Abstract
Donation after cardiac death (DCD) kidney transplantation developed rapidly in recent years, because of the shortage of deceased donors. DCD donation process should be performed according to the guidelines of different countries. DCD kidneys are associated with higher risk of primary non-function (PNF) and delayed graft function (DGF) compared to donation after brain death (DBD) kidneys; however, long-term patient and graft survival, as well as graft function were all comparable between DCD and DCD kidneys. Donor age, body mass index (BMI), hypertension, diabetes, high donor creatinine, cause of death, and cold ischemia time may affect the outcome of a DCD kidney transplant. Hypothermic machine perfusion (HMP) and normothermic machine perfusion (NMP) may reduce the PNF and DGF rate after transplant. Carefully selection of the DCD donor kidneys, pre-transplantation (zero time) biopsy, carefully management of fluid and monitor of immunosuppressive drugs, such as using ATG and low dose calcineurin inhibitor (CNI) may reduce DGF and improve the long-term outcome of DCD kidney transplantation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Kidney transplantation is the best treatment for patients with end-stage kidney diseases (ESRD). However, due to the shortage of donors, many patients died while waiting for suitable donors. In 2017, about 136,000 kidney transplants were performed worldwide, but according to WHO estimates, this activity is sufficiency only to meet 10% of transplant need. The average waiting time for a deceased donor kidney transplant in the UK is over 3 years. Owing to ill health, 12% of listed patients die or are removed from the waiting list within 3 years of listing [1]. In China, it is estimated that the ratio of donors and patients on the waiting list is about 1:30 [2]. Therefore, how to increase the deceased donors source for saving the lives of patients on the waiting list is a major problem worldwide.
Traditionally, the deceased donors were divided into donation after brain death (DBD) donors and donation after cardiac death (DCD) donors. The majority of kidney transplant recipients receive their kidney from brain dead (DBD) donors, but in recent years there has been a marked increase in the number of transplants using kidneys from donation after cardiac death (DCD) donors. In the UK, DCD donor numbers increased sixfold within ten years from 84 cases in 2004 to 527 cases in 2013 [1]. DCD donors has become a major way to expand the deceased donor pool over the last decade.
However, DCD kidney transplantation is associated with a higher risk of primary non-function (PNF) and delayed graft function (DGF), although the higher incidence of DGF after DCD kidney transplantation is not associated with the poorer graft survival as in DBD grafts [3, 4]. Meanwhile, the methods to improve the quality of DCD kidneys keep developing over the past decades, including hypothermic machine perfusion (HMP), premortem cannulation, normothermic machine perfusion (NMP) [5].
6.1 Current Situation and Trends of Kidney Transplantation from Cardiac Death Donors
In recent years, DCD kidney transplantation has developed rapidly worldwide. In Europe, especially the UK, the Netherlands, and Belgium have very successful DCD donor programs with 7.0–9.5 DCD donors per million population (pmp) in 2013. The USA, Australia, and Croatia also have well-developed DCD programs with 2.1–3.8 DCD donors per million population. DCD kidneys accounted for 11% of all kidney transplants in the USA and make up 30–50% of all deceased donors in some European countries. From 2006 to 2017, the proportion of DCD donors increased dramatically in both the UK and the USA (Fig. 6.1) [6]. However, there is still huge potential for expanding DCD donor pool. In Europe, there are approximately 350,000 cases of cardiopulmonary resuscitation a year. Only 40% of such cases are successfully resuscitated. However, for the remaining 60% that do not recover, these deaths become a potential for DCD donors. In a study in the USA between 2013 and 2016, it was estimated that there were 9828 potential DCD donors per year in the USA. If only 15% of the potential DCD donors could donate their kidneys, that would increase about 3000 cases of DCD kidney transplantation [7]. In China, DCD donor programs have begun in 2005 and developed rapidly in the last decade. In 2010, there were only 0.17 DCD donors per million population in DCD donor programs, while in 2019, the number increased to about 0.8 DCD donors per million population. Although there is still a huge gap in the DCD donor program between China and the Western countries, there is large potential for further increasing the DCD donation rate in China in the future.
