Abstract
The accurate diagnosis of urinary calculi is essential for treatment planning. Non-contrast-enhanced computed tomography (NCCT) is considered the gold standard for adults to diagnose urolithiasis in acute flank pain. Generally, CT has also overtaken the role of intravenous urography in stone diagnosis and treatment planning. Lower dose CT seems to be as accurate as NCCT for the same purpose. Ultrasonography (US) is considered first-line imaging for urolithiasis in paediatric and pregnancy groups of patients. Various iterations of US, especially with the Doppler setting, can improve diagnostic accuracy, whereas magnetic resonance imaging may be an alternative investigation tool for pregnant women. Plain radiographs and US scans can be combined for stone surveillance purposes. The study of stone composition can be inferred from double-energy CT scans. Differential kidney function is conventionally derived from nuclear renogram, but recently, CT-derived parameters have been shown to be a promising alternative.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The prevalence of urolithiasis is increasing worldwide, and in Asia, it is estimated to be around 1–5% [1]. The rising incidence of stone disease is largely attributable to changing climate and lifestyle modification. Consequently, there has been a rise in emergency department visits due to acute urolithiasis complications, while stone recurrence is also not uncommonly encountered [2].
Therefore, it is imperative that appropriate imaging is selected to accurately diagnose urinary calculi as it not only helps with treatment planning but also reduces the harm of ionising radiation to the patient. Imaging modalities are also used to help follow-up patients after conservative measures or definitive treatment. Finally, the output from imaging techniques can be used as a surrogate for renal function.
2 Utility of Imaging
The radiological diagnosis of urolithiasis in an emergency setting helps to confirm the presence of stones in acute abdomen presentations. Non-contrast-enhanced computed tomography (NCCT) is now considered the gold standard in the diagnosis of urolithiasis in adults with acute renal colic. Ultrasonography (US) is preferred for the paediatric and pregnant groups of patients. In the elective setting, treatment planning for stones utilises various imaging modalities for stone localisation, assessment of stone fragility and estimating differential renal function. Plain radiographs, along with intravenous urography (IVU), still play a role in identifying stones and outlining the upper urinary tract. Magnetic resonance imaging (MRI) is usually reserved for situations where ionising radiation and intravenous contrast studies are contra-indicated. Radio-isotope scans provide information about the relative renal function, which can aid in decision-making for urolithiasis intervention. Lately, many new iterations from plain radiograph, CT and US scans have been developed to increase the accuracy of stone detection whilst reducing exposure to ionising radiation.
3 Hazards of Imaging
There are risks associated with the use of imaging modalities, particularly those emitting ionising radiation. Risks can be divided into deterministic or stochastic effects. Deterministic effects of ionising radiation occur at a given threshold, and the effect is therefore proportional to the dose. Examples include skin erythema and cataract generation [3]. Stochastic effects relate to the induction of secondary cancers or hereditary effects. This can occur at any dose of radiation. Thus, the probability for the stochastic effect to occur increases with the dose. The severity, however, is dose-independent. In general, deterministic effects are rarely encountered in diagnostic imaging radiation doses [3].
The effective dose (measured in milli-Sievert, mSv) is a way of quantifying the risk of radiation exposure to human beings. It estimates the potential adverse biologic effect of the sum of equivalent doses of radiation to the exposed organs [3, 4].
Imaging modalities that utilise intravenous contrast (iodine or gadolinium) also have associated risks such as allergic reactions, impaired renal function, nephrogenic systemic fibrosis and death. It is therefore prudent that imaging studies are selected based on the As Low As Reasonably Achievable (ALARA) principle, i.e. using the lowest ionising radiation modality to answer a clinical question [3].
4 What Do the Guidelines Say?
Guidelines from the American College of Radiology (ACR) [4], American Urological Association (AUA) [3, 5], European Association of Urology (EAU) [6] and Societe Internationale d’Urologie–International Consultation of Urological Disease (SIU-ICUD) [7] provide recommendations on the utility of imaging in urolithiasis. For children, additional recommendations are available from the European Society for Paediatric Urology (ESPR) [8].
4.1 Adults
-
For acute flank pain suspicious of urolithiasis, all guidelines recommend performing an NCCT [Level A]. The EAU guidelines advocate NCCT after the initial US assessment.
-
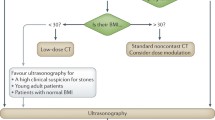
Low-dose NCCT should be performed when evaluating for ureteral and renal stones [ACR], especially in patients with BMI <30 [SIU-ICUD: Level A].
-
For a young patient and known stone former with previous radio-opaque stones, the AUA and ACR recommend US combined with kidney–ureter–bladder radiography (KUBXR) [Level C].
-
In complex stones or anatomy, additional contrast imaging can be obtained if the further definition of the collecting system and ureteral anatomy is needed [AUA: Grade C].
-
A focused area re-imaging can be performed prior to surgery if the passage of stone is suspected or stone movement will change management [AUA: Principle].
4.2 Pregnancy
-
US is the preferred method for imaging a pregnant woman with suspected urolithiasis.
-
The second-line option would be magnetic resonance imaging [AUA/EAU: Level 3].
-
Low-dose NCCT should be used as the last option in pregnant women [AUA/EAU].
4.3 Paediatrics
-
US is the first-line imaging modality for children with suspected urolithiasis, but it should include kidneys, fluid-filled bladder and ureter [EAU/AUA/ACR/ESPR: Level 2b].
-
KUBXR or low-dose NCCT is an option if US does not provide the relevant information [EAU/AUA Level 2b].
-
For non-obstructing renal stones, active surveillance can be pursued using periodic ultrasonography [AUA: Expert Opinion].
-
Prior to performing PCNL, a low-dose NCCT should be obtained [AUA: Grade C].
-
However, ESPR is unable to recommend the general use of low-dose NCCT in paediatric patients.
4.4 Surgical Planning
-
A functional imaging study (DTPA or MAG-3) may be obtained if clinically significant loss of renal function in the involved kidney or kidneys is suspected [AUA: Level C].
