Abstract
Food flavor is the combination of all sensory attributes experienced while ingesting food, with gustatory and olfactory perceptions being the most important attributes. This chapter mainly focuses on common types of flavor substances present in food, the interactions between these different flavor substances, and the influencing factors and flavor perception mechanisms for flavor substances. This chapter also introduces aromas present in different foods, formation of aromas, and the influence of processing on aroma composition and stability.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Overview
10.1.1 An Overview of Flavor
In addition to providing necessities to human beings, food also provides people with psychological enjoyment and sensory pleasure. Food flavor is a comprehensive yet broad concept; it is the overall impression kept in the brain by all sense organs while consuming food, mainly through gustatory and olfactory perceptions, but also including pain, touch, temperature sensations, and those sensations triggered by the trigeminal nerves (Fig. 10.1). Flavor substances in food are mostly categorized into taste substances received by gustatory perceptions and aroma substances received by olfactory perceptions.
Gustatory perception is the feeling from food in the mouth stimulating the gustatory organs. This stimulus sometimes comes from only one aspect of taste perception, but in most cases, it comes from a complex taste perception, including psychological taste perception (shape, color, brightness, etc.), physical taste perception (degree of hardness, sliminess, temperature, chewiness, texture, etc.), and chemical taste perception (sourness, sweetness, bitterness, saltiness, and other tastes).
Taste classifications are not uniform around the world. For example, it is divided into 5 tastes namely salty, sour, sweet, bitter, spicy in Japan; in Europe and the United States, it is divided into 6 flavors namely sweet, sour, salty, bitter, spicy, metal taste; it is divided into 8 flavors: sweet, sour, salty, bitter, spicy, light, astringent, and abnormal in India; while in China, in addition to the five flavors of sour, sweet, bitter, spicy and salty, there are also flavors of the fresh and astringent.
But physiologically, the four basic tastes are sweet, sour, salty, and bitter. In recent years, the concept of umami has become more accepted by the public and it has been named as the fifth basic taste. Spicy taste is caused by food stimulating the mucous membranes of the mouth and causing pain in both the nasal mucosa and the skin. Astringency taste refers to the astringent effect of the mucous membrane on the tongue.
However, in terms of food seasoning, spicy and astringent taste should be regarded as two separate tastes. As for umami and other flavors, they can make the whole flavor of food has a more delicious special effect, therefore, in Europe and the United States, umami substances can be used as flavor intensifier or synergistic agent, not as an independent taste of umami. It can be said that umami should also be regarded as an independent flavor in the flavoring of food.
Looking at human reactions to the basic tastes, saltiness is tasted first, while bitterness comes last. When looking at the sensitivity of tastes, people are most sensitive to bitterness; bitterness can be detected easily. Sensitivity for taste substances is often measured with threshold concentration as the standard. Threshold refers to the lowest concentration that could be detected. Because humans have different distribution of taste buds and different sensitivity to gustatory substances, the threshold and sensitivity for taste compounds will be different as well. For basic tastes, such as sour, sweet, bitter, and salty, the threshold for each representing substance is generally considered to be 0.3% for sucrose, 0.02% for citric acid, 16 mg/kg for quinine, and 0.2% for sodium chloride. (Table 10.1).
Olfactory perception is an important sense to the human body; it is produced by volatile substances stimulating the olfactory nerve cells in the nasal cavity and triggering a sensation by the central nervous system. Aromas in food are created by a combination of multiple aroma substances; it is rarely created by a single aroma compound. When these substances are combined in the perfect proportions, they can emit an attractive aroma. If not properly combined, the smell of food can be unbalanced, even causing off-flavors. Similarly, the relative concentration of fragrant substances in food can only reflect the strength of food aroma, but cannot completely and truly reflect the degree of pros and cons of food aroma. Therefore, the value of determining the role of an aromatic substance in a food aroma is called the aroma value (fragrance value). The aroma value is the ratio of the concentration of the aromatic substance to its threshold, that is
In general, when the aroma value is less than 1, people’s smelling organs will not cause sensation to this fragrant substance.
Flavor is one of the important aspects in assessing food quality and plays a decisive role in the selection, acceptance, and intake of food. Since flavor is a sensory attribute, the understanding and evaluation of flavor are often strongly biased depending on the individual, region, and ethnicity. Although modern analytical techniques provide a great convenience for in-depth study of flavor chemistry, it is difficult to accurately determine and describe the flavor of food by either qualitative or quantitative methods, because flavor is the physiological result of certain compounds acting on the human sensory organs. Therefore, sensory evaluation is still an important measure to flavor research.
10.1.2 Characteristics of Flavoring Substances
The flavor compounds reflecting the flavor of food generally are called flavor substances. There are generally many kinds of flavor substances interacting with each other in food, some of which play a leading role, others as a supporting role. If one or more compounds in food represent its food flavor, these compounds are called characteristic compounds. For example, the characteristic compound of sweet taste in a banana is isoamyl acetate, the characteristic compounds of cucumber are 2, 6- nonadienal, etc. The number of characteristic compounds in food is limited, existing at very low concentrations and sometimes not very stable. However, their existence provides an important basis for us to study the chemical basis of food flavor. The flavor substances reflecting the flavor of food generally have the following characteristics:
-
(1)
There are many kinds of flavor substances that can interact obviously each other. For example, flavor substances reached more than 500 in the cooked coffee. In addition, the antagonistic or synergistic effects among flavor substances make it difficult to reproduce the original flavor with recombined monomer components.
-
(2)
Small amount but significant effect. The content of flavoring substances in food varies greatly and the proportion is very low, but the flavor produced is obvious. For example, the aroma characteristic compounds of banana will give water a banana flavor at a concentration of only 5 × 10.6 mg per kilogram.
-
(3)
Many flavoring substances are easily decomposed by oxidation, heating, and so on, with poor stability. For example, the flavor of tea will become worse due to the automatic oxidation of its flavor substances.
-
(4)
The molecular structure of flavoring substances lacks general regularity. The molecular structure of flavoring substances is highly specific, a slight change in structure can make a big difference in flavor, even it is difficult to find regularity among the molecular structure of compounds with the same or similar flavor.
-
(5)
Flavor substance is also affected by its concentration, medium, and other external conditions.
10.2 Food Taste
10.2.1 Physiological Basis of Taste
Gustatory perception is produced by food’s soluble substances dissolved in saliva or food liquid stimulating taste receptors inside the oral cavity and then transmitted to the gustatory cortex in the brain through the gustatory sensory system. Lastly, through analysis by the brain’s central nervous system, gustation or taste is produced.
Taste bud is the part where the taste receptors and taste substances interact. Human’s taste buds are mainly located on the papillae, found on the surface of the tongue; only a small portion of taste buds are distributed in the soft palate, throat, pharynx, and other places. Filiform papillae, located on the surface of the tongue, is responsible for the sensation of touch but does not contain any taste buds. Near the filiform papillae, especially at the tip and sides of the tongue, fungiform papillae can be found. Along the two sides of the tongue, from the tip of the tongue to two-thirds after the end of the tongue, are foliate papillae, and circumvallate papillae are located at the end of the tongue (Fig. 10.2). Taste buds are elliptical, with supporting cells on the outer layer and multiple spindle-shaped gustatory cells on the inside. Gustatory hair is on top of gustatory cells, and nerve endings are distributed throughout the bottom of a taste bud (Fig. 10.3). Gustatory cell surface consists of protein, fat, and small amount of sugars, nucleic acids, and inorganic ions that can combine with different taste substances.
Different taste substances interact with different components on the receptors of taste cells. For example, the receptor for sweet substances is a protein, while the receptor for bitter and salty substances is a lipid. Some people think that the receptors of bitter substances are also may be related to protein. The experiments also showed that different taste substances have different binding sites on taste buds, especially sweet, bitter, and delicious substances. Their molecular structure has strict spatial specificity requirements, which reflects that different parts of the tongue have different sensitivity. There are different sensitivities. At the same time, the papillae on the surface of the tongue can be divided into fungiform papillae, filiform papillae, and foliate papillae according to their shapes. They exist in different parts of the tongue, respectively. Due to the uneven distribution of nipples, the perceptibility and sensitivity of different parts of the tongue to taste are also different (Fig. 10.4).
Only when the taste substances are dissolved in water, they can enter the orifice of taste buds and stimulate taste cells. When a piece of very dry sugar is placed on the surface of the tongue dried with filter paper, the sweetness of the sugar will not be felt. The saliva secreted by parotid gland, submandibular gland, sublingual gland, and numerous small salivary glands is the natural solvent of food. The activity of the secretory glands and the composition of saliva is also adapted to the type of food to a large extent. The drier the food is, the more saliva is secreted per unit time. When you eat egg yolk, the saliva secreted is thick and rich in proteases, while when you eat a sour plum, it secretes thin saliva with less enzymes. Saliva can also wash the mouth, so that the taste buds can distinguish taste more accurately. Therefore, saliva also has a great relationship with taste.
Experiments have shown that it takes only 1.5 to 4.0 ms for human taste from stimulating taste buds to perceiving taste, which is much faster than vision (13–15 ms), hearing (1.27–21.5 ms) or touch (2.4–8.9 ms). This is because taste is transmitted by nerves, almost reaching the limit speed of nerve transmission, while vision and hearing are transmitted by sound waves or a series of secondary chemical reactions, so they are slow. The bitter taste is the slowest, so in general, the bitter taste is always felt at the end. But people are often more sensitive to bitter substances than sweet ones.
The physiological mechanism of taste production has been basically confirmed, as shown in Fig. 10.5. For sweet compounds, the results showed that the taste receptors were combined with G-proteins (also for umami and bitterness). Once the sweet compounds are combined with the proteins of receptors on the surface of taste cells, the configuration of receptor proteins would change and then interact with G-proteins, activates adenyl cyclase to synthesize 3 ‘, 5’- cyclic AMP (cAMP) from ATP. After that, cAMP stimulates the cAMP-dependent kinase, which leads to the phosphorylation of K+ channel protein, and the K+ channel is finally closed. As a result, the reduction of K+ delivered to the cell leads to depolarization of the cell membrane, which activates the potential-dependent calcium channel and Ca2+ flows into the cell, releasing neurotransmitters (norepinephrine, norepinephrine) at the synapse. Therefore, an action potential is generated in the nerve cells, thereby generating corresponding conduction, and finally forming a corresponding sensation in the central nervous system.
10.2.2 The Main Factors that Affect Taste
10.2.2.1 Taste Substance Structure
The structure of taste substance is an internal factor affecting gustatory perception. Sometimes, a minute change in molecular structure can cause magnificent changes in taste. In general, sugars, such as glucose and sucrose, mostly provide sweet taste; carboxylic acids, such as acetic acid and citric acid, mostly provide sour taste. Salts, such as sodium chloride and potassium chloride, give off a salty taste, while alkaloids and heavy metals give off mostly bitter taste. However, there are many exceptions: saccharin, lead acetate, and other non-sugar organic salts are sweet, oxalic acid is not sour but astringent, potassium iodide is bitter but not salty, and so on.
10.2.2.2 Temperature
In general, gustatory perception is sensitive between 10 to 40 °C, with 30°C having the highest sensitivity. All gustatory perceptions will weaken with higher or lower temperature. At 50°C, all gustatory perceptions become slower. Sweet and sour tastes have an optimum perception temperature between 35 to 50 °C. Salty taste have an optimum temperature between 18 to 35 °C; however, bitter taste have an optimum temperature at 10°C. Every gustatory perception threshold will change as temperature changes and temperature will have varying effects on different gustatory perceptions, but the change is regular over a range of temperatures. The degree that different feeling of flavors is affected by temperature varies, generally, saccharin had the greatest effect on sweetness, while hydrochloric acid had the least effect.
10.2.2.3 Taste Substances’ Concentration
Odorant substances in an appropriate concentration will usually give a pleasant feeling, and inappropriate concentrations will generate unpleasant feelings. Taste substances’ concentration has varying effects on different gustatory perceptions. Usually, sweetness gives a pleasant feeling at perceived concentration, and pure bitterness will almost always be unpleasant. Sourness and saltiness, at low concentration, will bring happiness, but at high concentration, they will make people feel unpleasant.
10.2.2.4 Taste Substances’ Solubility
Flavoring substances can only stimulate the taste buds after being dissolved. Therefore, solubility and dissolution rate will affect gustatory sensation’s perceived rate and duration. For example, sucrose can be easily dissolved; therefore, sweetness is perceived quickly but also disappears quickly. On the other hand, saccharin is more difficult to dissolved; therefore, gustation appears slower while lasting longer. Because flavoring substances can only diffuse to taste receptors when they are dissolved to create a taste sensation, taste is also influenced by the medium in which the flavoring substance is located. The viscosity of solvent can influence taste substances’ contact with taste receptors. Different solvents could lower taste substances’ solubility or prevent the release of taste substances.