6.2 Classification of Donation After Cardiac Death
DCD donors are divided into four categories according to Maastricht classification of DCD donors [8]. Category I is defined as dead on arrival. Patients are from out-of-hospital accidents who are not resuscitated. Category II is defined as unsuccessful resuscitation. Patient is brought to the emergency room while being resuscitated by the emergency medical services (EMS), and is declared dead after cardiopulmonary resuscitation (CPR) is unsuccessful. Category III is defined as awaiting cardiac or circulatory death. Patient occurs circulatory death after a planned withdrawal of life-sustaining therapies (WLST). Category IV is defined as cardiac arrest in a brain dead donor. Patients suffer an unexpected cardiac arrest after diagnosis of brain death and during donor management but prior to the organ retrieval. Category I and II are defined as uncontrolled DCD donors, while category III to IV are defined as controlled DCD donors. The majority of DCD donors in Belgium, The Netherlands, the UK, and the USA are category III, whereas category II donors predominate in France and Spain. In China, there is a special category for DCD donors. China Category III. Organ donation after brain death is followed by circulatory death. Donor in this category has been diagnosed of brain death and organ procurement is conducted when cardiac arrest appears after a planned withdrawal of life-sustaining therapies because the relatives of the donor do not accept brain dead [9].
6.3 Donation Process of Cardiac Death Donors
Donation process of DCD donors includes withdrawal of life support from donors and harvesting the kidneys. Withdrawal of life-supporting treatment typically involves discontinuation of inotropes and ventilatory support. In China, a period of 5 minutes of observation is required after cardiac arrest before death can be confirmed and organ retrieval can be begun [9]. The time period deemed necessary from cessation of circulation to the start of organ procurement varies from 2 to 20 min internationally [10, 11].
Currently, the acceptable criteria for DCD donors in most of Chinese hospitals were as follows: (I) age <60; (II) warm ischemia time <25 min; (III) agonal time from withdrawal of mechanical, ventilated, or organ-perfusion support treatment to cardiac arrest <4 h and (IV) no history of systemic sepsis, diabetes mellitus, malignancy, or renal diseases [9].
Procurement of the kidneys from DCD donors in China is undertaken similar to that used in most other countries. Rapid laparotomy and arterial cannulation is performed, the abdominal organs are perfused with cold organ preservation solution such as UW or HTK solution, and ice slush is placed intraperitoneally to aid topical cooling of the organs. The warm ischemic time is controlled within 20 minutes. After in situ cooling, the kidneys are excised and delivered to the organ retrieval team, then are subjected to further cold perfusion on the back-table before cold storage. After procurement, the majority of DCD kidneys are subjected to simple static cold storage, and about 20% DCD kidneys in China undergo hypothermic machine perfusion, according to the preference of the retrieving and implanting surgeons [11].
There are three perfusion techniques including rapid laparotomy with direct aorta cannulation, in situ perfusions, and extracorporeal regional perfusion, which are commonly used to preserve kidneys before procurement. After the consent for donation is obtained and withdrawal of life support is performed, rapid laparotomy and direct aorta cannulation can be performed in Maastricht category III donors [12]. Before laparotomy is done and topical cooling of the organs is performed, in situ perfusion can be used in both controlled donors and uncontrolled donors, if consent for donation has been obtained [13]. Regional perfusion uses extracorporeal machine oxygenation circuit to selectively perfuse the abdominal organs after cannulation of the femoral vessels. This technique can be used to cool organs down both in uncontrolled DCD donors and DBD donors [14]. In recent years, it has been used to reperfuse the organs at body temperature (normothermic machine perfusion, NMP). This technique can further reduce the warm ischemia time of donor kidneys and reduce the PNF and DGF rate after DCD kidney transplantation [15].
