Abstract
Food allergy has increasingly become a public issue throughout the world. Consequently, scientific innovations have been taken to induce chemical modifications to decrease the anaphylaxis symptoms in sensitive individuals or to develop hypoallergenic foods utilizing food processing technologies. Conventional processing techniques covering heat treatment are widely exploited, but to some extent, high temperature may impose undesirable physicochemical changes in food quality with loss of nutrients. Therefore, nonthermal processing technologies could be the alternatives for mitigating the allergenicity. The current knowledge and recent studies are presented in this chapter, especially on the application of cold plasma technology for the abatement of food allergens. Compared with the heat-based methods, cold plasma treatment can adequately retain food quality, while weakening the allergenicity. Food allergen sources, classification, clinical manifestations, and epitope characterization are also introduced in detail. Further research efforts should be made for using cold plasma technology to replace traditional techniques for susceptible individuals who will benefit from diverse hypoallergenic foods and related products handled with innovative nonthermal technologies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Food allergy is an adverse immunological response mediated by Immunoglobulin-E (IgE) that occurs when exposed to certain foods. It is regarded as rapidly emerging public health concerns, affecting approximately 8% of children and 2% of adults in developed countries (Gomaa and Boye 2015; Jerschow et al. 2014; Ayuso et al. 2010). In 2013, according to the investigation of the US centers Centers for Disease Control and Prevention (CDC), the number of the allergic children was increased by 50% from 1997 to 2011 (Gupta et al. 2013). Likewise, over 150 million Europeans endure years of food allergy and presumably at least one suffer from one or more allergies in a breathtaking number of 44% adults living in Britain, rapidly growing by around two million between 2008 and 2009 (Food matters 2010). In addition, from 1995 to 2007, the cases of food allergy among children were reported to have increased up to five times in Australia (Mullins et al. 2009). Generally speaking, more than 170 foods can cause sensitive reactions, and 90% of the cases are thought to be induced by a certain protein in a group of eight allergenic foods, covering milk, soy, crustacean/shellfish, egg, fish, tree nut, and wheat (Thompson et al. 2006; Shriver and Yang 2011). However, incidental ingestions usually happen to allergenic patients, especially if they are unaware that they are allergic to certain foods before consuming. Notably, food allergy-related clinical symptoms cover respiratory issues (e.g., asthma, conjunctivitis, and rhinitis), skin reactions (e.g., erythema, urticaria, rash, eczema, angioedema, and pruritus), and gastrointestinal (GI) tract diseases (e.g., nausea, vomiting, diarrhea, reflux, and abdominal pain) (Cianferoni and Spergel 2009). Meanwhile, clinical symptoms of non-IgE-mediated food allergies include celiac disease, weight depreciation, food protein-induced enterocolitis syndrome, enteropathy and proctitis/proctocolitis, and food-induced pulmonary hemosiderosis (also called Heiner syndrome) (Cianferoni and Spergel 2009; Kim and Burks 2015; Henderson et al. 2012). Other severe clinical manifestation of food allergy might be associated with the life-threatening anaphylaxis such as shock and death. A clinical epidemiological survey of large scale conducted in the United States showed that annually about 125,000 cases of emergency and 53,700 cases of anaphylaxis were caused by food allergies, resulting in hospitalizations of 2000 and deaths of 200 patients (Sicherer and Sampson 2010). Sadly, the best way to avoid allergic symptoms in patients with food allergy is to avoid allergenic ingredient as much as possible.
Antigenic proteins are commonly glycoproteins with a molecular weight of 10–70 kDa, which contain specific sites called epitopes. These epitopes can be recognized based on their three dimensions(3D)structure and the interaction with allergen receptors. In terms of proteins characteristic nature and structure, any processing will not only induce the structure changes of proteins conformation but also simultaneously induce changes of epitopes structural characteristics, so as to influence the recognition of epitopes, thereby reducing or increasing the sensitization. Actually, after some processing like heating, new allergenic compounds called neo-allergens could be produced via interaction with intercomponents or intracomponents. (Kumar et al. 2012).