10.2.2.5 Age, Gender, and Physiological Conditions
Age influences taste sensitivity, which is mostly seen in people over 60. Generally, there was no significant change in taste sensitivity under the age of 60. Because taste buds, located on the tongue’s papillae, will decrease as age increases. This decrease in taste buds will reduce gustatory perception sensitivity. Under normal circumstances, when above 60 years of age, gustatory sensation sensitivity for salty, sour, sweet, bitter, and other tastes will be significantly reduced.
There are two different views on the effect of gender on taste. Some researchers believe gender does not have any influence on basic gustatory perceptions, while others believe gender does not influence bitterness, but women are more sensitive to saltiness and sweetness compare to men. Men are more sensitive to sour taste.
To some extent, gustatory perception sensitivity is dependent on body conditions; when the human body suffers from certain diseases or abnormalities, it can lead to lost or dullness of taste and changes in gustatory perception. For example, in the case of jaundice, the perception of bitterness is significantly reduced or even lost; when suffering from diabetes, the sensitivity of the tongue to sweet stimuli is significantly reduced; if ascorbic acid is lacking for a long time, the sensitivity to citric acid is significantly increased; after the increase of blood sugar content, the sensitivity to sweet feeling will be reduced. These facts also prove that, in a sense, taste sensitivity depends on the body’s needs. Changes in gustatory perception due to illness can be temporary or permanent,taste can return to normal after the disease is cured, but some are permanent changes.
People’s taste sensitivity increases when they are hungry, but has little effect on the preference of certain taste. Some experiments showed that the sensitivity of the four basic flavors reached the highest at 11:30 a.m. The degree of decrease was related to the caloric value of the food. People have high taste sensitivity before eating, which proves that taste sensitivity is closely related to the physiological needs of the body. One reason for the decrease in taste sensitivity after eating is that the intake of food meets the physiological needs; the other reason is that the eating leads to fatigue of taste receptors, thereby reducing taste sensitivity.
10.2.3 Interactions Between Taste Substances
The formation of taste, in addition to physiological phenomena, is also related to the chemical structure and physical properties of flavoring substances. Just as a substance may not taste the same because of its optical properties, while different substances can present the same taste.
In terms of the speed of basic taste perception, salty taste is the fastest while bitter taste is the slowest. But in terms of sensitivity, bitterness is the most sensitive and more perceptible. Now we use the threshold, which is the minimum concentration at which the substance can be perceptible (mol/m3, %或mg/kg). Due to the differences among animal species, people, race, habits, etc., the threshold value of various literature will be different to some extent.
The composition of food varies widely, and the ingredients can interact with each other. The taste of each food component cannot be simply combined; various factors must be considered.
10.2.3.1 Synergistic Taste Effect
The taste of one substance can be significantly enhanced by the presence of another substance, and this phenomenon is called the synergistic taste effect. For example, sodium glutamate (MSG) and 5’-inosinic acid (5’-IMP) can work synergistically to enhance umami taste. Maltol can synergize almost any other flavor; adding maltol to beverages and juices can enhance sweetness.
10.2.3.2 Antagonistic Taste Effect
One substance weakening or inhibiting the effect of another substance’ taste is known as the antagonistic taste effect. For example, when mixing any two of the following, sucrose, citric acid, sodium chloride, and quinine, in adequate concentration, will weaken each individual taste perception.
10.2.3.3 Taste Contrast Effect
The existence of two taste substances at the same time can influence feelings and psychology, and this is called the contrasting taste effect. For example, when salt is present in MSG, umami flavor is enhanced; when a small amount of salt is sprinkled onto watermelon, its sweetness can be enhanced. Coarse granulated sugar sometimes tastes sweeter than pure granulated sugar due to the presence of impurities.
10.2.3.4 Taste Alteration Effect
It has been found that the leaves of the tropical plant Gymnema sylvestre contain gymnemic acid. Sweet and bitter food can no longer be detected after chewing Gymnema sylvestre leaves, it can suppress sweet and bitter tastes for hours. However, these leaves have no alteration effect on sourness and saltiness. Furthermore, the interaction between two substances can sometimes change gustatory perception, such as the “miracle fruit” from Africa. This fruit contains an alkaline protein that will make sour substances taste sweet after consumption. Sometimes a sour orange gives you a sweet taste in your mouth, this phenomenon is known as the taste alternation effect or inhibitory effect. Alteration effect is a change in taste substance, while contrast effect is a change in the intensity of taste substance.
10.2.3.5 Taste Fatigue
After being stimulated by certain gustation substances for a long period of time, when consuming the same gustation substance afterwards, the intensity of the taste will often reduce; this phenomenon is known as taste fatigue. The phenomenon of taste fatigue involves psychological factors, for example, the second piece of candy will not taste as sweet as the first piece. Some people have a habit of eating MSG; even with more MSG added to a dish, the perception of umami can decrease.
When all kinds of sweeteners are used together, they can improve each other’s sweetness. For example, although D.E.42 starch syrup is much less sweet than sucrose at the same concentration, the sweetness of the mixture of 26.7% sucrose solution and 13.3% D.E.42 starch syrup was equal to that of 40% sucrose in the liquid phase.
A small amount of polysaccharide thickener was added to the sugar solution, for example, the sweetness and viscosity can be slightly improved when 2% starch or a small amount of gum is added to 1 ~ 10% sucrose solution. Sweeteners at appropriate concentrations (especially below the threshold) often have the effect of improving flavor when they are used with salty, sour, and bitter substances. However, when the concentration is high, the effect of other flavor sensitive substances on sweetness is not regular. For example, if 0.5% salt is added to 5 ~ 7% sucrose, the sweetness will increase, while 1% salt will give the opposite effect.
In addition, there is also an interaction between gustation substances and olfaction substances. Physiologically, although gustatory and olfactory perceptions are completely different, the complex feelings created by the mixture of tastes and aromas during food chewing and the transformation by taste substances and aroma compounds make the two perceptions promote each other.
In a word, the various flavoring substances and their taste sensation interacts with each other, the psychological effects they cause are all very subtle, but much remains unclear and needs further study.
10.3 Food Taste and Taste Substances
10.3.1 Sweet Taste and Sweet Taste Substances
Sweet taste is one of the most popular basic tastes, and sugars are the most common natural sweetening substances. Besides sugar and its derivatives, there are many non-sugar natural compounds, derivatives of these natural compounds, and synthetic compounds that can also possess a sweet taste.
10.3.1.1 Sweetening Mechanism (Shay’s Theory)
There are several hypotheses explaining the mechanisms of sweet taste perception. Earlier hypothesis believed sweet taste perception is related to the presence of multiple hydroxyl groups on sugar molecules, but this hypothesis was quickly rejected because the sweetness of different hydroxyl-containing compounds varied greatly. Many amino acids, certain metal salts, and non-hydroxyl-containing compounds, such as chloroform and saccharin, also have a sweet taste.
Currently, the AH/B theory proposed by Shallenberger et al. has the greatest influence; it explains the relationship between sweetness and its molecular structure (Fig. 10.6). This theory believes there is a hydrogen bond forming group (-AH), such as -OH, -NH2, = HN, in the molecule of a sweet compound called a proton donor. There is also an electronegative atom (-B), such as oxygen and nitrogen, called a proton acceptor; this atom is 0.25–0.4 nm from the -AH group. These two groups in sweet taste compounds must meet stereochemistry requirements to bind with receptors. Inside the sweet taste receptor, there are also AH/B structural units, and the distance between -AH and -B is 0.3 nm. When sweet taste compounds’ AH/B structure hydrogen bonds the AH/B structure on the receptor, gustatory nerves become stimulated and produce sweetness. The AH-B structure of other compounds, such as chloroform, saccharin, and glucose, can be represented by Fig. 10.7.
Shellenberger’s theory explains from a molecular level whether a substance can have a sweet taste but cannot explain the intrinsic factors causing compounds with AH/B structure having vastly different sweetness intensities. Kier, later, modified and developed the AH/B theory. He believed there is a lipophilic region for specific steric structures in sweet taste compounds besides just having the- AH and -B groups. There is a hydrophobic group (-X), such as -CH2CH3 and -C6H5, existing 0.35 nm from -AH group and 0.55 nm from -B group; this group can form hydrophobic interactions with sweet taste receptor’s lipophilic parts to produce a third contact point forming a triangular contact surface (Fig. 10.8). The -X group seems to promote interactions between certain molecules with sweet taste receptors, thus affecting perceived sweetness intensity. Therefore, the site of X is an extremely important property of a strongly sweet compound, it may be an important explanation for the difference in the quality of sweetness among sweet compounds. After this addition, the theory now becomes the AH-B-X theory.
10.3.1.2 Sweetness Intensity and Its Influencing Factors
The intensity of sweetness can be expressed by “sweetness”, but the sweetness cannot be measured quantitatively by physical or chemical methods at present, and can only be judged by human taste. It is usually based on the non-reducing natural sucrose that is more stable in water (for example, the sweetness of 15% or 10% sucrose aqueous solution at 20 °C is 1.0 or 100) when compared to other sweeteners at the same temperature and concentration. This kind of sweetness is called relative sweetness (Table 10.2). Because of the great influence of subjective factors, the results obtained by this method are often inconsistent and sometimes vary greatly in different literature.
The main external factors that affect the sweetness of sweet compounds:
-
(1)
Concentration. The sweetness increases with the increase in the concentration of sweet compounds, but the degree of sweetness of various sweet compounds was different. Most of the sugars and their sweetness were higher with the increase in the concentration of sugar, especially glucose. For example, when the concentration of sucrose and glucose is less than 40%, the sweetness of sucrose is higher; but when the concentration of both is greater than 40%, the sweetness of sucrose is almost the same. However, the bitterness of synthetic sweeteners becomes very prominent when the concentration is too high, so the use of sweeteners in food has a certain range of dosages.
-
(2)
Temperature. The effect of temperature on the sweetness of sweeteners is shown in two aspects. One is the effect on taste organs, the other is the effect on the structure of compounds. Generally, the sensitivity of sensory organs is the highest at 30 °C, so the evaluation of taste is more appropriate at 10 ~ 40 °C. The taste perception becomes dull under high and low temperature, which cannot truly reflect the actual situation. For example, ice cream has a high sugar content, but because we eat it at a low temperature, it doesn’t feel very sweet. In the lower temperature range, the temperature has little effect on sucrose and glucose, but the sweetness of fructose is significantly affected by temperature. This is because in the equilibrium system of fructose, with the increase of temperature, the percentage of high sweetness β - d-fructopyranose decreases, while the content of unsweetened β - d-fructofurane increases (Fig. 10.9).
-
(3)
Dissolution. Sweet compounds, like other flavor compounds, can interact with receptors on taste cells only when they are dissolved, thus producing corresponding signals and being recognized. Therefore, the solubility of sweet compounds will affect the production speed and maintenance time of sweetness. Sucrose produced sweetness quickly but maintained for a short time, while saccharin produced sweetness slowly but maintained for a longer time.
-
(4)
The interaction of sweet substances also affects the sweetness.
10.3.1.3 Common Sweeteners and Their Applications
There are numerous kinds of sweeteners. Common sweeteners include monosaccharides and disaccharides (such as glucose, fructose, xylose, sucrose, maltose, and lactose), sugar alcohols (including xylitol, sorbitol, maltitol, lactitol, D-mannitol, isomalt, erythrose), corn syrup, stevioside, neotame, sodium cyclamate, aspartame, etc. Sweeteners can be divided into two categories according to their sources: one is natural sweeteners, such as sucrose, starch syrup, fructose, glucose, maltose, glycyrrhizin, and stevioside; the other is synthetic sweeteners, such as sugar Alcohol, saccharin, cyclamate, palatinose, etc. Synthetic sweeteners have low calorific value and no fermentability, which are beneficial to diabetics and cardiovascular patients. According to their physiological and metabolic characteristics, sweeteners can also be divided into nutritional sweeteners and non-nutritional sweeteners.
-
1.
Monosaccharide and disaccharide in monosaccharide,
Among monosaccharides, glucose has a cool feeling, and its sweetness is 65 ~ 75% of that of sucrose. It is suitable for direct consumption and intravenous injection. Fructose (fructose) exists in fruits and honey together with glucose. It is sweeter than other sugars, and can be directly metabolized in the human body without pressure, so it is suitable for children and diabetic patients. Xylose is produced by the hydrolysis of xylan. It is soluble in water and has a sweet taste like fructose. Its sweetness is about 65% of that of sucrose. It has high solubility and permeability but low hygroscopicity. It is easy to cause a browning reaction and cannot be fermented by microorganisms. It is a sweetener that does not produce heat in the human body and can be consumed by patients with diabetes and high blood pressure.
