than that in diploids and tetraploids.
The pituitary structure of triploid Xiangyun crucian carp was different from diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂), which was related to the fast growth and sterility characteristics of the triploid Xiangyun crucian carp. In addition, a comparative study to gene structure and expression characteristics of GtHβ also explained the fertility of tetraploid fish and sterility of triploid fish (Long et al. 2009a; Long et al. 2009b).
In situ hybridization was used to make a research on the localization of Gnrh2 genes in the brains of different ploidy fishes. Localization of Gnrh2 gene in allotetraploids of red crucian carp (♀) × common carp (♂) was shown in Fig. 4.4a–d. The results showed that Gnhr2 genes displayed similar localization in diploid red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂), mainly in the posterior of midbrain or between the midbrain and epencephalon.
The localization observation of Gnrh2 genes in the midbrain of allotetraploids of red crucian carp (♀) × common carp (♂) (in situ hybridization). (a) The hematoxylin-eosin (HE) staining observation of sagittal section of allotetraploids of red crucian carp (♀) × common carp (♂) brain tissue. TEL: telencephalon. MB: midbrain. CE: epencephalon. MO: medulla oblongata. (b) Gnhr2 gene expression in allotetraploids of red crucian carp (♀) × common carp (♂) by antisense RNA probe. Arrows indicated positive signals of Gnhr2 mRNA localized in the posterior of midbrain or between midbrain and epencephalon. (c) Allotetraploids of red crucian carp (♀) × common carp (♂) brain tissue in situ hybridization by sense RNA probe. No positive signal was found in it. (d) Detection of Gnhr2 gene expression in the brain of allotetraploids of red crucian carp (♀) × common carp (♂) by antisense probe. Arrows indicated positive signals of Gnhr2 mRNA localized in the posterior of midbrain. Bar is 1000 μm in (a, b, and c) and 500 μm in (d)
In addition, we also analyzed the localization of Fshr and Lhr genes in ovaries and testes of different ploidy fishes during the breeding season by using in situ hybridization (Long et al. 2009a).
The results of in situ hybridization revealed that Fshr mRNA was localized in the follicle cells, granule cells, and outer space of radiation membrane of ovaries in diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂) while occurring in the sustentacular and interstitial cells of testes. The localization of Lhr gene in gonads of diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂) was similar to that of Fshr genes. However, the localizations of Fshr and Lhr genes in triploid Xiangyun crucian carp were quite different from diploids and tetraploids. There were only a few stage III oocytes in triploid ovaries. And the positive signals of Fshr and Lhr genes only could be observed in the follicle cells, granule cells, and outer space of radiation membrane of stage III oocytes. These signals were weaker than in diploids and tetraploids. But in triploid testes, no positive signal was observed in the sustentacular cells and interstitial cells.
4.2.1.6 Molecular Features of Sterile Allotriploid Fish
Dmc1 (disrupted meiotic cDNA) was a specific gene in the meiotic prophase I. The Dmc1-encoded protein is the necessary part for homologous chromosome pairing in meiosis. In the breeding season, Dmc1 genes were cloned from ovary and testis of diploid red crucian carp, triploid Xiangyun crucian carp, and allotetraploid red crucian carp (♀) × common carp (♂). The results showed that Dmc1 gene in the testes of different ploidy fishes was all expressed. Dmc1 gene of triploid Xiangyun crucian carp was highly expressed in the ovary, but only low expression of Dmc1 gene was found in diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂).
The results of real-time PCR showed that in the breeding season, Dmc1 gene had higher expression in the testis compared to that in the ovary at the same period. Among the different ploidy fishes, the highest expression of Dmc1 gene was found in the testis and ovary in triploid fish. Diploid fish had the medium expression of Dmc1 gene in the testis and ovary. Allotetraploid fish had the lowest expression of Dmc1 gene in the testis and ovary. During the nonbreeding season, higher expression of Dmc1 gene was found in the testis compared to the ovary in the same kind of fish. Among the three different ploidy fishes, the highest expression was found in allotriploid ovary, followed by tetraploid ovary. Diploid ovary had the lowest expression of Dmc1 gene. In the testis, expressions of the Dmc1 gene in the three fishes were similar. (Tao et al. 2008).
The results of real-time PCR showed that Dmc1 gene was expressed the most in the testis and ovary in triploid Xiangyun crucian carp among different ploidy fishes, followed by diploid red crucian carp, while the allotetraploids of red crucian carp (♀) × common carp (♂) had the lowest expression. Especially the expression of Dmc1 gene was higher in allotriploids than that in diploid and allotetraploid fish, significantly. According to the normal development, gonads of diploid and allotetraploid fish in the breeding season were generally well-developed. Most of the cells were no longer in primary oocytes and primary spermatocytes. But gonadal cells of triploid Xiangyun crucian carp could not reach the mature phase most especially in the ovary, which are usually undeveloped. Thus, it could be proposed that Dmc1 expression in different ploidy fishes had no relationship with ploidy levels but related to the development degree of gonads. Because triploid Xiangyun crucian carp was sterile, cells in meiotic prophase were the most expressed in the breeding season. According to previous studies, the sexual maturity of allotetraploids of red crucian carp (♀) × common carp (♂) was earlier than that of diploid red crucian carp and its sperm and eggs were formed earlier. Thus, allotetraploids of red crucian carp (♀) × common carp (♂) had the lowest number of cells in meiotic prophase I. Combined with the results of the real-time PCR, these pieces of evidence suggested that Dmc1 genes in different ploidy fishes were specifically expressed in meiotic prophase I which was similar to those in other species. Meanwhile, Dmc1 gene was overexpressed in the triploid Xiangyun crucian carp which was significantly higher than that in diploid and allotetraploid fishes. This may relate to the disorder of homologous chromosome pairing and sterility in allotriploid fish (Tao et al. 2008).
In addition, fish gonadal development and gamete maturation were mainly controlled by the hypothalamic-pituitary-gonadal axis (HPG axis) which is the crucial axis in regulation by nervous and endocrine systems. Brain-derived gonadotropin releases hormone (GnRH) to stimulate the pituitary to secrete gonadotropins (GTH) which combines with specific gonadotropin receptors (GtHR) in gonads to promote the secretion of sex steroids and induces gonadal maturation. Comparison of Gnrh, Gth, and Gthr gene expression would be helpful for discussing the sterile mechanism of triploid Xiangyun crucian carp.
