Abstract
The demand for fossil fuel and its price is rising every year, and hence, the dominant fuel source, i.e., petroleum-based fuel, is depleting at a faster rate leading to harmful pollution. The usage of vegetable oil eradicates these two drawbacks as fuel. But the main disadvantage of vegetable oil is low performance and combustion characteristics due to its higher value of viscosity. The main objective of this research is to reduce the vegetable oil viscosity by the transesterification process and to blend with diesel oil and to study its fuel properties, performance, combustion and emissions characteristics in detail. Here, Jatropha oil is experimented as fuel due to its availability in plenty. The Jatropha biofuel is blended with diesel in various proportions (J20, J40, J60, J80 & J100) by the transesterification process. An experimental study was conducted with fuel samples in an unmodified single-cylinder compression ignition engine. From the experimental information, it was observed that brake thermal efficiency decreased while brake specific fuel consumption increased as the proportion of Jatropha increased in biodiesel. However, biodiesel blend J20 had a higher brake thermal efficiency of 31.1% at 80% load conditions, and it was closer to that of diesel, which is 32.5%. Jatropha blend had a closer combustion characteristic such as heat release rate and rate of pressure rise to diesel oil. Most of the primary exhaust pollutants, such as carbon monoxide, carbon dioxide and hydrocarbons, were comparatively lower for Jatropha oil, but an increase in NOx and smoke opacity was observed. However, blend J20 had value comparable with diesel. The outcome of the experimental investigation on performance, combustion and emission on Jatropha biodiesel indicated that J20 could be the best alternative fuel as it gave better efficiency and emissions similar to those of diesel, then it could be directly used in CI engine without any modification.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The compression ignition engine is used for an extensive range of applications in the transportation division, and it also has applications in agricultural and power generation sectors due to its higher thermal efficiency. But due to fossil fuel depletion, researchers all over the world are impelled to develop an alternative fuel source that has comparable properties with diesel oil. Biogas, vegetable oils and methanol have been considered as alternative fuels for diesel. The previous experimental studies suggested that esterified vegetable oil has been recognized as the best alternative for diesel [1,2,3]. It also has a higher cetane number, calorific value and latent heat of vaporization compared with diesel. Table 1 shows the various vegetable oil biodiesel fuels whose chemical properties are closer to those of diesel. The availability, plantation and extraction of oil from seeds or crops do not pose a problem. The vegetable oils have the advantage of being biodegradable, non-toxic and pollution-free. The oils extracted from seeds or plants can simply be transesterified to produce biodiesel. Pure vegetable oil also has the potential to run a diesel engine restricted to lower viscosity [4, 5].
For better combustion and performance, the viscosity of vegetable oil is decreased by the transesterification process. During this process, the viscosity of the biodiesel gets reduced and turns equivalent to diesel [10,11,12,13,14]. The effect of viscosity will influence combustion, performance and emissions through fuel droplets, vaporization and atomization. Some researchers have reported that preheated oils also lead to better performance and emission characteristics [15,16,17,18,19]. It has been observed that preheating gives better emissions of HC, CO and particulate matter emissions due to the reduction of viscosity. It has also been noted that for higher compression ratios, biodiesel improves performance. In recent years, biodiesel has achieved significant consideration as an alternative renewable fuel. Biodiesel has several advantages over other petroleum products. Recent researches have shown that exhaust gases have less unburned hydrocarbon, particulate matter, carbon monoxide and sulfur levels while using biodiesel as a fuel. But the oxides of nitrogen have increased. Nowadays, to reduce smoke and NOx with improved performance, considerable research is going on with water emulsion fuel, EGR and SCR.
Priyabrata Pradhan [20] investigated on the impact of Jatropha on the performance, emission and combustion on CI engine and reported that Jatropha biodiesel had a marginal decrease in brake thermal efficiency compared with mineral diesel, and it also had a reduction in CO2, HC and NOx and increased in CO emissions. Anand et al. [21] investigated the Jatropha biodiesel for injection pressure that ranges from 200 bar and 250 bar on diesel engine.
It was reported that there was a negligible decrease in BTE and a rise in emissions of emissions for Jatropha blends. Higher density, viscosity and molecular weight make it challenging to atomize the biodiesel at low temperatures and the low loads, causing more CO emissions. A significant cause for the lower CO emissions at high loads from the combustion of biodiesel is the inbuilt oxygen content, which makes the burning of biodiesel more complete when the engine works at higher loads.
