Abstract
The liver receives its abundant blood supply from both the systemic arterial and the portovenous systems. After going through the liver, the blood then drains through the hepatic venous system into the inferior vena cava and hence back to the heart. Blood loss from the raw surfaces of the liver during liver parenchymal transection is inevitable, and the amount of blood loss significantly affects post-operative morbidity, mortality and long-term survival. Minimizing intraoperative blood loss significantly improves patient outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
12.1 Blood Loss During Liver Resectional Surgery
The liver receives its abundant blood supply from both the systemic arterial and the portovenous systems. After going through the liver, the blood then drains through the hepatic venous system into the inferior vena cava and hence back to the heart. Blood loss from the raw surfaces of the liver during liver parenchymal transection is inevitable, and the amount of blood loss significantly affects post-operative morbidity, mortality and long-term survival. Minimizing intraoperative blood loss significantly improves patient outcomes.
12.2 Applied Anatomy and Physiology
Bleeding from the inflow blood coming from the hepatic artery and the portal vein can be controlled readily with porta hepatis clamping (the Pringle’s manoeuvre). However, backflow bleeding still occurs from the divided branches of the outflowing major hepatic veins (the right, middle and left) and the short hepatic veins (Fig. 12.1). The rate of this bleeding is directly proportional to the presumed gradient across the divided vessels. On one side is the hepatic venous and sinusoidal pressure which is directly rated to the central venous pressure (CVP). On the other side, it is open to air which is atmospheric pressure. In other words, the rate of blood loss is proportional to the pressure gradient between the CVP and the atmospheric pressure. Another factor that determines the rate of blood loss is the diameter of the divided vessel, with a larger divided vessel losing blood at a faster rate (Fig. 12.2). Thus, it is logical to assume that haemodynamic manipulation to maintain a low CVP is one of the important techniques to decrease blood loss during liver transection. With less blood loss, there is a better view of the transected area, and larger vessels can be controlled and ligated before they bleed, thus entering into a virtuous cycle of less bleeding → clearer view → less bleeding → clearer view.
12.3 Advantages of a Low CVP in Liver Resectional Surgery
The advantages of a low CVP which leads to a decrease in intraoperative blood loss include:
-
1.
Decreases the percentage of patients who requires blood transfusion
-
2.
Shortens operative time
-
3.
Decreases post-operative morbidities and mortalities
-
4.
Shortens hospital stay
-
5.
Faster recovery, especially the post-operative liver and renal functions
12.4 Why Are Some Anaesthesiologists Still Reluctant in Using Low CVP?
This mainly arises out of the lack of communication between the anaesthesiologists and the liver surgeons and in some sense, a mistrust of the anaesthesiologist on the ability of the surgeon in avoiding major bleeding during liver resectional surgery. These anaesthesiologists are worried about the narrow safety margin for the patient’s cardiovascular system being able to provide adequate perfusion to the patient’s major organs during an episode of major bleeding. Thus, this mutual trust has to develop gradually between the anaesthesiologist and the surgeon. Even if an anaesthesiologist declines to use low CVP, he/she should realize that a high CVP makes bleeding during liver transection worse and should avoid doing so.
The second reason is a relative lack of knowledge by the anesthesiologist in this area. Physiological studies have shown that a mean arterial blood pressure of >50 mmHg is able to maintain perfusion to major organs. Moreover, adequate oxygenation to major organs is not only dependent on the perfusion pressure in the cardiovascular system, but is also dependent on the oxygen content of the blood. This is calculated by the following equation:
Where:
-
SO2 = percentage saturation of Hb with oxygen.
-
Hb = haemoglobin concentration in grammes per 100 mL blood.
-
PO2 = partial pressure of oxygen (0.0225 = mL of O2 dissolved per 100 mL plasma per KPa, or 0.003 mL per mmHg).
In order to maintain good oxygen supply to major organs in a relatively low perfusion pressure, the haemoglobin level should be ensured by high inspiratory oxygen. Avoiding hypothermia also significantly improves the oxygen-carrying capacity. A careful study of the oxygen dissociation curve in Fig. 12.3 helps the reader to understand more about the oxygen-carrying capacity of haemoglobin.
12.5 Low CVP: How Low Is Low?
The CVP in a normal person in the supine position is 5–12 mmHg (or 7–16 cm H2O) during liver resection. Some surgeons even request a CVP near zero.
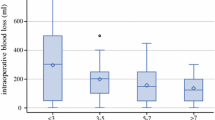
What is the optimal CVP in liver resection? The impact of the variations in CVP on the rate of blood loss and on the perfusion of major organs has been studied. An animal study using Bama miniature pigs aimed to evaluate the haemodynamic and oxygen transport changes during liver resection at different CVP levels was carried out. The CVP was controlled from 0 to 8 cm H2O during liver resection. The rates of blood loss and the hepatic venous pressures during liver resection were almost linearly related to the CVP. A significant drop in mean arterial pressure, cardiac output, cardiac index and in oxygen delivery happens when the CVP was less than 2 cm H2O. In addition, there was a significant drop in oxygen consumption and oxygen extraction ratio when the CVP was less than 1 cm H2O. Oxygen delivery, oxygen consumption and oxygen extraction ratio remained relatively constant between CVP from 2 to 8 cm H2O. Based on this animal study, the optimal CVP for liver resection is 2–3 cm H2O. A later study showed that these findings could also be applied to human beings.