By real-time PCR and Western blot, the Gnrh2 gene expressions in brains from diploid red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂) were analyzed from mRNA and protein levels. From Fig. 4.5a, b and Fig. 4.6a, b, during the nonbreeding season, the mRNA and protein expressions of Gnrh2 were the highest in the brain from triploid Xiangyun crucian carp, followed by diploid red crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂) had the lowest expression. During the breeding season, mRNA and protein expression of Gnrh2 had the highest expression in brains from allotetraploids of red crucian carp (♀) × common carp (♂), followed by diploid red crucian carp, and the lowest expression was found in triploid Xiangyun crucian carp. Thus, compared to diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂), the expression of Gnrh2 in triploid Xiangyun crucian carp was lowest, which may lead to the unnormal release of secretory granules, and finally contribute to dysfunctional gonadal development and even sterility. However, during the nonbreeding season, high expression of Gnrh2 in triploid Xiangyun crucian carp was found which may be due to the fact that the gene could not be downregulated normally by some unknown factors (Long et al. 2009b; Wang 2013).
The results of real-time PCR showed the gene expression of Gnrh2 in brains of different ploidy fishes (partially quoted from reference) (Long et al. 2009a). (a) During the nonbreeding season, the expression profiles of Gnrh2 in the brain of different ploidy fishes. (b) During the breeding season, the expression profiles of Gnrh2 in the brain of different ploidy fishes. In (a) and (b), 1 indicated the midbrain of diploid red crucian carp, 2 indicated the midbrain of triploid Xiangyun crucian carp, and 3 indicated the midbrain of allotetraploids of red crucian carp (♀) × common carp (♂) (the midbrain of diploid fish was the calibrator sample that had a relative quantification value of 1)
The Western blot result of GnRH2 protein in brains of different ploidy fishes. (a) During the nonbreeding season, expression of GnRH2 protein in brains of different ploidy fishes. (b) During the breeding season, expression of GnRH2 protein in brains of different ploidy fishes. In (a) and (b), 1 indicated diploid midbrain, 2 indicated the midbrain of triploid Xiangyun crucian carp, and 3 indicated the midbrain of allotetraploids of red crucian carp (♀) × common carp (♂)
In bony fishes, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) have similar structures with FSH and LH in mammals, respectively. Both FSH and LH are composed of α subunit and β subunit. The two gonadotropins (GTHs) share the same α subunits, while they have different β subunits. By real-time PCR, the expressive differences of Fshβ and Lhβ of the breeding and nonbreeding season in the pituitaries of different ploidy fishes were analyzed. Fshβ of allotetraploids of red crucian carp (♀) × common carp (♂) had lower expression than that of diploids during the nonbreeding season but a higher expression than that of diploids during the breeding season. However, Fshβ expression of the pituitary in triploid Xiangyun crucian carp was the highest during the nonbreeding and breeding season. The Lhβ expressions in the three different ploidy fishes were similar with Fshβ expression (Long et al. 2009a; Long et al. 2009b).
It was noteworthy that regardless of nonbreeding or breeding season, the expressions of Fshβ and Lhβ in pituitary from triploid Xiangyun crucian carp were higher than those of diploid and tetraploid fishes. From the observation of pituitary ultrastructure, it could be found that after breeding season, the secretory granules and globules in GTH cells from triploid Xiangyun crucian carp could not be normally expelled like diploids and tetraploids (Long et al. 2009a). Fshβ and Lhβ mRNA content of triploid Xiangyun crucian carp after breeding season could not reduce normally which was consistent with its morphological traits.
Through in situ hybridization, the location of Fshr and Lhr in the ovaries and testes of different ploidy fishes in breeding season was analyzed. Fshr was located in follicle cells, granulosa cells, and outside layer of zona radiata in ovaries. While Fshr was distributed in Sertoli cells and Leydig cells in the testes from diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂) oocytes. The Lhr distributions in diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂) were similar to the distribution of Fshr. However, the location of Fshr and Lhr in the gonad of triploid Xiangyun crucian carp was different from that in diploid and tetraploid fishes. There were only a few oocytes in stage III in the ovary from triploid Xiangyun crucian carp. Positive hybridization signals of Fshr and Lhr could only be found in follicular cells, granulosa cells, and outside layer of zona radiata in oocytes in stage III. And these signals were weaker in triploid fish than those in diploids and tetraploids. In testicular lobules from triploid Xiangyun crucian carp, positive signal could not be observed in Sertoli cells and Leydig cells as that in the testis in diploid and tetraploid fish.
Vasa is a member of the DEAD-box family which encodes an ATP-dependent RNA helicase and plays an important role in processes of germ cell proliferation and differentiation (Linder et al. 1989). The research on vasa gene in red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂) would provide data to explore the molecular mechanism of sterile triploid fish. By real-time PCR, the expression of vasa in different tissues from triploid Xiangyun crucian carp was carried out. The result showed that vasa was specifically expressed only in the testis and ovary (Yu et al. 2015). While no expression signal was detected in the pituitary, heart, brain, spleen, liver, kidney, gills and muscle, which indicated vasa played an important role in the process of gonadal development of triploid fish (Liu 2012a).
In addition, real-time PCR was used to make a comparative study on vasa expression levels in gonads from diploid common carp, diploid red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂) (Liu 2012a). The result indicated that differences were found by comparing the sterile triploid fish and fertile fishes including common carp, red crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂). Vasa gene were expressed in testes from common carp, red crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂), and the expression level in the nonbreeding season was higher than that in the breeding season. Interestingly, comparing the breeding and nonbreeding seasons, there was no significant difference in testicular expression of vasa gene in triploid Xiangyun crucian carp, while triploid Xiangyun crucian carp had significantly lower expression compared to common carp, red crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂) in testes (Fig. 4.7a). Vasa was expressed in ovaries from common carp, red crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂), and the expression levels in the nonbreeding season were all higher than that in the breeding season. However, in the ovary from triploid Xiangyun crucian carp, the vasa gene had higher expression during the breeding season than that during the nonbreeding season (Fig. 4.7b).
The results of real-time PCR of vasa expression in the gonad of different ploidy fishes during breeding and nonbreeding season. (a) Vasa gene expression in testes in different ploidy fishes. (b) Vasa gene expression in the ovary in different ploidy fishes. Note: Different letters showed significant differences among groups (P < 0.05)
Previous study (Liu et al. 2000) showed that during the breeding season, the testis of triploid fish was full of immature spermatids but no mature sperm was found. Meanwhile, it also showed that the transcript of vasa gene in sperm from oligozoospermia patients was 1/5 of those of vasa gene in normal sperm (Xu et al. 2005) Therefore, it could be speculated that the sterility of male triploid fish had a certain relevance with the low expression of vasa gene. In addition, the previous study showed that the ovary of triploid Xiangyun crucian carp could not develop normally (Liu et al. 2000). During the breeding season, there were a large number of cystically arranged oogonia with small volumes and a few primary oocytes in the ovary of triploid Xiangyun crucian carp. The oocytes were mainly stagnated in stage I and stage II, while only a few oocytes in stage III and stage IV could be found. In diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂), the oocytes were mainly in stage I, stage II, and stage IV during the breeding season. During the nonbreeding season, oocytes of triploid crucian carp were mainly in stage I, while in red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂), oocytes were mainly in stage I and stage II. Another study in catfish, Clarias gariepinus, showed that the vasa gene was expressed mainly in stages I and II with the highest expression in stage II (Raghuveer and Senthilkumaran 2010). The abnormal increase in vasa expression in ovary of triploid fish was associated with the main components of stage I and stage II oocytes. Compared to fertile red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂), the vasa gene expression in triploid Xiangyun carp was significantly abnormal with the reproductive cycle. Thus, the abnormal expression of vasa gene was related to the sterility of female triploid fish.