In human daily life, raw food materials are subjected to different cooking methods to enhance organoleptic and nutritional quality as well as for preservation and detoxification. Thermal processing techniques such as baking, steaming, frying, and boiling are usually selected to achieve these objectives. As for proteins, with the increase of heating treatment temperature, the inherent structure of proteins is gradually destroyed, which may have a positive or negative effect on its allergenicity or even no effect at all. Generally, heating treatment will cause the hydrolysis of peptide bonds and the denaturation and aggregation of disulfide bonds and noncovalent bonds, affecting sensitization of allergenic proteins, manifested in the destruction of existing conformational epitopes, generation of neo-epitopes, or exposure of existing internal epitopes (Gupta et al. 2013). For instance, by the analysis of SDS-PAGE patterns, the immunoreactivity of extracted proteins in lentil and chickpea was both reduced after boiling for 60 and autoclaving for 30 min (Cuadrado et al. 2009). This implies that positive results require severe processing conditions; however the losses in sensorial and nutritional profile are generated by comparatively high temperature.
As a promising nonthermal tool, cold plasma technique can induce conformational changes and reduce the allergenicity of allergic proteins, while retaining the flavor and quality of the food materials. Hence, researchers have explored the effect of nonthermal-based processing techniques on certain proteins and the potential of manufacturing hypoallergenic foods using cold plasma technology. On this basis, this chapter summarizes research findings focusing on cold plasma processing technique to modify and abate food allergens, which means to give a comprehensive and updated overview on current research about cold plasma processing technology to the control of food allergens.
6.2 Food Allergens and Their Properties
Food allergies are mediated by different immune mechanisms, generally sorted into IgE-mediated, non-IgE-mediated, and mixed reaction types. Among them, the IgE-mediated type of allergenic reactions occurs when allergenic peptides or proteins permeate into the respiratory lining, gut, or skin and then IgE antibodies are produced in patients bodies (Mansouri 2015). As shown in Fig. 6.1 (example with seafood allergen), after digested through the GI tract, the resulting peptides from food proteins are processed by antigen-presenting cells and released to T cells in predominantly complex constrained manner of histocompatibility complex (MHC), which activates T cells to produce the corresponding cytokines. Subsequently, food-specific IgE antibodies are produced, which attach to the IgE receptor present on the basophil receptor. When re-contact with the same food allergen, the hypersensitivity reaction triggered. It could cause a relative swift immune response, which provokes the degranulation of effectors cells and the liberation of pre-formed mediators such as histamine, and prompts the immediate symptoms of allergic reactions (Cianferoni and Spergel 2009). Non-IgE type is usually mediated by cellular immunity, associated with delayed symptoms (either subacute or chronic in nature), which is primarily restricted to the GI tract and occurs within hours or days (Henderson et al. 2012). And the mixed reaction type mediated by a mixture of IgE and non-IgE consists of an overlap between immediate and delayed onset, and its clinical manifestation includes either atopic dermatitis or eosinophilic gastroenteropathy (Mansouri 2015).
6.2.1 Characteristics of Food Allergens
Food allergens are antigenic molecules, usually referred to as proteins or glycoproteins, which provoke allergic reactions in the patients’ body. Most food allergens are highly stable, resistant not only to high temperature and pressure but also to hydrolysis and digestion (Sicherer and Sampson 2010). However, this variation in the nature of food allergens is possible because different proteins have their own structural properties and may be modified at different sites during processing. For example, vicilins and TMs are both food allergens, but the former is oligomer while the latter contains repeat peptide units, which result in increased IgE epitope valence and higher immunogenicity. In addition, allergenic proteins (e.g., lipid transfer proteins and 2S albumins) with extramolecular or intramolecular disulfide bonds may be more allergenic and more resistant to heat treatment or acid denaturation. Similarly, binding of ligands (such as metal ions, lipids, and steroids) to allergenic proteins (such as casein, albumin, beta-lactoglobulin, betv1, etc.) may stabilize the structural conformation of the protein and enhance its stability against enzymolysis; the action of most proteases depends on the properties of protein substrate (Breiteneder and Mills 2005; Sathe et al. 2016). It is important to mention that ligands can bind to a specific protein involving a series of complex interactions. Caseins (αs1-, αs2-, and β-caseins), for example, often contain residues of phosphate groups such as phosphoserine or phosphothreonine, which can chelate calcium mental ions to form the microstructures of nanoclusters. About 1000 of these nanoclusters combine to form casein micelles in milk (Breiteneder and Mills 2005). Meanwhile, PV protein is globular in shape and contains six helices; also, it is an EF-hand protein, which means the protein is characterized by the presence of a helix, a loop, and a second helix, with the two helices arranged like thumb and index finger of people’s one hand (Moonesinghe et al. 2016; Ruethers et al. 2018). There are three helix-loop helical structures in PV structure, and two are able to bind divalent cations, such as Ca2+ and Mg2+, while third domain covers the hydrophobic surface of these functional regions (Bugajskaschretter et al. 2000). PV is a low molecular weight calcium-binding protein that participates in calcium signaling pathways. And releases of the protein-bound calcium induce conformational changes and accompanying loss of its sensitization (Virginie 2020). Therefore, the use of antacids in patients who are allergic to PV is inappropriate because neutralization of the acid reduces calcium removal and may result in PV resistance to pepsin, increasing the susceptibility of sensitive individuals (Untersmayr et al. 2003).