Among disaccharides, sucrose has pure sweetness and high sweetness. It is rich in cane sugar and beet sugar. In industry, sucrose is often used as raw material to produce sucrose, which is the most used natural sweetener. Maltose has the highest nutritional value among the sugars. It is sweet, refreshing, and mild. Unlike sucrose, maltose can stimulate the gastric mucosa. Its sweetness is about 1/3 of that of sucrose. Lactose is a special sugar in milk. Its sweetness is 1/5 of that of sucrose. It is a kind of sugar with low sweetness and poor water solubility. After eating, lactose is decomposed into galactose and glucose in the small intestine and absorbed by the human body, which is conducive to the absorption of calcium. It has strong adsorption to gas and colored substances and can be used as a protective agent for meat flavor and color; It is easy to react with protein by Maillard reaction and form attractive golden yellow when added to baked food.
-
2.
Starch syrup
Starch syrup (starch syrup), also known as conversion syrup, is made up of glucose, maltose, oligosaccharide, and dextrin. Glucose value (D.E.) is commonly used to express the degree of starch conversion in the industry. D.E. refers to the percentage of dry matter of invert sugar (in terms of glucose) contained in starch conversion solution. D. If E. is less than 20%, it is called low conversion syrup; if D.E. = 38 ~ 42%, it is called medium conversion syrup; when D.E. is more than 60%, it is called high conversion syrup. Medium conversion syrup, also known as ordinary syrup or standard syrup, is the main product of starch syrup. D. Syrups with different E. values are different in sweetness, viscosity, thickening, hygroscopicity, permeability, and storability, and can be selected according to the use. Isomeric syrup is a part of glucose isomerization to fructose under the action of isomerase, also known as fructose syrup. At present, the conversion rate of fructose of isomeric syrup is generally over 42%, even more than 90% (called high fructose syrup). The isomeric syrup has pure sweetness, good crystallinity, fermentability, permeability, moisture retention, and storability, and has developed rapidly in recent years.
-
3.
Glycyrrhizin
Glycyrrhizin (glycyrrhizin) is formed by the condensation of glycyrrhizic acid and two molecules of glucuronic acid, with a relative sweetness of 100 to 300, and its disodium or trisodium salt is commonly used. It has a good fragrance-enhancing effect, can alleviate the salty taste of table salt, is not fermented by microorganisms, and has detoxification and liver protection effects. However, it is rarely used alone because of its slow sweetness and long retention time. When it is used together with sucrose, it is helpful to develop sweetness and save about 20% sucrose. When it is combined with saccharin, the ratio of glycyrrhizin/saccharin (3 ~ 4): 1, plus sucrose and sodium citrate, the sweetness is better. It can be used for flavoring dairy products, cocoa products, egg products, beverages, soy sauce, pickles, etc.
-
4.
Stevioside
Stevioside exists in the stem and leaf of stevioside, which is the dried powder of water extract of Stevia rebaudiana leaves. The sugar base is sophorose and glucose, and the aglucone is diterpene steviol. The specific sweetness is 200–300, which is one of the sweetest natural sweeteners. The sweetness of stevioside is close to that of sucrose. It is stable to heat, acid, and alkali, has good solubility, no bitterness and foaming, and has curative effects in lowering blood pressure, promoting metabolism, and treating hyperacidity. It is suitable for sweeteners and low energy food for diabetic patients.

-
5.
Sugar alcohol
At present, there are four kinds of sugar alcohol sweeteners (alditols) put into practical use, mainly including D-Xylitol, D-sorbitol, D-Mannitol, and maltitol. Their absorption and metabolism in the human body are not affected by insulin, nor do they hinder the synthesis of glycogen. They are a kind of sweeteners that do not increase blood sugar. They are ideal sweetener for patients with diabetes, heart disease, and liver disease. They all have moisture retention and can maintain certain moisture content in food and prevent drying. In addition, sorbitol can prevent the crystallization of sucrose and salt from the food, keep the balance of sweet, sour, and bitter taste, maintain food flavor and prevent starch aging. Xylitol and mannitol have cool taste and aroma, and can also improve food flavor; they are not easy to be used and fermented by microorganisms, so they are good anticaries sweeteners.
Sugar alcohol sweeteners also have a common feature, that is, excessive intake can cause diarrhea, so it has the effect of defecation with moderate intake.
-
6.
Saccharin
Saccharin is currently the most used synthetic sweetener. Its molecules have a bitter taste, but the anions dissociated in water have a sweet taste, with a relative sweetness of 300 to 500, and a slightly bitter aftertaste. When the concentration is greater than 0.5%, the bitter taste of the molecule is easy to appear. After people consume saccharin, it will be excreted in feces and urine as it is, so it has no nutritional value.
-
7.
Sodium cyclamate
Sodium cyclamate is a kind of non-nutritive sweetener. Its chemical name is Sodium cyclamate. It is a safe food additive with less toxicity. Its sweetness is 30 ~ 50 times of that of sucrose, slightly bitter. It is soluble in water, stable to heat, light, and air, and slightly bitter after heating. It is widely used in the production of beverage, ice cream, candied fruit, candy, and medicine.

-
8.
Aspartame
Aspartame (AMP) is also known as protein sugar and aspartame. The chemical name of the active ingredient is aspartyl phenylalanine methyl ester. Its sweetness is 100 to 200 times that of sucrose, and its sweetness is refreshing. Pure, soluble in water, white crystals. But the stability is not high, it is easy to decompose and lose its sweetness. Aspartame is safe and has a certain degree of nutrition. It is widely used in the beverage industry. It can be added according to normal production needs in China.

-
9.
Parachine
Palaginose, also known as isomaltulose, is a white crystal with a sweet taste and no peculiar smell. Its biggest characteristic is anticaries, slow absorption by the human body, and slow rise of blood glucose, which is beneficial for the prevention and treatment of diabetes patients and the prevention of excessive accumulation of fat. As anti-caries and functional sweetener, palaginose is widely used in chewing gum, high-grade candy, sports drinks, and other food.
-
10.
Others
Honey is the nectar collected from honeybees’ nectaries. It is a light yellow to red yellow strong viscous transparent paste and crystallizes at low temperature. The total sugar content is about 80%, in which glucose is 36.2%, fructose is 37.1%, sucrose is 2.6%, dextrin is about 3.0%. Honey has its own special flavor because of the different kinds of flowers which contains more fructose. Due to its feature that hard to crystallize and easy to absorb moisture in the air, it can prevent food from drying and is mostly used in the processing of cakes and pills.
In addition to the above sweeteners, there are also some natural derivatives sweeteners, such as some amino acid and dipeptide derivatives, dihydrochalcone derivatives, Perillaldehyde derivatives, sucralose, etc.
10.3.2 Sour Taste and Sour Taste Substances
Sour taste is a chemical gustation caused by taste receptors stimulated by hydrogen ions. Any compounds that can release H + in solution will have a sour taste. Vinegar is regarded as one of the representatives and reference materials to distinguish the taste of food. Since human beings have adapted to acidic foods, proper sourness can give people a refreshing feeling and promote appetite. Sour taste intensity can be evaluated by some evaluation methods, such as tasting method or measuring salivary flow rate. The subjective equivalent value (P.S.E) is often used in the tasting method, which refers to the concentration of acid when the same sour taste is felt; the flow rate of saliva secretion is expressed by measuring the number of milliliters of saliva flowing out of each parotid gland within 10 min.
Different acids have different tastes, and there is not a simple relationship between acid concentration and sourness. The sour taste is related to the characteristics of acidic groups, pH, titer acidity, buffering effect, and other compounds, especially the presence or absence of sugar. The main factors affecting sourness:
-
(1)
Hydrogen ion concentration. All the acid taste agents can dissociate hydrogen ion, which shows that the acid taste is related to the concentration of hydrogen ion. When the concentration of hydrogen ion in the solution is too low (pH > 5.0 ~ 6.5), it is difficult to feel a sour taste; when the concentration of hydrogen ion in the solution is too high (pH < 3.0), the intensity of sour taste is too strong to be tolerated; however, there is no functional relationship between the concentration of hydrogen ions and the acid taste.
-
(2)
Total acidity and buffering effect. Usually, when the pH value is the same, the sour agent with greater total acidity and buffering effect has stronger sourness. For example, succinic acid is more acidic than malonic acid because the total acidity of succinic acid is stronger than that of malonic acid at the same pH value.
-
(3)
The properties of anions in acidizing agents. The anions of acidizing agents have a great influence on the strength and quality of acid taste. When the pH value is the same, the acidity of organic acids is stronger than that of inorganic acids. Adding hydrophobic unsaturated bonds in the structure of anions, the acidity is stronger than that of carboxylic acids with the same carbon number; if hydrophilic hydroxyl groups are added to the structure of anions, the acidity is weaker than the corresponding carboxylic acids.
-
(4)
Other factors. When sugar, salt, and ethanol are added to the acidizing agent solution, the acidity will be reduced. The proper mixing of sour and sweet taste is an important factor to form the flavor of fruits and drinks; the appropriate salty and sour taste is the flavor characteristic of vinegar; if a proper amount of bitter substances is added into the acid, the special flavor of food can also be formed.
Different acids have different gustatory perceptions, and there is a complex relationship between the concentration of acid and the taste of sourness. The taste of sourness is related to the concentration of hydronium ion, total acidity, action of buffer, nature of anions, and other factors. When a solution’s hydronium ion concentration is too low (pH > 5.0–6.5), sourness cannot be detected; when hydronium ion concentration in solution is too high (pH < 3.0), sourness becomes unbearable. Usually, when pH values are the same, the acidifier with higher total acidity and higher buffering capacity will taste sourer. When pH values are the same, organic acids will have stronger sourness compare to inorganic acids. If hydrophobic unsaturated double bonds are added to an anion, then sourness taste will be stronger than carboxylic acid with the same carbon number. If hydrophilic hydroxyl groups are added to an anion, then sour taste will be weaker compare to the corresponding carboxylic acid. Furthermore, when sugar, salt, or ethanol is added to acidic solutions, the taste of sourness will be reduced.
10.3.2.1 Mechanism of Acid Formation
It is currently believed that H+, in an acidifying agent HA, is the taste determining group and A− is the taste assisting group. Sour taste receptors are the phospholipids on the taste buds. Sour sensation occurs when the cation interacts and exchanges with the phospholipid heads in the receptor. When pH is the same, organic acids will have stronger sourness compared to inorganic acids, It is due to the strong adsorption of the taste assisting group A- of organic acids on the surface of phospholipid receptors, which can reduce the positive charge density on the membrane surface, that is to say, the repulsion to H + is reduced. For diprotic acids, the longer the carbon chain, the longer the sourness sensation will last. This long-lasting sourness sensation is caused by anion A− forming intramolecular hydrogen-bonded cyclic chelate or metal chelate that can adhere to the lipid membrane, thus reducing positive charges on the membrane surface. If a carboxyl or hydroxyl group is added to A−, it will weaken the lipophilicity of A− and reduce sourness. On the contrary, if a hydrophobic group is added to A−, A− can adhere better onto lipid membranes. Anions have an influence on sourness sensation as well. Organic acid anions usually provide a refreshing acidity, Of course, there are certain exceptions.
The order of acid intensity obtained by taste method and saliva flow rate method is not consistent, so some people think that the two reactions come from different parts of stimulation. It has also been proved that most of the protons bound to the acid receptor membrane are ineffective and can not cause local conformation changes on the membrane. Since the unsaturated hydrocarbon chain in the membrane structure is easy to combine with water, the proton in the acid also has a tunneling effect. So, some people also believe sour taste reception might not be located on the phospholipid head, but on the double bonds of the phospholipid chain. This is because conformational changes in sections of lipid membrane require strong electrostatic repulsion created by π complex formed after protonation of the phospholipid double bonds.
Although the previous mechanism explained some sour taste sensations, it is not enough to justify whether H+, A−, or HA has the most influence on sourness perception. There are many properties of acidulating molecules, such as molecular weight, molecular structure, and polarity, and their influence on sourness perception is still unknown. Sour taste perception mechanism has yet to be clarified.
10.3.2.2 Important Acidifiers and Their Application
Common acidifiers include vinegar, citric acid, malic acid, tartaric acid, lactic acid, ascorbic acid, gluconic acid, and many more. Vinegar is the most common acidifiers used in China; its main component is acetic acid. Citric acid and malic acid are mainly present in fruits and vegetables, while tartaric acid content is higher in fruits, such as grapes. Lactic acid in fermented kimchi and sauerkraut is not only for flavoring, but also to prevent the growth of undesired microorganisms. Ascorbic acid can be used as an acidifier in foods and can also prevent oxidation and browning. Gluconic acid is a sugar acid formed by the oxidation of aldehyde group in glucose; it is easily dehydrated in dry environments forming gluconolactone, and this reaction is reversible. Gluconolactone could be used as a coagulator in making lactone tofu and as a swelling agent for baking cookies.