Nabi et al. [22] investigated the influence of cottonseed oil on single-cylinder water-cooled, four-stroke, DI diesel engines. It was noted that preferred biodiesel resulted in lower CO, particulate matter, smoke and higher NOx emissions than diesel at all load conditions. At full load condition, it also resulted, in B10, in lower smoke emission and particulate matter by 14 and 24% to mineral diesel. Blend B30 showed that the CO emissions decreased by 24% and increased NOx emissions of 10%, and it was owing to the existence of oxygen in their molecular structure. Cottonseed blend has slightly lower thermal efficiency than mineral diesel due to its more moderate calorific content. However, higher density, higher volatility and higher viscosity might be the reasons for its reduction in its efficiency. Table 2 shows the study of various biodiesel fuels and their performance, emission and combustion characteristics.
From Table 1, it can be observed that properties such as calorific value, cetane number, kinematic viscosity and relative density of Jatropha oil are highly relative to mineral diesel compared to other biofuels. So these properties will influence in better performance, combustion and emission characteristics of Jatropha diesel blend used as fuel in the CI engine. The government of India launched the biofuel mission in 2003 to develop the Jatropha biodiesel industry. The planning commission reported that 13.4 million hectares of land available for Jatropha plantation [31]. Jatropha is an off-seasonal crop. So it can be cultivated at a slack agricultural season.
From the literature analysis, it can be perceived that only very limited researches have investigated in the field of Jatropha biodiesel with preheating of oil using the engine exhaust. In the current investigation, raw Jatropha oil was transformed into biodiesel using the transesterification process and converted into the fuel of various proportions on a volume basis. And during its operation in the engine, it was preheated with the engine exhaust for the betterment of engine behavior. The chemical and the physical properties of the fuel models have been analyzed on ASTM biodiesel standards. The objective of the present investigation was to determine the extent to which biodiesel blending could improve the combustion and emission characteristics without sacrificing its performance in an unmodified diesel engine. For that purpose, the experiment was conducted with a biodiesel blend in an unmodified diesel engine at all load conditions. The performance characteristics such as BTE, BSFC and emission characteristics such as CO2, CO, HC, NOx and smoke opacity were measured for Jatropha biodiesel and compared with mineral diesel. The combustion features such as rate of heat release and peak in-cylinder pressure graphs were constructed against the crank angle to find the effectiveness of combustion.
2 Materials and Methods
2.1 Jatropha
Jatropha is one of the flowering plants in the spurge family, & it is also called Euphorbiaceae. The Jatropha fruits are yellow color, and their dried seeds are black in color and oval in shape. The oil extracted from the seeds is golden yellow in color and fragrance-free. Jatropha has usually been used in basket creation and dye production. It had origin from tropical America and also many portions of the jungles in Africa [32, 33]. It is a drought-resistant, permanent plant, grown-up to fifty years, and it grows on any kind of soil. It has a yield of about 1 kg per square meter per year [34]. The toxin is the only drawback in it. In World War II, it was used as biodiesel for engines [35, 36]. In Jatropha seed oil, phorbol esters are considered toxic. The phorbol esters are destroyed by chemical refining (degumming, neutralization, silica/ bleaching, mild deodorization) and physical refining (stripping at 240° and vacuum). The Jatropha seeds oil is highly viscous used in the manufacture of soap and candles. In cosmetics production, it is used as a diesel/paraffin additional or extender [37, 38]. It has significant inferences for fixing the demand for rural energy usage and also exploring useful substitutes for fossil fuels to reduce greenhouse gas addition in the atmosphere. Figure 1 shows the jatropha plant and its fruit.
2.2 Biodiesel Preparation Process—Transesterificaton
The energy content of the vegetable oils is similar to that of diesel. The vegetable oils cannot be used as fuels in unmodified diesel engines as they are too viscous due to their high molar mass. Triglycerides react with methanol in the presence of a catalyst to form glycerol, and methyl ester is called biodiesel, and the same is shown in Fig. 2. Vegetable oil can be converted into a usable fuel, known as biodiesel, in a transesterification reaction. The triglycerides in Jatropha oil react with methanol, which is one of the alcohols, in the existence of a strong base such as sodium hydroxide to produce glycerol and Jatropha methyl ester, which is also known as Jatropha biodiesel. The chemical reaction between them is shown in Fig. 3. In this reaction, an excess of alcohol is used to drive the position of equilibrium to the right in favor of the products. The three alkyl ester molecules produced have similar energy content to the triglyceride but are less viscous due to their lower molar mass, and they are suitable for use in diesel engines.
Biodiesel is much less toxic and more biodegradable than regular diesel. In the industrial sector, the alkali-catalyzed transesterification process is followed for mass production [39, 40]. The whole process, which contains the conversion of Jatropha seeds into Jatropha biofuel, is as shown in Fig. 4. Table 3 shows the weight composition of fatty acid [41].