Specific chemical groups called epitopes can react with antibodies or be recognized by antigen receptors, thus triggering immune responses, which can be categorized into linear and conformational epitopes. Also it can be classified into T cells and B cells epitopes according to their receptors. Linear epitopes are composed of successive short peptides, while conformational epitopes are specific three-dimensional structures of amino acid residues formed in spatial proximity to each other that can be recognized by immunoactive substances. Various techniques have been used to map B cell and T cell epitopes, providing important information to advance the pathogenesis of food allergy and the mechanisms of immune tolerance. Specifically, methods for determining IgE-binding linear epitopes include polypeptide microarrays and overlapping peptide sequences (Martínez-Botas et al. 2013). Relatively complex conformational epitopes are available using techniques such as nuclear magnetic resonance (NMR), X-ray crystallography, and silicon analysis. In addition, hydrogen/deuterium exchange footprint analysis may also be used for analysis but is not suitable for conformational epitopes recognized by human polyclonal IgE receptors. For helper and regulatory T cells, their epitopes can be mapped by peptides with 10–20 residues, which is the overlapping area of target allergens and specific T cell sequences. Alternatively, T cell epitopes can be identified by silico analysis, despite the fact that some synthetic epitopes are ought to be verified by T cells from allergenic patients, to ensure their biological reactivity (Nielsen et al. 2010).
6.2.2 The Most Common Allergenic Foods and Related Allergens
The main seafood allergens consist of parvalbumin (PV), collagen, tropomyosin (TM), arginine kinase (AK), sarcoplasmic calcium-binding protein (SCP), triosephosphate isomerase (TIM), and myosin light chain (MLC) and the crystal structures of main allergens in seafood, which are shown in Fig. 6.2. Also, the most common allergenic food resources and their associated allergens are given in Table 6.1.
6.3 Cold Plasma Technology for Food Allergy Control
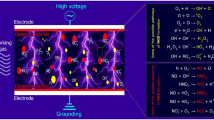
With the increasing requirements of health and safety of food, plasma technology has gained immerse attention in recent years. As a potent technology, plasma technology has been proved suitable for effective preservation, microbial decontamination, and modification of food products. Plasma can be divided into high temperature plasma and cold plasma according to the system temperature (Ekezie et al. 2018). The discharge ways of cold plasma include dielectric barrier discharge (DBD), radio frequency (RF) discharge, corona discharge, plasma jet, and so on (Han et al. 2019). The electrical conductivity of plasma is extremely high and different from ordinary gases in electromagnetic properties; hence it is commonly regarded as the fourth state of matter, which is an aggregate of reactive particles generated following excitation and ionization of gas, i.e., UV photons, ions, electrons, free radicals, neutral molecules, and excited atoms. These active constituents form active oxidation system waves: reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Surowsky et al. 2015). It has been reported that these plasma reactive components could induce certain interactions with proteins and then their conformational structures changed (Tammineedi et al. 2013; Gavahian and Khaneghah 2020). Because food allergens are mostly proteins, cold plasma can react with allergens in a similar way through these above active particles (Tolouie et al. 2017). Currently, many conjectures about the mechanism of controlling allergens by cold plasma have been posed; although the exact pathway is not clear, all depend on the linear and conformational epitopes alterations induced by active particles. To be specific, conformational epitopes can be destroyed by the aggregation or crosslinking of proteins, while linear epitopes can be altered by fragmentation. Besides, active substances may lead to the breakage of peptide bonds and the oxidation of amino acids, resulting in the destruction of protein integrity (Surowsky et al. 2013). Disulfide bonds in proteins can also be attacked by free hydroxyl radical in active particles to form RSH and RSO (Surowsky et al. 2013; Meinlschmidt et al. 2016). All the above possible mechanisms reflect that, to a certain extent, the active oxidizing substances in the cold plasma will affect the protein structures, thereby incapacitating the antibody binding sites and achieving the purpose of controlling allergenicity.