-
1.
Vinegar. Vinegar is the most commonly used sour flavor material in China. In addition to 3 ~ 5% acetic acid, it also contains a small amount of other organic acids, amino acids, sugars, alcohols, esters, etc. Its sour taste is mild, and in addition to being used as a seasoning agent in cooking, it also has the functions of preventing corruption and removing fishy smell. Acetic acid is highly volatile and has a strong sour taste. The industrially produced acetic acid is a colorless irritating liquid, which can be mixed with water as well, and can be used to prepare synthetic vinegar, but it lacks the flavor of vinegar. Acetic acid with a concentration above 98% can freeze into an ice-like solid, so it is called glacial acetic acid.
-
2.
Citric acid. Citric acid is one of the most widely distributed organic acids in fruits and vegetables. It can be completely dissolved in water and ethanol at 20 °C, and is more soluble in cold water than in hot water. Citric acid can form three forms of acid salts, but most of them are insoluble or hardly soluble in water except alkali metal salts. The sour taste of citric acid is round, nourishing, refreshing, and delicious. It reaches the highest acidity immediately after entering the mouth, and the aftertaste lasts for a short time. It is widely used in the preparation of cool drinks, canned fruits, candies, and so on, with a normal dosage of 0.1 ~ 1.0%. It can also be used to prepare fruit juice powder as a synergist of antioxidants. Citric acid has good anti-corrosion performance, anti-oxidation, and synergistic effect with high safety.
-
3.
Malic acid. Malic acid usually coexists with citric acid. It is a colorless or white crystal, and easily soluble in water and ethanol, and can be soluble in 55.5% at 20 °C. Its sour taste is 1.2 times stronger than citric acid, refreshing, slightly irritating, slightly bitter, and astringent, and has a long taste time. When combined with citric acid, it has the effect of strengthening sour taste. Malic acid is highly safe and is often used in beverages, especially jelly. Sodium malate has a salty taste and can be used as a salting agent for kidney patients.
-
4.
Tartaric acid. Tartaric acid is widely present in many fruits, and it dissolves 120% in water at 20 °C. Tartaric acid has a stronger sour taste, about 1.3 times that of citric acid, but it has a slightly astringent feeling. Tartaric acid is highly safe, and its use is the same as citric acid, and it is mostly used in combination with other acids, but it is not suitable for preparing foaming beverages or as a food expander.
-
5.
Lactic acid. Lactic acid is rarely found in fruits and vegetables. It is mostly artificial synthetic products. It is soluble in water and ethanol and has an antiseptic effect. The acid taste is slightly stronger than citric acid. It can be used as a pH regulator. Used in refreshing drinks, synthetic wine, synthetic vinegar, spicy soy sauce, etc. Use it to make kimchi or sauerkraut, not only for seasoning, but also to prevent the reproduction of bacteria.
-
6.
Ascorbic acid. Ascorbic acid (ascorbic acid) is white crystals, easily soluble in water, has a refreshing sour taste, but is easily oxidized. It can be used as a sour agent and vitamin C additive in food, and it also has the effect of preventing oxidation and browning and can be used as an auxiliary sour agent.
-
7.
Gluconic acid. Gluconic acid is a colorless or light yellow liquid, easily soluble in water, slightly soluble in ethanol, because it is not easy to crystallize, its products are mostly 50% liquid. It is easy to be dehydrated to produce γ- or δ-gluconolactone when it is dried, and this reaction is reversible. Using this characteristic, it can be used in some foods that cannot be acidic at first but need acidity after being heated in water. For example, adding gluconolactone to soy milk will generate gluconic acid when heated to coagulate soy protein to obtain lactone tofu. In addition, gluconolactone is added to biscuits, which becomes a bulking agent during baking. Gluconic acid can also be used directly in the preparation of refreshing drinks, vinegar, etc., can be used as a preservative flavoring agent for instant noodles, or as a substitute for lactic acid in nutritious foods.
10.3.3 Bitterness and Bitter Taste Substances
Bitter taste has a very low threshold concentration but is a ubiquitous taste in food. Bitter taste lasts longer than sweetness, saltiness, and sourness. Although pure bitterness is unpleasant, when combined in proper proportions with sweetness, sourness, and other tastes, it can create a special flavor. For example, Momordica charantia, ginkgo, tea, coffee, and so on all have a certain bitter taste, but are regarded as delicious food. Most bitter substances have pharmacological effects and can regulate physiological functions. For example, some people with digestive disorders and taste weakening or declining often need strong stimulation of receptors to return to normal. Because of the minimum threshold of bitterness, it is easy to achieve this purpose.
10.3.3.1 The Mechanism of Bitterness
The mechanisms of bitter taste perception can mainly be explained by steric effect theory, intramolecular hydrogen bond theory, and the three-point contact theory.
-
1.
Steric effect theory. Shallenberger et al. believe that, bitter taste and sweet taste, are both depended on the stereochemistry of molecules; these two taste perceptions can be stimulated by similar molecules. Some molecules can produce both sweetness and bitterness.
-
2.
The intramolecular hydrogen bond theory. Kubota et al. in the investigation of enmein molecular, believe any hydrogen bond separated by 0.15 nm will have a bitter taste. Intramolecular hydrogen bonds can increase the hydrophobicity of molecules and form a chelate with transition metal ions easily which have similar structures with bitter-tasting molecules.
-
3.
The three-point contact theory. Lehmann et al. find that there is a linear relationship between the sweetness intensity of several D-amino acids and the bitterness intensity of their L-isomers. So, it is believed that, like the sweet taste, bitter taste molecules and bitter taste receptors produce bitterness by three-point contact; however, the third contact point of bitter taste substances is in the opposite direction of that for sweet taste substances.
Although the above-mentioned bitterness theories can explain the production of bitterness to a certain extent, most of them break away from the structure of the taste cell membrane and only focus on the molecular structure of the stimulus, and do not consider the existence of some bitter inorganic salts.
-
4.
Theory of Induced Adaptation Guangzhi Zeng proposed the bitter taste molecular recognition theory based on his taste cell membrane induction adaptation model. The main points are as follows:
-
(1)
Bitter taste receptors are “water holes” formed by polyene phospholipids on the membrane surface, which provide a nest for the coupling between bitter substances and proteins. At the same time, inositol phospholipids (PI) can generate PI-4-PO4 and PI-4, 5-(PO4)2 through phosphorylation, and then combine with Cu2+, Zn2+, Ni2+, etc., to form the “lid” of the acupoint. The bitter molecules must first push the lid off before they can enter the hole and interact with the receptor. In this way, the inorganic ions bound to the lid in the form of salt bonds, which become the monitoring indicator of molecular recognition. Once it is replaced by some transition metal ions, the lid on the taste receptor no longer receives the stimulation of the bitter substance, resulting in an inhibitory effect.
-
(2)
The receptor acupoints composed of coiled polyene phospholipids that can form various multipolar structures and interact with different bitter substances. The results showed that the taste of quinine sulfate did not affect the bitter taste of urea or magnesium sulfate, and vice versa. If quinine and urea are tasted together, the synergistic effect will be produced and the bitterness will be enhanced. It is proved that quinine and urea have different action sites or water holes on taste receptors. However, if you drink coffee after tasting quinine, the bitterness of coffee will be weakened, which indicates that the two have the same action site or water hole on the receptor, and they will produce competitive inhibition.
-
(3)
The receptor acupoints composed of polyene phospholipids have a side that adheres to the surface protein and has a wider contact with the lipid block. Compared with the specificity requirements of sweet substances, the requirements of polar base position distribution and three-dimensional direction order of bitter substances are not very strict. Any stimulant that can enter any part of the bitter receptor will cause “hole closure”, which changes the conformation of phospholipid and produces bitter information through the following ways.
-
(1)
-
①
Salt bridge conversion. Cs+, Rb+, K+, Ag+, Hg2+, R3S+, R4N+, RNH-NH3+, Sb(CH3)4, etc., belong to structure destroying ions. They can destroy the ice crystal structure around the hydrocarbon chain, increase the water solubility of organic matter, and freely enter and exit the biofilm. When they open the salt bridge into the bitter receptor, they can induce conformational changes. Although Ca2+, Mg2+, etc., have the same structure as Li+ and Na+ to produce ions, they have a salting-out effect on organic matter, but Ca2+ and Mg2+ can make phospholipids agglomerate with some anions. Facilitate structural destruction of ions to enter the receptor, and produce a bitter taste.
-
②
The destruction of hydrogen bonds. (NH2)2C = X, RC(NH2) = X, RC = NOH, RNHCN, etc., where X or O, NH or S group, can be used as hydrogen bond donors.

And the above structures can be used as hydrogen bond acceptors. Since the bitter receptor is a curly polyene phospholipid hole, there is no obvious spatial selectivity, so that the above stimulus with a multipolar structure can also open the lid salt bridge to enter the receptor (larger bitter peptides can only have a part of the side chain to enter) Then destroys the hydrogen bonds and lipid-protein interactions, which gives a great impetus to the change of receptor conformation.
-
③
The formation of hydrophobic bonds. Hydrophobic bond stimulants are mainly esters, especially lactones, thiols, amides, nitriles and isonitriles, nitrogen heterocycles, alkaloids, antibiotics, terpenes, amines, etc. Hydrophobes without polar groups cannot enter the receptor, because the ligands of the salt bridge and the phospholipid head have chirality, which makes the receptor surface have a certain degree of selectivity for the hydrophobe. However, once these hydrophobes penetrate deep into the pore lipid layer, they do not have any spatial specificity requirements, and can cause the conformation of the receptor to change through the action of hydrophobic bonds.
The theory of induced adaptation has further developed the bitterness theory and made a great contribution to the explanation of the complex phenomenon of bitterness.
For examples:
-
(1)
It broadly summarizes various types of bitter substances, which provides convenience for further research on the relationship between structure and taste.
-
(2)
The view that there are transition metal ions on the receptor provides an explanation for the fact that thiols, penicillamine, acidic amino acids, oligopeptides, etc., can inhibit bitterness and certain metal ions can affect bitterness.
-
(3)
A possible explanation is made for the phenomenon that the sweet blind cannot feel any sweeteners, while the bitter blind cannot perceive the few bitter substances with conjugated structure. Bitterness blindness is inherited congenitally. When Cu2+, Zn2+, and Ni2+ form a strong complex with the protein on the patient’s receptor, when the receptor surface is used as a monitoring ion, some bitter substances are difficult to open the lid to enter the acupuncture point.
-
(4)
The view that the bitter taste receptor is mainly composed of phospholipid membrane also provides an explanation for the intensity of bitter taste. Because bitter substances have a cohesive effect on the lipid membrane and increase the surface tension of the lipid membrane, there is a corresponding relationship between the two; the greater the surface tension produced by the bitter substance, the greater the intensity of its bitterness.
-
(5)
Explain the phenomenon that the intensity of bitterness increases with the decrease of temperature, which is just the opposite of the effect of temperature on sweetness and spiciness. Because the process of bitter substances condensing the lipid film is an exothermic effect, which is opposite to the endothermic effect of sweet and spicy substances that make the film expand.
-
(6)
It also explains why the effect of anesthetics on various taste receptors is the fastest disappearance of bitterness and the slowest recovery. This is because polyene phospholipids have greater solubility for anesthetics, and the receptor loses the law of changing conformation after swelling, and can no longer trigger bitter information, and so on.
10.3.3.2 Common Bitter Substances and Their Applications
There are 4 major bitter taste substances derived from plants that are present in food and medicine, including alkaloids, anthraquinones, glycosides, and bitter peptides. The animal sources include picric acid, formanilide, formamide, phenylurea, and urea.
Almost all alkaloids are bitter, and bitterness increases as alkalinity increases. Quinine is the most commonly used bitter reference substance. There are as many as tens of thousands of terpenoids, generally containing lactones, internal acetals, internal hydrogen bonds, glycoside hydroxyl groups, and other structures that can form chelates and have a bitter taste. For example, the bitter component of hops is terpenoids. Most of the glycosides have a bitter taste, such as amygdalin and glucosinolate. Flavonoids and flavanones are widely found in citrus peels and Chinese herbal medicines, most of which are bitter molecules. When the amino acid side chain group has more than 3 carbon atoms and has a base, it is a bitter molecule. When the side chain group is not hydrophobic, its bitterness is not strong.