2.3 Experimental Setup
A water-cooled, single-cylinder and four-stroke direct-injection compression engine with a compression ratio of 17.5:1 and power of 5.2 kW run at a speed of 1500 rpm was used for the present research work. The high viscosity is the major drawback of using Jatropha oil in an unmodified compression ignition engine. So, it is imperative to reduce the fuel viscosity before its use in the engine. Its higher viscosity is reduced by preheating the Jatropha biodiesel up to 90 °C by the heat exchanger, which utilizes the heat from exhaust gases that pass through it. Its viscosity is also reduced by blending with diesel. Experiments were conducted on various proportions of Jatropha biodiesel blend (J20, J40, J60, J80 and J100) and mineral diesel. The properties of diesel and Jatropha blends at 90 °C were measured, as given in Table 4 and other technical specifications of the engine used for this work are given in Table 5.
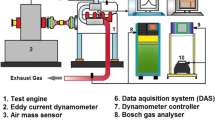
The performance, combustion and emission of the Jatropha oil with diesel in various proportions were measured. The eddy current dynamometer was used for multiple loading conditions. The schematic experimental setup for our investigation is shown in Fig. 5.
The Jatropha and diesel oils were filled in separate fuel tanks, which are our major components. The heat exchanger, exhaust gas analyzer, smoke meter, dynamometer and data acquisition system were also used as main components. The engine was started with diesel first and then with Jatropha oil for the purpose of warm-up. It also reduced deposits and cold-starting problems in the fuel line and injection system. The voltage and the current spent by the load were measured by voltmeter and ammeter. The exhaust gas composition was measured using an exhaust gas analyzer. Figure 5 represents the schematic diagram of the experimental setup test rig.
The physical, thermal and chemical properties of the Jatropha and diesel are given in Table 4. Jatropha oil has a high pour point, density and cloud point compared with diesel. The fire and flash points are higher for Jatropha oil than diesel and make it safe to use. Increased carbon deposit is due to the higher carbon content from the Jatropha oil. The emissions and combustion properties are enhanced by the existence of oxygen, but the calorific value of the Jatropha oil decreases. The Jatropha oil has around 80% calorific value compared with diesel. Higher viscosity in Jatropha oil is the main issue of its use as a fuel in the diesel engine. In this experimental examination, viscosity was minimized by preheating and blending the Jatropha oil with diesel. The viscosity of Jatropha oil was maintained at 90 °C in this investigation because, at this temperature, it had a relative viscosity closer to diesel. Hence, Jatropha must be heated up to 90 °C before its injection into the engine to attain fuel properties close to diesel. Viscosity decrease depends upon its concentration of the combination.
2.4 Analysis of Uncertainty
The range of error in the experimental results represents the uncertainty analysis. It arises due to multiple factors such as instrument selection, condition, calibration, environment, reading, observation and test procedure. The experiments were performed thrice to minimize the effect of errors in the results. The uncertainty of various measuring units is given in Table 6. The uncertainty percentage was calculated by the square root of the sum of squares of the uncertainty values of brake power, brake specific fuel consumption, brake thermal efficiency, total fuel consumption, carbon monoxide, hydrocarbon, oxides of nitrogen, smoke, temperature and pressure [42].
±2.26% was calculated as uncertainty values for the current experimental setup. The experimental uncertainty of various measuring units is given in Table 6.
3 Results and Discussion
3.1 Analysis of Performance Characteristics
3.1.1 Brake Thermal Efficiency (BTE)
The BTE of Jatropha oil and diesel blends increases with increases in engine load, as presented in Fig. 6. It is witnessed that the calorific value of Jatropha oil is lesser than that of diesel oil. As a result of increasing the proportion of Jatropha biodiesel in blend decreases, the calorific value is proportional, which affects in increased BSFC, and it reduces BTE. BTE of Jatropha blends lower than that of diesel. However, BTE of J20 stays very nearer to diesel, and all other combinations have lesser BTE compared with J20 and mineral diesel. The combustion characteristics of the fuel molecules were increased by the presence of oxygen content in the biodiesel.
The higher viscosity and reduced volatility of the Jatropha biodiesel oil affect the atomization and combustion characteristics [43]. Therefore, BTE was found to be lesser for higher blend concentrations related to diesel. The BTEs of diesel and its blends were found increased with increases in load, but it tended to decrease with further increase in peak load. The maximum BTE of 31.1% was attained for J20, while for diesel, it was 32.5% at 80% of its full load condition.