By using real-time PCR and Western blot, the expression of Piwi in different ploidy fishes like diploid red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂) was investigated (Zhou et al. 2012; Zhou et al. 2014). PIWI-interacting RNAs (piRNAs) as a new class of non-coding RNA were only found to be expressed in gonad specifically. By combining piRNA with Piwi proteins, they regulated gene expression in transcriptional and protein translation levels. Currently, the study on piRNA was only limited to a small number of animals (Burgess 2013). Similar to zebrafish, Piwi families contained two genes including Piwil-1 and Piwil-2 in common carp and crucian carp. The expression patterns of Piwil-1 and Piwil-2 in common carp and crucian carp were similar to those of Piwil-1 and Piwil-2 in zebrafish (Houwing et al. 2007; Zhou et al. 2012). The tissue expression of Piwi study showed Piwi was specifically expressed only in the testis and ovary from different ploidy fishes (Zhou et al. 2014). In addition, the Piwi expression in the testis of triploid Xiangyun crucian carp was lower than that in diploid red crucian carp both in the breeding season and nonbreeding season, while allotetraploids of red crucian carp (♀) × common carp (♂) had lower expression of Piwi only during the nonbreeding season. In ovaries, higher expression of Piwi in triploid Xiangyun crucian carp was observed compared to diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂) either during breeding season or nonbreeding season (Zhou et al. 2014).
Except for the abnormal expression of Piwi, piRNA expression in female triploid Xiangyun crucian carp also significantly increased. By cloning small RNA sequences in ovaries, 61, 72, and 66 piRNAs were obtained from diploid red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂), respectively.
Seven piRNA sequences were identified in the three fishes simultaneously. Interestingly, although only 12 identified piRNA could map to the transcribed mRNA sequence, the located gene functions were varied. For example, ikbkg plays an important role in immune function. Zp2.5 is associated with the egg development. Tll10 is a gene that encodes a tubulin tyrosine ligase. Ttnb encodes the protein which is a component of muscle fiber. Zbtb48 encodes the protein which is a transcription factor and belongs to zinc finger protein. These genes have wide functions in some biological activities. Among them, zp2.5 is directly involved in the final maturation of egg development. After studying on the ovaries of three fishes during the breeding season by real-time PCR, five of the seven piRNAs in triploid Xiangyun crucian carp were significantly higher than that in diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂), while the other two piRNAs had no significant expression difference in the three fishes. These results demonstrated that the expression amount of piRNAs had a correlation with the Piwi expression. In addition, in vivo injection and in vitro culture proved that E2 suppressed the expression of piRNA (Zhou et al. 2014) .
A quantitative analysis of Lhr mRNA in ovaries from different ploidy fishes showed that Lhr expression in triploid Xiangyun crucian carp was significantly lower than in diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂) (Zhou et al. 2014), which was consistent with the result of in situ hybridization of Lhr previously reported (Long et al. 2009b).
These clues suggested that the abnormal increase of Piwi-piRNA signal pathway in sterile female triploid Xiangyun crucian carp was driven by the dysfunction of HPG axis, especially the abnormal expression of Lhr in the ovary. The HPG axis suppressed Piwi-piRNA signal pathway. During the breeding season, the relevant factors of the HPG axis were activated which inhibited the expression of piRNA. The inhibition of piRNA relieved the suppression of gonadal developed genes by piRNAs and ultimately led to the success of ovulation. In triploid Xiangyun crucian carp, the expression of HPG axis was dysfunctional and E2 could not normally be released, and therefore, HPG axis could not inhibit Piwi-piRNA signaling pathway normally. Even in the breeding season, piRNA and Piwi were both highly expressed in triploid fish which led to inhibition of transcriptional or genomic activities. Finally, oocytes could not be mature and successfully ovulated (Zhou et al. 2012; Zhou et al. 2014).
4.2.1.7 The Epigenetic Features of Sterile Allotriploid Fish
Histone post-modifications, including phosphorylation, acetylation, methylation, ubiquitination, SUMOylation, and crotonylation, are important epigenetic marks. Usually, they involve different biological processes via two main models, one is regulating gene expression by altering interactions between histones and DNA to influence the structure of chromatin (i.e., its compactness or looseness), and the other is recruiting different proteins (Strahl and David 2000; Tan et al. 2011; Xu et al. 2009). Till now, few studies have been reported on histone modifications involved in regulating meiotic impairment during oocyte early development. Comparative analysis was performed between the sterile female allotriploid fish and fertile female diploid red crucian carp (Zhou et al. 2019).
Twenty different post-modifications on various residues of histone H3 were detected by ELISA. Most histone H3 post-modifications levels were comparable. The levels of H3K4me3, H3K9me3, H3K79me, and H3K79me3 were higher in the ovaries of allotriploid fish than in those of red crucian carp (Zhou et al. 2019). In yeast, H3K4me3 occurs near double-strand break that initiate homologue recombination during the prophase stage of meiosis (Valérie et al. 2009). As a heterochromatin marker, H3K9me3 is enriched at unsynapsed trivalents during impaired SC formation (Naumova et al. 2013). In mice, during normal meiosis prophase I, histone H3K79me is uniformly present, while when meiosis is perturbed or arrested, its level increases (Ontoso et al. 2014). H3K79me3 increased from pachynema onward (Ontoso et al. 2014). Therefore, different histone modification detected above was consistent with the characteristics of pachytene arrest in allotriploid fish. However, to understand how these post-modifications regulate different gene expression in oocytes during developmental arrest in fish, more studies, such as chromatin immunoprecipitation (ChIP), are needed.
The previous study showed that a variety of reproduction-related genes (Dmc1, Gnrh2, Gth, Gthr, vasa, etc.) had abnormal expression in triploid Xiangyun crucian carp, Piwi and piRNA in triploid Xiangyun crucian carp were also abnormally expressed, and the histone modification state of ovary in triploid Xiangyun crucian carp changed. This was closely related to the sterile phenotype of triploid Xiangyun crucian carp. In terms of sterility, triploid Xiangyun crucian carp and other sterile triploid fishes were similar in organisms, cells, and tissues as well as molecular characteristics. As for individuals, the sterility of triploid Xiangyun crucian carp showed that it could not form and ovulate normal eggs and sperm resulting in no progenies. As for cell and tissue levels, the sterility of triploid Xiangyun crucian carp was represented as dysfunctional pairing of homologous chromosomes and could not form gametes with reproductive function. As for gonads, the ovary, testis, and fat-like gonad were all abortive. As for pituitary, during the breeding season, the sterility showed that hormonal granules in pituitary could not be released and represented as characteristics of endocrine disorders. As for the molecular level, several genes such as Dmc1, Gnrh2, Gth, Gthr, vasa, Piwi, etc., had abnormal expression in triploid Xiangyun crucian carp. As for the epigenetic state, some histone modifications, such as H3K4me3, H3K9me3, H3K79me, and H3K79me3, had changed in the ovary of triploid Xiangyun crucian carp. Therefore, the sterility mechanism of sterile triploid fish was associated with its abnormality in genetic (chromosome), epigenetic (histone modification), endocrine (HPG axis), and reproduction (gonadal development).