6.4 Early Graft Function of DCD Kidney Transplantation
Delayed graft function (DGF) is the most striking difference in outcome between DCD and DBD donor kidneys, which is most commonly defined as the need for dialysis in the first 7 days post-transplant. Uncontrolled DCD kidneys have a much higher incidence of delayed graft function rate than controlled DCD kidneys and controlled DCD kidneys have a higher DGF rate compared to DBD kidneys. In a French study of uncontrolled DCD, delayed graft function occurred in 92% of recipients. The incidence of DGF after controlled DCD kidney transplantation in the UK is 49%. Hoogland reported that the incidence of PNF and DGF was substantially high in both type II (n = 128) and the type III (n = 208) groups (22% vs. 21% and 61% vs. 56%, respectively) [16]. Analyzing an American database of 78,001 kidney donations, of which 2136 were from DCD donors, the results showed that although delayed graft function was more common in kidneys from DCD donors, particularly if the donation was uncontrolled, the 1-year graft survival was similar in all groups [17]. Primary non-function (PNF) rate after kidney transplant was low in controlled DCD kidneys. A study analyzing the data from the UK showed that the rate of PNF for both controlled DCD and DBD kidneys was similarly low, although the incidence was slightly higher for DCD than for DBD kidneys (4 vs. 3%, respectively, adjusted odds ratio of 1.49, P = 0.04) [18]. Our initial 71 DCD kidney transplants showed that the incidence of PNF and DGF were 2.8% and 28.2%, respectively. The PNF and DGF rates were significantly higher in DCD kidney transplants than DBD kidney transplants, which were lower than 1% and 10%, respectively [19].
In order to reduce the DGF rate of DCD kidney transplantation, usage of hypothermic machine perfusion (HMP) is increased for preserving the DCD kidneys in the recent years. A meta-analysis including both DBD and DCD kidneys suggested that HMP was associated with a relative risk of DGF of 0.804 (0.672–0.961) and that the reduction in DGF associated with HMP predicted a modest improvement in 10-year graft survival of 3% [20]. However, more randomized controlled trials of machine perfusion for DCD kidneys have produced conflicting results with respect to DGF. The results of two large, randomized controlled trials of static storage vs. machine perfusion of human DCD kidneys, in which one kidney from each donor was stored without perfusion, and the other was machine perfused, confirmed that pretransplant machine perfusion had no effect on 1-year patient, graft survival, and estimated post-transplant GFR. Decreased incidence and duration of DGF after machine perfusion was identified in 82 pairs of DCD kidneys [21], whereas the other study showed no beneficial effect on DGF [22]. A meta-analysis of multiple studies of hypothermic machine perfusion in DCD showed reduced DGF rates than kidneys placed in cold storage (Odds ratio = 0.64, P = 0.03) but no difference in 1-year graft survival [23]. Another meta-analysis comparing 175 machines perfused DCD kidney grafts with 176 cold storage grafts showed that machine perfused kidneys suffered less DGF (Odds ratio = 0.56, P = 0.008) but no differences in PNF and 1-year graft or patient survival [24]. Given the increased cost of machine perfusion, similar intermediate-term graft, and patient survival, the benefit of machine perfusion is unclear. Therefore, further studies are required before machine perfusion could be recommended over static cold storage as a better way to reduce DGF.