3.1.2 Brake Specific Fuel Consumption (BSFC)
Brake specific fuel consumption has been found to increase with a higher proportion of Jatropha biodiesel in the blend-related diesel in the various engine load range (Fig. 7). It is owing to the collective effects of the fuel density, viscosity and heating value of the blends. BSFC has increased owing to the high density of the Jatropha biodiesel oil blends based on its proportion levels. The higher bulk modulus has resulted in higher fuel discharge for the same value in BSFC. The usage of a low percentage of Jatropha biodiesel in diesel has resulted in lesser BSFC compared with diesel in all loads. The low BTE for J100 could be owing to lower calorific value and an increase in fuel consumption as related to J20. However, on the whole, by running the engine with Jatropha biodiesel, BSFC is always higher than the biodiesel as well as diesel.
3.2 Analysis of Emission Characteristics
3.2.1 Emissions of Nitrogen Oxides (NOx)
NOx emission is a highly harmful gaseous emission compared with other engine exhaust emissions. Hence, its reduction in an engine has always been one of the key aims of engine researchers all over the world. The NOx emissions of diesel fuel were related to various proportions of Jatropha biodiesel at different loads as shown in Fig. 8. It is observed that the emission of oxides of nitrogen increases with increases in load for all fuels. It is also observed that if the amount of Jatropha in the blend increases, then emissions of NOx also increase. This increase is due to Jatropha oil being an oxygenated fuel and that leads to improved combustion, and therefore, higher combustion temperature is attained. The higher temperature promotes NOx formation. The NOx emissions have increased enormously between 40 and 60% of the rated load for all blends and mineral diesel. However, the emissions of NOx of blend J20 are nearer to diesel compared with others.
3.2.2 Carbon Monoxide
The development of CO emission mainly depends upon the physical and chemical properties of the fuel used. Figure 9 shows that the engine discharges lesser CO for Jatropha blends when related to diesel. When the proportion of Jatropha biodiesel in the blend increases, the percentage of emission of CO decreases. But all Jatropha blends are far below the percentage of CO emissions than that of mineral diesel. The decrease in CO emission of Jatropha blend is due to the high cetane number and oxygen present in the molecular structure of Jatropha oil, and it supplies the necessary oxygen to convert CO to CO2 when combustion takes place. Kumar et al. [44], in his investigation, reported that an increase in cetane number, there was a reduction in ignition delay, increased the injection pressure, thereby making the fuel particles finer, giving lower CO emissions. The emissions of CO for all blends are closer to those of diesel at 20% and 40% of the rated load. But the variation increases as 0.2% for diesel and 0.08% for J100 at full load conditions.
3.2.3 Hydrocarbon
The HC emission variation for various proportions of blends is shown in Fig. 10. It is observed that for diesel and Jatropha blends, the HC emissions are showing a decreasing trend first up to 40% of its load and then increasing trend with increasing load. It is owing to the existence of a rich fuel mixture at higher loads. It is witnessed that an increasing proportion of Jatropha in the blend decreases HC emissions. It is due to the presence of oxygen in the Jatropha oil, and the higher combustion temperature promotes the oxidation of HC. Elango and Senthilkumar [45] concluded that the effect of viscosity increased the HC emissions level of the blend at higher load conditions. The emissions of HC for all Jatropha blends are closer to those of diesel at 40% rated load as the emission values are 15% for diesel and 12.5% for J100. However, the variations of HC are maximum at its full load condition as the emissions values are 34.2% for diesel and 24.4% for J100.
3.2.4 Carbon Dioxide
The emission intensities of CO2 for various proportions of biodiesel and diesel is shown in Fig. 11. At full load conditions, the CO2 emissions for diesel, J20, J40, J60, J80 and J100 are found to be 11.5%, 11.01%, 10.4%, 10.21%, 10.15% and 10.11%, respectively. It is shown that the CO2 emission of Jatropha blends is less than that of diesel at all load conditions. It may be pointed out that Jatropha blends contain lower carbon content than diesel. The Jatropha oil contains more oxygen content, which is also one of the reasons for lower CO2 emissions of Jatropha blend compared with diesel.
3.2.5 Smoke Density
The emissions of smoke opacity with the varying load are shown in Fig. 12. It indicates that as Jatropha biodiesel concentration increases, the smoke opacity also increases irrespective of the load condition. At full load conditions, the smoke opacity for diesel, J20, J40, J60, J80 and J100 are found to be 18.2, 19.1, 22.4, 25.2, 32.7 and 45.1 HSU, respectively. It is owing to reduced volatility and improper mixing of fuel droplets with air because of the higher viscosity of the Jatropha blends. At full load condition, the value of the smoke density of Jatropha biodiesel J100 is two times the value of diesel at the same load condition. The NOx and smoke emissions are controlled by combustion treatments (exhaust gas recirculation, emulsified biodiesel), exhaust after treatments (selective catalytic reduction, lean NOx traps) and fuel treatments (fuel additives) [46].