4.2.2 The Rapid Growth Mechanism of Triploid Xiangyun Crucian Carp and Triploid Xiangyun Carp
Since the dysfunction of the sterile gonad in triploid Xiangyun crucian carp, the energy used in gonadal development might be transferred to the energy for growth which led to fast growth. Several specificities of triploid Xiangyun crucian carp may relate to their sterility and fast growth.
Growth and development of fish were controlled by the growth hormone/insulin-like growth factor axis. Growth hormone (GH) secreted by pituitary cells was combined with tissue-specific growth hormone receptor (GHR) to activate a series of biological cascades which induced transcription of downstream target genes. Insulin-like growth factor 1 (IGF-1) in the liver could stimulate tissue growth, especially muscle.
GH promotes growth, gonadal development, energy metabolism, osmoregulation, social behavior, and immunity as well as other physiological activities that were mediated by IGF-1 in the liver. Through a comparative study of Gh, Ghr, and Igf-1 expression, the endocrine mechanism of fast growth in triploid Xiangyun crucian carp was investigated.
The tissue expression of Gh, Ghr, and Igf-1 in triploid Xiangyun crucian carp was analyzed by real-time PCR. Gh was only specifically expressed in the pituitary, not in other tissues. Ghr and Igf-1 had the same expression patterns expressed in all tested tissues with the highest expression in the liver. The liver was the major secretion tissue for IGF-1. IGF-1 was produced by biological cascade from the combination of GH and GHR; thus, the GHR content in the liver was higher in the liver compared with other tissues. In other tissues, IGF-1 primarily acted as an autocrine and paracrine hormone (Zhong et al. 2012).
The results of real-time PCR revealed the different expression levels of Gh in the pituitary, Ghr, and Igf-1 in the liver as well as Igf-1 in muscle in different ploidy fishes. Before and after breeding season, the highest expression of Gh in the pituitary was found in triploid Xiangyun crucian carp, while the lowest expression was observed in allotetraploids of red crucian carp (♀) × common carp (♂). As for the Ghr and Igf-1 in the liver, triploid Xiangyun crucian carp had the highest expression, following medium expression in diploid red crucian carp, and the lowest in allotetraploids of red crucian carp (♀) × common carp (♂). Similar results were found in Igf-1 from muscle. The highest expression of Igf-1 in muscle was observed in triploid Xiangyun crucian carp, while the lowest expression was shown in allotetraploids of red crucian carp (♀) × common carp (♂) (Zhong et al. 2012).
The result of in situ hybridization demonstrated that Igf-1 was widely expressed in the liver; however, stronger signals were found in triploid fish compared to the other two fishes (diploids and allotetraploids) (Fig. 4.8a–f). The clues above proved that Gh, Ghr, and Igf-1 had a higher expression in triploid Xiangyun crucian carp, which may be related to the pituitary structure differences of triploid Xiangyun crucian carp (Zhong et al. 2012).
The distribution of Igf-1 expression in livers from different ploidy fishes. (a) Detection of Igf-1 expression using probes of antisense RNA in the liver from diploid red crucian carp liver. (b) Detection of Igf-1 expression using probes of antisense RNA in the liver from triploid Xiangyun crucian carp. (c) Detection of Igf-1 expression using probes of antisense RNA in the liver from allotetraploids of red crucian carp (♀) × common carp (♂). (d) Control of Igf-1 expression using probes of sense RNA in the liver from diploid red crucian carp liver. (e) Control of Igf-1 expression using probes of sense RNA in the liver from triploid Xiangyun crucian carp. (f) Control of Igf-1 expression using probes of sense RNA in the liver from allotetraploids of red crucian carp (♀) × common carp (♂). Bar = 20 μm
The results of real-time PCR and tissue in situ hybridization showed that the expressions of Gh in pituitary, Ghr in liver, and Igf-1 in the liver and muscle were the highest in triploid Xiangyun crucian carp, followed by diploid red crucian carp, and the lowest expressions of these genes were found in allotetraploids of red crucian carp (♀) × common carp (♂). Especially the expressions of Gh in the pituitary and Igf-1 in the liver in triploid Xiangyun crucian carp were significantly higher than that in other two fishes (diploids and allotetraploids). The somatotrophs (STH) cells (STH cells are the key cells which secrete GH) had different proportion in the pituitary in experimental fish which were determined by analyses of pituitary structures in diploid red crucian carp, triploid Xiangyun crucian carp, and allotetraploids of red crucian carp (♀) × common carp (♂). During the breeding season, STH cells accounted for 25.0%, 38.0%, and 20.0% in diploids, triploids, and tetraploids, respectively. Compared with the diploid fish, the number of STH cells in triploid fish was 1.5 times that of diploid fish. Previous shreds of evidence showed that the growth rate of triploid Xiangyun crucian carp was 21.8% and 70.8% faster than that of Japanese white crucian carp and Pengze crucian carp, respectively. While allotetraploids of red crucian carp (♀) × common carp (♂) grew slower than diploid red crucian carp and triploid Xiangyun crucian carp which demonstrated that the proportion of STH cells in the pituitary may relate to the growth rate. The growth hormone was considered to be secreted by STH cells in the pituitary. In addition, the expression of GH in triploid Xiangyun crucian carp was much higher than that of diploid red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂), which may due to the different proportion of pituitary in different ploidy fishes. Presumably, more STH cells may secrete excessive GH, resulting in rise of GH concentration in the pituitary. To bind the excessive GH, the liver tissue secreted more GHR, thereby promoting excessive secretion of downstream factor IGF-1 in cascade reaction. With IGF-1 being released into the blood, the IGF-1 concentration was increased in muscle which promoted the growth of muscle and led to a faster growth rate of triploid Xiangyun crucian carp.
In addition, from the perspective of nutriology, the rapid growth characteristic of triploid Xiangyun crucian carp could also be explained. Glutamate dehydrogenase (GDH) was one of the important joint points connecting amino acid metabolism and glucose metabolism and played an important role in amino acid metabolism. The research showed that during breeding and nonbreeding seasons, Gdh gene mRNA expression in the liver of triploid Xiangyun crucian carp was higher than in red crucian carp, indicating triploid Xiangyun crucian carp had a higher metabolic efficiency for amino acids compared to red crucian carp with amino acid metabolism advantage (Liu et al. 2012; Liu 2012b).