6.3.1 Cold Plasma Technology for Plant Food Allergy Control
Cold plasma has been increasingly used for a lot of purposes in the food industry, including sterilization, modification of foodstuff, degradation of fungal toxins and pesticide residues, enhancement of the germination and mutation breeding of grains, and packaging surface sterilization (Thirumdas et al. 2015; Alves Filho et al. 2016). In the past decades, the effects of cold plasma on plant food allergen have been reported on the immune reactivity of soybean (Meinlschmidt et al. 2016), peanuts (Venkataratnam et al. 2019), and wheat (Sun et al. 2020). To explore the influence of plant foods with cold plasma treatment, Meinlschmidt et al. (2016) found out that after direct or remote cold plasma treatment of soy allergen (e.g., β-conglycinin and glycinin), the SDS-PAGE profile of the protein bands became less intense with the extension of treatment time, and new protein bands formed at 50 kDa after 5 min and 60 min, respectively. In addition, ELISA results using specific mouse monoclonal antibodies showed that cold plasma treatment of soybean protein isolates reduced its immunoreactivity by as much as 89–100% over time (Meinlschmidt et al. 2016). The loss of protein bands may be due to the decrease of protein solubility after cold plasma treatment and the formation of insoluble aggregates, while SDS-PAGE could only detect soluble proteins. And the generation of new bands can be explained by the formation of aggregates formed by cross-linkage between free amino acids. Similarly, in a previous study on proteins extracted from Pisum sativum, its solubility reduced after plasma treatment (Bußler et al. 2015). Likely, wheat allergenicity was reduced by 37% when subjected to DBD plasma (Nooji 2011). In a recent study, Venkataratnam et al. (2019) investigated the effects of cold atmospheric plasma on the antigenicity of protein Ara h 1 with dry, defatted peanut flour (DPF),and whole peanut (WP) samples at voltage of 80 kV for different treatment durations (0, 15, 30, 45, and 60 min). The SDS-PAGE results revealed no changes in the protein intensity bands corresponding to Ara h 1 for both DPF and WP. Competitive ELISA of samples showed a reduction in antigenicity up to 43% for DPF and 9.3% for WP. Circular dichroism studies revealed modifications in secondary structure induced by plasma reactive species (Venkataratnam et al. 2019). Nevertheless, conversely to the above, Alves Filho et al. (2016) investigated the influence of atmospheric plasma processing on cashew nut allergenicity. To determine the effect of plasma processing on cashew nut allergens, direct ELISA using protein extracts from processed cashew nuts was used to compare antibody binding. Extracts from processed nuts were first evaluated by SDS-PAGE. The results showed that there was not a statistically significant difference in binding of either the rabbit anti-cashew or human cashew allergic IgE to the processed cashew nut extract samples, indicating the plasma processing steps evaluated here would not be useful in the generation of cashew nuts with reduced potential to cause allergic reactions (Alves Filho et al. 2016). Conceivably, this failure to achieve the expected decrease in immunoreactivity might be responsible for the low efficiency of remote plasma. More studies aimed at validating the potency of cold plasma are needed to control plant food allergy.