Many of the salts have a bitter taste, which may be related to the sum of their anion and cation radius. As the sum of ionic radii increases, the saltiness decreases and the bitterness increases. For example, NaCl and KCl have a pure salty taste, the sum of their radii is less than 0.658 nm, while KBr is salty and bitter, the sum of radii is 0.658 nm, and the bitterness of CsCl and KI is large, and the sum of radii is greater than 0.658 nm.
-
1.
Caffeine and Theobromine. Both caffeine and theobromine are purine derivatives and the main alkaloid bitter substances in food. When the concentration of caffeine in water is 150–200 mg/kg, it has a moderate bitter taste, and it is present in coffee, tea, and kola nuts. Theobromine (3,7-dimethylxanthine) is like caffeine and has the highest content in cocoa, which is the cause of the bitter taste of cocoa.
-
2.
Amygdalin. Amygdalin is a glycoside formed by cyanobenzyl alcohol and gentiobiose. It is found in the cores, seeds, and leaves of many Rosaceae plants such as peaches, plums, and apricots. Especially bitter almonds are the most. The seed kernel also contains enzymes that decompose it. Amygdalin itself is non-toxic and has antitussive effect. Too much raw almonds and peach kernels can cause poisoning, because the ingested amygdalin is decomposed into glucose, benzaldehyde, and hydrocyanic acid under the action of emulsions in the body at the same time.
-
3.
Naringin and nehoesperidin. Naringin and nehoesperidin are the main bitter substances in citrus peels. The bitterness of naringin pure product is bitter than quinine, and the detection threshold can be as low as 0.002%. The type of glycoside in the flavonoid glycoside molecule has a decisive relationship with whether it has a bitter taste. After hydrolysis, the bitterness disappears. Using this principle, enzyme preparations can be used to remove the bitterness of orange juice (Fig. 10.10). Rutose and hesperetose are normally in rhamnose glucoside form, rutose is presented as rhamnose (1 → 6) glucose while hesperetose is presented as rhamnose (1 → 2) glucose.
-
4.
Bile. Bile is a liquid secreted by the liver of animals and stored in the gallbladder, with a very bitter taste. The initially secreted bile is a clear and slightly viscous golden yellow liquid with a pH between 7.8 and 8.5. Due to dehydration and oxidation in the gallbladder, the color turns green and the pH drops to 5.50. The main components in bile are cholic acid, chenodecholic acid, and deoxycholic acid.
-
5.
Quinine. Quinine is a widely used standard substance for bitterness. The bitterness threshold of quinine hydrochloride is about 10 mg/kg. Bitter substances have lower taste thresholds than other taste substances and are less soluble in water than other taste active substances. In soft drinks with the characteristics of sweet and sour taste, the bitterness can be reconciled with other tastes, so that this type of drink has a cooling and exciting effect.
-
6.
Bitter hops. Hops are widely used in the beer industry to give the beer a characteristic flavor. The bitter substance of hops is a derivative of humulone or lupulone. Humulone is the most abundant in beer. When the wort is boiled, it is converted into isohumulone through an isomerization reaction. Isohumulone is the precursor of the skunk odor and sun-flavored compounds produced by beer under light irradiation. When there is hydrogen sulfide produced by yeast fermentation, the ortho-carbon atom of the ketone group on the isohexene chain occurs. The photocatalytic reaction produces a 3-methyl-2-butene-1-thiol (isoprenethiol) compound with a skunk smell. In the pre-isomerized hop extract, the selective reduction of ketones can prevent this reaction from happening, and the use of clean brown glass bottles to package beer will not produce skunk smell or sun smell. Whether the volatile hop aroma compounds remain in the malt boiling process is a question that has been debated for many years. It has now been fully proved that the compounds that affect the flavor of beer do remain in the process of full boiling of the wort. Together with other compounds formed by the bitter hop substances, the beer has a flavor.
-
7.
Protein hydrolysate and cheese. Protein hydrolysate and cheese have an obvious unpleasant bitter taste, which is caused by the total hydrophobicity of the side chains of peptide amino acids. All peptides contain a considerable number of AH-type polar groups, which can meet the requirements of the position of polar receptors, but the size of each peptide chain and the nature of their hydrophobic groups are very different. Therefore, the ability of these hydrophobic groups to interact with the main hydrophobic positions of the bitter taste sensor is also different. It has been proved that the bitterness of peptides can be predicted by calculating their hydrophobic value. Figures 10.12 characterize the bitter peptides of αs1 casein derivatives, and their hydrophobic amino acids trigger strong hydrophobicity.
-
8.
Hydroxylated fatty acids. Hydroxylated fatty acids often have a bitter taste. The bitter taste of these substances can be expressed by the ratio of the number of carbon atoms in the molecule to the number of hydroxyl groups or the R value. The R value of sweet compound is 1.00 ~ 1.99, the value of bitter compound is 2.00 ~ 6.99, and there is no bitterness when it is greater than 7.00.
-
9.
The bitter taste of salts. The bitter taste of salts is related to the sum of the ion diameters of the salt anions and cations. The salt whose ion diameter is less than 0.65 nm shows a pure salty taste (LiCl = 0.498 nm, NaCl = 0.556 nm, KCl = 0.628 nm). Therefore, KCl has a slightly bitter taste. As the sum of ion diameter increases (CsCl = 0.696 nm, CsI = 0.774 nm), the bitter taste of its salt gradually increases, so magnesium chloride is (0.850 nm) quite bitter salt.
Quinine is an alkaloid and is also recognized as a standard for bitter taste perception. The bitterness threshold of quinine hydrochloride is about 10 mg/kg (Fig. 10.10). Terpenoid compounds are abundant in plants, and the ones containing lactone, aldolactol, intramolecular hydrogen bond, or glycosidic hydroxyl group, and other groups capable of forming chelates are bitter. For example, bitterness produced by some isoprenoid derivatives in hops is an important feature of its flavor profile,these bitter compounds are mainly derivatives of humulone or lupulone. Most ligands in glycosides have a bitter taste, such as amygdalin and glucosinolate. Amygdalin is composed of cyanbenzyl alcohol and gentiobiose; it is non-toxic, but can be toxic when excessive intake because it can be broken down by amygdalase in the body into glucose, benzaldehyde, and hydrocyanic acid. Naringin and nehoesperidin are flavanone glycosides; they are the main bitter taste substances present in citrus fruit peels. Bitter taste disappears when nehoesperidin glycosides are hydrolyzed (Fig. 10.11). Some L-amino acids have bitter tastes, such as leucine, phenylalanine. When the side chain is not strongly hydrophobic, bitterness is not strong either. Protein hydrolysates and fermented hard cheese have a distinct bitter taste; this is related to the peptide side chain’s total hydrophobicity and relative molecular mass.
10.3.4 Salty Taste and Salty Taste Substances
Salt taste is very important in food seasoning. Salty taste is the taste displayed by neutral salt. Only sodium chloride produces a pure salty taste. It is not easy to simulate this salty taste with other substances. Such as potassium bromide, ammonia iodide, etc., in addition to salty taste, but also has a bitter taste, which is not a simple salty taste, the taste is present in coarse salt. The taste characteristics of various salt solutions with a concentration of 0.1 mol/L are shown in Table 10.3.
10.3.4.1 Patterns of Saltiness
The saltiness is jointly determined by the dissociated anions and cations. Although the production of salty taste is related to the interdependence of cations and anions, the cations are easily adsorbed by the carboxyl or phosphate groups of the protein of taste receptors and present a salty taste. Therefore, salty taste is more closely related to the cations dissociated from salt, while anions affect the strength and side taste of salty taste, cations are the positioning groups of salts, and anions are the taste assisting groups. The strength of salty taste is related to the relative size of the taste nerve’s induction of various anions. From the comparison of several salty substances, it is found that the salt with a small anion and cation radius has a salty taste, the salt with large radius has a bitter taste, and the salt in the middle has a salty bitter taste. The larger the atomic weight of the cation and anion of the salt, the more it tends to increase the bitterness.
10.3.4.2 Common Salty Substances
The salt used for food seasoning should be pure salt. Table salt is often mixed with other salts such as potassium chloride, magnesium chloride, and magnesium sulfate, and their content increases, which in addition to salty taste, but also brings bitterness; but if they are present in trace amounts, they are beneficial to the appearance when processed or eaten directly. Taste effect. Therefore, table salt needs to be refined to reduce the content of these bitter salts.
Although many neutral salts show salty taste, their taste is not as pure as sodium chloride, and most of them have bitter or other tastes.
Due to the adverse effects of excessive salt intake on the body, people are interested in salt substitutes. In recent years, there have been many varieties of table salt substitutes. Several organic sodium salts such as sodium gluconate and sodium malate also have the same salty taste as table salt. They can be used as salt free soy sauce and salt food for patients with kidney disease to limit the intake of salt.
In addition, the salt of amino acid also has a salty taste, such as adding 15% of 5’-nucleotide sodium with 86% H2NCOCH2N+H3Cl−, its salty taste is no different from table salt, which may become a food salty agent in the future. Potassium chloride is also a relatively pure salty substance, which can partially replace NaCl in athlete drinks and low-sodium foods to provide salty taste and supplement potassium in the body. However, there is still a big difference between the taste of food using salt substitutes and the taste of foods using NaCl, which will limit the use of salt substitutes.
Neutral salts provide salty taste. Sodium chloride is the most common salty taste substance. Although saltiness is present in other neutral salts, their salty flavor is not as pure as sodium chloride; many exhibit bitter flavor and other flavors. Bitterness in salt is related to the sum of ion diameters in between anions and cations. When the sum of ion diameter is less than 0.65 nm, salt will have a pure salty taste. As the sum of ion diameter increases, bitterness in salt gradually intensifies as well.
Salty taste perception mechanism is mainly the interaction of hydrated anion–cation complex with AH/B receptor. The cations and anions in salt can both bind to the receptor, and the cation is more likely to bind to the taste perception receptor producing a salty taste. Anions have an influence on salty taste intensity and food flavors.
10.3.5 Umami Taste and Umami Taste Substances
Umami taste is a complex gustatory perception; umami mainly refers to the taste of specific chemicals such as glutamic acid, inosinic acid and aspartic acid, sodium L-glutamate, commonly known as MSG. Umami was recognized as the fifth basic taste of food in recent years. When an umami taste substance is used in concentration higher than the threshold, food’s umami flavors can be enhanced. When used in concentration below the threshold, it can only enhance flavors that are already present. In Europe and the United States, umami taste substances are referred to as flavor enhancers. Common umami taste substances can be distinguished by their chemical structures, mainly including amino acids, nucleotides, peptides, and organic acids.
10.3.5.1 Mechanism of Umami
The backbone structure of umami taste substances is −O-(C)n-O−, n = 3-9. When n = 4-6, umami taste becomes noticeable; when n = 5, umami taste is the strongest. The aliphatic chain is not limited to a straight chain, but can also be a part of an alicyclic ring; the C can be substituted by O, N, S, P, etc. Maintaining the negative charge at both ends of the molecule is very important for the umami taste. If the carboxyl group is esterified, amidated, or heated to form lactones and lactams, the umami taste will be reduced. However, the negative charge at one end can also be replaced by a negative dipole, such as tricholic acid and ibotenic acid, whose umami taste is 5-30 times stronger than monosodium glutamate. This general formula can summarize all the peptides and nucleotides with umami taste. At present, for reasons of economic efficiency, side effects, and safety, the main commercial umami flavor agents are glutamic acid type and nucleotide type.
10.3.5.2 Common Umami Agents
Umami agents can be divided into amino acids, peptides, nucleotides, and organic acids if they are distinguished from their chemical structure characteristics.
-
1.
Amino acids and peptides
Among the natural amino acids, the sodium salt of L-glutamic acid and L-aspartic acid and their amides have umami taste. Sodium L-glutamate is commonly known as monosodium glutamate, which has a strong meat flavor. Glutamic acid-type umami flavors (MSG) are aliphatic compounds, which have spatial specificity requirements in structure. If they exceed the specificity range, they will change or lose their taste. Their taste determining groups are the negatively charged functional groups at both ends, such as –C=O, –COOH, –SO3H, and –SH. Taste assisting groups have some hydrophobic properties, such as α–L–HN2 and –OH. The electrostatic attractions between NH3+ and COO− are what give MSG its savory flavor. When pH reaches its isoelectric point of 3.2, umami taste will be at its minimum. When pH is at 6, the compound almost completely dissociates, and umami taste intensity will be at its maximum. When pH is above 7, umami taste disappears due to the formation of disodium salt. Table salt is an adjuvant of MSG, MSG also has the effect of alleviating saltiness, sourness, and bitterness, so that food has a natural flavor. The sodium salt and amide of L-aspartic acid also have umami taste and are the main umami taste substances in plant foods such as bamboo shoots.
