Abstract
Pleomorphic xanthoastrocytoma is a rare glial tumor that often arises from the temporal lobe of young adults. This is considered a low-grade tumor but has an aggressive variant which may arise de novo or after progression from grade II tumors. Surgery is the cornerstone of therapy and confers survival advantage when a GTR is accomplished. Adjuvant radiation therapy is advocated in higher grade tumor or in salvage setting. Recent molecular studies have identified BRAF mutation in nearly 60% patients and BRAF inhibitors are in use with promising results in a subset of patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction [1,2,3,4]
-
Pleomorphic xanthoastrocytoma (PXA) is a low-grade glial tumor constituting 1% brain tumors.
-
Kepes et al. described it for the first time in 1993.
-
Arises from subpial astrocytes.
-
Most commonly affects second and third decade of life (10–30 years); Median age—33 years (Range 4.5–75).
-
No gender predilection.
-
Associated with seizure in young adults and considered a part of epileptogenic tumors spectrum.
-
Nearly 50% of patients present with seizure and one-third present with headache.
-
These tumors commonly arise from the temporal lobe (nearly 50%). Frontal, multi-lobar, and spinal location has also been described.
-
Mostly localized but isolated reports of leptomeningeal dissemination.
-
Most of the tumors are supratentorial, less than 10% are infratentorial.
-
Tumor is superficial, proximal to dura but without involvement.
2 Classification
-
Pleomorphic xanthoastrocytoma—WHO grade II tumors (80%)
-
Anaplastic pleomorphic xanthoastrocytoma (APXA)—WHO grade III tumor (Anaplastic may be de novo or as progression from grade II)
-
Criteria
-
>5 mitoses per 10 high-power fields and increased cellularity
-
Presence of necrosis
-
MIB-1 labeling index >4%
-
3 Investigation
Anaplastic cases appear as high-grade glioma.
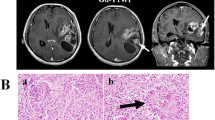
3.1 Appearance on CT Scan
-
Solid and cystic component
-
Appears hypodense or isodense
-
Calcification rare
-
With little or no surrounding edema
-
Cause scalloping of the overlying bone
3.2 MRI
-
Appears solid-cystic tumor with homogenous to heterogeneous contrast enhancement
-
Peripheral eccentric cystic component (50–60%)
-
Exhibit dural tail which is mostly reactive
-
T1: Solid component iso to hypointense
-
T1 post contrast: enhancement
-
T2: Solid component iso- to hyperintense
-
T2 FLAIR: Cystic areas show hyperintensity
4 Pathology
-
Tumors are moderately cellular, predominantly pleomorphic and show foci of lymphoplasmacytic infiltration.
-
Necrosis and mitoses rare.
-
The tumor has been named pleomorphic as there is variable histological feature including spindle cells, polygonal cells, and multinucleated cells.
-
Pathogenesis is driven by MAPK pathway.
-
Nearly 60–70% patients harbor BRAF mutations; less common in anaplastic PXA.
-
Mutation of BRAF confers better survival compared with wild variant.
-
Immunohistochemistry
-
GFAP: Positive
-
S100: Positive
-
Vimentin: Positive
-
Reticulin: Positive
-
PAS: Positive
-
Synaptophysin, MAP2 and neurofilament: variable
-
Ki-67 proliferation index: <1%
-
5 Treatment
5.1 Surgical Excision [5]
-
Surgical excision standard of care and a maximal safe resection is aim of surgery.
-
Nearly 60% patients can undergo GTR.
-
Patients with a GTR have good long-term survival.
GTR (months) | STR (months) | |
|---|---|---|
PFS | 48 | 14 |
OS | NR | 62 |
5.2 Radiation [2, 3, 6,7,8,9,10]
-
Data on adjuvant radiation is limited.
-
Conflicting data about survival advantage in PXA.
-
Radiation has been utilized more in salvage setting (76%).
-
In APXA radiation may be more effective as these patients experience early failure.
5.2.1 Indications for Adjuvant RT
-
PXA with GTR: Not required.
-
PXA with STR or recurrence: RT should be considered.