-
In planning for stone surgery, a contrast study can be performed to evaluate the anatomy of the renal collecting system [EAU].
-
For shockwave lithotripsy (SWL), careful fluoroscopic and/or ultrasonographic monitoring during SWL facilitates good outcomes [EAU: Level 2a].
-
In planning for endourological procedures, pre-procedural imaging of the kidney with US or CT scan, including contrast medium where possible or retrograde pyelographic study when starting a procedure, can be performed to assess stone comprehensiveness and anatomy of collecting system [EAU: Level 1a].
-
A low-dose NCCT may be obtained prior to performing percutaneous nephrolithotomy on paediatric patients [AUA: Level C].
-
Deferred imaging is performed after SWL, ureteroscopy or percutaneous antegrade lithotripsy to determine the presence of residual fragments [EAU: Level 3].
-
Paediatric patients with asymptomatic or non-obstructing renal stones may be actively surveyed with periodic US [AUA: Expert Opinion].
4.5 Stone Workup
-
In patients with unknown stone composition, US is performed in the case of suspected urolithiasis, which is then followed by NCCT with determination of Hounsfield units to provide information about stone composition [EAU].
5 Imaging Modalities
5.1 Plain Radiograph/X-Rays
Plain X-ray involves the use of a single energy source to produce photons. These pass through tissues, which then encounter a contralateral receiver. Historically, kidney–ureter–bladder radiograph (KUBXR) was used to complement intravenous urography studies [9].
A plain KUBXR is commonly used in patients with renal colic, as most stones contain calcium salts and hence are radio-opaque. It can reveal the cause of renal colic if radiopacity is detected at the expected location of the kidney or ureter based on the patient’s symptoms. Nevertheless, not all stones are radio-opaque and not all calcifications are phleboliths. The sensitivity and specificity of KUBXR have been reported to be 57% and 76%, respectively [9]. When assessing for new stones, the effective radiation dose per KUBXR study ranges between 0.7 and 1.5 mSv [10].
The advantages of KUBXR include (a) low ionising radiation exposure, (b) widespread availability, (c) not dependent on the expertise and (d) low cost (it is about 10% of the cost of an ultrasound study) [9].
In addition to its relatively low accuracy in diagnosing urinary calculi, KUBXR also does not detect all stones (radiolucent) such as uric acid, xanthine and drug stones [6].
Nevertheless, KUBXR remains useful in (a) the treatment planning for radiopaque stones in extra-corporeal shockwave lithotripsy (ESWL), (b) the evaluation of ureteral stent placement and (c) the follow-up of residual stone burden after treatment [11].
To improve its accuracy, when combined with abdominal US for initial evaluation of acute colic, the sensitivity and specificity for the diagnosis of ureteral stones are 96% and 91%, respectively [12]. One study on 66 patients comparing US–KUBXR with NCCT found that the combination affords a sensitivity of 79% versus 93% of NCCT in detecting stone. All the missed cases had reported spontaneous stone passage. Therefore, it was suggested that after a negative combined US–KUBXR evaluation, NCCT would not add further information [13]. Lipkin and Ackerman suggested that KUBXR should be done before the US as the former can detect calcifications, direct the US examination and confirm the diagnosis [14]. The American College of Radiology proposed that this combined imaging modality may be able to detect all clinically significant stones and hence should be considered in young patients and those with known stone diseases [4].
Scout films on CT are performed at a lower mA than a standard KUBXR. However, NCCT scout image is not equivalent to a KUBXR [3]. The former can miss up to 25–51% of stones detected on KUBXR [10]. When a ureteral stone is detected on NCCT, the stone is only visualised on CT scout images about half the time. Thus, KUBXR should still be used if the stone is not seen on CT scout, as the stone will be detected in 10% of these patients [3]. For ureteral stone, the AUA recommends that KUBXR has a role for follow-up, for stones seen on CT scout image or initial KUBXR, in those patients undergoing medical expulsion therapy [11]. Surveillance oblique KUBXR films may be considered in stones located in the sacro-iliac area, which was not visible on CT scout or initial KUBXR [3].
KUBXR findings were also found to be able to significantly change the surgical management in 17% of renal stones initially detected on NCCT [15].
When assessing for new stones, KUBXR was found to have a sensitivity of 37.0% for stones <5 mm, and this increased to 87.5% for larger stones. Therefore, in the follow-up of stone formers, this may be a cost-effective modality for monitoring stone size [9].
Overall, although KUBXR may confer a lower ionising radiation dose, multiple radiographs performed over time, especially for young stone formers, may expose a patient to an effective dose similar to a low-dose CT scan [4].
5.2 Digital Tomosynthesis
This modality of imaging integrates KUB radiograph scout films taken via a 60-degree arc around the patient, with a digital detector and special computational software system for integration of imaged data on the opposing end detector [9, 14, 16]. Coronal section images are taken whilst overlying structures are subtracted to produce an image for the area of interest [9, 14]. The enhanced visualisation of digital tomosynthesis (DT) in the antero-posterior axis is of advantage over conventional KUBXR [16]. DT has a lower resolution than a CT but at a reduced dose of radiation compared with standard or low-dose CT. It outperforms KUBXR or intravenous urography in diagnostic accuracy whilst preserving image quality regardless of the patient’s BMI [16]. DT is also less costly compared to conventional CT [14].
There is emerging evidence that DT is more sensitive in detecting renal rather than ureteral stones in ex vivo studies [16]. In an in vivo study, DT was found to be significantly more sensitive than digital radiography for detecting kidney stones but not ureteral stones. The sensitivity of detection for stone sizes between 2 and 5 mm was reported as 64% and for larger stones, 76%. Radiation dose was only slightly increased when compared to digital radiography but substantially lower than standard and low-dose CT [14].