4.3 The Application of Triploid Xiangyun Crucian Carp and Triploid Xiangyun Carp
Allotriploid hybrids not only have advantages including faster growth and stronger disease resistance but also exhibit sterility which is important for controlling the excessive multiplication of farmed fish and protecting natural germplasm resources. After more than 20 years of research and application, the allotriploid fish such as triploid Xiangyun crucian carp and triploid Xiangyun carp have advantages such as fast growth, good meat quality, high edible rate, strong disease resistance, and infertility. After popularization in China, the triploid Xiangyun crucian carp and triploid Xiangyun carp are popular among most farmers and consumers which produce significant economic and social benefits.
4.3.1 The Characteristics of Triploid Xiangyun Crucian Carp and Triploid Xiangyun Carp
4.3.1.1 Fast Growth Rate
Cultivation experiments showed that the growth rate of triploid Xiangyun crucian carp was 3–5 times faster than that of the local crucian carp. Compared to Japanese white crucian carp and Pengze crucian carp, the growth rate of triploid Xiangyun crucian carp was 21.8% and 70.8% faster than that of those, respectively. The farming experiment performed by Beijing Aquatic Product Technology Promotion Department showed that the growth rate of triploid Xiangyun crucian carp was 1.5 times faster than that of normal crucian carp. The cultivation test of Daxian County in Sichuan Province demonstrated that the growth rate of triploid Xiangyun crucian carp was 3 times faster than that of normal crucian carp. The triploid Xiangyun crucian carp fry (the fry had been cultivated in summer which was born in spring) grew to 300–400 g in regions south of the Yangtze River and 200–250 g in regions north of the Yangtze River (e.g., Beijing City and Liaoning Province) at the end of the same year, while juveniles (the fish had been cultivated in winter which was born in spring) attained 400–450 g when they become marketable fish. The maximum individual of triploid Xiangyun crucian carp was 2700 g. The polyculture generally yielded 150–200 kg per mu (666.7 m2). If triploid Xiangyun crucian carp served as main farmed fish, the yield was 600–1200 kg per mu.
The growth rate of triploid Xiangyun carp was 30.0% to 40.0% faster than that of common carp. Comparative cultivation in the Qionghu Lake Fishery of Yuanjiang City in Hunan Province showed that the growth rate of triploid Xiangyun carp cultured in the cage was 45.8% faster than that of common carp. Cultivation in different ponds showed that the growth rate of triploid Xiangyun carp was 29.4% faster than that of common carp. The cultivation test of Daxian County in Sichuan Province showed that the growth rate of triploid Xiangyun carp was 40.0% faster than that of common carp. The triploid Xiangyun carp fry (the fry had been cultivated in summer which was born in spring) generally grew to 500–600 g at the end of the same year, while juveniles (the fish had been cultivated in winter which was born in spring) attained 600–750 g when they became marketable fish. The polyculture yielded 150–350 kg per mu. According to market requirements in Hunan Province, farmers would control the size of triploid Xiangyun carp within 600–700 g to improve yield and benefits by increasing breeding density.
4.3.1.2 Sterility
Triploid Xiangyun crucian carp and triploid Xiangyun carp are allotriploid fish with chromosome sets as 3n = 150. Their gonads are sterile, which could not produce mature sperm and eggs and also could not reproduce itself. This characteristic makes triploid Xiangyun crucian carp and triploid Xiangyun carp unable to mate with other common carp or crucian carp. Therefore, they would not cause differentiation and degeneration of quality. Meanwhile, they could not interfere with the germplasm resources of common carp, crucian carp, and other fish in natural waters.
4.3.1.3 Strong Feeding Ability
The triploid Xiangyun crucian carp has an omnivorous and plankton feeding habit. The feed coefficient is 1.3–1.4 when fed with pellet feed containing 30.0% to 37.0% proteins which contributes to the low farming cost. This is associated with the sterility of triploid Xiangyun crucian carp. Due to its sterility, the nutrition intake transfers to the energy for growth in maximum and decreases the energy for gonadal development. Although triploid Xiangyun carp is omnivorous, the ability to filter plankton is not obvious.
4.3.1.4 Strong Disease Resistance and High Survival Rate
The survival rates of triploid Xiangyun crucian carp and triploid Xiangyun carp fry (the fry had been cultivated in summer which was born in spring) are over 90.0%. The survival rates of juveniles (the fish had been cultivated in winter which was born in spring) are over 95.0%. When silver carp, bighead carp, grass carp, and crucian carp cultured in the same pond died due to hypoxia, few triploid Xiangyun crucian carp would die simultaneously.
4.3.1.5 The Beautiful Shape and High-Quality Meat
The edible parts of triploid Xiangyun crucian carp and triploid Xiangyun carp are 10.0% to 15.0% more than that of the general crucian carp and common carp and have few intercostal spinules. The nutritive values of fish mainly depend on protein and fat contents in muscle. In addition, the evaluation of protein quality depends on the amino acid composition and essential amino acid (EAA) content for humans. By comparison with other fish, triploid Xiangyun crucian carp and triploid Xiangyun carp are newly economical fish which contain low water content, low ash content and high nutritional value.
4.3.2 The Promotion and Application of Triploid Xiangyun Crucian Carp and Triploid Xiangyun Carp
Years of aquaculture prove that sterile triploid Xiangyun crucian carp and triploid Xiangyun carp have advantages such as fast growth, nice meat, high edible rate, strong disease resistance, low temperature resistance, and hypoxia resistance. They are also easy to catch and fish. Therefore, they can be cultivated in ponds, lakes, reservoirs, rice fields, net cages, and intensive and industrial freshwaters. They have been promoted nationwide and produced significant social and economic benefits.
4.4 The Triploid Fish Derived from Hybridization of Allotetraploid Fish [Red Crucian Carp (♀) × Common Carp (♂)] with Other Diploid Fish
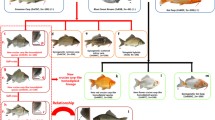
Goldfish (C. auratus), red crucian carp, and Japanese white crucian carp all belong to the genus Carassius, Cyprinidae family, with 100 somatic chromosomes. Their biological characteristics have similarities as well as differences. For example, they are similar in genetic and physiological characteristics, but their appearances are significantly different. The red goldfish and red crucian carp are all in red, but red goldfish have double bifurcated tails and higher dorsal. The body colour of Japanese white crucian carp is silver. The paternal parent allotetraploids of red crucian carp (♀) × common carp (♂) respectively match the three maternal parents, and then we obtain three kinds of triploid crucian carps which are similar in genetic and physiological characteristics. For example, they are all sterile allotriploid fish, but different in respect to appearance.