3.3 Analysis of Combustion Characteristics
3.3.1 Heat Release Rate Versus Crank Angle
The heat release rates (HRR) versus crank angle for all Jatropha blends and diesel at full loading conditions are shown in Fig. 13. HRR attains negative value at the beginning of the combustion due to the vaporization of the fuel. After the start of the combustion phase (SOC), HRR turns into positive. All the proportions of Jatropha blends have almost identical combustion as mineral diesel at all stages, such as ignition delay, premixed combustion and diffusion combustion.
From Fig. 13, it is witnessed that at full engine load condition, combustion starts earlier for Jatropha blends. At the time of fuel injection, thermal cracking happens due to high cylinder temperature. It leads to shorter ignition delay for Jatropha blend compared to diesel. At the premixed combustion stage, the HRR is greater for diesel due to its high volatility and improved air–fuel mixing characteristics. It is also because of the longer ignition delay, which leads to a large amount of fuel addition. At diffusion combustion, Jatropha blends have high HRR in higher load conditions. The higher HRR for mineral diesel is 145.1 J/degree, and for J100, it is 76.3 J/degree, which is two times that of mineral diesel. However, the combustion duration for diesel is less than that of Jatropha blends.
3.3.2 In-Cylinder Pressure Versus Crank Angle
The variations of cylinder pressure for the crank angle for diesel and Jatropha blends J20, J40, J60, J80 and J100 at full engine loads are shown in Fig. 14. From these figures, it is observed that cylinder pressure rates are almost comparable with diesel for all fuel blends at low engine load conditions.
At higher load conditions, Jatropha blends have earlier pressure rise than diesel, and mineral diesel had higher peak pressure. The Jatropha blends had initial combustion compared to diesel, and the pressure rise rate is slower for Jatropha blends at full load conditions. Hence, it is due to the slower burning characteristics. The starting point of combustion for all fuels gets advanced as the engine load is increased. The combustion starts earlier for Jatropha blends. It is because of shorter ignition delay and partly due to advanced injection timing and also due to higher density and higher bulk modulus of Jatropha oil. It is due to the complex and pre-flame chemical reaction at high temperature. In the high cylinder temperature existing during fuel injection, Jatropha attained thermal cracking, and it leads to formation of lighter compounds [26]. Therefore, it causes in earlier ignition and shorter ignition delay for Jatropha blend compared to diesel.
Jatropha blend J40 has higher peak pressure of 58.5 bar, and it is higher compared with all blends and diesel. J100 has a peak pressure of 54.2 bar, and it is lower compared with all blends and mineral diesel.
3.3.3 Exhaust Gas Temperature
The influence of BTE and BSFC was reflected in exhaust gas temperature as well. The exhaust gas temperature with blends having a higher proportion of biodiesel blend was higher than that of diesel at greater loads, as shown in Fig. 15. When biodiesel concentration was increased, the exhaust gas temperature rose by a small value. Upon using 100% Jatropha oil, a higher value of exhaust gas temperature was obtained, which indicated more loss of energy. Elango et al. suggested that combustion is delayed for the blends, and more of the heat is released during mixing controlled combustion phase in which higher amount of heat goes with exhaust gas [45]. Hence, exhaust gas temperatures are higher. The EGT improved with an increase in load for all blends. The rise in EGT with load was evident, and it showed that more fuel was required to take additional capacity. EGT is a sign of the range of conversion of heat into work, which occurs inside the cylinder. It was found that EGT for various fuel blends at different load conditions were almost identical. EGT increased with a rise in power for all fuel blends. As the biodiesel fuel concentration was improved, the exhaust gas temperature also got enhanced. The highest exhaust gas temperature was 340 °C at higher power for J100.
4 Conclusion
The principal objective of the current investigation was to measure the performance, combustion and emission characteristics of the compression ignition engine fueled with various proportions of Jatropha blends with diesel and mineral diesel. Accordingly, an experimental investigation was conducted. The investigation revealed that if the viscosity of Jatropha could be nearer to diesel, it would give better performance and emission characteristics. Hence, the viscosity of the Jatropha oil was reduced by the transesterification process and then by blending it with diesel with a preheating technique from the exhaust gas.
-
The experimentation was conducted for raw diesel and various proportions of Jatropha diesel blends (J20, J40, J60, J80 and J100) at different load conditions and constant speed (1500 rpm).
