Abstract
Fusion imaging opens up new possibilities for intervention planning, implementation, and control, but also offers the opportunity to better assess remission or progress in the follow-up of liver tumor treatment. Contrast enhanced ultrasound (CEUS) with image fusion enables the experienced radiological examiner to dynamically record the tumor microvascularization down to the capillary level. It can facilitate the diagnosis and therapy control after liver interventions. In addition to the primary applications of image fusion in the diagnosis and treatment of liver lesions, further useful indications can be integrated into the daily work routine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Development of Ultrasound Fusion Imaging
The first examinations with an image fusion ultrasound (US) with the CT, later also the MRI, were very time consuming and could only be implemented with special additional US navigation technology. The fusion technique on US devices was initially only applicable to very experienced investigators.
The next steps were to increasingly integrate the technology into high-performance ultrasound devices and to make the technology more readily available. The examination times could be shortened significantly and advantages, such as improved tumor detection and characterization, became apparent in liver tumors. The faster and more precise the systems became, the more they were used to carry out targeted punctures, biopsies, and especially ablative therapies and transarterial chemoembolization (TACE), in order to plan them and to monitor them in the follow-up.
The fusion techniques are expanding to more and more indications and are regarded as the basis of automated navigation procedures. Fusion imaging opens up new possibilities for intervention planning, implementation, and control, but also offers the opportunity to better assess remission or progress in the follow-up of liver tumor treatment [1].
13.2 Basics of Image Fusion
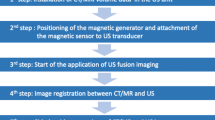
For a dynamic image fusion between ultrasound and a slice image process in real time, a magnetic field generator and a corresponding transducer sensor are required as hardware. A magnetic location system enables the transducer sensor position to be detected, and thus the exact spatial position of the sensor in the room can be calculated. For image fusion, digital imaging, and communications in medicine (DICOM) data sets of all common slice image methods (CT, MRI, PET-CT) can be used. For this purpose, the DICOM data are loaded into the ultrasound system and the data records are then registered manually using anatomical landmarks or automatically based on image recognition features. After a successful data fusion, the registered sectional image data move simultaneously to the sonographic sectional plane. Various presets are optionally available, which optionally display the registered images in the overlay technique or in the side-by-side view. The color-coded duplex sonography (FKDS), Power Doppler (PD), or CEUS can be easily integrated into the merged image. Thus, the simultaneous use of the CEUS and the image fusion gives the possibility of a tumor-related assessment of the microvascularization in direct comparison to the contrast medium-enhanced CT or MRI [2, 3].
13.3 Clinical Application of Image Fusion
CEUS with image fusion enables the experienced radiological examiner to dynamically record the tumor microvascularization down to the capillary level. In addition to the primary applications of image fusion in the context of diagnostics, targeted biopsies, drainage systems, radio frequency ablation (RFA), microwave ablation (MWA), irreversible electroporation (IRE), and chemoembolization (TACE) can also be planned and carried out. New possibilities open up for effective diagnosis and therapy of oncological diseases by integrating all image modalities on a modern ultrasound device [1].
Currently, with the fast development of medical imaging, the detectability of HCC in early stages has been significantly improved [4]. Ultrasound is regarded as the first-line real-time guidance imaging method for percutaneous ablation. Due to some small or isoechoic HCC lesions in liver cirrhosis background, or due to intervening factors such as the bowel gas or diaphragm, various challenges may exist during the real-time ultrasound guidance. By merging the real-time images from ultrasound/CEUS with a previously obtained CT/MRI, fusion imaging methods expands the feasibility and success rates of ablation procedures [5,6,7,8]. Ultrasound has been improved in the detection and characterization of focal liver lesions [4]. In addition, fusion imaging method can also be used for real-time guidance of intervention procedures. The reported success rates of fusion-guided biopsies or CEUS-guided tumor ablation were between 80% and 100% [3, 4, 9].
13.4 Contrast Enhanced Ultrasound and Image Fusion
With modern ultrasound technology, contrast enhanced sonography (CEUS) enables dynamic detection of microvascularization at the capillary level. If sulfur hexafluoride microbubbles (SonoVue®/Bracco) are used as ultrasound contrast signal amplifiers, the oscillation of the microbubbles with a low mechanical index (MI) <0.2 and the corresponding contrast agent software can be used to dynamically record liver blood flow and tumor vessels. CEUS is becoming increasingly important for the detection and characterization of malignant liver lesions and allows percutaneous treatment when surgery is not possible. CEUS imaging fusion with CT and MRI opens up further options for targeted and modified tumor treatments (Figs. 13.1 and 13.2).