6.5 Graft and Patient Survival of DCD Kidney Transplantation
DCD kidneys show a comparable patient and graft survival to DBD kidneys and show a survival benefit to recipients over waiting for DBD kidneys [25]. A study from the UK including 739 DCD and 6759 DBD kidney transplant recipients, showed no difference in graft survival up to 5 years (hazard ratio = 1.01, P = 0.97) or in eGFR at 1 to 5 years after transplantation (at 12 months: −0.36 ml/min per 1.73 m2, P = 0.66) [26]. A cohort from US Mycophenolic Renal Transplant Registry including 133 DCD kidney transplants and 415 DBD transplants. The incidence of DGF was 29.4% and 23.5% in the DCD group and the DBD group, respectively (P = 0.1812). The incidence of BPAR at 12 months was 9.0% and 9.9% respectively (P = 0.7713). The 1-year graft loss rate in the DCD group was higher than that in the DBD group (7.5% vs. 3.1%, P = 0.0283), and the 4-year graft loss rate and patient death rate were not significantly different between the DCD and DBD groups [27]. By comparing the long-term outcome of kidney transplantation from uncontrolled (n = 128) and controlled (n = 208) DCD donor kidneys procured, Hoogland et al. found that ten-year graft and recipient survival are similar in both groups (50% vs. 46%, p = 0.74 and 61% vs. 60%, p = 0.76, respectively). The outcome of kidney transplantation from uncontrolled and controlled donors after cardiac death is equivalent [16]. Another study from the Netherlands, including 2711 DCD kidney transplants and 3611 DBD kidney transplants, showed that despite higher incidences of early graft loss (+50%) and delayed graft function (+250%) in DCD grafts, 10-year graft and recipient survival were similar for the two graft types (10-year graft survival: 73.9%, 10-year patient survival: 64.5%). Long-term outcome equivalence was explained by a reduced impact of delayed graft function on DCD graft survival (RR: 0.69, 95% CI 0.55–0.87, p < 0.001). Mid and long-term graft function (eGFR), and the impact of delayed graft function on eGFR were similar for DBD and DCD grafts [28]. Our data from 71 DCD kidney transplants showed that the 1- and 3-year graft survival was 95.7% and 92.4%, respectively, which were comparable to DBD kidney transplants [19].
6.6 Graft Function of Recipients of DCD Kidney Transplantation
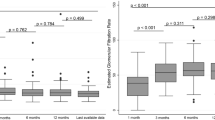
Recipients with DCD kidneys have similar graft survival compared to DBD donor, which has been reported by many studies. There is still some concern by some clinicians that graft function may be inferior in recipients of DCD kidneys, because ischemic injury incurred at the time of donation and transplantation may affect the long-term outcome. A study from UK compared graft function between 1768 DCD and 4127 DBD kidney transplant recipients. The results showed that graft function (eGFR) at 1 year was lower in DCD kidneys group compared to DBD kidneys group (eGFR 48 ml/min per 1.73 m2 vs. 50 ml/min per 1.73 m2, P = 0.01). There was no difference in graft function between DCD and DBD groups at 5 years after transplantation (49.6 ml/min per 1.73 m2 vs. 48.1 ml/min per 1.73 m2, P = 0.97) [18]. In a Chinese cohort study compared 325 DCD kidney transplants with 409 living donors (LD) kidney transplant. The graft function in the DCD group was better than that of the LD group at 3 years after transplant (eGFR: 71.14 ± 22.28 vs. 64.29 ± 16.76 mL/min/1.73 m2; P < 0.001). There was no significant difference between the paired DCD and LD group (eGFR: 62.22 ± 18.50 vs. 66.99 ± 17.81 mL/min/1.73 m2; P = 0.068) when matching donor age [28]. Therefore, there is no evidence that long-term graft function is inferior in kidney recipients from DCD donors than DBD donors or living donors.
6.7 Risk Factors Associated with Outcome of DCD Kidney Transplantation
There are several risk factors that may affect the outcome of DCD kidney transplantation. Donor age is the most important factor, which may affect graft survival no matter the recipients received kidneys from DCD or DBD donors. In a study of deceased kidney transplantation, the recipients who received kidneys from donors >60 years had more than twice the risk of graft failure compared to those transplanted with kidneys from donors <40 years in 3 years of transplantation (HR 2.35, 95% CI 1.85–3.0, P < 0.0001) [18]. In a study from Italy including young (<60 years) and old (≥60 years) DCD kidney transplants and old DBD kidney transplants, the results showed that compared to young DCD recipients, old DCD kidney transplant recipients had lower patient survival (66% vs. 85%; P = 0.014), death-censored graft survival (63% vs. 83%; P = 0.001), and eGFR (34 ml/min per 1.73 m2 vs. 45.0 ml/min per 1.73 m2; P = 0.021) after 5 years. In addition, old DCD recipients had higher incidence of DGF (70% vs. 47.2%; P = 0.029) and graft thrombosis (12.5% vs. 1.4%; P = 0.021) than young DCD recipients. There was similar 5-year patient survival (66% vs. 67%; P = 0.394) and death-censored graft survival (63% vs. 69%; P = 0.518) when compared to old DCD kidneys and old DBD kidneys. However, old DCD transplant had higher DGF (70% vs. 37.5%; P = 0.007) and lower estimated glomerular filtration rate (34 mL/min per 1.73 m2 vs. 41 mL/min per 1.73 m2; P = 0.029) than old DBD group [29].