-
Brake thermal efficiency (BTE) decreased as Jatropha concentration in the blend increased. In contrast, maximum brake thermal efficiency of 31.1% was achieved for J20, while for diesel, it was 32.5% at full load conditions. At the same time, the brake specific fuel consumption (BSFC) and exhaust gas temperature were higher for Jatropha biodiesel blends than for diesel.
-
Jatropha blends had higher peak pressure at lower loads, and mineral diesel had higher peak pressure at higher loads. NOx and smoke emissions were higher than diesel for Jatropha blends because of reduced volatility and higher viscosity of mixtures. In contrast, the emissions CO, HC and CO2 were lower for Jatropha blends than for diesel due to low carbon content, high oxygen content and high cetane number of biodiesel.
From the detailed experimental investigation on performance, combustion and emission on Jatropha biodiesel, it could be inferred that J20 was the best alternative fuel, and it gave better efficiency and emissions similar to those of diesel. And hence, it can be directly used in the CI engine without any engine modification.
Abbreviations
- ASTM:
-
American Society for Testing Materials
- CI:
-
Compression ignition
- CO:
-
Carbon monoxide
- HC:
-
Hydrocarbon
- CO2:
-
Carbon dioxide
- NOx:
-
Oxides of nitrogen
- HRR:
-
Heat release rate
- J20:
-
20% transesterified Jatropha oil and 80% diesel in volume
- J40:
-
40% transesterified Jatropha oil and 60% diesel in volume
- J60:
-
60% transesterified Jatropha oil and 40% diesel in volume
- J80:
-
80% transesterified Jatropha oil and 20% diesel in volume
- J100:
-
100% volume of transesterified Jatropha oil in volume
References
Rehman, A., Phalke, D. R., & Pandey, R. (2011). Alternative fuel for gas turbine: Esterified jatropha oil–diesel blend. Renewable Energy, 36(10), 2635–2640.
Balasubramanian, D., Kamaraj, S., & Krishnamoorthy, R. (2020). Synthesis of biodiesel from waste cooking oil by alkali doped calcinated waste egg shell powder catalyst and optimization of process parameters to improve biodiesel conversion (No. 2020-01-0341). SAE Technical Paper.
Kaisan, M. U., Abubakar, S., Ashok, B., Balasubramanian, D., Narayan, S., Grujic, I., & Stojanovic, N. (2018). Comparative analyses of biodiesel produced from jatropha and neem seed oil using a gas chromatography–mass spectroscopy technique. Biofuels, 1–12.
Mat, S. C., Idroas, M. Y., Hamid, M. F., & Zainal, Z. A. (2018). Performance and emissions of straight vegetable oils and its blends as a fuel in a diesel engine: A review. Renewable and Sustainable Energy Reviews, 82, 808–823.
EL-Seesy, A. I., He, Z., Hassan, H., & Balasubramanian, D. (2020). Improvement of combustion and emission characteristics of a diesel engine working with diesel/jojoba oil blends and butanol additive. Fuel, 279, 118433.
Gautam, A., & Agarwal, A. K. (2013). Experimental investigations of comparative performance, emission and combustion characteristics of a cottonseed biodiesel-fueled four-stroke locomotive diesel engine. International Journal of Engine Research, 14(4), 354–372.
Ali, M. H., Mashud, M., Rubel, M. R., & Ahmad, R. H. (2013). Biodiesel from neem oil as an alternative fuel for diesel engines. Procedia Engineering, 56, 625–630.
da Silva, M. A. V., Ferreira, B. L. G., da Costa Marques, L. G., Murta, A. L. S., & de Freitas, M. A. V. (2017). Comparative study of NOx emissions of biodiesel-diesel blends from soybean, palm, and waste frying oils using methyl and ethyl transesterification routes. Fuel, 194, 144–156.
Leung, D. Y., Wu, X., & Leung, M. K. H. (2010). A review on biodiesel production using catalyzed transesterification. Applied Energy, 87(4), 1083–1095.
Sahoo, P. K., Das, L. M., Babu, M. K. G., Arora, P., Singh, V. P., Kumar, N. R., et al. (2009). Comparative evaluation of performance and emission characteristics of Jatropha, karanja, and polanga based biodiesel as fuel in a tractor engine. Fuel, 88(9), 1698–1707.
Gog, A., Roman, M., Toşa, M., Paizs, C., & Irimie, F. D. (2012). Biodiesel production using enzymatic transesterification–current state and perspectives. Renewable Energy, 39(1), 10–16.