6.3.2 Cold Plasma Technology for Seafood Allergy Control
Seafood such as fish, crustacean, and mollusk is favored by individuals due to its high value of nutrition, valuable source of protein, physiologically active substances (e.g., docosahexaenoic acid), and minerals (e.g., iron, calcium, magnesium) (Kubota et al. 2016; Sicherer and Sampson 2018). Therefore, seafood is of great significance in human nutrition and health, but with the rapidly increasing consumption of seafood, allergy mediated by IgE is turning into a severe problem around the world, especially in coastal areas. Seafood allergy is one kind of the serious causes of hypersensitivity reactions such as diarrhea, vomiting, and life-threatening anaphylaxis (Ruethers et al. 2018). Seafood allergy can be caused not only by ingestion but also by inhaling aerosolized proteins during capture or cooking process (De Muto et al. 2020). In addition, different seafood allergens have cross-reactivity; thus, reducing the allergens at the sources can effectively avoid allergic reaction (Davis et al. 2020).
As for the influence of seafood with cold plasma treatment, the current studies focused on the modification of protein configuration to reduce allergenicity (Venkataratnam et al. 2019). It was reported that when exposed to free radicals of cold plasma, the secondary structure of protein is damaged, including the changes in percentages of α-helix and β-sheet, decomposition of amide bonds and side chains, which might mask or disrupt the conformational binding epitopes, leading to the decrease of allergenicity (Sarangapani et al. 2018). It was believed that hydroxyl radicals produced by cold plasma had the capacity of oxidization. Then hydroxyl radicals disrupted the antibodies binding sites, resulting in the reduction of allergenicity (Wu et al. 2014). In our research group, Ekezie et al. (2019a) ascertained the impact of atmospheric pressure plasma jet treatment (APPJ) as a function of treatment times (0, 2, 4, 6, 8, and 10 min) on the conformation and physicochemical properties of myofibrillar proteins (MPs) extracted from king prawn (Litopenaeus vannamei). The results showed that the pH and protein solubility were decreased after 10-min treatment of APPJ. These results were also confirmed by dynamic light scattering (DLS) analysis, which indicated an increase in the mean particle diameter of MPs from 654 to 2297 nm. Complementary methodologies used to characterize the structural changes confirmed the exposure of hydrophobic groups and promotion of protein-protein interactions. These occurrences were particularly more intense at the longest treatment duration. The high energetic and oxidizing species contained in the plasma gas may have considerable implications on the physicochemical and structural characteristics of MPs from king prawn. After that, Ekezie et al. (2019b) explored the changes of the IgE-binding capacity of king prawn (Litopenaeus Vannamei) TM through conformational changes induced by cold argon-plasma jet (CAPJ). The result showed that the level of α-helix structures declined as the treatment time progressed, while the level of β-sheets and random coils increased. The free sulfhydryl groups decreased as CAPJ treatment progressed due to the formation of disulfide bonds while surface hydrophobicity increased until equilibrium values. In addition, after 15 min of plasma exposure, the maximum reduction recorded for IgE and IgG binding capacities were 17.6% and 26.87%, respectively, as revealed by ELISA test. These results were attributed from the unfolding and denaturation of proteins, which was indicated by complementary structural analysis. The study indicated that the nonthermal plasma might be a hopeful means for developing hypoallergenic food products. In addition, Shriver (2011) found that when the treatment conditions were set as 30 kV, 60 Hz, and 5 min, the DBD plasma reduced the hypersensitivity of prawn by 67%, which may be explained by the changes of protein configuration caused by active particles and then the changes of the epitopes, so that the IgE antibody could no longer recognize them and could not provide any conditions for the immune response. Thus, more experiments are needed to confirm the effectiveness of cold plasma, and the optimization of processing parameters needs further research.