The dipeptides and tripeptides with hydrophilic amino acids connected to the carboxyl end of glutamic acid also have umami taste, such as L-α-aminoadipate, disodium succinate, glutathione-glycerine, and glutathione Silk tripeptide, tricholic acid, etc. If it connects to a hydrophobic amino group, it will produce a bitter taste. In addition to the two amino acids mentioned above, L-alanine, glycine, theanine, and other amino acids all have unique savory flavors.
Umami peptides are umami taste producing micromolecular peptides; they can be extracted from food or synthesized by amino acids. The taste characteristics of umami peptides are related to their amino acid composition and generally contain one or two acidic groups from glutamic acid and aspartic acid. Umami peptides’ flavor profile is not only related to the type of amino acid, but also its primary structure and spatial arrangement. Studies have found that umami peptides have good thermal stability. In addition to having umami taste, it can also mask or weaken bitterness and improve food flavor.
-
2.
Nucleotides
Nucleotide based umami taste substances are aromatic heterocyclic compounds that are structurally spatially specific. Its food determining group is the hydrophilic ribose phosphate, and the flavor assisting group is the hydrophobic substituent on the aromatic heterocyclic ring. Common nucleotide substances exhibiting savory tastes are 5’-IMP, 5’-GMP, and 5’-xanthic acid; the first two compounds have the most intensive umami taste (Fig. 10.13), which, respectively, represent the umami taste of fish and mushrooms. Furthermore, 5’-deoxyinosinic acid and 5’-deoxyguanosic acid also have umami taste. Inosinic acid-type umami flavor (IMP) is an aromatic heterocyclic compound, and its structure also requires space specificity. Its positioning group is hydrophilic ribose phosphate, and the taste assisting group is a hydrophobic substituent on the aromatic heterocyclic ring. When these 5’-nucleotides are combined with sodium glutamate, they can significantly increase the umami taste of sodium glutamate (Table 10.4). For example, the umami taste of a mixture of 1% IMP +1% GMP + 98% MSG is simply MSG 4 times. These 5’-nucloetides have synergistic effects with MSG; when used together, savory taste intensifies as concentration increases.
-
3.
Organic acids
The main organic acid having umami taste is succinic acid and its sodium salts; it is the main umami taste substance present in shellfish. There is also a small amount of food fermented by microorganisms such as soy sauce, sauce, rice wine, and so on. They can be used as seasonings, and used for the flavoring of alcoholic refreshing drinks and candy, and their sodium salts can be used for brewing and meat food processing. If used in combination with other umami agents, it will help the freshness effect.
Maltol and ethyl maltol are commercially used as flavor enhancers in fruits and sweets. Commercial maltol is a white or colorless crystalline powder, and its solubility in water at room temperature is 1.5%. When heated, its solubility in water and oil increases. The appearance and chemical properties of ethyl geritol are like maltol. Both two substances have the structure of o-hydroxy ketene, and a small number of isomers formed by o-diketone exist in equilibrium with it. Because the structure is like phenols, it has some chemical properties like phenols. For example, maltol can react with terpene salts to become purple-red, and can form salts with alkalis. The high concentration of maltol has a pleasant caramel aroma, while the dilute solution has a sweet taste. 50 mg/kg of maltol can make the juice have a round and soft taste. Both maltol and ethyl maltol can match the AH/B part of the sweetness receptor, but as a sweetness enhancer, ethyl maltol is much more effective than maltol. Maltol can reduce the detection threshold concentration of sucrose by half. The actual flavor enhancement mechanism of these compounds is still unclear.
In addition, the umami taste of the compound can change with the change of structure. For example, although sodium glutamate has umami taste, glutamic acid and disodium glutamate have no umami taste.
10.3.6 Spicy Taste and Spicy Taste Substances
Spicy taste is the combination of a special burning sensation and a sharp tingling sensation caused by the consumption of certain compounds. Spicy taste substances can not only stimulate the tactile nerves of the tongue and the oral cavity, but also stimulate the nasal cavity and sometimes causing burning sensations on the skin. Appropriate level of spiciness can increase appetite and promote the secretion of digestive fluid.
10.3.6.1 Mechanism of Spicy Taste
Common spicy taste substances are generally amphiphilic molecules with both polar and non-polar groups. The polar head is the flavor determining group, while the non-polar tail is the flavor assisting group. Studies have shown that increasing the chain length of the non-polar tail will increase the molecule’s overall perceived spiciness. When the chain length (Cn) in the non-polar tail is n = 9, then spicy taste will reach its maximum intensity and dramatically drops afterwards (Figs. 10.14 and 10.15), known as the C9 law. The spiciness of several substances such as capsaicin, piperine, xanthophylline, gingerin, cloves, allicin, and mustard oil conforms to the C9 hottest law.
For spicy taste substances containing double bonds, the more cis double bonds present, the spicier the substance will be; trans-double bonds have little effect on spiciness intensity. Double bond has the greatest influence when on C9 position. When a spicy taste molecule’s end chain contains no cis double bonds or branched chains, the spicy taste will be completely lost as the number of carbons in the molecule becomes greater than 12. Similarly, the hydrocarbon chain lengths of aliphatic aldehydes, alcohols, ketones, and carboxylic acids can have changes in spicy taste. If the chain length exceeds C12 but there is a cis double bond near ω-position, then there can still be spicy taste present. Some of the less polar molecules, such as BrCH = CHCH2Br, and CH2 = CHCH2X(X being NCS, OCOR, NO2, and ONO),and (CH2 = CHCH2)2Sn(n = 1,2,3), Ph(CH2)nNCS, etc., can also have spicy taste.
The polarity and position of the polar groups of the spicy substances have a great relationship with the taste. When the polarity of the polar head is large, it is a surfactant; when the polarity is small, it is an anesthetic. Symmetrical molecules with a central polarity such as

Their spiciness is only equivalent to half a molecule, and its spiciness is greatly reduced because of its reduced water solubility. Symmetrical molecules with polar groups at both ends such as:

In this case, their taste becomes weaker. Increase or decrease the hydrophilicity of the polar head, such as the transform from
 to
to
 , where the spiciness is reduced. Even the position change of the hydroxyl group may lose the spiciness and produce sweetness or bitterness.
, where the spiciness is reduced. Even the position change of the hydroxyl group may lose the spiciness and produce sweetness or bitterness.
10.3.6.2 Common Spicy Substances
Common spices and vegetables containing spicy taste substances include chili pepper, black pepper, ginger, nutmeg, clove, garlic, onion, chives, wasabi, and radish.
1. Hot and spicy substance Hot and spicy substance is a non-aromatic spiciness that can cause a burning sensation in the mouth. There are:
-
(1)
Capsicum. The main spicy taste substance in capsicum is capsaicin, a class of unsaturated monocarboxylic vanillyl-amides with different carbon chain lengths (C8-C11); a small amount of dihydrocapsaicin, a linear saturated carboxylic acid, is
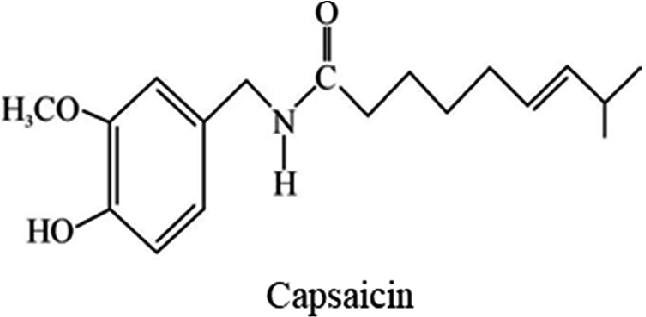
also present. Capsaicinoids have different spiciness intensities, with the spiciest having a side chain of C9-C10. The double bond is not necessary for spiciness. The capsaicin content of different peppers varies greatly. Sweet peppers are usually very low in content. Generally, red peppers contain 0.06%, horn red peppers contain 0.2%, Indian sam peppers contain 0.3%, and Uganda peppers can be as high as 0.85%.
-
(2)
Pepper. There are two common types of black pepper and white pepper, both of which are processed from fruits. Among them, black pepper can be made from unripe green fruits; white pepper can be made from mature fruits harvested when the color changes from green to yellow but not red. In addition to a small amount of capsaicinoids, the spicy components of pepper are mainly piperine. Piperine is an amide compound, and its unsaturated hydrocarbon group has cis-trans isomers. Among them, the more cis double bonds it has, the hotter it is; the all-trans structure is also called isopiperine. The spicy taste of pepper will decrease after exposure to light or storage, which is caused by the isomerization of cis-piperine to trans structure. Synthetic piperine has been used in food (Fig. 10.16).
-
(3)
Xanthoxylum. The main spicy ingredient of Xanthoxylum is sanshool, which is an amide compound. The amides found in Xanthoxylum are shown in Table 10.5. In addition, there is a small amount of alkyl propyl isothiocyanate and so on. Like pepper and chili, it also contains some volatile fragrance components in addition to spicy components.
Table 10.5 The amides in Xanthoxylum
2. Aromatic spicy substances Aromatic spicy substances are a class of substances that are accompanied by strong volatile aromatic substances in addition to the spicy taste, and are ingredients that have the dual effects of taste and smell.
-
(1)
Ginger. The pungent ingredient of fresh ginger is a type of o-methoxyphenol alkyl ketone, the most representative of which is 6-gingerol. The length of the carbon chain outside the hydroxyl group on the side chain of the ring in the molecule is different (C5 ~ C9). Fresh ginger is dried and stored, gingerol will be dehydrated to produce gingerol compounds, which are more pungent than gingerol. When ginger is heated, the side chain on the gingerol ring breaks to produce gingerone, and the pungent taste is milder. Among gingerol and gingerenol, the spicy taste is strongest when n = 4 (Fig. 10.17).
The spiciness in fresh ginger is produced by a class of o-methoxyl-phenol alkyl ketones; the most commonly known compound is 6-gingerol. Dehydrated gingerol can form shogaol phenolic compounds, which are spicier compare to gingerol. When ginger is heated, the side chain of the shogaol ring breaks to form zingerone; zingerone has a moderate spicy taste. Spicy taste intensity is strongest when n = 4 in gingerol and shogaol (10.11). The main spicy taste substances in nutmeg and clove are eugenol and isoeugenol; these compounds also contain o-methoxyl-phenol groups.
-
(2)
Nutmeg and clove. The pungent components of nutmeg and cloves are mainly eugenol and isoeugenol, and these compounds also contain o-methoxyphenol groups.
-
(3)
Mustard glycosides. There are two types of glucosin, sinigrin and sinalbin, which produce glucose and mustard oil during hydrolysis. Myrosin is present in the seeds of mustard (brassica juncea), black mustard (sinapic niqra) and horseradish (horse radish), and other vegetables. Glucosinolates are found in white mustard seeds (sinapis alba).
S-methyl-cysteine-S-oxide is also contained in cruciferous vegetables such as glycoside blue, radish, and cauliflower.
3. Stimulating spicy substances stimulating spicy substances is a class of substances that can stimulate the nasal cavity and eyes in addition to stimulating the tongue and oral mucosa, with taste, smell, and tearing properties. Mainly includes:
-
(1)
Garlic, green onion, and leeks. The main spicy components of garlic are allicin, diallyl disulfide, and propyl allyl disulfide. Among them, allicin has the largest physiological activity. The main spicy components of green onions and onions are dipropyl disulfide and methyl propyl disulfide. Leek also contains a small amount of the above disulfide compounds. These disulfides will decompose to produce mercaptan when heated, so garlic and shallots will not only weaken the spicy taste, but also produce sweetness after being cooked.
-
(2)
Mustard and radish. The main spicy component is isothiocyanate compounds, among which propyl isothiocyanate is also called allyl mustard oil, which has a strong pungent spicy taste. They are hydrolyzed to isothiocyanate when heated, and the spicy taste is weakened.
Looking at garlic, onions, and chives, the main spicy taste substances in garlic are allicin, diallyl disulfide, and propyallyl disulfide, and of the three, allicin has the highest physiological activity. Leeks and onions’ main spicy taste compounds are dipropyl disulfide and methyl propyl disulfide. Chives also have a small amount of disulfide compounds. Disulfides decompose to form the corresponding mercaptan when heated; therefore, garlic and onions’ spiciness intensity weakens after cooking and sweetness is produced. The main spicy taste substances in wasabi and radish are isothiocyanate compounds, and one of the compounds, propyl isothiocyanate, is also called allyl mustard oil; it has a pungent spiciness. When heated, these compounds hydrolyze to isothiocyanic acid and reduce the spiciness.