Lee, A. F., Bennett, J. A., Manayil, J. C., & Wilson, K. (2014). Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chemical Society Reviews, 43(22), 7887–7916.
Kouzu, M., & Hidaka, J. S. (2012). Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel, 93, 1–12.
Ramalingam, K., Balasubramanian, D., Chellakumar, P. J. T. J. S., Padmanaban, J., Murugesan, P., & Xuan, T. (2020). An assessment on production and engine characterization of a novel environment-friendly fuel. Fuel, 279,.
Lingesan, S., Annamalai, K., Parthasarathy, M., Ramalingam, K. M., Dhinesh, B., & Lalvani, J. I. J. (2018). Production of Garcinia gummi-gutta methyl ester (GGME) as a potential alternative feedstock for existing unmodified DI diesel engine: Combustion, performance, and emission characteristics. Journal of Testing and Evaluation, 46(6), 2661–2678.
Khalid, A., Tajuddin, A. S. A., Jaat, N., Manshoor, B., Zaman, I., Hadi, S. A. A., et al. (2017). Performance and emissions of a diesel engine fuelled with preheated biodiesel fuel derived from crude palm, Jatropha, and waste cooking oils. International Journal of Automotive and Mechanical Engineering, 14, 4273–4284.
Chauhan, B. S., Kumar, N., Du Jun, Y., & Lee, K. B. (2010). Performance and emission study of preheated Jatropha oil on a medium-capacity diesel engine. Energy, 35(6), 2484–2492.
Hazar, H., & Aydin, H. (2010). Performance and emission evaluation of a CI engine fueled with preheated raw rapeseed oil (RRO)–diesel blends. Applied Energy, 87(3), 786–790.
Jeyakumar, N., Narayanasamy, B., Balasubramanian, D., & Viswanathan, K. (2020). Characterization and effect of Moringa Oleifera Lam. antioxidant additive on the storage stability of Jatropha biodiesel. Fuel, 281, 118614.
Pradhan, P., Raheman, H., & Padhee, D. (2014). Combustion and performance of a diesel engine with preheated Jatropha curcas oil using waste heat from the exhaust gas. Fuel, 115, 527–533.
Anand, B. P., Saravanan, C. G., & Srinivasan, C. A. (2010). Performance and exhaust emission of turpentine oil powered direct injection diesel engine. Renewable Energy, 35(6), 1179–1184.
Nabi, M. N., Akhter, M. S., & Shahadat, M. M. Z. (2006). Improvement of engine emissions with conventional diesel fuel and diesel–biodiesel blends. Bioresource Technology, 97(3), 372–378.
Tarabet, L., Loubar, K., Lounici, M. S., Khiari, K., Belmrabet, T., & Tazerout, M. (2014). Experimental investigation of DI diesel engine operating with eucalyptus biodiesel/natural gas under dual fuel mode. Fuel, 133, 129–138.
Gogoi, T. K., & Baruah, D. C. (2011). The use of Korach seed oil methyl ester blends as fuel in a diesel engine. Applied Energy, 88(8), 2713–2725.
Haiter, L. A., Ravi, R., Arumugham, S., & Thyagarajan, K. (2012). Performance, emission, and combustion evaluation of diesel engines using methyl esters of Mahua oil. International Journal of Environmental Sciences, 3(1), 639–649.
Agarwal, A. K., & Dhar, A. (2013). Experimental investigations of performance, emission, and combustion characteristics of Karanja oil blends fuelled the DICI engine. Renewable Energy, 52, 283–291.
Nithyananda, B. S., Anand, A., & Prakash, G. V. N. (2013). Experimental investigation of neem and mixed Pongamia-coconut methyl esters as biodiesel on CI engine. International Journal of Mechanical Engineering & Technology, 4, 232–242.
Sanjid, A., Masjuki, H. H., Kalam, M. A., Abedin, M. J., & Rahman, S. A. (2014). Experimental investigation of mustard biodiesel blend properties, performance, exhaust emission, and noise in an unmodified diesel engine. APCBEE Procedia, 10, 149–153.
Nalgundwar, A., Paul, B., & Sharma, S. K. (2016). Comparison of performance and emissions characteristics of DI CI engine fueled with dual biodiesel blends of palm and Jatropha. Fuel, 173, 172–179.
Kakati, J., & Gogoi, T. K. (2016). Biodiesel production from Kutkura (Meyna Spinosa Roxb. Ex.) fruit seed oil: Its characterization and engine performance evaluation with 10 and 20% blends. Energy Conversion and Management, 121, 152–161.
Datta, A., & Mandal, B. K. (2014). Use of Jatropha biodiesel as a future sustainable fuel. Energy Technology & Policy, 1(1), 8–14.