With regard to planning, implementation and control, as well as the follow-up, CEUS has already taken on a fixed diagnostic role for detection and characterization of liver tumors. The decisive advantage is that when using ultrasound contrast agents, repeated intravenous contrast agent applications can be carried out without stressing the kidneys, and moreover, there is no impairment of thyroid function. CEUS, therefore, is particularly suitable in situations in which the administration of contrast media on CT or MRI for liver tumor diagnosis, intervention preparation, or control after interventions is restricted due to contraindications considering contrast media. These application options are furthermore also available for the fusion of CEUS with MRT, CT, or PET CT [10,11,12].
13.4.1 CEUS Image Fusion with CT or MRI for Intervention Planning, Treatments, and Follow-Up
The possibilities to perform a fusion by existing CT or MRI data in DICOM format stored on high-performance ultrasound devices are now available from almost all device manufacturers. The tumor findings in CT or MRI can be transferred to the real-time image in ultrasound. For CEUS, malignancy criteria include irregular arterial hypervascularization and an increasing wash-out in the late phase. With CEUS, it is possible to compare the dynamic microvascularization of the early arterial phase after 10–15 s after i.v. bolus injection until to the late phase of 3–5 min. Thus, before tumor treatment by surgery, or if this is not possible by an ablative procedure or embolization, the degree of dynamic hypervascularization and the exact vascular relationship with the fusion CEUS for CT or MRI could be performed.
In cases of difficult conditions, navigation systems facilitate a biopsy to histologically secure even small suspicious tumor lesions. Targeted ablation can be facilitated under difficult angulated puncture conditions by the tracking systems with global positioning systems (GPS) control. Initial studies on control after interventions show advantages of a CEUS fusion with CT or MRI for early detection of tumor recurrences. In addition, a merger can enable targeted intervention or ablation and contribute valuable information about the location of the tumor herd, even if it is not or only partially visible in the B-mode [2].
The use of this technique in combination with further sectional imaging can result in new aspects or a modified tumor treatment for the patient at tumor conferences. Whether additional examinations can really be avoided if existing CT or MRI data sets are used would be an interesting cost aspect of the fusion technology on the US device to be investigated. However, the technology has so far been reserved for individual centers and is only used for improved training and educational purposes to learn targeted punctures and drainages.
13.4.2 Diagnosis of Liver Tumors and Fusion Imaging
The combination of CEUS and CT lends itself to the characterization and detection of unclear liver foci and enables improved detection and assessment of unclear focal liver lesions. A “wash-out” of the liver lesion beginning in the portal venous phase and increasingly in the late phase is considered a malignancy criterion in the CEUS; an increasing contrast agent enhancement of the liver lesion characterizes a benign lesion. The dynamic contrast agent effects of CEUS can be optimally used by a fusion CEUS/CT if no contrast agent can be applied for tumor detection in CT or if, for example, the contrast is not optimal due to circulatory effects or the contrast agent protocol does not show arterial (15–45 s) or late phase (3–5 min). Fusion imaging can be used for both, lesion detection and characterization, and finally supports consecutive therapy [2, 13].
Oncological tumor boards discuss whether surgery is possible or whether percutaneous interventional intervention makes sense, e.g., TACE, RFA, MWA, or, if necessary, selective internal radiotherapy (SIRT). Image fusion of CEUS with CT allows a reliable, highly specific post-interventional evaluation of TACE success with good sensitivity and without any further radiation exposure (Fig. 13.3). It can detect residual viable tumor at an early stage, resulting in a close patient monitoring or re-therapy [14].
Fusion imaging enables improved liver segment allocation and allows assessment of the vascular reference. Interventions can be planned and post-interventional controls can be optimized. Intraoperatively, the removal of smaller liver tumors <10 mm can be facilitated. A broad intraoperative application is currently opposed to the technical effort.
Image fusion with volume navigation (V Nav) of CEUS with ceCT or ceMRI frequently allows a definitive localization and diagnosis of hepatic lesions in patients with primary hepatic carcinoma or metastatic diseases. This might cause a change of the therapeutic strategy in many patients with hepatic lesions [15].