High donor body mass index (BMI) is another risk factor for DGF and graft failure. A study showed that DCD kidneys from donors with BMI > 45 kg/m2 had a 1.84 times higher risk of graft loss [30]. Hypertension, diabetes, high donor creatinine and donor cause of death may also affect the outcome of DCD kidney transplant; the donors with these risk factors are defined as expanded criteria donors (ECD). The ECD donors usually have a poor outcome after kidney transplants than standard donors. In a UK Transplant Registry analysis study, ECD donors occurred in 31.5% of DBD and 34.9% of DCD transplants. There was no difference in graft survival between DCD and DBD transplants, although recipients from ECD donors had inferior graft survival compared to recipients from standard criteria donors. In addition, the risk-adjusted analysis showed that there was no significant interaction between standard criteria donors/ECD status and donor type when adjusting with HLA mismatch, recipient age, CIT, and recipient cause of the renal disease (P = 0.45). The primary non-function rate was higher in ECD DCD kidneys group compared to the standard criteria DCD kidneys group (4.1% and 2.7%, respectively, P = 0.02) [18].
Cold ischemia time (CIT) is another important risk factor that affects the outcome of a DCD kidney transplant. Kidneys from DCD donors are particularly vulnerable to long cold ischemia time. A study from the UK showed that relative risk for graft loss was 2.36 times (HR 1.39–4.02, P = 0.004) higher in DCD kidneys with a CIT of >24 h compared to kidneys with CIT of <12 h. The graft survival at 5 years after transplant was also lower in recipients with >24 h of CIT compared to recipients with <12 h of CIT (82.6% and 88.6%, respectively). There was no significant interaction between prolonged CIT and increasing donor age (P = 0.96). There were 22% of DCD donor kidneys used cold pulsatile machine perfusion; however machine perfusion did not show the impact on improving graft survival for deceased donor kidneys (adjusted HR 0.97, 95% CI 0.8–1.2, P = 0.80) [18]. Because cold ischemia time greatly impacts graft loss in DCD kidney transplant, CIT should be kept as short as possible (preferably <12 h).
6.8 Selection of DCD Kidneys
DCD kidneys have higher PNF and DGF rates after transplant. How to select or decline a DCD kidney is an important question in clinic. The decision to accept or decline a DCD donor kidney is usually made by transplant surgeons in the transplant centers based on the quality of the DCD donor kidney. The most common reason to decline a kidney by a transplant surgeon is the donor age, particularly the donor is too elderly. In recent years, many transplant centers have relaxed their criteria for using DCD kidneys from marginal donors because the experience of using DCD kidneys has accumulated step by step. Many DCD kidneys from old donors and donors with diabetes or cardiovascular disease have been used in the experienced centers. However, the discard rate of kidneys from DCD donors is still high, especially for kidneys from the elderly and ECD donors. Other factors may also cause surgeons to decline DCD kidneys, such as a protracted agonal period before asystole, unfavorable gross appearance following perfusion, and high resistant index during cold pulsatile perfusion. We evaluated the quality of 58 DCD and ECD donor kidneys using hypothermic machine perfusion. The results showed that the parameters of hypothermic machine perfusion might be useful non-invasive tools for evaluating the quality of DCD/ECD kidneys. One hour resistant index (RI) of machine perfusion >0.4 is correlated with DGF rate and 1 year graft function in DCD or ECD kidney transplantation [31]. Other reasons to decline kidneys from DCD donors include surgical damage to the organs during procurement, having risk of transmitting infection or malignancy of donor.