5.3 Intravenous/Intraluminal Urography and Pyelography
Before the widespread availability of CT scans, intravenous urography (IVU) was the standard imaging technique for diagnosing and planning stone surgery. It provides information on renal function, anatomy of the collecting system and the level of obstruction [6]. Each examination confers an effective radiation dose between 1.5 and 3.5 mSv [10].
In acute flank pain assessment, IVU has a sensitivity of 85.2% and specificity of 90.4% in detecting stone [14]. The advantages of IVU are its ability (a) to delineate challenging renal anatomy, particularly before percutaneous nephrolithotomy (PCNL), and (b) to provide relative renal function information, in addition to evaluating for obstruction. All these pieces of information are useful for surgical planning [10].
However, IVU has the disadvantages of (a) higher effective radiation dose compared to standard radiograph, (b) longer acquisition time than CT, especially in the evaluation of obstruction, (c) inability to identify alternative diagnoses compared to CT scan, (d) higher cost and (e) higher risk due to contrast use [4].
IVU is also useful in equivocal situations of calcific density, which may represent a phlebolith or ureteral stone [4]. It has been shown that when IVU is added to DT, the diagnostic quality of standard IVU for urolithiasis rises from 46.5% to 95.5%, with a mean radiation dose reduction of 56% [17]. IVU along with an excretory CT scan can help to characterise the lower pole anatomy in urolithiasis to prognosticate the success of ESWL [10].
IVU is contraindicated in renal insufficiency, dehydration, pregnancy and in patients with past reactions to iodinated contrast agents. Currently, the availability of non-iodinated contrast material has reduced the risk of contrast allergies [4].
Retrograde pyelography performed prior to percutaneous nephrolithotomy (PCNL) or flexible ureteroscopy procedures in an anaesthetised patient helps to provide an on-table assessment of the upper urinary tract collecting system. This review may lead to a change in operative strategy [5, 10]. In addition, the placement of the nephrostomy tube after PCNL can be facilitated by antegrade pyelography.
In general, NCCT and contrast-enhanced CT have supplanted the use of IVU in the management of nephrolithiasis.
5.4 Ultrasonography
Ultrasonography (US) is commonly used as first-line imaging for suspected urolithiasis. Generally, it visualises the renal parenchyma, renal collecting systems and the bladder well but is poor in delineating the ureter due to overlying bowel gas or thick adipose tissue [11]. Apart from kidney and bladder stones, US is also able to detect calculi at pyelo-ureteral and vesico-ureteral junctions, as well as to detect upper urinary tract dilatation [6]. The sensitivity and specificity for the detection of ureteral stone are 45% and 94%, respectively. For renal stones, the accuracy is 45% and 88%, respectively [9, 16]. However, sensitivity can be reduced if the stone size is <3 mm, as it may not produce a shadow or miss out due to a decompressed urinary system [9]. In addition, US is useful in picking up secondary signs of urinary obstruction (i.e. hydronephrosis, hydroureter and perinephric fluid) and identifying other sources of flank or abdominal pain [3, 4].
The advantages of US include (a) its portability, (b) its ubiquitousness, (c) no radiation exposure (hence it is suitable for subsets of patients, i.e. pregnant women and paediatric patients) and (d) its reproducibility [6, 14].
However, US is disadvantageous because of (a) reduced sensitivity and specificity compared to CT scan, (b) inaccuracy in stone size determination, (c) reduced accuracy in stone detection for obese patients [14], (d) the need for skilled/medical personnel to perform, (e) significantly more time-consuming and (f) its variable findings as it is operator-dependent [10].
In obese patients, US can overestimate stone sizes compared to NCCT, up to 1 mm in stones smaller than 5 mm [10, 16]. Furthermore, US cannot differentiate dilatation without obstruction from true obstruction [4].
In a randomised controlled study comparing the role of US and CT in the assessment of suspected obstructive urolithiasis, no differences were reported in the sensitivity (~85%) and specificity (~50%), or complication rates, between the two intervention arms, at the time of discharge from the emergency department. However, a follow-up CT scan was performed for 40.7% of patients who had initial point-of-care US scan and in 27% of those who had radiology departmental US. Overall healthcare costs were also not significantly different between the groups [18]. Therefore, it has been proposed that US can be safely used as first-line imaging in emergency settings for patients with symptoms of urolithiasis [19].
Furthermore, for patients presenting to the emergency department, especially with solitary kidney, fever or doubt regarding the diagnosis of renal colic, the EAU recommends US as the initial evaluation [6].
There are many iterations to an US study that can potentially improve its accuracy in detecting stone and obstruction. Colour Doppler US adds value to grey-scale US alone in the evaluation of urolithiasis [11, 16].
When the urinary bladder is visualised in the transverse view using colour Doppler, ureteral jets appear as intermittent bursts of fluid on each side of the bladder. The unilateral absence or reduced jet flow rate with continuous jet flow pattern due to decreased peristalsis is specific for the presence of an obstructing ureteral stone [9, 14, 20].
The sonographic twinkling artefact is characterised by the appearance of alternating colours located deep to the stone on colour Doppler (typically seen as shadows on grey-scale US). Imaging with high pulse repetition frequency has been shown to increase the sensitivity of urolithiasis diagnosis from 66% on grey-scale to 97% as compared to NCCT [10]. Nevertheless, there is a high false-positive rate of about 50% [10, 11], which, in the acute setting, may have implications for confirmatory NCCT scan [16]. Therefore, the artefact should be evaluated with other parameters.
Doppler US can also be used to calculate the resistive index (RI) of the renal artery in a kidney with hydronephrosis. This measurement has been proposed as an indicator of ureteral obstruction when elevated unilaterally in a hydronephrotic kidney. Typically an RI value of 0.70 or a RI difference of ≥10% between the two kidneys indicates obstruction [10, 11]. However, the estimated RI has not been widely accepted due to conflicting results [11].
In patients presenting with acute flank pain, US has been found to be up to 100% sensitive and 90% specific for the diagnosis of ureteral obstruction. However, it is worth noting that about 11–15% of patients with urolithiasis may not show hydronephrosis on US [20]. This may be due to dehydration or that the hydronephrosis has not developed, typically only visible within 2 h of clinical presentation [4].