6.4 Advantages and Limitations of Cold Plasma for Food Allergy Mitigation
Cold plasma for food allergy mitigation mentioned in this chapter affects structural characteristics and physicochemical nature of food proteins in a variety of ways, thus influencing gastrointestinal digestion, bioavailability, and alleviating their sensitization. During thermal processing, heat reduces allergenic potency by changing protein structure, affecting antibody binding epitopes and improving digestibility, but it may have adverse influence on quality attributes. Currently, the hypoallergenic food products marketed in stores are mainly produced by enzymatic hydrolysis; however, because the degree of enzymatic hydrolysis is difficult to control, the amino acids, dipeptides, and tripeptides obtained from excessive hydrolysis as well as their hydrophobicity are closely related to the bitter taste, making the protease hydrolysates often contain bitter peptides and unacceptable by consumers. In addition, the influence of food allergen processing methods on allergen sensitization is also affected by many complex factors, such as the influence of other food components, the properties of allergen, and the selection of processing conditions. The cumulative effect of all these factors has also led to inconsistent results in the treatment and remission of food allergens using cold plasma technology. In spite of this, many of the existing studies have shown cold plasma in alleviating food allergy potential in this field; currently the main challenge and the most urgent need is to focus on the introduction of cold plasma standardization. Certainly, a comprehensive understanding of the mechanisms of cold plasma treatment and the properties of the allergen is the key to further implementation and application.
6.5 Conclusions
Food allergy is a matter of global human health concerns. With good prospect in application of reducing allergenicity, cold plasma processing technology induces protein aggregation or crosslinking to change allergic protein conformational epitopes, promotes the fragments of peptide bond fracture or fraction amino acid sequences to change the linear epitopes, and helps to reduce food protein allergy. Although it is not possible to completely eliminate food allergens through processing at present, threshold attenuation can be achieved through optimization of processing conditions. Therefore, developing evaluated and validated methods to confirm the application potential of cold plasma is necessary.
For further development, more studies about the alleviation of allergens are required. All clinically relevant seafood allergens should be identified as much as possible. The cross-reactivity between different types of seafood allergens or with other food allergens also requires more work to do. The allergen mitigation mechanism by cold plasma should be fully understood in order to achieve precision optimization of the treatment parameters. With the knowledge, we can better understand the characteristics of seafood allergens and choose the appropriate technology to reduce allergens. Moreover, to reliably use cold plasma in the food industry, adequate toxicity studies such as animal and human tests should be conducted to determine whether ingestion of foods treated with cold plasma can cause toxicity in vivo. These key areas of research in future will provide valuable support for the development of low-allergenic or no-allergenic products.
References
Alves Filho EG, Cullen PJ, Frias JM, Bourke P, Tiwari BK, Brito ES, Rodrigues S, Fernandes FAN (2016) Evaluation of plasma, high-pressure and ultrasound processing on the stability of fructooligosaccharides. Int J Food Sci Technol 51(9):2034–2040
Ayuso R, Sanchez-Garcia S, Lin J, Fu Z, Dolores Ibanez M, Carrillo T, Blanco C, Goldis M, Bardina L, Sastre J, Sampson HA (2010) Greater epitope recognition of shrimp allergens by children than by adults suggests that shrimp sensitization decreases with age. J Allergy Clin Immunol 125(6):1286–1293
Breiteneder H, Mills ENC (2005) Molecular properties of food allergens. J Allergy Clin Immunol 115(1):14–23