Two kinds of triploid crucian carps from the crossing of male allotetraploids of red crucian carp (♀) × common carp (♂) with female diploid goldfish and red crucian carp, respectively, will be introduced later. The authors make a systematical research on the appearance, growth rate, chromosome number, karyotype, gonadosomatic index (GSI), and gonadal structure of these two triploid crucian carps which further enrich the research about polyploidy fishes.
4.4.1 The Allotetraploid Fish [Red Crucian Carp (♀) × Common Carp (♂)](♂) × Goldfish (♀)
4.4.1.1 The Early Embryonic Development of Allotetraploid Fish [Red Crucian Carp (♀) × Common Carp (♂)](♂) × Goldfish (♀)
Triploid crucian carp was obtained after crossing of allotetraploid fish [red crucian carp (♀) × common carp (♂)](♂) with goldfish (♀). The early embryonic development of triploid crucian carp was observed. The mature eggs of goldfish were yellow, round, and sticky with egg diameters ranging from 1.3 to 1.4 mm. The cleavage began 60 min after fertilization, in which the water temperature was (20 ± 1)°C. And the cleavage time lasted 60–180 min. It gradually entered into the early, middle, and late blastula period after 3 h 30 min, 10 h later into the gastrula period, and 15 h 30 min later into the neurula period. The organs began to form after 19 h 30 min and 63–75 h after fertilization the fry hatched out. In addition, the section observation of embryos of triploid crucian carp showed that cell division in blastula period was vigorous, and cells in the gastrula period exhibited normal division, cytomorphosis, and migration. Combined with observation of the appearance development of triploid crucian carp embryos, it indicated that the triploid fish had a normal early embryonic development which provided an important biological basis for studying developmental biology of triploid fish and their application in production (Yi et al. 2006).
Zygote period: Mature eggs of goldfish were yellow, round, and sticky with egg diameters in 1.3–1.4 mm. They were smaller than the allotetraploid eggs [allotetraploid egg diameter (1.7 ± 0.1) mm]. The ooplasm was distributed evenly. Fertilized eggs expanded after absorbing water and became shining bright. The perivitelline space was amplified. The egg cytoplasm began to focus on the animal pole and gradually uplifted to form a blastoderm, 40 min after fertilization.
Cleavage period: The animal pole of cells began to crack and the blastoderm split into two similar blastomere lengthways and then it entered into the two-cell period approximately 60 min after fertilization. From 70 to 85 min, the blastoderm continued its second longitudinal crack along the vertical direction of the first division and this was the four-cell period. The blastoderm continued twice longitudinal cracks and entered into the eight-cell period in which eight blastomeres were arranged neatly into two rows after 110 min. The embryos were in the 16-cell period when blastomeres were arranged in a square shape in blastoderm about 110–144 min later. Embryos in multicellular period were found, and most of them were in the 64-cell period after 180 min later. The embryos entered into the multicellular period, and few were in the early blastula period after 3 h 45 min.
Blastula period: Cells continued to split with an increasing number, 4 h 30 min after fertilization. The size of cells also became correspondingly smaller. The embryos gradually entered into the morula period. When it came to 6 h, a small number of embryos died. In the 7 h 30 min, more than half of the embryos reached the late blastula period.
Gastrula period: Germ layer began to conduct epiboly approximately 10 h after fertilization. The embryos slowly entered into the gastrula period and the germ ring occurred. In the mid-gastrula period, embryonic shield continued to extend after 12 h 30 min.
Neurula period: Embryos entered into the early neurula period when yolk plug was not fully coated, 15 h 30 min after fertilization. In the 17 h 20 min, the yolk was completely coated, and yolk plug disappeared.
Organogenetic period: When it came to 19 h 30 min, embryos entered into the organogenetic period. And 20 h later, optic vesicle appeared. Sarcomere appeared after 21 h 30 min later. The brain was already differentiated into forebrain, midbrain, and afterbrain and the tail bud appeared after 22 h 30 min. In the 28 h, blastokinesis appeared and it moved into the muscle effector period. In the beginning the blastokinesis was with low frequency, but gradually it began to accelerate. Embryos curled and extended with the reduction of yolk 31 h later. After 34 h, the heart began to beat with an average frequency of 39 beats/min and it gradually increased to 70 beats/min later. Blood started to flow after 47 h 30 min. And 49 h 30 min later, all embryos began their blood circulation.
Hatching period: The embryo tail was so considerably twisted out of the egg membrane during 63–75 h after fertilization. However, the newly hatched juvenile fish had a lot of yolk which could only swim for a short time in water. Finally, 1–2 days after fertilization, juveniles developed into free-swimming larvae.
Histological observation of early embryonic development: Section results showed that 6 h after fertilization, the cells in blastula period underwent vigorous mitosis and cell proliferation was normal. Cells deformed and readied to migrate under the section observation in the early gastrula period about 11 h after fertilization. After 15 h later, some cells in the late gastrula period of embryos were still proliferated under the section observation and some cells already deformed and migrated. And the migration direction was very clear (Yi et al. 2006).
4.4.1.2 The Appearance and Ploidy of Allotetraploid Fish [Red Crucian Carp (♀) × Common Carp (♂)](♂) × Goldfish (♀)
In appearance, the triploid crucian carp was steel gray with a single tail. Liu et al. studied the morphological traits of this triploid crucian carp, allotetraploids of red crucian carp (♀) × common carp (♂), goldfish, diploid Japanese white crucian carp, and triploid Xiangyun crucian carp (Liu et al. 2004b). From the ratio of body depth and body length, the length and number of barbels, and the number of dorsal fins and lateral lineage scales, the appearance of triploid crucian carp was just between its parent allotetraploids of red crucian carp (♀) × common carp (♂) and goldfish which showed the characteristics of hybridization. And the ratio of body depth and body length, barbel length and number of triploid Xiangyun crucian carp also presented the same trait, while the ratio of body depth and body length of new triploid crucian carp was significantly higher than that of the triploid Xiangyun crucian carp, reflecting the body of triploid crucian carp was wider than the triploid Xiangyun crucian carp, and it enriched the triploid fish types. After detecting renal cell chromosomes, the authors found the new triploid crucian carp had 150 chromosomes with karyotype 33 m + 51sm + 33st + 33 t (Liu et al. 2004b).
4.4.1.3 The Growth and Fertility of Allotetraploid Fish [Red Crucian Carp (♀) × Common Carp (♂)] (♂) × Goldfish (♀)
The average quality of allotetraploid fish [red crucian carp (♀) × common carp (♂)] (♂) × goldfish (♀) at 1 year of age was 350 g, and the largest one reached up to 550 g. From December of that year to the second year in July, all triploid crucian carps would be checked monthly, but no sperm or eggs could be squeezed out. The sex ratio of male and female triploid crucian carp was 1:1 which was detected in breeding season. The average quality of females was about 387.3 g, while the average quality of males was 328.6 g. This showed that females grew faster than males at 1 year old.