10.3.7 Other Gustatory Perceptions
10.3.7.1 Refreshing Taste
Refreshing taste is produced by the stimulation of special taste receptors in the nasal cavity and oral cavity by some compounds. The typical refreshing taste is mint flavor, including spearmint and wintergreen oil flavor. With menthol and D-camphor as representatives (Fig. 10.18), they have both a refreshing smell and a refreshing taste. Among them, menthol is a commonly used cooling flavor in food processing, and it is widely used in candies and cooling drinks. The mechanism by which such flavor products produce a cooling sensation is unclear. Menthol can be obtained by steam distillation from the stems and leaves of peppermint. It has 8 optically active bodies, and L-menthol exists in nature.
Some sugars also have a cooling sensation when they are crystallized into the mouth, but this is because they absorb a lot of heat when they dissolve in saliva. For example, the heat of dissolution of sucrose, glucose, xylitol, and sorbitol crystals are 18.1, 94.4, 153.0, and 110.0 (J/g), respectively, and the latter three sweeteners obviously have this cool flavor.
10.3.7.2 Astringency
When the oral mucosa protein is coagulated, it will cause convergence, and the taste is astringency. Therefore, astringency is not produced by the action of taste buds, but by the stimulation of tactile nerve endings, which is characterized by astringency and dryness in the mouth.
The main chemical components that cause the astringency of food are polyphenols, followed by iron metals, alum, aldehydes, phenols, and other substances. Some fruits and vegetables also cause astringency due to the presence of oxalic acid, coumarin, and quinic acid. The astringent effect of polyphenols is directly related to the property of being hydrophobically bound to proteins. For example, tannin molecules have a large cross-section and are easy to have hydrophobic interactions with protein molecules. Phenol groups can also cross-link with proteins. Generally, tannins with a moderate degree of condensation have this effect, but when the degree of condensation is too large, the solubility will no longer appear astringent.
The astringency of immature persimmon is a typical astringency, and its astringent components are glycosides with leucoanthocyanins as the basic structure, which are polyphenolic compounds and are easily soluble in water. When the cell membranes of astringent persimmon and immature persimmon rupture, the polyphenol compounds gradually dissolve in water and present an astringent taste. In the process of persimmon ripening, intermolecular respiration or oxidation causes the polyphenols to oxidize and polymerize to form water-insoluble substances, and the astringency disappears.
Tea also contains more polyphenols. Due to the different processing methods, the polyphenols contained in the various teas made are different, so their astringency is also different. In general, green tea contains a lot of polyphenols, while black tea is oxidized after fermentation, and its content is reduced, and the astringency is not as strong as green tea.
Astringency is the desired flavor in some foods, such as tea and red wine. In some foods, it has an impact on the quality of the food. For example, when there is protein, there will be precipitation between the two.
10.3.7.3 Metallic
Since there may be an ion exchange relationship between the metals in contact with food and the food, there is often an unpleasant metal taste in canned foods that have been stored for a long time, and some foods will also have peculiar smells due to the introduction of metals into the raw materials.
Refreshing taste is produced by certain compounds being in contact with oral tissue or nerves stimulating special receptors. The most common refreshing taste is mint flavor, including menthol and camphor; they are considered as both refreshing aroma and refreshing taste. The mechanism by which these flavor products are produced is not known. Some sugar crystals can produce refreshing feels after entering the mouth, but that is due to heat absorption during dissolution of crystals.
Astringent taste is not produced by taste buds, but by the stimulation of tactile nerve endings causing the oral cavity to feel astringent and dry. The main chemical composition of astringent taste in food is polyphenolic compounds, and others include ferrous metals, alums, aldehydes, and phenols. Some fruits and vegetables contain oxalic acid, coumarin, and quinic acid, which can cause astringent taste.
Due to possible ion exchange occurring between metal in contact with food and the food, canned foods with long storage times often have an unpleasant metallic taste. Some foods may also have off-flavors due to raw materials contaminated with metals.
10.4 Olfaction
10.4.1 Olfactory Organs
Olfaction is an important sensation to the human body. It is a sensation caused by the central nervous system as volatile substances stimulate the olfactory nerve cells in the nasal cavity. Among them, the pleasant sense of smell is called fragrance, and the unpleasant sense of smell is called stink. Smell is a more complex and sensitive sensory phenomenon than taste.
According to the anatomical structure, the olfactory system can be divided into 3 parts, the olfactory epithelium, the olfactory bulb, and the olfactory cortex. The olfactory epithelium is composed of olfactory cells, supporting cells, basal cells, and mucous glands. There are specific receptors on the olfactory epithelial ciliary membrane called the olfactory receptor. The first step of aroma perception begins with olfactory receptors interacting with taste substances and the olfactory receptor molecules will determine the specificity of olfactory perception signals. When the olfactory receptor is activated, electrical signal will be generated; this signal will reach the olfactory center through the olfactory conduction pathway and cause a sense of smell. Current research suggests there are at least four different systems involved in the sensation and conduction of olfactory signals, including the main olfactory system, the accessory olfactory system, terminal nerve system, and the trigeminal nervous system. The main olfactory system mostly senses aroma-containing and volatile substances and is also the major focus in flavor chemistry research. The accessory olfactory system is mainly composed of vomeronasal organs, and it is an independent olfactory system that senses odorless substances and substances that are difficult to volatilize, such as pheromones. Terminal nerve system is an independent chemosensory system present in all vertebrates and is accompanied by the main olfactory system and vomeronasal organs. In addition to feeling pain, coldness, temperature, and touch, the trigeminal nervous system is also involved in olfaction. Some taste substances can stimulate the trigeminal nerve to produce a sensation, which is often considered as part of the olfactory perception.
10.4.2 Theories of Olfaction
Several olfaction theories have been proposed based on aroma substances’ molecular characteristics and the relationship between their odors. The theories with the most influences are sterochemical theory and vibrational theory of olfaction.
Olfactory stereochemical theory was proposed by Amoore in 1952. It is the first time that the olfaction produced by substances is related to its molecular shape, and the concept of primary odors is proposed for the first time in olfactory research. Therefore, this theory is also called the main aroma theory, which is like the visual perception of color Amoore’s theory holds that the odor of different substances is a different combination of a limited number of dominant odors, and each dominant odor can be perceived by a different primary odor receptor in the nasal cavity. According to the frequency of various odors in the literature, Amoore proposed seven dominant odors, including ethereal, camphoraceous, musty, floral, minty, pungent, and putrid. To prove that dominant odors do exist and how to distinguish them, Amoore also conducted a “specific anosmia” experiment. After that, guillott’s analysis of amoore’s experimental results suggested that the lack of a certain dominant odor receptor was the cause of the specific olfactory anosmia with the lack of a certain dominant odor receptor. So, Olfaction stereochemical theory believes aroma characteristics are determined by taste substances’ molecular weights and structure. The reaction of different molecularly sized, shaped, and charged taste substances with corresponding receptors of the olfactory system is a lock and key mechanism. When a gas molecule can be properly embedded into a receptor like a key in a lock, this gas molecule’s unique smell can then be detected. The theory of olfactory stereochemistry explains to some extent that substances with similar molecular shapes have different odors because they have different functional groups.
The vibrational theory of olfaction was first proposed by Dyson in 1937 and further developed by Wright in the 1950s and 1960s. The theory holds that olfactory receptor molecules can resonate with aroma molecules. This theory is based primarily on the comparative study of optical isomers and isotopic substitution. In general, enantiomers have the same far-infrared spectrum, but their aromas can vary widely. The replacement of aroma molecules with hydrazine can change the vibrational frequency of the molecules, but this has little effect on the aroma of the substance.
10.4.3 Characteristics and Classification of Olfaction
10.4.3.1 Characteristics of Olfaction
-
1.
Acuity. People have a very acute sense of smell. Some odor compounds can be detected even at very low concentrations. It is said that individual trained experts can distinguish 4000 different odors. Some animals have an acuter sense of smell, and sometimes even modern instruments can’t catch up. The olfactory sensitivity of dogs is well known, while the olfactory ability of eels is almost equal to that of dogs, and they are about 1 million times more sensitive than that of humans.
-
2.
Fatigue and adaptation. when the olfactory central nervous system falls into negative feedback status due to the long-term stimulation of some odors, the sensation is inhibited and adaptation is produced. Perfume, though fragrant, is not known for a long time, but it can endure for a long time even though it is smelly. This indicates that olfactory cells are prone to fatigue and are not sensitive to specific odors. In addition, when people’s attention is distracted, they will not feel the smell, and they will form a habit of the smell when they are stimulated by a certain smell for a long time. Fatigue, adaptation, and habit work together and are difficult to distinguish.
-
3.
Great individual differences. Different people have different senses of smell, even those who have a keen sense of smell will also vary according to the smell. The extreme case of being insensitive to smell forms olfactory blindness, which is also genetic. Some people think that women’s sense of smell is sharper than men’s, but there are different opinions.
-
4.
The threshold will change with people’s physical condition. When people are tired or malnourished, their olfactory function will be reduced; when they are sick, they will feel that the food is not fragrant; women may have hyposmia or hypersensitivity during menses, gestation or menopause, etc. All these indicate that the physiological condition of human beings also has an obvious influence on olfaction.
10.4.3.2 Classification of Olfaction
In fact, olfactory classification is to divide the odor like substances into a group and make semantic description of their characteristic odors. At present, there is no authoritative olfactory classification method. However, Amoore analyzed the odors of 600 compounds and their chemical structures and proposed that there were at least seven basic odors: light smell, camphor smell, moldy smell, flower smell, mint smell, pungent smell, and rotten smell. Many other odors may be caused by the combination of these basic odors. However, in the study of structure–odor relationship, some people often divide the odor into ambergris, bitter almond, musk, and sandalwood. Boelens studied 300 kinds of aroma compounds and found that the odorants could be classified into 14 basic odors, while Abe classified 1 573 odorants into 19 categories by cluster analysis. In the classification of smell, the most important thing is how to measure the similarity between two kinds of smell, that is, the standard of classification, which is also an important reason for the different classifications of odor.
10.4.3.3 Olfaction Substances
There are many unique types of olfaction substances in foods, mainly from flavor substances present in food raw materials. These flavor substances can also come from the processing and storage of food and be produced by microorganisms. Although most flavor substances only require a low usage concentration, this low concentration can still have an important influence on the food product’s flavor and a person’s appetite.
Olfaction substances usually have a low boiling point and high volatility. Some are water-soluble and others are fat-soluble. They can pass through the mucous membrane of the olfactory receptor and the lipid membrane of receptor cells. The molecular weight of these substances is relatively small, normally not greater than 300. Most aroma substances are organic; only a small number of inorganic substances have aromas, such as H2S and NO2. The smell of food is usually the result of a combination of different volatile substances, and different types of flavor substances will produce different aromas.
Odor activity value (OAV) is the detection of an olfaction substance having an effect in food aroma. OAV is the ratio of aroma substance concentration and its threshold value. The aroma threshold value refers to the concentration of aroma compounds in air or water causing an odor detection. The smaller the threshold concentration, the stronger the odor detection. It is commonly believed that when OAV is lower than 1, human’s olfactory organs will not be able to detect this substance. The larger the OAV, the more contributions it will bring to food aroma.
There are numerous kinds of olfaction substances, and the perceptions created by them are very different. It is difficult to accurately classify these substances. Currently, there are physicochemical classification, psychological classification, and olfactory blindness classification. Looking at the properties of aroma substances, they can be classified into alcohol, ester, acid, ketone, terpene, heterocyclic (such as pyrazine, pyrrole, pyrroline, and imidazole), sulfur containing matter, and aromatic hydrocarbon. The relationship between the structure of aroma substances and their aroma is extremely complicated and is still inconclusive.
10.5 Aroma Components in Food
10.5.1 Aroma Components in Fruits and Vegetables
The flavor of fruits and vegetables is one of the main factors contributing to their quality. The types and quantities of flavor substances present in different fruits and vegetables vary. The aroma components of fruits are produced during the metabolic processes of plants and increase in concentration as fruits ripen. Aroma components are mainly organic acid esters, aldehydes, anthraquinones, and volatile phenols, followed by alcohols, ketones, and volatile acids.
Small esters are the main components of many fruit aromas, such as apples, strawberries, pears, melons, bananas, and cherries. 78 to 92% of the volatile substances in apple are esters formed by reactions of acetic acid, butyric acid, and carpoic acid with ethanol, butanol, and hexanol, respectively. Among the volatile components in pineapple, esters account for 44.9%; ethyl acetate accounts for more than 50% of muskmelon’s volatile components. Some of the small esters in fruit aromas are methyl or methylthio-branched esters; for example, apple volatile substances contain 3-methylbutyl acetate, tertiary 3-methylbutyrate, and butyl 3-methylbutyrate, and they create the typical apple aroma with low threshold concentration. In these compounds, ethyl 3-methylbutyrate’s threshold concentration is only at 1 × 10−7 mg/kg and is recognized as one of the most important components of apple aroma. The six important thioester aroma components in melons are methyl methylthioacetate, ethyl methylthioacetate, 2-methylthioethyl acetate, methyl 3-methylthiopropionate, ethyl 3-methylthiopropionate, and 3-methylthioacetate. Methyl 3-methylthiopropionate and ethyl 3-methylthiopropionate have the most influence on pineapple aroma.