Bugajskaschretter A, Grote M, Vangelista L, Valent P, Sperr WR, Rumpold H, Pastore A, Reichelt R, Valenta R, Spitzauer S (2000) Purification, biochemical, and immunological characterisation of a major food allergen: different immunoglobulin E recognition of the apo- and calcium-bound forms of carp parvalbumin. Gut 46(5):661–669
Bußler S, Steins V, Ehlbeck J, Schlüter O (2015) Impact of thermal treatment versus cold atmospheric plasma processing on the techno-functional protein properties from Pisum sativum ‘Salamanca’. J Food Eng 167:166–174
Cianferoni A, Spergel JM (2009) Food allergy: review, classification and diagnosis. Allergol Int 58(4):457–466
Cold plasma as a tool for the elimination of food contaminants: recent advances and future trends. Crit Rev Food Sci Nutr 60(9):1581–1592
Cuadrado C, Cabanillas B, Pedrosa MM, Varela A, Guillamón E, Muzquiz M, Crespo JF, Rodriguez J, Burbano C (2009) Influence of thermal processing on IgE reactivity to lentil and chickpea proteins. Mol Nutr Food Res 53(11):1462–1468
Davis CM, Gupta RS, Aktas ON, Diaz V, Kamath SD, Lopata AL (2020) Clinical management of seafood allergy. J Allergy Clin Immunol Pract 8(1):37–44
De Muto M, Trambusti I, Bini G, D’Elios S, Franchetti E, Rosati S, Costagliola G, Di Cicco M, Comberiati P, Peroni D (2020) Anaphylaxis to shellfish by inhalation of cooking vapor in a child. Pediatr Allergy Immunol 31:71
Ekezie F-GC, Cheng J-H, Sun D-W (2018) Effects of nonthermal food processing technologies on food allergens: a review of recent research advances. Trends Food Sci Technol 74:12–25
Ekezie F-GC, Cheng J-H, Sun D-W (2019a) Effects of atmospheric pressure plasma jet on the conformation and physicochemical properties of myofibrillar proteins from king prawn (Litopenaeus vannamei). Food Chem 276:147–156
Ekezie F-GC, Sun D-W, Cheng J-H (2019b) Altering the IgE binding capacity of king prawn (Litopenaeus vannamei) tropomyosin through conformational changes induced by cold argon-plasma jet. Food Chem 300:125143
Foods Matter (2010) Mintel’s allergy and allergy remedies UK. Retrieved 27th November, 2017, from http://www.foodsmatter.com/allergy_intolerance/miscellaneous/articles/mintel_allergy_report_2010.html
Gavahian M, Khaneghah AM (2020) Cold plasma as a tool for the elimination of food contaminants: recent advances and future trends. Crit Rev Food Sci Nutr 60(9):1581–1592
Gomaa A, Boye J (2015) Impact of irradiation and thermal processing on the immunochemical detection of milk and egg allergens in foods. Food Res Int 74:275–283
Gupta RS, Holdford DA, Bilaver L et al (2013) The high 7 economic burden of childhood food allergy in the United States. J Allergy Clin Immunol 131(2):AB223
Han Y, Cheng J-H, Sun D-W (2019) Activities and conformation changes of food enzymes induced by cold plasma: a review. Crit Rev Food Sci Nutr 59(5):794–811
Henderson CJ, Abonia JP, King EC, Putnam PE, Collins MH, Franciosi JP, Rothenberg ME (2012) Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol 129(6):1570–1578
Jerschow E, Lin RY, Scaperotti MM, Mcginn AP (2014) Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. J Allergy Clin Immunol 134(6):1318
Kim EH, Burks W (2015) Immunological basis of food allergy (IgE-mediated, non-IgE-mediated, and tolerance). Chem Immunol Allergy 101:8–17
Kubota H, Kobayashi A, Kobayashi Y, Shiomi K, Hamada-Sato N (2016) Reduction in IgE reactivity of Pacific mackerel parvalbumin by heat treatment. Food Chem 206:78–84
Kumar S, Verma AK, Das M, Dwivedi PD (2012) Molecular mechanisms of IgE mediated food allergy. Int Immunopharmacol 13(4):432–439
Mansouri M (2015) Food allergy: a review. Archiv Pediatr Infect Dis 3(3):e22470
Martínez-Botas J, Cerecedo I, Zamora J, Vlaicu C, Dieguez MC, Gómez-Coronado D, De Dios V, Terrados S, De La Hoz B (2013) Mapping of the IgE and IgG4 sequential epitopes of ovomucoid with a peptide microarray immunoassay. Int Arch Allergy Immunol 161(1):11–20
Meinlschmidt P, Ueberham E, Lehmann J, Reineke K, Schlüter O, Schweiggert-Weisz U, Eisner P (2016) The effects of pulsed ultraviolet light, cold atmospheric pressure plasma, and gamma-irradiation on the immunoreactivity of soy protein isolate. Innovative Food Sci Emerg Technol 38:374–383