The mean value of triploid crucian carp ovary index was 4.1, and the mean value of testis index was 3.5. It could be viewed with eyes about the surface of triploid crucian carp where only a few egg granules could be observed. But mature egg granules were spread on the surface of red crucian carp ovary. Triploid crucian carp testis was white. No semen flew out when the testis was cut with scissors. Histological section showed that most triploid crucian carp ovaries were occupied by small volume of underdeveloped oogonia which usually grouped together to form cystic structures. Among these small cells, there were some primary oocytes in which no mature eggs were observed. The triploid crucian carp testis was mainly made up of many small fine tubes, some of which contained no spermatids, but spermatids can be seen in some other tubes in irregular shapes. They were not clear and began to degrade and disintegrate. Mature sperm could not be observed.
The ovary, testis, and fat gonad index of triploid crucian carp were lower than the ovary and testis gonad index and the two mean values of Japanese white crucian carp, respectively, which indicated that this triploid crucian carp was similar to triploid Xiangyun crucian carp in the gonadal development and they were both suppressed (Liu et al. 2004b).
4.4.1.4 The Meat Quality of Allotetraploid Fish [Red Crucian Carp (♀) × Common Carp (♂)] (♂) × Goldfish (♀)
The analysis of nutrient and amino acids in the new triploid crucian carp muscle showed that the content of amino acids of dry sample was 102.4 mg/g and the content of essential amino acids (Thr, Val, Met, Ile, Leu, Phe, Lys, Trp) for humans was 45.2 mg/g, accounting for 44.2% of the total amino acid content. The content of two kinds of semi-essential amino acids (His, Arg) was 11.68 mg/g, accounting for 11.4% of the total amount. And the content of four kinds of flavor amino acids (Asp, Glu, Gly, Ala) was 31.6 mg/g, accounting for 30.8% of the total amount. By comparing the nutrient content in the muscle of new triploid crucian carp and its paternal parent allotetraploids of red crucian carp (♀) × common carp (♂), maternal parent goldfish, and other types of fish, the content of four kinds of flavor amino acids was found to be much higher than those in its parents. The content of essential amino acids for humans was also much higher than in triploid Xiangyun crucian carp (triploid Xiangyun carp) and other similar fish (Xiang et al. 2006).
4.4.2 The Allotetraploid Fish [Red Crucian Carp (♀) × Common Carp (♂)](♂) × Red Crucian Carp (♀)
A new triploid crucian carp was made by crossing between male allotetraploids of red crucian carp (♀) × common carp (♂) and female diploid red crucian carp. This triploid crucian carp was flat in its body side in the shape of fusiform. Its body color was steel gray with no barbels. Most of their measurable and accountable traits were between their parents and some of the measurable traits were beyond its parents which reflected the hybridization characteristics.
The analysis of karyotype showed the new triploid crucian carp chromosome number was 150 which was consistent with the chromosome number of triploid Xiangyun crucian carp.
The gonadal development of the new triploid crucian carp lagged behind common diploid fish and their germ cells showed degradation. Two types could be observed from the new triploid crucian carp gonads: (1) The gonad in the belt was located on both sides of the swim bladder. It was pale yellow, and petal-shaped leaflet occurred after fixation and it took on ovarian features. But most of the germ cells in the ovary were in the oogonia period, and in some cells there were vesicular structures and they began to degenerate. (2) Only two adipose tissues were in the gonad. Neither testis nor ovary was found (Shen et al. 2006). In this new triploid fish, no testis gonad was found which might result from the randomness of sampling.
Regarding genetic composition, this triploid crucian carp was obtained from fertilization of diploid sperm of allotetraploid fish [red crucian carp (♀) × common carp (♂)] and haploid eggs of red crucian carp. So they both had genomes from common carp and red crucian carp and red crucian carp genomes might account for the majority. Genetic heterozygosity led to some distinct hybridization characteristics in their appearances. For example, the ratios of body depth/body length, caudal peduncle depth/caudal peduncle length, and head height/body depth were between their parents. Because genetic genes of red crucian carp accounted for the most, many appearance features were close to the maternal parent, such as measurable traits like ratios of body length/overall length, head length/body length, and head height/head length. Therefore, their body size was closer to that of red crucian carp. In the new triploid crucian carp, no individuals with barbels were detected, which was a noteworthy and interesting phenomenon. The crossing of red crucian carp and allotetraploids of red crucian carp (♀) × common carp (♂) was similar to the process of backcross. The progenies only had genetic genes from common carp and red crucian carp and the red crucian carp took the advantages. Therefore, it had a large possibility to own the red crucian carp characteristics (without barbels). It could be concluded that species with a closer genetic relationship with red crucian carp had a large possibility to have no barbels. Compared with several hybrids, we could find that hybrids had no barbels when its cytoplasm and nucleus both contained red crucian carp genetic materials. The formation of barbels whether they had a relationship with the genetic materials in red crucian carp or not required further research. The formation of the triploid fish provided good material for studying the barbel development (Shen et al. 2006).