Another role of US is providing sonographic guidance for percutaneous access for nephrolithotomy procedures. In experienced hands, the success rate of access is as high as 88–99% with US guidance. For obese patients who require higher effective radiation dose under fluoroscopic guidance for comparable image quality, US is more advantageous [16].
Given its performance in detecting renal stones, US can be used as an alternative to CT as a follow-up imaging for patients with distal ureteric stones or renal stones undergoing conservative management [6, 7, 10].
5.5 Multidetector Computed Tomography
Helical/spiral non-contrast-enhanced CT (NCCT) was initially studied for flank pain by Smith et al. in the early 1990s [21]. This imaging technique relies on the relative absorption of radiation by body tissues and stones, where the 3-D image of stone and the surrounding anatomy are then reconstructed into multi-planar views [9]. Thinner transverse slices (1–3 mm) are usually preferred with improved sensitivity in stone detection. However, 5 mm axial slices with 3 mm coronal and sagittal re-formatted images also provide adequate stone detection with a lower radiation dose [11].
The most commonly applied iteration of CT scan for urolithiasis is NCCT or CT-KUB. It is now regarded as the first-line imaging for acute flank pain suspicious of urolithiasis in the emergency department. NCCT confers high sensitivity (95–100%) and specificity (96–98%) in detecting stones [5, 6, 10]. Furthermore, it is useful in detecting secondary signs of obstruction due to ureteral stones such as hydronephrosis, hydroureter, peri-ureteral oedema and renal enlargement (Fig. 2.1) [4]. It can also demonstrate other organic causes of flank pain in 9–15% of scans (Fig. 2.2). NCCT is also considered the gold standard for detecting residual stone fragments post-therapy [11].
Apart from stone diagnosis, NCCT provides other qualitative measurements such as stone size and location, as well as inference on stone composition and density, expressed in Hounsfield units (see later) [4,5,6]. Coronal views of CT accurately provide maximal stone size estimation, which may be a factor in treatment decision and predicting stone passage [4, 22].
NCCT also conveys skin-to-stone distance (SSD) measurement, which is useful in treatment planning using extra-corporeal shockwave lithotripsy (ESWL). SSD greater than 9–11 cm has been associated with lower stone-free rates [10]. This parameter is derived from the mean of three measurements (lateral skin-to-stone, posterior skin-to-stone and 45 degrees between the initial two measurements) [11]. Other anatomical parameters derived from NCCT, which are predictive of successful ESWL stone treatment, include unfavourable factors such as narrow infundibulo-pelvic angle (<70°), long infundibular length (>3 cm) and narrow infundibular width (<5 mm) [10].
Differentiating stones that are intramural or have already passed into the bladder in supine NCCT during acute renal colic can be challenging [22]. In symptomatic patients who are suspicious of having distal ureteral stones, a prone NCCT can be very helpful [10]. Furthermore, prone positioning allows for anatomic determination of kidneys with surrounding organs and pleura in planning for PCNL [14].
NCCT can also be used to estimate stone volume in relation to the pelvicalyceal system and surrounding organs. This can be calculated from the water displacement method, which is comparable to volumetric stone measurement using software [22]. Such information is vital for pre-operative evaluation of the site and direction of percutaneous renal access, for example. It can also be used to predict the success of ESWL and flexible ureteroscopy [10].
The advantages of NCCT include (a) the ease of performing in the emergency department with faster image acquisition by non-medical staff [10], (b) no requirement for IV contrast, (c) ability to assess other abdomino-pelvic viscera and pathologies [14] and (d) ability to identify radiolucent stone [16]. Interestingly, about one-third of NCCT scans for urolithiasis resulted in observations for other findings, while up to 70% of acute flank pain requiring NCCT resulted in non-urolithiasis aetiologies [14].
The disadvantages include (a) the use of ionising radiation, which is of concern in those at risk of stone recurrence, thus requiring multiple lifetime imaging, and (b) the inability to image protease-inhibitor-related stones such as indinavir.
At times, delineation of the collection system using contrast-enhanced CT with excretory phase is useful when stone removal is planned [5, 6, 11]. This is recommended for complex renal or ureteral anatomy (e.g. horseshoe kidney, cross-fused ectopia) and unusual patient body habitus (refer to Chap. 25) [5]. Although IVU can provide this information, a randomised clinical trial found that, for supine PCNL planning, CT scan resulted in easier access into the pelvicalyceal system and reduced operating time [23].
There are valid concerns regarding cumulative exposure to radiation, especially in young patients with urolithiasis who may undergo repeat scans over the years, as well as obese patients who may require three times the effective radiation dose compared with non-obese patients [16]. Another relevant concern is the induction of secondary cancer, with one case in every 660 patients having received a single CT of the abdomen [22].
5.6 Measures That Are Taken to Lower the Emission of Ionising Radiation During CT
Several advancements have been made in CT technology to address the radiation dose concern. This includes modification of scan parameters, modulation of scan parameters according to the patient’s characteristics and the use of automatic dose-modulation software or X-ray filters, which adjust the radiation based on the scout images and according to the thickness and density of various anatomic regions [10, 16]. Limiting the range of view to the kidney, ureter and bladder also reduces radiation dose [4].
Standard CT evaluation involves radiation dose of up to 9.6 mSv for men and 12.6 mSv for women, per examination. Recent advancements in CT technology allow for low-dose CT (LDCT) to be performed (with effective radiation doses of 0.7–2.3 mSv) per examination. Even low-density stones such as uric acid stone are well detected by LDCT [14]. Similar sensitivities and specificities have been reported between standard- and low-dose CT regimens for the diagnosis of urolithiasis [10]. A meta-analysis of prospective studies found a pooled sensitivity of 93.1% and pooled specificity of 96.6% for LDCT detection of urolithiasis [24]. LDCT has been shown to produce equivalent stone measurements as compared to standard-dose CT [4]. However, LDCT performs poorly for obese patients (BMI > 30 kg/m2) and smaller stones (<3 mm) [14]. Thus, LDCT is preferred for BMI ≤ 30, while reducing ionising radiation dose and maintaining both sensitivity and specificity at 90% and higher [3, 6].