Moonesinghe H, Mackenzie H, Venter C, Kilburn S, Turner P, Weir K, Dean T (2016) Prevalence of fish and shellfish allergy: a systematic review. Ann Allergy Asthma Immunol 117(3):264–272
Mullins RJ, Dear K, Tang MLK (2009) Characteristics of childhood peanut allergy in the Australian capital territory, 1995 to 2007. J Allergy Clin Immunol 123(3):689–693
Nielsen M, Lund O, Buus S, Lundegaard C (2010) MHC class II epitope predictive algorithms. Immunology 130(3):319–328
Nooji JK (2011) Reduction of wheat allergen potency by pulsed ultraviolet light, high hydrostatic pressure, and nonthermal plasma. University of Florida, M.Sc thesis, University of Florida, Florida, United States
Ruethers T, Taki AC, Johnston EB, Nugraha R, Le TT, Kalic T, McLean TR, Kamath SD, Lopata AL (2018) Seafood allergy: a comprehensive review of fish and shellfish allergens. Mol Immunol 100:28–57
Sarangapani C, Patange A, Bourke P, Keener K, Cullen PJ (2018) Recent advances in the application of cold plasma technology in foods. In: Doyle MP, Klaenhammer TR (eds) Annual Review of Food Science and Technology, pp 609–629
Sathe SK, Liu C, Zaffran VD (2016) Food Allergy. Annu Rev Food Sci Technol 7(1):191–220
Shriver SK (2011) Effect of selected nonthermal processing methods on the allergen reactivity of Atlantic white shrimp (Litopenaeus setiferus), MSc Thesis, University of Florida, Florida, United States
Shriver SK, Yang WW (2011) Thermal and nonthermal methods for food allergen control. Food Eng Rev 3(1):26–43
Sicherer SH, Sampson HA (2010) Food allergy. J Allergy Clin Immunol 125(2):S116–S125
Sicherer SH, Sampson HA (2018) Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 141(1):41–58
Sun F, Xie X, Zhang Y, Duan J, Ma M, Wang Y, Qiu D, Lu X, Yang G, He G (2020) Effects of cold jet atmospheric pressure plasma on the structural characteristics and immunoreactivity of celiac-toxic peptides and wheat storage proteins. Int J Mol Sci 21(3):1012
Surowsky B, Fischer A, Schlueter O, Knorr D (2013) Cold plasma effects on enzyme activity in a model food system. Innovative Food Sci Emerg Technol 19:146–152
Surowsky B, Schlueter O, Knorr D (2015) Interactions of non-thermal atmospheric pressure plasma with solid and liquid food systems: a review. Food Eng Rev 7(2):82–108
Tammineedi CVRK, Choudhary R, Perez-Alvarado GC, Watson DG (2013) Determining the effect of UV-C, high intensity ultrasound and nonthermal atmospheric plasma treatments on reducing the allergenicity of α-casein and whey proteins. LWT Food Sci Technol 54(1):35–41
Thirumdas R, Sarangapani C, Annapure US (2015) Cold plasma: a novel non-thermal technology for food processing. Food Biophys 10(1):1–11
Thompson T, Kane RR, Hager MH (2006) Food allergen labeling and consumer protection act of 2004 in effect. J Am Diet Assoc 106(11):1742–1744
Tolouie H, Hashemi M, Mohammadifar MA, Ghomi H (2017) Cold atmospheric plasma manipulation of proteins in food systems. Crit Rev Food Sci Nutr 58(15):2583–2597. https://doi.org/10.1080/10408398.2017.1335689
Untersmayr E, Scholl I, Swoboda I, Beil WJ, Forsterwaldl E, Walter F, Riemer AB, Kraml G, Kinaciyan T, Spitzauer S (2003) Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol 112(3):616–623
Venkataratnam H, Sarangapani C, Cahill O, Ryan CB (2019) Effect of cold plasma treatment on the antigenicity of peanut allergen Ara h 1. Innovative Food Sci Emerg Technol 52:368–375
Virginie D (2020) What we know about fish allergy by the end of the decade? J Investig Allergol Clin Immunol 29(6):414–421
Wu Y, Liang Y, Wei K, Li W, Yao M, Zhang J (2014) Rapid allergen inactivation using atmospheric pressure cold plasma. Environ Sci Technol 48(5):2901–2909
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Zhejiang University Press
About this chapter
Cite this chapter
Cheng, JH., Wang, H., Ekezie, FG.C., Li, J. (2022). Abatement of Food Allergen by Cold Plasma. In: Ding, T., Cullen, P., Yan, W. (eds) Applications of Cold Plasma in Food Safety. Springer, Singapore. https://doi.org/10.1007/978-981-16-1827-7_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-1827-7_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1826-0
Online ISBN: 978-981-16-1827-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)