-
APXA: adjuvant radiation may be considered upfront even after GTR.
5.2.2 Target Volume
-
In adjuvant or salvage setting:
-
Local radiation only (post-operative cavity plus any residual enhancing mass with 1 cm isotropic expansion as CTV and 3–5 mm additional as PTV)
-
-
In CSF positive cases: CSI should be considered.
5.2.3 Dose
Local radiation: 54–60 Gy in conventional fractionation
CSI dose 30 Gy to the entire cranio-spinal axis followed by boost to the primary up to 56–60 Gy
5.3 Chemotherapy
-
Role of chemotherapy controversial.
-
V600E BRAF mutation in nearly 70% patients which constitutively activates RAS/RAF/MEK/ERK signaling pathway.
-
BRAF inhibitor monotherapy or BRAF+MEK inhibitor are used in recurrent tumors.
-
BRAF inhibitors, Vemurafenib and Dabrafenib showed promising result.
7 Follow-up
-
Nearly 50% patients experience progression within 3 years.
-
Local recurrence is most common pattern of recurrence. Leptomeningeal spread seen in 7% patients.
-
Follow-up should be done with clinical examination and CEMRI of brain every 3 months for first 3 years and thereafter every 6 months.
-
Re-surgery followed by radiation or chemotherapy may be used to salvage.
8 Treatment Algorithm

References
Patibandla MR, Nayak M, Purohit AK, Thotakura AK, Uppin M, Challa S. Pleomorphic xanthoastrocytoma with anaplastic features: a rare case report and review of literature with reference to current management. Asian J Neurosurg. 2016;11(3):319.
Mallick S, Benson R, Melgandi W, Giridhar P, Rath GK. Grade II pleomorphic Xanthoastrocytoma; a meta-analysis of data from previously reported 167 cases. J Clin Neurosci. 2018;54:57–62.
Mallick S, Giridhar P, Benson R, Melgandi W, Rath GK. Demography, Pattern of care, and survival in patients with xanthoastrocytoma: a systematic review and individual patient data analysis of 325 cases. J Neurosci Rural Pract. 2019;10(3):430–7.
Herpers MJ, Freling G, Beuls EA. Pleomorphic xanthoastrocytoma in the spinal cord. J Neurosurg. 1994;80(3):564–9.
Fouladi M, Jenkins J, Burger P, Langston J, Merchant T, Heideman R, et al. Pleomorphic xanthoastrocytoma: favorable outcome after complete surgical resection. Neuro-Oncology. 2001;3(3):184–92.
Nakamura M, Chiba K, Matsumoto M, Ikeda E, Toyama Y. Pleomorphic xanthoastrocytoma of the spinal cord. J Neurosurg Spine. 2006;5(1):72–5.
Gill M, Pathak HC, Madan R, Bhattacharya S, Choudhary GS. Primary spinal pleomorphic xanthoastrocytoma. Neurol India. 2010;58(5):771–3.
Simal-Julián JA, Sanchis-Martín R, Prat-Acín R, Miranda-Lloret P, Conde-Sardón R, Cárdenas-Ruiz-Valdepeñas E, et al. Spinal pleomorphic xantoastrocytoma. Neurocirugia (Astur). 2010;21(5):390–5.
Das S, Yip S, Hukin J, Cochrane D, Dunham C. Pleomorphic xanthoastrocytoma of the spinal cord: case report and literature review. Clin Neuropathol. 2014;33(3):190–6.
Jeong JY, Suh YL, Hong SW. Atypical teratoid/rhabdoid tumor arising in pleomorphic xanthoastrocytoma: a case report. Neuropathology. 2014;34(4):398–405.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mallick, S., Anjali, V.R. (2021). Pleomorphic Xanthoastrocytoma. In: Mallick, S., Giridhar, P., Rath, G.K. (eds) Evidence based practice in Neuro-oncology. Springer, Singapore. https://doi.org/10.1007/978-981-16-2659-3_19
Download citation
DOI: https://doi.org/10.1007/978-981-16-2659-3_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2658-6
Online ISBN: 978-981-16-2659-3
eBook Packages: MedicineMedicine (R0)