Advances in ultra-LDCT (i.e. effective radiation dose ~1 mSv) showed that combined with model-based iterative reconstruction, stones of 3 mm or larger can be detected [14, 22]. The sensitivity and specificity of this modality are 74% and 77% for stone size <3 mm and 92% and 82% for stones ≥3 mm, respectively. Ultra-LD CT was also inferior to LDCT in detecting secondary signs [14].
Limitations of ultra-LDCT are the detection of stones less than 3 mm and patients with BMI > 30 [22]. An in vivo study by Rob et al. compared ultra-LDCT (effective dose ≤1.9 mSv) or LDCT (<3.5 mSv) versus standard-dose CT (4.5–5 mSv). They reported sensitivity of 90–100% and specificity of 86–100% for ultra-LDCT and LDCT, respectively [25].
Despite the benefits of low-dose CT, the uptake of LD protocol has been less than 10% based on cross-sectional studies performed in the United States [22].
5.7 Magnetic Resonance Imaging
This imaging modality provides a comprehensive review of soft tissues in the abdomino-pelvic region. However, magnetic resonance urography (MRU) cannot be used to directly detect urolithiasis [6, 10]. Using standard magnetic resonance imaging (MRI) sequences, stones will appear as non-specific signal void [9].
MRI is able to detect secondary effects of obstruction due to urolithiasis, but this could be non-specific, as filling defects in the ureter could be due to a blood clot or tumour [10]. The T2-weighted sequences are able to reveal signs of obstruction such as hydronephrosis and perinephric oedema [4, 10]. Compared to CT scan, MRI performed in acute ureteral obstruction has a greater sensitivity (77%) in detecting perinephric fluid compared to perinephric stranding on CT scan (45%) [14]. Diffusion-weighted sequence allows for the detection of pathophysiological changes to renal perfusion and diffusion in patients with unilateral ureteral obstruction and for monitoring treatment progress [10]. Nevertheless, MRI does not provide quantitative information on the renal function that could assist management in the setting of obstructive uropathy [14].
The sensitivity of MRI for urolithiasis detection is variable. It has a reported median sensitivity of 82%, which is higher than that of US and KUBXR but lower than CT scan [3]. In diuretic-enhanced excretory MRU in patients with obstructive uropathy, MRI accuracy was reported as 93% [20].
One utility of MRI is the detection of protease-inhibitor (Indinavir) stones in HIV patients, which is radiolucent and not visible on CT or KUBXR [14].
The advantages of MRI include (a) no ionising radiation, thus making it desirable for paediatric patients, pregnant women and nephropathy patients who must avoid contrast [14]; and (b) its ability to provide 3-D images without radiation [9].
The disadvantages of MRI are (a) restricted access, (b) higher cost (i.e. three times more than a CT scan), (c) lower accuracy and (d) longer image acquisition time [9]. In addition, the use of high-dose paramagnetic contrast may be teratogenic, as shown in animal studies [10].
6 Imaging in Special Groups
6.1 Pregnancy
The risk of ionising radiation for investigative procedures during pregnancy is dependent on the gestational age of the foetus (the lowest risk is before 8th and after 23rd week) and radiation dose (<50 mGy is considered safe) [6]. Radiological exposure carries a risk of <1 in 5000 (1 in 33,000 per mGy) for fatal childhood cancers and <1 in 10,000 (1 in 40,000 mGy) for induced heritable diseases. Hence, stochastic effects of ionising radiation on the foetus are of particular concern. In pregnancy, radiation harm can be reduced further by (a) imaging only the affected side, (b) shielding the maternal pelvis and (c) keeping the exposure time or number of radiographs to a minimum [26].
In pregnant women with flank pain suspicious of urolithiasis, transabdominal or transvaginal US is regarded the best initial study [4,5,6,7]. To increase the accuracy in US detection, Doppler US measurement of the resistive index (RI), using a cut-off of 0.70 or a change in RI of 0.06, is useful in the diagnosis of acute unilateral ureteric obstruction if the scan is performed within 6–48 h of presentation [26]. However, detection rates can be compromised if done outside this time window in patients with renal disease and with non-steroidal anti-inflammatory drugs on board [26].
The twinkling artefact of US, using B-mode and Doppler, can improve the sensitivity of stone detection by differentiating stones from other echogenic structures [9]. Colour Doppler can be utilised to detect ureteral jets, or the passage of urine, at the uretero-pelvic junction where an absence of jets represents complete ureteral obstruction. However, false positives can occur due to the ureteral compression by a gravid ureter. Hence, this study should be confirmed in contralateral decubitus patient position [26].
If US is equivocal in detecting stones, MRI is proposed as the second-line imaging modality. This investigation defines the level of obstruction, and in some situations, it provides an estimate of stone size [6]. Although it has no harmful ionising radiation to the foetus, MRI should be avoided in the first trimester of pregnancy due to limited data on safety during foetal organogenesis [26]. Nevertheless, there is inadequate data to prove the deleterious effects of MR exposure to a developing foetus. Non-contrast MRI at 1.5 T should be used on the basis that medical benefits outweigh any unknown potential risks [27]. Furthermore, there is widespread consensus that gadolinium-based contrast agents should be avoided during pregnancy [27].
The MRI also serves as a useful adjunct for US in pregnant women. Kidneys do undergo physiological dilatation 90% of the time, especially on the right side, usually seen as early as 6 weeks gestation and resolves by 6 weeks postpartum [9]. Hydronephrosis can be attributable to a compressed ureter between the gravid uterus and the linea terminalis [4]. Hence, MRI is useful if stones cannot be visualised on US, but clinical suspicion of obstructing urolithiasis persists [9].