13.4.3 CEUS with Image Fusion for Performing Punctures, Biopsies, and Drainage
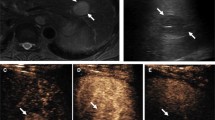
Current developments in fusion imaging make it easier to guide biopsy needles to the liver foci identified in other sectional imaging methods using GPS-like navigation techniques. If these lesions can partially not or hardly be recognized in conventional B mode ultrasound (BMUS) scan, with the GPS marking, the target lesion can be marked with a target point in order to implement a targeted puncture “in-plane” or “ex-plane.” This is particularly advantageous for small and near-diaphragmatic tumor sites. If tumors are clearly recognizable in the B-mode or by CEUS, the experienced examiner will usually puncture them with puncture sound probes or with freehand technique. In the same way, GPS technology can also be used for inflammatory behavior to place a drainage percutaneously [16]. An in vitro study showed significantly less time needed for the simulated interventions in all examiners when V Nav was used (P < 0.05). Percutaneous biopsies and drainages, even of small lesions involving complex access pathways, could be accomplished with a high success rate by using 3D real-time image fusion together with real-time needle tracking [13] (Fig. 13.4).
Fusion CEUS and contrast enhanced CT (ceCT) for planning interventional procedure of an abscess on the right liver lobe using GPS markers with a virtual tracking line for the puncture. Green point in the center of the abscess. After successful placement of the drainage contrast agent was applied into the drainage for visualization of the correct placement without complications
With image fusion of CEUS with CT/MRI, accompanying by the use of GPS navigation systems, small foci can be punctured even in difficult locations (Fig. 13.5). If the tumor lesion cannot be visualized by the fundamental B-mode, the location is determined according to the contrast medium dynamics of the tumor focus or is determined by using a marker [17].
CEUS can also be helpful for diagnosis of the type and for the targeted puncture or ultrasound-controlled drainage of suspected inflammatory fluids. Fine septal structures, which are only reproduced by CEUS, within these fluid contents allow a more targeted puncture to be performed on abscesses. Diagnostic accuracy of complicated, inflammatory, or suspected tumor cysts can be done in a targeted manner. Differentiation of complicated cysts in suspected echinococcus is much better, but so is the assessment of cystic tumors with partial necrosis. A puncture needle or drainage can also be used to make an exact representation of the drainage access and its location using the smallest amounts of contrast medium. For this purpose, less than 0.5 ml of ultrasound contrast medium with saline solution was applied via the drainage. This can also be used to evaluate connections to neighboring organs via fistulas. The percutaneous execution of targeted biopsies, punctures, and drainages can also be used in combination with fusion imaging.
13.4.4 Liver Interventions with the CEUS Fusion: RFA, MWA, and IRE
Planning, implementation, and monitoring after ablation of malignant liver tumors are of great diagnostic importance in liver interventions. In the case of HCC or liver metastases, MWA or IRE are used in addition to RFA, if no operation is possible. With the imaging fusion, the localization of smaller tumor sites for intervention planning and implementation could be much easier (Fig. 13.6). Therapeutic outcomes of RFA under CEUS added fusion guidance for HCC in correlation to fusion with B-mode were also evaluated. Adding CEUS to fusion imaging was useful for improving the conspicuity of HCC inconspicuous on fusion imaging alone, thus enabling successful percutaneous RFA with excellent therapeutic outcomes [18].
Intervention planning includes the exact registration of all tumor areas, their localization, and the relation to the liver vessels, the capsule, and the diaphragm. The use of contrast agent imaging enables dynamic detection of the liver vessels and the tumor vessels. In the case of the ablative procedures, treatment of the tumor lesion with a safety margin of >5 mm in all planes must be achieved in accordance with the tumor extent and localization (Fig. 13.7).
A prospective non-randomized study was performed to evaluate CEUS-CT/MR fusion imaging for assessment of treatment response in the ablation procedure. The cumulative local tumor progression (LTP) rate and overall survival (OS) rate were not significantly different between fusion imaging group and routine CEUS group. However, for large lesions (>3 cm) or lesions located close to major vessels, the cumulative LTP rate was significantly lower in fusion imaging group. Intraprocedural CEUS-CT/MR fusion imaging might be a potentially efficient method in reducing LTP during HCC thermal ablation, especially for difficult ablation liver lesions [19].
The assessment of ablative procedures can be sonographically restricted during the intervention due to gas development, but still offers the possibility of being available as contrast agent imaging in the further course if the use of contrast agents in CT or MRI is limited by the kidney function. In the case of ablative procedures, the RFA or microwave needle must be unplaced in the case of larger tumors; here, the fusion technique can be used in order to deliver the untreated portion of the tumor to the therapy. The same applies to the monitoring of an IRE (Fig. 13.8). In the IRE, the main vessels that cross the tumor, especially the main branches of the hepatic artery and the portal vein, are preserved, the tumor capillaries are switched off by the procedure. CEUS has a very high diagnostic value in the success control according to RFA, MWA, and IRE and is also suitable for further follow-up [8] (Fig. 13.9).