Buyukkaya, E. (2010). Effects of biodiesel on a DI diesel engine performance, emission, and combustion characteristics. Fuel, 89(10), 3099–3105.
Silitonga, A. S., Ong, H. C., Mahlia, T. M. I., Masjuki, H. H., & Chong, W. T. (2014). Biodiesel conversion from high FFA crude jatropha curcas, Calophyllum inophyllum, and Ceiba pentandra oil. Energy Procedia, 61, 480–483.
Henning, R. K. (1994). Fighting desertification by integrated utilisation of the Jatropha plant. www.Jatropha.org.
Xiao, J., Zhang, H., & Niu, L. (2015). Effect of detoxification on conformational and functional properties of Jatropha curcas proteins. International Journal of Food Properties, 18(7), 1524–1534.
Rani, C., Kajla, S., Pal, M., Poonia, A. K., & Kharb, P. (2013). Jatropha curcas: A potential source for biofuel production. Renewable Energy Sources and Their Applications, 155.
Silitonga, A. S., Atabani, A. E., Mahlia, T. M. I., Masjuki, H. H., Badruddin, I. A., & Mekhilef, S. (2011). A review of the prospect of Jatropha curcas for biodiesel in Indonesia. Renewable and Sustainable Energy Reviews, 15(8), 3733–3756.
Kim, H., & Choi, B. (2010). The effect of biodiesel and bioethanol blended diesel fuel on nanoparticles and exhaust emissions from the CRDI diesel engine. Renewable Energy, 35(1), 157–163.
Padhi, S. K. (2010). Preparation and characterization of biodiesel from non-edible oils (Doctoral dissertation).
Ahmed, W., Nazar, M. F., Ali, S. D., Rana, U. A., & Khan, S. U. D. (2015). Detailed investigation of optimized alkali catalyzed transesterification of Jatropha oil for biodiesel production. Journal of Energy Chemistry, 24(3), 331–336.
Kawakami, K., Oda, Y., & Takahashi, R. (2011). Application of a Burkholderia cepacialipase-immobilized silica monolith to batch and continuous biodiesel production with a stoichiometric mixture of methanol and crude Jatropha oil. Biotechnology for Biofuels, 4(1), 42.
Karthickeyan, V., Thiyagarajan, S., Ashok, B., Geo, V. E., & Azad, A. K. (2020). Experimental investigation of pomegranate oil methyl ester in ceramic coated engine at different operating condition in direct injection diesel engine with energy and exergy analysis. Energy Conversion and Management, 205,.
Özener, O., Yüksek, L., Ergenç, A. T., & Özkan, M. (2014). Effects of soybean biodiesel on a DI diesel engine performance, emission, and combustion characteristics. Fuel, 115, 875–883.
Kumar, P., Sharma, M. P., & Dwivedi, G. (2014). Impact of biodiesel on combustion, performance, and exhaust emissions of diesel engines. Journal of Integrated Science and Technology, 2(2), 57–63.
Elango, T., & Senthilkumar, T. (2011). Combustion and emission characteristics of a diesel engine fuelled with jatropha and diesel oil blends. Thermal Science, 15(4), 1205–1214.
Jeevahan, J., Mageshwaran, G., Joseph, G. B., Raj, R. D., & Kannan, R. T. (2017). Various strategies for reducing Nox emissions of biodiesel fuel used in conventional diesel engines: A review. Chemical Engineering Communications, 204(10), 1202–1223.
Acknowledgements
The authors would like to convey their heartfelt thanks to Dr. S. Saravanan, Principal, CK College of Engineering and Technology, Cuddalore, for encouraging this research work and to the authorities of Mepco Schlenk Engineering College, Sivakasi, for their support.
Declaration of Competing Interest
The authors announce that they have no recognized competing financial interests or personal relationships that could have seemed to influence the work described in this article.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Balasubramanian, D., Rajarajan, A., Krishnamoorthy, R., Quoc, T.D. (2021). Experimental Investigation of Unmodified Diesel Engine on Performance, Combustion and Emission with Various Proportions of Jatropha Biofuel in Diesel. In: Singh, A.P., Kumar, D., Agarwal, A.K. (eds) Alternative Fuels and Advanced Combustion Techniques as Sustainable Solutions for Internal Combustion Engines. Energy, Environment, and Sustainability. Springer, Singapore. https://doi.org/10.1007/978-981-16-1513-9_7
Download citation
DOI: https://doi.org/10.1007/978-981-16-1513-9_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1512-2
Online ISBN: 978-981-16-1513-9
eBook Packages: EnergyEnergy (R0)