In the second and third trimesters of pregnancy, low-dose CT (LDCT) scan can be considered the last option for stone detection if US and MRI cannot achieve a diagnosis [4,5,6]. LDCT has a higher positive predictive value (95.8%) than MRI (80%) and US (77%); thus this can potentially avoid unnecessary negative interventions such as ureteroscopy [6]. The American College of Obstetrics and Gynaecology stated that radiation exposure of less than 50 mGy, which is well below the average for low-dose CT, is not associated with the development of foetal anomalies or foetal loss [3, 5].
6.2 Paediatrics
Children with urolithiasis represent a group with a higher risk of stone recurrence. Hence, with the prospect of repeated imaging throughout their lifetime, the ALARA principle should be adhered to [6, 9]. Furthermore, imaging procedures may require their co-operation, anaesthesia and exposure to ionising radiation. Adult protocols cannot be applied to children because (a) their stones are small and poorly calcified, (b) they have smaller ureters surrounded by fat, which can reduce the diagnostic accuracy of CT scan [6, 8], and (c) they have 10 times higher sensitivity to radiation than adults, thus higher chance of developing malignancies later in life [10].
US scan should be the first choice in investigating urolithiasis in children. This modality can visualise the kidney and the rest of the urinary tract rather well, with adequate hydration and good bladder volume. Most of the stones in children are located in the pelvicalyceal junction or in the proximal and/or distal ureter. Sometimes, small concretions are detected by US, which may be missed by IVU or low-dose CT [8]. US also has a higher accuracy in stone detection in children due to small body size and shorter stone to probe distance [8].
The US features for stones in children include echogenic foci with posterior shadowing, ureteral and pelvicalyceal system dilatations, and increased renal echogenicity and size, which are more conspicuous than in adults [8]. Nevertheless, small stones and modern US machines with harmonic and spatial compounding imaging features may fail to cast an acoustic shadow [28]. Although less sensitive (70%) than CT, it is an adequate screening tool to diagnose most clinically significant stones [28]. In addition, US can be used for surveillance for asymptomatic and non-obstructive renal stones in children [5]. US detection of stones during acute obstruction can be enhanced by the twinkling artefact and measurement of the resistive index using colour Doppler, such as in pregnant women [8]. However, US fails to detect >40% of stones in children, and it provides limited information regarding renal function [6]. US is advantageous as it does not require anaesthesia and no radiation is involved.
The use of plain X-ray can assist in localising stone before lithotripsy procedures, and it is useful for follow-up, too [6, 8]. IVU should be used judiciously for specific indications, and usually it supplements US findings. The IVU should be limited to three or four views, including KUBXR, and with adequate coning, this should be adequate for diagnosis with a lowered radiation dose [8].
There is now widespread use of CT as a first-line study given its wide availability in the United States. Between 2003 and 2011, about 63% of children underwent CT scans compared to US (24%) as first-line imaging in the United States. NCCT confers near 100% sensitivity and specificity for urolithiasis. Low-dose CT (radiation dose <3 mSv) using stone protocol has been introduced, and this achieved a diagnostic sensitivity of 96.6% for nephrolithiasis. Nevertheless, the accuracy of low-dose CT for paediatric nephrolithiasis has not been confirmed [28]. Thus, low-dose CT can be considered an alternative if US cannot provide information on urolithiasis [6].
7 Stone Composition and Fragility
Pre-procedural determination of stone composition can assist in optimal stone management. Traditionally, the stone composition is deduced from chemical analysis utilising sophisticated spectrometry, which can be costly and is not widely available. No other chemical analysis can determine in vivo stone composition. Stone fragility can be assessed to predict the likelihood of fragmentation.
Historically, stone density has been regarded as a surrogate of its composition. This is measured using NCCT and expressed in Hounsfield units (HU). In addition, HU can be used to predict success rates for stone treatment [16]. For ESWL treatment, stones with HU of between 900 and 1200 were found to be independent factors for treatment failure. In practice, the association between ESWL failure rate and HU values is not linear. Furthermore, most stones have mixed composition, resulting in overlap in their attenuation values, thus making the response to ESWL less predictable [16]. Other limitations of HU values include variability between CT scanner models and the high radiation dose involved in deriving its value [29].
A Turkish study on 115 patients with renal stones who had HU measurements and subsequent stone analyses found that HUdiff (the difference between maximal and minimal HU for a particular stone) and the mean HU value (HUave) can reliably predict stone mineral complexity. HUdiff < 341.5 showed 81.8% sensitivity and 67.2% specificity for identifying mono-mineral stones [30]. Other studies identified that HUave < 900 predicts uric acid stone, HUave > 1000 favours a calcium-based stone, whilst HU of 900–1000 is associated with other stones (cystine, struvite and calcium oxalate monohydrate–uric acid) [30].
Double-energy CT (DECT), which is performed by scanning an object with two scanners at two different energies (80 and 140 kV), thus producing two sets of data, which are then merged into a CT image, is an alternative method to predict in vivo stone composition [16]. The different X-ray attenuation obtained from the two scanners for various stone elements with different atomic numbers can be used to infer stone composition by measuring their differences [11, 16].
In vivo characterisation of urinary stones and sub-characterisation of calcium stones are now possible with DECT. It has been shown that DECT is better than conventional CT in differentiating uric acid from non-uric acid stones [11]. Lately, the distinction between struvite and cysteine stone has also been made by DECT. This will facilitate the selection of struvite stone patients for ESWL who are more likely to achieve treatment success [11]. DECT can also generate a low-to-high energy ratio. Differences in the ratios of different stone types can be used to predict its composition. For example, a ratio of 1.13 to 1.24 predicts cysteine stone, whereas a ratio more than 1.24 is likely to be a calcium salt [16].