12.6 Methods to Lower CVP
There are two main ways to lower the CVP during liver resectional surgery.
-
1.
Physiological Way
With the reverse Trendelenburg position (around 15 degrees head-up), the blood inside the venous compartment is pooled in the lower extremities, thus lowering the CVP (Fig. 12.4).
This method is not often used because there is always a theoretical risk of air embolism by using this position. Many surgeons do not like to operate with the patient in this position because the instruments tend to fall off the operating table.
-
2.
Pharmacological Ways
Both diuretics and/or venodilators have been frequently used.
-
(a)
Diuretics
The intravascular volume, especially the venous compartment, can be depleted with an intravenous bolus of loop diuretics (e.g. Frusemide 5–40 mg i.v.), which lowers the CVP. The major disadvantage is the activation of the renin-angiotensin-aldosterone system and finally raises the plasma anti-diuretic hormone, thus resulting in oliguria in the post-operative period, which may persist up to 24–48 h.
-
(b)
Venodilators
The use of venodilators (e.g. glycerol trinitrate 0.5–5 mg/h) seems to be a more practicable and titratable method in achieving a low CVP. It relaxes the smooth muscle in the vessel wall and reduces the pressure inside the vessel provided that the intravascular volume remains the same. Glycerol trinitrate is the drug of choice. At a low plasma level, it has more vasodilatation effect in the venous system than the arterial system. The effect is immediate, and the half-life is short. Therefore, the CVP can be titrated to the desired level gradually by adjusting the rate of infusion.
-
(a)
12.7 Measures to Take When Using Low CVP for Liver Resectional Surgery
-
1.
Preoperative Assessment
Adequate assessment of the cardiopulmonary function to make sure that the patient can tolerate a period of low blood pressure. A history on whether the patient has been put on long-term glycerol trinitrate is important to rule out possible drug tolerance.
-
2.
Intraoperative Measures
After the patient is put under general anaesthesia, an intravenous drip should be set up to give normal saline at a rate of 100 mL/h, aiming to maintain urine output of >30 mL/h. A double lumen catheter should be inserted into the right internal jugular vein for CVP monitoring. Except in patients who are under 50 years of age or ASAI, an arterial line should be set up to monitor the blood pressure.
At the start of liver parenchymal transection, an intravenous infusion of glycerol trinitrate 0.5–6 ng/h should be given to maintain a CVP at 2–3 cm H2O. At a low CVP of 2–3 cm H2O, there are concerns about hypoperfusion and tissue hypoxia of the major organs. This problem can be readily tackled by increasing the inspiratory oxygen concentration (FiO2) to 80% and with the use of a vasopressor (e.g. phenylephrine 0.05 mg intravenously) to maintain a mean blood pressure of 50 mmHg, which would ensure adequate oxygen delivery to the vital organ. If excessive bleeding occurs, further fluid load can be given to replace the volume loss.
After completion of hepatic parenchymal transection, the Pringle’s manoeuvre is unclamped and adequate haemostasis is done by the surgeon on the raw liver surface. The CVP can then be brought up to more than 100 mmHg by stopping the intravenous nitrate infusion and by increasing the intravascular volume by infusion of 1–2 L of colloid solution (e.g. Gelofusine). Haemostasis is then secured one more time by the surgeon.
12.8 Conclusion
Although there is no randomized controlled trial to evaluate the relative risks and benefits of maintaining a low CVP in liver resectional surgery, it would be logical to avoid using a high CVP in liver surgery. With meticulous use, the low CVP technique is definitely helpful in liver resectional surgery to significantly reduce intraoperative blood loss.
Further Reading
Guo Y, Lin CX, Lau WY, et al. Hemodynamics and oxygen transport dynamics during hepatic resection at different central venous pressures in a pig model. Hepatobiliary Pancreat Dis Int. 2011;10:516–20.
Huntington JT, Royall NA, Schmidt CR. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol. 2014;109:81–8.
Lin CX, Guo Y, Lau WY, et al. Optimal central venous pressure during partial hepatectomy for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:520–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd. and People's Medical Publishing House Co. Ltd.
About this chapter
Cite this chapter
Lau, W.Y. (2021). Low Central Venous Pressure in Liver Resectional Surgery. In: Applied Anatomy in Liver Resection and Liver Transplantation. Springer, Singapore. https://doi.org/10.1007/978-981-16-0800-1_12
Download citation
DOI: https://doi.org/10.1007/978-981-16-0800-1_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0799-8
Online ISBN: 978-981-16-0800-1
eBook Packages: MedicineMedicine (R0)