Pre-transplantation (Zero time) biopsy histology is an important predictor for the outcome of DCD kidneys and can improve transplant outcome if those kidneys are not transplanted that are identified as probable failures after transplant. The most commonly used criteria for pre-transplantation is “Remuzzi Score.” The Remuzzi Score has four components, including glomerular sclerosis, tubular atrophy, interstitial fibrosis, and atherosclerosis. After histological evaluation, the severity of chronic kidney injury in DCD donor kidneys can be quantified from scoring for each of these four components. Each component is scored 0–3, and provide a summed composite Remuzzi score of 0–12. It was recommended that kidneys from DBD donors with a score of >6 should be discarded, those kidneys with a score of 4–6 should be used as dual kidney transplantation, and those kidneys with a score of 0–3 should be used as single transplants [32]. A large retrospective multicenter analysis from Italian transplant centers has confirmed the value of pre-transplantation biopsy in transplantation of marginal kidneys according to Remuzzi score [33]. It has been shown in several large cohort of marginal deceased donor kidneys that pre-transplantation donor biopsy allowed safe allocation and transplantation of marginal kidneys. Some of those marginal kidneys might have been discarded on the basis of their high kidney donor profile index, which usually indicating a higher risk of post-transplant graft failure. However, acceptable transplant outcomes have been accomplished.
6.9 Pediatric DCD Kidney Transplantation
DCD kidney transplantation is often associated with an inflammatory reaction and oedema due to longer warm ischemia time; therefore, DCD kidneys may need a higher arterial blood pressure to get an adequate perfusion pressure. Many research have shown that pediatric DCD kidney transplantation is associated with a higher rate of DGF and reduced graft survival rate compared to pediatric DBD kidneys, and the hazard ratio is more than doubled. A retrospective cohort study from the Netherlands comparing 91 pediatric DCD kidney transplants with 405 pediatric DBD kidney transplants [34]. The results showed that the grafts from DCD donors were associated with higher rate of delayed graft function (48% vs. 8%, P < 0.001) and primary non-function (9% vs. 2%, P < 0.01) compared to DBD donors. There was no difference in estimated glomerular filtration rate between the two groups (57 ± 17 vs. 58 ± 21 ml/min at 1 year and 62 ± 14 vs. 57 ± 22 ml/min at 5 years, respectively). The risk of graft failure was higher in the DCD group than the DBD group (HR 2.440, 95% CI 1.280–4.650, P = 0.007) after adjusting for several confounding variables. Patient survival was similar between two groups (HR 1.559, 95% CI 0.848–2.867, P = 0.153). Therefore, it should weigh the slightly higher risk of graft failure by accepting a DCD kidney against the risks of staying on the waiting list for a long period when the surgeons decide whether or not to allocate a DCD kidney to a child.
6.10 Postoperative Management of DCD Kidney Transplantation
6.10.1 Peri-Operative Fluid Management
Fluid depletion in peri-operative period of DCD kidney transplantation may decrease initial graft function and increase the DGF rate after transplant. It has been shown that pre-operative and operative fluid loading may reduce the DGF rate after transplant. In a study including recipients of DCD kidneys, the results showed that for those recipients from DCD kidneys, low central venous pressure and low blood pressure during operation might increase the risk of PNF [35]. Therefore, it is important to monitor venous pressure immediately after the surgical procedure, keep the recipients well hydrated, and avoid immediate post-transplant dialysis. These methods may reduce the DGF and PNF rate after a DCD kidney transplant.
6.10.2 Post-Transplant Monitoring
After DCD kidney transplantation, patients with DGF should undergo regular ultrasonography, renal angiography, or both to rule out other causes other than acute tubular necrosis, usually due to temporary renal insufficiency. In addition, it is difficult to diagnose rejection in patients with DGF. Therefore, biopsies should be performed when necessary. In our center, Acute rejection was clinically diagnosed if serum creatinine increased 10% or more per day, and at the same time, ultrasound examination for the allograft showed the resistant index greater than 0.8. Most patients with the clinical diagnosis of acute rejection were further proven by standard percutaneous kidney allograft biopsy [19].