Initially, DECT required a higher effective radiation dose. Thus, Nestler et al. proposed stratifying patients with uric acid stone in which patients with urine pH < 5.5 should undergo DECT, while those with urinary pH > 5.5 should receive standard CT [22]. Recently, the radiation dose for DECT was reported to be comparable to standard CT (2.6 vs. 2.7 mSv). In fact, by further reducing the current in the scanner, DECT can still produce compositional stone analysis at 40% lower radiation dose, equivalent to that of low-dose CT [16]. Currently, ultra-low-dose DECT has managed to produce excellent differentiation between uric acid (sensitivity and specificity 100%) and non-uric acid stones (sensitivity 100%, and specificity 79%) [16].
The limitations of DECT include (a) higher costs of the scanner, (b) challenges in clinical workflow if prospective patient selection becomes necessary, (c) variability in reporting radiation dose and (d) indeterminate best energy levels for imaging as well as post-processing algorithms [4, 22].
In addition, a high-resolution CT scan producing thin (<5 mm) slices, viewed in the bone window, can be used to assess the internal architecture of urolithiasis. Using magnification, stones that appear homogeneous in architecture are less likely to fragment during ESWL compared to stones with heterogeneous profile [11]. However, studies on urinary stone fragility are still limited.
8 Differential Renal Function
Urolithiasis can have an impact on renal function. Differential renal function should be ascertained in situations where treatment decisions can be made more accurately, particularly when standard anatomical imaging reveals potential loss of renal parenchyma [5, 31]. The functional information will help to prioritise the treatment side in situations of bilateral urolithiasis and assist in deciding if kidney preservation or removal is indicated in chronic stone disease [31]. Also, baseline kidney function can be ascertained in the following treatment outcomes of upper urinary tract stone disease [5].
Although parenchymal thickness, measured by US or CT scan, can estimate renal function, there are situations such as chronic kidney disease or staghorn/complex stones, where the renal function cannot be properly determined (Fig. 2.3). Furthermore, in the past, the demonstration of contrast excretion on X-ray films, such as in IVU or excretory phase in contrast-enhanced CT or MR urography, are relied upon to provide functional information of the kidneys. However, this has now been brought into question [31].
Nuclear renal scan is regarded as the gold standard for evaluating differential renal function. This study can also evaluate for obstruction. Commonly used radio-isotope tracers include the purely glomerular-filtered 99m-technitium-diethylene-triamine-pentaacetic acid (DTPA) and tubular-secreted, more efficient, 99m-technitium-mercapto-acetyl-triglycerine (MAG3) [32]. The value of differential renal function was proposed by Sreenevasan in 1974. In bilateral renal calculi, renography provided differential renal function information whereby the better kidney was operated on first, with positive post-operative outcomes [33]. However, renal isotope scans are not widely available, are costly, involves radiation exposure, are operator dependent and has a prolonged acquisition time, and in stone surgery, it does not contribute any anatomical information. The ability to assess obstruction via nuclear renography is compromised in cases of moderate-to-severe chronic kidney disease. Similarly, the assessment of renal function is limited in the setting of obstruction; thus, any obstruction needs to be alleviated first [5].
In view of those limitations, various derivatives of CT scans have been used to estimate renal function. Feder et al. studied the ratio of the parenchymal area of both kidneys and compared them with the MAG3 renal scan. Both showed a very high correlation between predicted and observed renal function, with an average difference of 4.7% between the two [34]. Samar et al. investigated 21 patients with unilateral obstructive uropathy and derived the percentage total renal volume of both normal and obstructed kidneys from helical CT scans. This was compared with percentage renal function determined from DTPA. Again, they demonstrated strong agreement between the two parameters, for both normal and obstructed kidneys [35]. In conclusion, CT-derived parameters seemed promising in predicting split renal function, although its utility needs to be tested in well-designed studies.
9 Conclusions
Imaging technology has improved over the years to improve accuracy in the detection of urinary calculi, and this helps with treatment planning. NCCT in adults and US scan in paediatric and pregnant patient groups have proven to be useful in detecting a majority of stones in the emergent and elective settings. The different iterations of NCCT with lower doses of ionising radiation have proven to be increasingly accurate compared to conventional imaging. Similarly for US scan, additional information on urolithiasis can be obtained via B-mode and Doppler features such as twinkling artefact, ureteral jets and resistive indices measurements. Using readily available scans, stone composition and hardness can now be deduced from advanced CT features such as double-energy CT. Furthermore, differential renal function can now be inferred from CT parameters, although this requires further validation.
References
Raheem IA, Khandwala YS, Sur RL, Ghani KR, Denstedt JD. Burden of urolithiasis: trends in prevalence, treatments, and costs. Eur Urol Focus. 2017;3:18–26.
Geraghty RM, Proietti S, Traxer O, Archer M, Somani BK. Worldwide impact of warmer seasons on the incidence of renal colic and kidney stone disease: evidence from a systematic review of literature. J Endourol. 2017;31(8):729–35.
Fulgam PF, Assimos DG, Pearle MS, Preminger GM. Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: AUA technology assessment. J Urol. 2013;189:1230.
Moreno CC, Beland MD, Goldfarb S, et al. Acute onset flank pain—suspicion of stone disease (urolithiasis). ACR appropriateness criteria. Reston, VA: American College of Radiology; 2015.
Assimos D, Krambeck A, Miller NL, Monga M, Murad MH, Nelson CP, et al. Surgical management of stones: American Urological Association/Endourological Society guideline, part 1. J Urol. 2016;196:1153–60.
Türk C, Skolarikos A, Neisius A, et al. EAU Guidelines on Urolithiasis. In: EAoUG Office, Editor. EAU Guidelines. Edn published as the 35th EAU Annual Meeting, Amsterdam. Arnhem, The Netherlands: European Association of Urology Guidelines Office; 2020.
Jung H, Andonian S, Assimos D, Averch T, Geavlete P, Kohjimoto Y, et al. Urolithiasis: evaluation, dietary factors, and medical management: an update of the 2014 SIU-ICUD international consultation on stone disease. World J Urol. 2017;35:1331–40.
