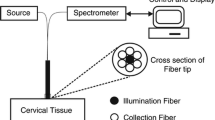
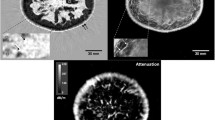
Abstract
Autofluorescence imaging is an emerging method for detection of cancer associated risks, depending on the variation in autofluorescence property of normal and cancer cells. In the present study, autofluorescence images of cervical cell were assessed for early cervical cancer risk prediction. For classification of cervical epithelial cells collected from normal and clinically diagnosed cancer patients, a set of spectral texture features (SPTF) were extracted from 2-dimensional (2-D) Fourier transform of autofluorescence images. To discriminate the normal and the abnormal cells, SPTFs were evaluated by two 1-D functions, i.e., radial function and angular function, accumulated from the frequency spectrum. The classification was assessed by four different types of support vector machines (SVMs), namely, Linear SVM, Quadratic SVM, Cubic SVM, and Gaussian SVM. The best accuracy was achieved by Gaussian SVM (0.95) with a sensitivity of 0.90 and specificity of 0.88. The overall accuracy of all the classifiers was more than 0.82. This experiment was also evaluated by receiver operating characteristics (ROC) curve and area under ROC curve (AUC) of each classifier, which reveals the comparative results for this database.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Cervical cancer, Early diagnosis screening, World Health Organization (WHO). https://www.who.int/health-topics/cervical-cancer. (2020). Accessed 10 Jan 2021
Thekkek, N., Richards-Kortum, R.: Optical imaging for cervical cancer detection: solutions for a continuing global problem. Nat. Rev. Cancer 8(9), 725–731 (2008). https://doi.org/10.1038/nrc2462
Combs, C.A.: Fluorescence microscopy: a concise guide to current imaging methods. Curr. Protoc. Neurosci. 50(1), 2.1.1–2.1.14 (2010). https://doi.org/10.1002/0471142301.ns0201s50.
Chankong, T., Theera-Umpon, N., Auephanwiriyakul, S.: Automatic cervical cell segmentation and classification in Pap smears. Comput. Methods Programs Biomed. 113(2), 539–556 (2014). https://doi.org/10.1016/j.cmpb.2013.12.012
Bora, K., Chowdhury, M., Mahanta, L.B., Kundu, M.K., Das, A.K.: Automated classification of Pap smear images to detect cervical dysplasia. Comput. Methods Programs Biomed. 138, 31–47 (2017). https://doi.org/10.1016/j.cmpb.2016.10.001
Arya, M., Mittal, N., Singh, G.: Texture-based feature extraction of smear images for the detection of cervical cancer. IET Comput. Vis. 12(8), 1049–1059 (2018). https://doi.org/10.1049/iet-cvi.2018.5349
Lorenzo-Ginori, J.V., Curbelo-Jardines, W., López-Cabrera, J.D., Huergo-Suárez, S.B.: Cervical cell classification using features related to morphometry and texture of nuclei. Lect. Notes Comput. Sci. 222–229 (2013).
Plissiti, M.E., Nikou, C., Charchanti, A.: Combining shape, texture and intensity features for cell nuclei extraction in Pap smear images. Pattern Recognit. Lett. 32(6), 838–853 (2011). https://doi.org/10.1016/j.patrec.2011.01.008
Ricketts, I.W.: Cervical cell image inspection - a task for artificial neural networks. Netw. Comput. Neural Syst. 3(1), 15–18 (1992). https://doi.org/10.1088/0954-898X_3_1_003
Chankong, T., Theera-Umpon, N., Auephanwiriyakul, S.: Cervical cell classification using fourier transform. In: 13th International Conference on Biomedical Engineering, pp. 476–480. Springer, Berlin (2009). https://doi.org/10.1007/978-3-540-92841-6_117
Chankong, T.: Automatic classifying of cervical cells using fourier spectral features. In: 2018 4th International Conference on Green Technology and Sustainable Development (GTSD), pp. 759–762 (2018). https://doi.org/10.1109/GTSD.2018.8595662.
Liu, X., Wang, D.: Texture classification using spectral histograms. IEEE Trans. Image Process. 12(6), 661–670 (2003). https://doi.org/10.1109/TIP.2003.812327
Pavlova, I., Sokolov, K., Drezek, R., Malpica, A., Follen, M., Richards, R.: Microanatomical and biochemical origins of normal and precancerous cervical autofluorescence using laser-scanning fluorescence confocal microscopy. Photochem. Photobiol. 77(5), 550–555 (2003). https://doi.org/10.1562/0031-8655(2003)077%3c0550:MABOON%3e2.0.CO;2
Smith-McCune, K.K., Weidner, N.: Demonstration and characterization of the angiogenic properties of cervical dysplasia. Cancer Res. 54(3), 800–804 (1994). http://www.ncbi.nlm.nih.gov/pubmed/7508337
Collier, T., Follen, M., Malpica, A., Richards, R.: Sources of scattering in cervical tissue: determination of the scattering coefficient by confocal microscopy. Appl. Opt. 44(11), 2072-2081 (2005). https://doi.org/10.1364/AO.44.002072
Nakano, T., et al.: Gaits classification of normal vs. patients by wireless gait sensor and support vector machine (SVM) classifier. Int. J. Softw. Innov. 5(1), 17–29 (2017). https://doi.org/10.4018/IJSI.2017010102
Branchitta, F.: Dynamic-range compression and contrast enhancement in infrared imaging systems. Opt. Eng. 47(7), 076401-1 – 076401-14 (2008). https://doi.org/10.1117/1.2956655
Naicker, N., Adeliyi, T., Wing, J.: Linear support vector machines for prediction of student performance in school-based education. Math. Probl. Eng. 2020, 1–7 (2020). https://doi.org/10.1155/2020/4761468
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Adhikary, S., Das, S., Naskar, T.K., Maity, S.P., Barui, A. (2022). Quantitative Autofluorescence Imaging Reveals the Potential for Cervical Cancer Detection. In: Das, A.K., Nayak, J., Naik, B., Dutta, S., Pelusi, D. (eds) Computational Intelligence in Pattern Recognition . Advances in Intelligent Systems and Computing, vol 1349. Springer, Singapore. https://doi.org/10.1007/978-981-16-2543-5_41
Download citation
DOI: https://doi.org/10.1007/978-981-16-2543-5_41
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2542-8
Online ISBN: 978-981-16-2543-5
eBook Packages: Intelligent Technologies and RoboticsIntelligent Technologies and Robotics (R0)