References
Burgess DJ (2013) Defining piRNA expression. Nat Rev Genet 14(5):301
Gui J, Liang S, Sun J, Huang W, Jiang Y (1990) Studies on genome manipulation in fish, I. induction of triploids transparent colored crucian carp (Carassius auratus transparent colored variety) by hydrostatic pressure. Acta Hydrobiol Sinica 14:336–344
He X (1999) Researches on tetraploid gene bank and chromosomes of triploid Xiangyunnesis crucian carp and common carp. Hunan Normal University, Changsha
Houwing S, Kamminga L, Berezikov E, Cronembold D, Girard A, Elst H, Filippov D, Blaser H, Raz E, Moens C (2007) A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell 129(1):69–82
Linder P, Lasko PF, Ashburner M, Leroy P, Nielsen PJ, Nishi K, Schnier J, Slonimski PP (1989) Birth of the D-E-A-D box. Nature 337(6203):121–122
Liu Y (1993) Propagation physiology of main cultivated fish in China. Agricultural Publishing House, Beijing, pp 22–23
Liu G (2012a) Vasa gene expression analysis in different polyploidy hybrids progeny of red crucian carp (♀) × common carp (♂). Hunan Normal University, Changsha
Liu Q, Wang Y, Liu S, Guo X, Luo K, Zhang C, Liu Y (2004a) A comparative study on blood and hemocyte of different polyploid crucian carp (♀) × common carp (♂). Prog Nat Sci 14(10):1111–1117
Liu S, Cao Y, He X, Li J, Liu Y (2001) The formation of tetraplpoid hybrids of common carp with red crucian carp and the evolutionary significance of teraploidzation in vertebrate. Eng Sci 3:33–41
Liu S, Feng H, Liu Y, Zhou G, Zhang X (1999) The measurement of DNA content of the tetraploids F3 - F4 hybrids of red crucian carp (♀) × common carp (♂) and their triploid offspring and other related diploid fish. J Nat Sci Hunan Norm Univ 22(4):61–68
Liu S, Hu F, Zhou G, Zhang X, He X, Feng H, Liu Y (2000) Gonadal structure of triploid crucian carp produced by crossing allotetraploid hybrids of Carassium auratus red var.(♀) × Cyprinus carpio (♂) with Japanese crucian carp (Carassius auratus cavieri). Acta Hydrobiol Sinica 24:301–306
Liu S, Sun Y, Li S, Feng H, Li J, Zhou G, Zhang X, Liu Y (2002) Analysis of gonadosomatic indexes of the triploid crucian carp. J Fish China 2:111–114
Liu S, Sun Y, Zhang C, Luo K, Liu Y (2004b) Triploid crucain carp-allotetraploid hybrids (♂) × goldfish (♀). Acta Genet Sin 31(1):31–38
Liu Z (2012b) Studies on cloning and expression of genes related to protein metabolism of different ploidy hybrids derived from red crucian carp (♀)× common carp (♂). Hunan Normal University, Changsha
Liu Z, Zhou Y, Liu S, Zhong H, Zhang C, Kang X, Liu Y (2012) Characterization and dietary regulation of glutamate dehydrogenase in different ploidy fishes. Amino Acids 43(6):2339–2348
Long Y, Liu S, Huang W, Zhang J, Sun Y, Zhang C, Song C, Liu J, Liu Y (2006) Comparative studies on histological and ultra-structure of the pituitary of different ploidy level fishes. Sci China Ser C Life Sci 49(5):446–453
Long Y, Tao M, Liu S, Zhong H, Chen L, Tao S, Liu Y (2009a) Differential expression of Gnrh2, Gthβ, and Gthr genes in sterile triploids and fertile tetraploids. Cell Tissue Res 338(1):151–159
Long Y, Zhong H, Liu S, Min T, Chen L, Xiao J, Liu Y (2009b) Molecular characterization and genetic analysis of Gnrh2 and Gthβ in different ploidy level fishes. Prog Nat Sci: Mater Int 11:119–129
Naumova A, Fayer S, Leung J, Boateng K, Camerini-Otero R, Taketo T (2013) Dynamics of response to asynapsis and meiotic silencing in spermatocytes from robertsonian translocation carriers. PLoS One 8:e75970
Ontoso D, Kauppi L, Keeney S, San-Segundo P (2014) Dynamics of DOT1L localization and H3K79 methylation during meiotic prophase I in mouse spermatocytes. Chromosoma 123(1–2):147–164
Raghuveer K, Senthilkumaran B (2010) Cloning and differential expression pattern of vasa in the developing and recrudescing gonads of catfish, Clarias gariepinus. Comp Biochem Physiol A Mol Integr Physiol 157(1):79–85
Shen J, Liu S, Sun Y, Zhang C, Luo K, Tao M, Zeng C, Liu Y (2006) A new type of triploid crucian crap - red crucian carp (♀) × allotetraploid fish (♂). Prog Nat Sci 16(12):1348–1352
Strahl B, David A (2000) The language of covalent histone modifications. Nature 6765(403):41–45
Tan M, Luo H, Sangkyu L, Fulai J, Yang J, Emilie M, Thierry B, Cheng Z, Rousseaux S, Rajagopal N, Lu Z, Ye Z, Zhu Q, Wysocka J, Ye Y, Khochbin S, Ren B, Zhao Y (2011) Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 6(146):1016–1028
Tao M, Liu S, Long Y, Zeng C, Liu J, Liu L, Zhang C, Duan W, Liu Y (2008) The cloning of Dmc1 cDNAs and a comparative study of its expression in different ploidy cyprinid fishes. Sci China Ser C Life Sci 51(1):38–46
Valérie B, Nicolas R, Waka L, Sandrine B, Vincent G, Alain N (2009) Histone H3 lysine 4 trimethylation marks meiotic recombination initiation sites. EMBO J 28(2):99–111
Wang D (2013) Expression and sex determination mechanism of reproductive genes of HPG Axis in different ploidy fish. Hunan Normal University, Changsha
Xiang B, Liu S, Zhang C, Sun Y, Duan W, Shen J, Luo K, Tao M, Zeng C, Liu Y (2006) The analysis of nutritional component and amino acid composition of muscle in a new type of triploid crucian carp (carassius auratus). J Nat Sci Hunan Norm Univ 29:85–88
Xu D, Bai J, Duan Q, Costa M, Dai W (2009) Covalent modifications of histones during mitosis and meiosis. Cell Cycle 8(22):3688–3694
Xu H, Gui J, Hong Y (2005) Differential expression of vasa RNA and protein during spermatogenesis and oogenesis in the gibel carp (Carassius auratus gibelio), a bisexually and gynogenetically reproducing vertebrate. Dev Dyn 233(3):872–882
Yi N, Zhang C, Wang Y, Liu S, Liu Y (2006) The early embryonic development of triploid crucain carp coming from allotetraploid hybrids (♂) × goldfish(♀). J Nat Sci Hunan Norm Univ 29:87–91
Yu F, Zhong H, Gang L, Liu S, Zhang Z, Zhou Y, Tao M, Liu Y (2015) Characterization of vasa in the gonads of different ploidy fish. Gene 574:337–344
Zhang C, Liu S, Sun Y, Xiao J, Liu Y (2008) Chromosomal studies of germ cells in diploid and polyploid fish produced by distant crossing. J Mol Cell Biol 41(1):53–60
Zhang C, He X, Liu S, Sun Y (2005) Chromosome pairing in meiosis I in allotetraploid hybrids and allotriploid crucian carp. Acta Zool Sin 51(1):89–94
Zhong H, Zhou Y, Liu S, Tao M, Long Y, Liu Z, Zhang C, Duan W, Hu J, Song C (2012) Elevated expressions of GH/IGF axis genes in triploid crucian carp. Gen Comp Endocrinol 178(2):291–300
Zhou R, Shang R, Gong D, Xu X, Liu S (2019) Characterization of H3 methylation in regulating oocyte development in cyprinid fish. Sci China Life Sci 62(6):829–837
Zhou Y, Wang F, Liu S, Zhong H, Liu Z, Tao M, Zhang C, Liu Y (2012) Human chorionic gonadotropin suppresses expression of Piwis in common carp (Cyprinus carpio) ovaries. Gen Comp Endocrinol 176(2):126–131
Zhou Y, Zhong H, Liu S, Yu F, Hu J, Zhang C, Tao M, Liu Y (2014) Elevated expression of Piwi and piRNAs in ovaries of triploid crucian carp. Mol Cell Endocrinol 383(1–2):1–9
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Science Press
About this chapter
Cite this chapter
Liu, S. et al. (2022). The Basic Biological Characteristics of Sterile Allotriploid Fish. In: Liu, S. (eds) Fish Distant Hybridization. Springer, Singapore. https://doi.org/10.1007/978-981-16-5067-3_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-5067-3_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-5066-6
Online ISBN: 978-981-16-5067-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)