Riccabona M, Avni FE, Blickman JG, Dacher J-N, Darge K, Lobo ML, Willi U. Imaging recommendations in paediatric uroradiology. Minutes of the ESPR uroradiology task force session on childhood obstructive uropathy, high-grade fetal hydronephrosis, childhood haematuria, and urolithiasis in childhood. ESPR Annual Congress, Edinburgh, UK, June 2008. Pediatr Radiol. 2009;39:891–8.
Brisbane W, Bailey MR, Sorensen MD. An overview of kidney stone imaging techniques. Nat Rev Urol. 2016;13(11):654–62.
Villa L, Giusti G, Knoll T, Traxer O. Imaging for urinary stones: update in 2015. Eur Urol Focus. 2016;2(2):122–9. https://doi.org/10.1016/j.euf.2015.10.007.
Masch WR, Cronin KC, Sahani DV, Kambadakone A. Imaging in urolithiasis. Radiol Clin North Am. 2016; https://doi.org/10.1016/j.rcl.2016.10.002.
Mitterberger M, Pinggera GM, Pallwein L, et al. Plain abdominal radiography with transabdominal native tissue harmonic imaging ultrasonography vs unenhanced computed tomography in renal colic. BJU Int. 2007;100:887–90.
Ripolles T, Agramunt M, Errando J, Martinez MJ, Coronel B, Morales M. Suspected ureteral colic: plain film and sonography vs unenhanced helical CT. A prospective study in 66 patients. Eur Radiol. 2014;14:129–36.
Lipkin M, Ackerman A. Imaging for urolithiasis: standards, trends, and radiation exposure. Curr Opin Urol. 2016;26:56–62.
Lamb AD, Wines MD, Mousa S, Tolley DA. Plain radiography still is required in the planning of treatment for urolithiasis. J Endourol. 2008;22:2201–5.
Koo K, Matlaga BR. New imaging techniques in the management of stone disease. Urol Clin North Am. 2019;46(2):257–63. https://doi.org/10.1016/j.ucl.2018.12.007.
Wells ITP, Raju VM, Rowberry BK, et al. Digital tomosynthesis—a new lease of life for the intravenous urogram? Br J Radiol. 2011;84:464–8.
Smith-Bindman R, Aubin C, Bailitz J, Bengiamin RN, Camargo CA Jr, Corbo J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100–10.
Mills L, Morley EJ, Soucy Z, Vilke GM, Lam SHF. Ultrasound for the diagnosis and management of suspected urolithiasis in the emergency department. J Emerg Med. 2018;54(2):215–20.
Gottlieb M, Long B, Koyfman A. The evaluation and management of urolithiasis in the emergency department: a review of the literature. Am J Emerg Med. 2018;36(4):699–706.
Smith RC, Rosenfield AT, Choe KA, et al. Acute flank pain: comparison of non-contrast-enhanced CT and intravenous urography. Radiology. 1995;194:789–94.
Nestler T, Haneder S, Hikamp NG. Modern imaging techniques in urinary stone disease. Curr Opin Urol. 2019;29:81–8.
El-Wahab OA, El-Tabey MA, El-Barky E, El-Baky SA, El-Falah A, Refaat M. Multislice computed tomography vs intravenous urography for planning supine percutaneous nephrolithotomy: a randomised clinical trial. Arab J Urol. 2014;12(2):1627.
Xiang H, et al. Systematic review and meta-analysis of the diagnostic accuracy of low-dose computed tomography of the kidneys, ureters and bladder for urolithiasis. J Med Imaging Radiat Oncol. 2017;61:582.
Rob S, Bryant T, Wilson I, Somani BK. Ultra-low-dose, low-dose, and standard dose CT of the kidneys, ureters, and bladder: is there a difference? Results from a systematic review of the literature. Clin Radiol. 2017;72:11–5.
Meher S, Gibbons N, DasGupta R. Renal stones in pregnancy. Obstet Med. 2014;7:103–10.
Kanal E, Greenberg T, Hoff MN, et al. ACR manual on MR safety. Reston, VA: American College of Radiology; 2020. p. 15–6.
Bowen DK, Tasian GE. Pediatric stone disease. Urol Clin North Am. 2018;45:539–50.
Bres-Niewada E, Dybowski B, Radziszeskiw P. Predicting stone composition before treatment—can it really drive clinical decisions? Cent European J Urol. 2014;67:392–6.
Celik S, Sefik E, Basmaci I, Bozkurt IH, Aydin ME, Yonguc T, Degirmenci T. A novel method for prediction of stone composition: the average and difference of Hounsfield units and their cut-off values. Int Urol Nephrol. 2018;50(8):1397–405. https://doi.org/10.1007/s11255-018-1929-3.
Nayyar R, Khattar N, Sood R. Functional evaluation before stone surgery: is it mandatory? Indian J Urol. 2012;28:256–62.
Taylor AT. Radionuclides in nephrourology, part 1: radiopharmaceuticals, quality control, and quantitative indices. J Nucl Med. 2014;55:608–15.
Sreenevasan G. Bilateral renal calculi. Ann R Coll Surg Engl. 1974;55:9–12.
Feder MT, Blitstein J, Mason B, Hoenig DM. Predicting differential renal function using computerized tomography measurements of renal parenchymal area. J Urol. 2008;180(5):2110–5.
Sarma D, Barua SK, Rajeev TP, Baruah SJ. Correlation between differential renal function estimation using CT-based functional renal parenchymal volume and 99mTc-DTPA renal scan. Indian J Urol. 2012;28:414–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ngoo, KS., Sothilingam, S. (2021). Imaging for Urinary Calculi. In: Ng, A.C.F., Wong, M.Y., Isotani, S. (eds) Practical Management of Urinary Stone. Springer, Singapore. https://doi.org/10.1007/978-981-16-4193-0_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-4193-0_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4192-3
Online ISBN: 978-981-16-4193-0
eBook Packages: MedicineMedicine (R0)