To compare the applicability of fusion imaging between CT/MRI-CEUS fusion imaging and US-CEUS fusion imaging in the assessment of treatment response during liver tumor ablation. The applicable rate of US-CEUS fusion imaging was lower than that of CT/MRI-CEUS fusion imaging, because of some inconspicuous lesions in conventional ultrasound. However, the registration success rate of US-CEUS fusion imaging was higher than that of CT/MRI-US fusion imaging, especially for patients with pre-ablation surgeries or procedures. Both CT/MRI-CEUS and US-CEUS fusion imaging are proved to be feasible means for immediate evaluation of treatment response for liver thermal ablation. US-CEUS fusion imaging showed distinguished advantages including convenience and a higher success rate of registration [20].
In the hands of the experienced examiner, the image fusion from the CEUS combined with the CT and MRI examination preceding the intervention can be used for the post-interventional follow-up. The image fusion of CEUS with MRI or CT is also suitable for lesions not detectable in the fundamental B-mode, but can be precisely localized by CEUS fusion and can then be punctually targeted and ablatively treated using navigation systems. In a randomized controlled trial, clinical application values of CEUS, CT, MRI, and three-dimensional ultrasound-CEUS fusion imaging techniques in the assistance of thermal ablation for HCC were compared. All the three techniques are proved to be feasible for intraoperative HCC thermal ablation [21].
In conclusion, ultrasound image fusion offers the potential for real-time imaging and can be combined with other cross-sectional imaging techniques as well as CEUS, which can facilitate the diagnosis and therapy control after liver interventions. In addition to the primary applications of image fusion in the diagnosis and treatment of liver lesions, further useful indications can be integrated into the daily work routine.
References
Kuorda H, Abe T, Fujiwara Y, Okamoto T, Yonezawa M, Sato H, Endo K, et al. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2019;25:2365–72.
Haimerl M, Brünn K, Poelsterl S, Beyer LP, Wiesinger I, Stroszczynski C, Jung EM, et al. Quantitative evaluation of real-time maximum liver capacity (LiMAx) and time intensity curve (TIC) analysis in CEUS-based microperfusion. Clin Hemorheol Microcirc. 2017;67:373–82.
Haimerl M, Poelsterl S, Beyer LP, Wiesinger I, Nießen C, Stroszczynski C, Wiggermann P, et al. Chronic liver disease: quantitative MRI vs CEUS-based microperfusion. Clin Hemorheol Microcirc. 2016;64:435–46.
Dong Y, Wang WP, Mao F, Ji ZB, Huang BJ. Application of imaging fusion combining contrast-enhanced ultrasound and magnetic resonance imaging in detection of hepatic cellular carcinomas undetectable by conventional ultrasound. J Gastroenterol Hepatol. 2016;31:822–8.
Minami T, Minami Y, Chishina H, Arizumi T, Takita M, Kitai S, Yada N, et al. Combination guidance of contrast-enhanced US and fusion imaging in radiofrequency ablation for hepatocellular carcinoma with poor conspicuity on contrast-enhanced US/fusion imaging. Oncology. 2014;87(Suppl 1):55–62.
Calandri M, Mauri G, Yevich S, Gazzera C, Basile D, Gatti M, Veltri A, et al. Fusion imaging and virtual navigation to guide percutaneous thermal ablation of hepatocellular carcinoma: a review of the literature. Cardiovasc Intervent Radiol. 2019;42:639–47.
Min JH, Lim HK, Lim S, Kang TW, Song KD, Choi SY, Rhim H, et al. Radiofrequency ablation of very-early-stage hepatocellular carcinoma inconspicuous on fusion imaging with B-mode US: value of fusion imaging with contrast-enhanced US. Clin Mol Hepatol. 2014;20:61–70.
Mauri G, Cova L, De Beni S, Ierace T, Tondolo T, Cerri A, Goldberg SN, et al. Real-time US-CT/MRI image fusion for guidance of thermal ablation of liver tumors undetectable with US: results in 295 cases. Cardiovasc Intervent Radiol. 2015;38:143–51.