6.10.3 Immunosuppressive Therapy Protocol
DCD kidneys are more susceptible to calcineurin inhibitor (CNI) nephrotoxicity compared to DBD kidneys. Immediately use of CNI after transplant may exacerbate ischemic injury of DCD kidneys, increase DGF rate, delay recovery from DGF and impair long-term graft function. Therefore, it is better to avoid or postpone the use of CNI drugs or use low dose CNI immediate after transplant. In some patients with severe CNI nephrotoxicity, mTOR inhibitors may be used to replace CNI. Polyclonal antibodies may be used in order to postpone the immediate use of CNI after DCD kidney transplants. Some studies showed that anti-thymocyte globulins (ATG) can protect donor kidneys from ischemia-reperfusion injury during operation [36]. In our center, patients were given rabbit anti-thymocyte globulin and methylprednisolone as induction therapy during the operation and the first two days after kidney transplantation. In our experience, thymoglobulin seemed to be more effective than ATG-F on reducing DGF in patients with increased risk factors for DGF. For the patients with increased risk factors for DGF, the DGF rate was 22.5% in the thymoglobulin group vs. 56.3% in the ATG-F group (P = 0.015) [37]. For the recipients who received DCD kidneys from old donors, the maintenance CNI dose should be kept in relatively low level, because these kidneys are more susceptible for CNI nephrotoxicity.
References
Callaghan CJ, Harper SJF, Saeb-Parsy K, et al. The discard of deceased donor kidneys in the UK. Clin Transpl. 2014;28:345–53.
Huang J. The “Chinese Mode” of organ donation and transplantation. Hepatobil Surg Nutr. 2017;6(4):212–4.
Weber M, Dindo D, Demartines N, et al. Kidney transplantation from donors without a heartbeat. N Engl J Med. 2002;347:248–55.
Brook NR, Waller JR, Nicholson ML. Non heart-beating kidney donation: current practice and future developments. Kidney Int. 2003;63:1516–29.
Tavares-da-Silva E, Figueiredo A. Renal procurement: techniques for optimizing the quality of the graft in the cadaveric setting. Curr Urol Rep. 2020;21(2):12.
Ibrahim M, Vece G, Mehew J, et al. An international comparison of deceased donor kidney utilization: what can the United States and the United Kingdom learn from each other? Am J Transplant. 2020;20:1309–22.
Boyarsky BJ, Jackson KR, Kernodle AB, et al. Estimating the potential pool of uncontrolled DCD donors in the United States. Am J Transplant. 2020 May 5; https://doi.org/10.1111/ajt.15981.
Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 2016;29(7):749–59.
Huang J, Wang H, Fan ST, et al. The national program for deceased organ donation in China. Transplantation. 2013;96(1):5–9.
Domínguez-Gil B, Haase-Kromwijk B, Van Leiden H, et al. Current situation of donation after circulatory death in European countries. Transpl Int. 2011;24:676–86.
Reich DJ, Mulligan DC, Abt PL, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant. 2009;9:2004–11.
Snoeijs MG, Dekkers AJ, Buurman WA, et al. In situ preservation of kidneys from donors after cardiac death: results and complications. Ann Surg. 2007;246:844–52.
Garcia-Rinaldi R, Lefrak EA, Defore WW, et al. In situ preservation of cadaver kidneys for transplantation: laboratory observations and clinical application. Ann Surg. 1975;182:576–84.
Koyama I, Shinozuka N, Miyazawa M, Watanabe T. Total body cooling using cardiopulmonary bypass for procurement from non-heart-beating donors. Transpl Proc. 2002;34:2602–3.
Net M, Valero R, Almenara R, et al. The effect of normothermic recirculation is mediated by ischemic preconditioning in NHBD liver transplantation. Am J Transplant. 2005;5:2385–92.
Hoogland ERP, Snoeijs MGJ, Winkens B, et al. Kidney transplantation from donors after cardiac death: uncontrolled versus controlled donation. Am J Transplant. 2011;11:1427–34.
Gagandeep S, Matsuoka L, Mateo R, et al. Expanding the donor kidney pool: utility of renal allografts procured in a setting of uncontrolled cardiac death. Am J Transplant. 2006;6:1682–8.
Summers DM, Johnson RJ, Hudson A, et al. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet. 2013;381:727–34.