Tranquart F, Dujardin PA, Bouche O, Marcus C, Borg C, Manzoni P, Douillard JY, et al. Value of contrast-enhanced ultrasound quantification criteria for identifying patients not responding to bevacizumab-based therapy for colorectal liver metastases. Ultraschall Med. 2018;39:544–58.
Schaible J, Stroszczynski C, Beyer LP, Jung EM. Quantitative perfusion analysis of hepatocellular carcinoma using dynamic contrast enhanced ultrasound (CEUS) to determine tumor microvascularization. Clin Hemorheol Microcirc. 2019;73:95–104.
Denis de Senneville B, Frulio N, Laumonier H, Salut C, Lafitte L, Trillaud H. Liver contrast-enhanced sonography: computer-assisted differentiation between focal nodular hyperplasia and inflammatory hepatocellular adenoma by reference to microbubble transport patterns. Eur Radiol. 2020;30:2995–3003.
Como G, Montaldo L, Baccarani U, Lorenzin D, Zuiani C, Girometti R. Contrast-enhanced ultrasound applications in liver transplant imaging. Abdom Radiol (NY). 2020;
Jung EM, Friedrich C, Hoffstetter P, Dendl LM, Klebl F, Agha A, Wiggermann P, et al. Volume navigation with contrast enhanced ultrasound and image fusion for percutaneous interventions: first results. PLoS One. 2012;7:e33956.
Wobser H, Wiest R, Salzberger B, Wohlgemuth WA, Stroszczynski C, Jung EM. Evaluation of treatment response after chemoembolisation (TACE) in hepatocellular carcinoma using real time image fusion of contrast-enhanced ultrasound (CEUS) and computed tomography (CT)–preliminary results. Clin Hemorheol Microcirc. 2014;57:191–201.
Rennert J, Georgieva M, Schreyer AG, Jung W, Ross C, Stroszczynski C, Jung EM. Image fusion of contrast enhanced ultrasound (CEUS) with computed tomography (CT) or magnetic resonance imaging (MRI) using volume navigation for detection, characterization and planning of therapeutic interventions of liver tumors. Clin Hemorheol Microcirc. 2011;49:67–81.
Mohr A, Jung EM, Stroszczynski C, Schacherer D, Klebl F. New economic training model for installing ultrasound-guided drainages. Z Gastroenterol. 2014;52:1257–62.
Kang TW, Lee MW, Song KD, Kim M, Kim SS, Kim SH, Ha SY. Added value of contrast-enhanced ultrasound on biopsies of focal hepatic lesions invisible on fusion imaging guidance. Korean J Radiol. 2017;18:152–61.
Lee MW, Lim HK, Rhim H, Cha DI, Kang TW, Song KD, Min JH, et al. Percutaneous radiofrequency ablation of small (1-2 cm) hepatocellular carcinomas inconspicuous on B-mode ultrasonographic imaging: usefulness of combined fusion imaging with MRI and contrast-enhanced ultrasonography. Can J Gastroenterol Hepatol. 2018;2018:7926923.
Ju JX, Zeng QJ, Xu EJ, He XQ, Tan L, Huang QN, Li K, et al. Intraprocedural contrast-enhanced ultrasound-CT/MR fusion imaging assessment in HCC thermal ablation to reduce local tumor progression: compared with routine contrast-enhanced ultrasound. Int J Hyperthermia. 2019;36:785–93.
Xu E, Long Y, Li K, Zeng Q, Tan L, Luo L, Huang Q, et al. Comparison of CT/MRI-CEUS and US-CEUS fusion imaging techniques in the assessment of the thermal ablation of liver tumors. Int J Hyperthermia. 2019;35:159–67.
Huang Q, Zeng Q, Long Y, Tan L, Zheng R, Xu E, Li K. Fusion imaging techniques and contrast-enhanced ultrasound for thermal ablation of hepatocellular carcinoma – A prospective randomized controlled trial. Int J Hyperthermia. 2019;36:1207–15.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jung, E.M., Dong, Y. (2021). Contrast Enhanced Ultrasound (CEUS) and Image Fusion for Liver Interventions. In: Wang, WP., Dong, Y., Dietrich, C.F., Jung, E.M. (eds) Contrast-Enhanced Ultrasound Imaging of Hepatic Neoplasms. Springer, Singapore. https://doi.org/10.1007/978-981-16-1761-4_13
Download citation
DOI: https://doi.org/10.1007/978-981-16-1761-4_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1760-7
Online ISBN: 978-981-16-1761-4
eBook Packages: EngineeringEngineering (R0)