Chen GD, Ko S-CD, Wang C, Qiu J, Han M, He X, Chen L. Kidney transplantation from donors after circulatory death: an initial report of 71 cases from China. Am J Transplant. 2013;13(5):1323–6.
Wight JP, Chilcott JB, Holmes MW, et al. Pulsatile machine perfusion vs. cold storage of kidneys for transplantation: a rapid and systematic review. Clin Transpl. 2003;17:293–307.
Jochmans I, Moers C, Smits JM, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg. 2010;252:756–64.
Watson CJ, Wells AC, Roberts RJ, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant. 2010;10:1991–9.
Bathini V, McGregor T, McAlister VC, et al. Renal perfusion pump vs. cold storage for donation after cardiac death kidneys: a systematic review. J Urol. 2012;189:2214–20.
Deng R, Gu G, Wang D, et al. Machine perfusion versus cold storage of kidneys derived from donation after cardiac death: a meta-analysis. PLoS One. 2013;8:e56368.
Snoeijs MG, Schaubel DE, Hene R, et al. Kidneys from donors after cardiac death provide survival benefit. J Am Soc Nephrol. 2010;21:1015–21.
Summers DM, Johnson RJ, Allen J, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet. 2010;376:1303–11.
Zhu D, McCague K, Lin W, et al. Outcome of kidney transplantation from donor after cardiac death: reanalysis of the US mycophenolic renal transplant registry. Transplant Proc. 2018;50(5):1258–63.
Zhang X, Lyu J, Yu X, et al. Comparison of graft outcome between donation after circulatory death and living-donor kidney transplantation. Transplant Proc. 2020;52(1):111–8.
Favi E, Puliatti C, Iesari S, et al. Impact of donor age on clinical outcomes of primary single kidney transplantation from Maastricht category-III donors after circulatory death. Transplant Direct. 2018;4(10):e396.
Ortiz J, Gregg A, Wen X, et al. Impact of donor obesity and donation after cardiac death on outcomes after kidney transplantation. Clin Transpl. 2012;26:E284–92.
Chen G, Wang C, Zhao Y, et al. Evaluation of quality of kidneys from donation after circulatory death/expanded criteria donors by parameters of machine perfusion. Nephrology (Carlton). 2018;23(2):103–6.
Remuzzi G, Grinyò J, Ruggenenti P, et al. Early experience with dual kidney transplantation in adults using expanded donor criteria. Double Kidney Transplant Group (DKG). J Am Soc Nephrol. 1999;10:2591–8.
Gandolfini I, Buzio C, Zanelli P, et al. The kidney donor profile index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: distribution and association with graft outcomes. Am J Transplant. 2014;14:2515–25.
de Vries EE, Hoogland PE, et al. Transplantation of kidneys from paediatric DCD donors: a comparison with DBD donors. Nephrol Dial Transplant. 2013;28(1):220–6.
Snoeijs MG, Wiermans B, Christiaans MH, et al. Recipient hemodynamics during non-heart-beating donor kidney transplantation are major predictors of primary nonfunction. Am J Transplant. 2007;7:1158–66.
Jose Perez-Saez M, Montero N, Redondo-Pachon D, et al. Strategies for an expanded use of kidneys from elderly donors. Transplantation. 2017;101:727–45.
Chen GD, Lai XQ, Ko DS, et al. Comparison of efficacy and safety between rabbit anti-thymocyte globulin and anti-T lymphocyte globulin in kidney transplantation from donation after cardiac death: a retrospective cohort study. Nephrology (Carlton). 2015;20(8):539–43.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 People's Medical Publishing House, PR of China
About this chapter
Cite this chapter
Chen, G., Li, Q. (2022). Kidney Transplantation from Cardiac Death Donors. In: He, X., Huang, J. (eds) Organ Donation and Transplantation after Cardiac Death in China. Springer, Singapore. https://doi.org/10.1007/978-981-16-0815-5_6
Download citation
DOI: https://doi.org/10.1007/978-981-16-0815-5_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0814-8
Online ISBN: 978-981-16-0815-5
eBook Packages: MedicineMedicine (R0)