Certain varieties of strawberry and citrus fruit also contain thioesters in their volatile substances. Alcohol in apples account for 6–12% of total volatile substances, and the main alcohols are butanol and hexanol. Ripe bananas contain large amount of syringol, syringol methyl ester, and its derivatives. Volatile substances in grapes contain benzyl alcohol, pheylethyl alcohol, vanillin, vanilone, and its derivatives. Derivatized esters of cinnamic acid are also found in ripe strawberries mainly including methyl esters and ethyl esters. Terpenoid compounds are an important part of grape aroma. Thirty-six monoterpenoid compounds are identified from grape volatiles, and it is believed that linalool and geraniol are the main aroma components.
Compared to fruits, the aroma of vegetables is not strong but some vegetables have unique aromas, such as garlic and onions. These vegetables contain sulfur compounds; when tissue cells are damaged, enzymes will combine with aroma precursor substrates in the cytoplasm and catalyze the production of volatile aroma substances. These enzymes are usually multi-enzyme complexes or multi-enzyme systems with differences in types and varieties. If dried cabbages are treated with enzymes from leaf mustard, these cabbages will have leaf mustard’s aroma. The volatile substances in tomatoes are mainly alcohols, ketones, and aldehydes, including cis-3-hexenal, hexenal, hexenol, cis-3-hexenol, 1-hepten-3-one, 3-methylbutanol, 3-methylbutanal, acetone, and 2-heptenal.
10.5.2 Meat Aroma and Aroma Components
The aroma of meat is mainly produced after processing, especially after heat treatment. Raw meat exhibits the rawness aroma present in animals and gamey odor from blood. Meat aroma is mainly formed by flavor precursors in meat through lipid thermal degradation, thiamine pyrolysis, Maillard reaction, and other pathways. The juice in meat contains many kinds of amino acids, peptides, nucleotides, acids, and sugars. Among these components, inosinic acid content is quite high and can be mixed with other compounds to create the aroma of meat. When fat is removed from different types of meat and heated afterwards, the aroma components produced are very similar. Sulfur-containing compounds, such as 2-methyl-3-furanthiol, furfuryl mercaptan, 3-mercapto-2-pentanone, and thiomethanine, are essential flavor substances in all meats. The aroma variation in different types of meat is mainly caused by fat composition. The aroma components produced by heating meat fat are mainly carbonyl compounds, lipids, and lactones. The main compounds in beef aroma are mercaptothiophene and mercaptofuran. The unique flavor in lamb is related to medium-chain fatty acids, especially those with methyl branches. 4-methyloctanoic acid is one of the most important fatty acids in generating lamb flavor. Pork contains a high content of γ-C5, C9, and C12 lactones, and they produce a sweet aroma. When the meat is heated in different ways, the resulting aroma can have either similar components or components with unique characteristics. The main characteristic components of boiled meat aroma are sulfides, furan compounds, and benzene ring compounds. The main characteristic components of barbecue aroma are pyrazine compounds; this aroma also contains ketone compounds and carbonyl compounds, such as isovaleraldehyde.
10.5.3 Dairy Products Aroma and Aroma Components
The aroma components in fresh milk are mainly short-chain saturated fatty acids and carbonyl compounds, such as 2-hexanone, 2-pentanone, methyl ethyl ketone, acetone, acetaldehyde, and formaldehyde. There is also trace amount of ether, ethanol, chloroform, acetonitrile, vinyl chloride, and methyl sulfide in milk. Although there is only small amount of dimethyl sulfide in cow’s milk, it is the main aroma component. The aroma threshold value for dimethyl sulfide in distilled water is approximately 1.2 × 10−4 mg/L; if present in a concentration higher than the threshold, there will be off-flavors and malt flavors present in cow milk.
The fat and lactose in milk have a strong ability to absorb external odors, especially cow’s milk. When the temperature is about 35 °C, its absorption capacity is the strongest, and the temperature of the milk that has just been expressed is exactly in this range. Therefore, it is necessary to prevent contact with materials with peculiar smell.
Milk contains lipase, which can hydrolyze dairy fat into short-chain saturated fatty acids; among these fatty acids, butyric acid has a strong rancid odor. When dairy cows are fed with green fodder, it can inhibit the hydrolysis-type rancid odor of milk. This may be related to the carotene content in the fodder, because carotene can inhibit hydrolysis. On the contrary, when fed with dry feed, the milk is prone to hydrolytic rancidity. In addition to feeding factors, the temperature fluctuations, lack of timely cooling, and long-term stirring that cause the hydrolysis-type rancid odor of cow’s milk promote the hydrolysis of milk fat, which makes the milk produce a rancid odor.
Long-term exposure of cow’s milk and other dairy products to air can also produce rancid odors, also known as oxidative odor. Rancidity is caused by the auto-oxidation of unsaturated fatty acids in milk fat that produces α-, β-unsaturated aldehydes (RCH = CHCHO) and unsaturated aldehydes with two double bonds. Among them, octadienal with 8 carbon atoms and nonadienal with 9 carbon atoms are the most prominent. Even if the two are below 1 mg/kg, the oxidative smell of dairy products can be smelled. Trace metals, ascorbic acid, and light all promote the oxidative odor of dairy products, especially the strongest catalytic effect of divalent copper ion. When the copper content of dairy products is one part per million, it can form a strong catalytic effect. Ferric ion also has a catalytic effect, but it is weaker than copper.
When cow’s milk is exposed to sunlight, it will produce a sun-burning smell. This is because methionine in cow’s milk undergoes oxidative decomposition under the action of vitamin B2 (i.e., riboflavin) to produce β-methylthiopropionaldehyde which has a cabbage odor. if it is highly diluted, it will have a sun odor. Even if β-methylthiopropionaldehyde in milk is diluted to 0.05 mg/kg, the smell can be felt. Cow’s milk must have the following 4 factors to produce the smell of sunlight: light energy, free amino acids or peptides, oxygen, and vitamin B2. β-Methylthiopropionaldehyde can be decomposed to produce methanethiol and dimethyldisulphide and other pungent odor compounds (Fig. 10.19).
In addition, the effect of bacteria can decompose leucine in milk into 3-methylbutanal, making milk produce a malty odor, and the reaction process is shown in Fig. 10.20.
The main aroma components in fresh butter are volatile acids (such as n-butyric acid, n-pentanoic acid, isovaleric acid, n-octanoic acid), alcohols (such as ethanol and isobutanol), isovaleraldehyde (1.0 ~ 10 mg/kg), diacetyl (0.001 46 mg/kg), and acetoin (0.004 47 mg/kg). Among these compounds, aldehydes are derived from amino acid degradation and ketones are derived from the oxidative decomposition of fatty acids such as oleic acid and linoleic acid. Diacetyl and acetoin are the main aroma components of fermented dairy products; they are formed by microbial fermentation of citric acid. The reaction process is shown in Fig. 10.21.
10.5.4 Aquatic Products Aroma and Aroma Components
The range of aquatic product aroma is broader than that of livestock and poultry meat. There are copious types of aquatic products, including not only animal species, such as finfish, shellfish, and crustaceans, but also aquatic plants. The aroma changes with freshness and processing. The smell of aquatic products can be roughly divided into the aroma of fresh products and aroma of processed products. Fresh aquatic products have plant-like scent and melon-like aroma like the aroma of C6, C8, and C9 compounds produced by lipoxygenase in plants; these compounds are mainly aldehydes, ketones, and alcohols. Fish will begin to have fishy off-flavor as it becomes less fresh, and this off-flavor is mainly caused by amines, including ammonia, dimethylamine, and trimethylamine as representing compounds. Compare to fresh raw fish, cooked fish has a higher content of volatile acids, nitrogenous compounds, and carbonyl compounds. The main aroma components in crab meat include pentanol, valeraldehyde, aldehyde, furfural, and trimethylamine; Aroma components in different kinds of crab will vary greatly. Shrimp contains hydrocarbons, alcohols, ketones, lipids, naphthalenes, and other compounds, with more than 65% of hydrocarbons giving shrimps a sweet aroma. 1-penten-3-ol, (cis, trans) 3,5-Octadien-2-one, (trans, trans) 3,3-octadien-2-one, and ester compounds impart a nice flavor to shrimps.
10.5.5 Tea Aroma and Aroma Components
Tea aroma is one of the important indicators of tea quality. The characteristic aroma of tea is related to various factors such as tea tree variety, harvesting season, climate conditions, and processing. Current research shows that aroma components in fresh tea are relatively small, and most of the aroma substances in tea are formed during processing. The aroma components in processed tea mainly include alcohols, aldehydes, ketones, esters, acids, nitrogen-containing compounds, and sulfur-containing compounds. Due to different processing techniques used on each unique variety of tea, the aroma components are also widely different.
The aroma components in each variety of green tea differ greatly due to variation in processing techniques. In roasted green tea, caramel aroma substances are high in concentration, such as benzyl alcohol, geraniol, pyrazine, and parole, and they usually have a chestnut aroma or fresh odor. There is a high concentration of linalool and its oxides in steamed green tea providing a strong grassy aroma. Black tea generally has floral and fruity aromas, and the main aroma substances are geraniol, linalool, and its oxides including benzyl alcohol, 2-phenylethyl alcohol, and methyl salicylate. Oolong tea is a semi-fermented tea with a floral aroma as its unique property. The main aroma substances in oolong tea include methyl jasmonate, hydrazine, linalool, and its oxides such as benzyl alcohol, phenylethyl alcohol, jasmone, jasmolactone, nerolidol, and geraniol.
10.5.6 Baked Goods Aroma and Aroma Components
The aroma of baked goods is mainly the products of carbonylation (Maillard reaction), carbohydrate pyrolysis, oil decomposition, and sulfur compounds (thiamine and sulfur-containing amino acids) decomposition during heat treatment.
The Maillard reaction not only produces brown-black pigments, but also forms a variety of aroma substances. Baked goods aroma is mostly produced by pyrazine compounds. Sugar is an important precursor to the formation of aroma. When the temperature is above 300 °C, sugar can be pyrolyzed to form a variety of aroma substances; the most important ones are furan derivatives, ketones, aldehydes, and diacetyl. Carbonylation reactions not only produce brown-black colored pigments, but also forms a variety of aroma substances. The product of carbonylation varies with the reactants, such as leucine, valine, lysine, and proline that can react during moderate heating with glucose to produce attractive odors. However, cystine and tryptophan can produce off-flavor aromas.
In addition to alcohols and esters formed during fermentation, the aroma substances of bread and other flour products also contain many carbonyl compounds produced during the baking process. Addition of leucine, valine, and lysine into fermented dough can enhance the aroma of bread. Heating dihydroxyacetone and proline together can produce cookie aromas.
Peanuts and sesame have a strong unique aroma after roasting. The flavor of roasted peanut is closely related to pyrazines, of which 2,5-diethylpyrazine is the most relevant substance to roasted peanut flavor. The main characteristic component of sesame aroma is sulfur-containing compounds.
10.5.7 Fermented Foods Aroma and Aroma Components
The effect of fermentation on food flavor is mainly shown in two aspects; first is flavor substances produced by microorganisms acting on raw material’s protein, sugar, fat, and other substances, and examples of these flavor substances include the acidic taste in vinegar and pleasant aroma in soy sauce. On the other hand, fermentation by microorganisms can convert non-flavor substances into flavor substances during the ripening and storage of products such as aroma compounds in Chinese spirits. The aroma components in fermented foods and seasonings are mainly alcohols, aldehydes, ketones, acids, and esters. Due to the large number of different metabolites produced by microorganisms in different proportions, the aroma of each fermented food is unique.
10.5.7.1 The Aroma of Alcohol
The aromatic components of various wines are very complex, and their components vary with the varieties. For example, the main aroma components of Maotai liquor are ethyl acetate and ethyl lactate; the main aroma producing substances of Luzhou Daqu are ethyl caproate and ethyl lactate; the contents of acetaldehyde and isoamyl alcohol are relatively high in the two kinds of liquor; in addition, there are dozens of other microscales and trace volatile components identified in the liquor.
10.5.7.2 The Aroma of Sauce and Soy Sauce
The sources of flavor substances in sauce and soy sauce include: aroma components produced by raw material components; aroma components produced by microbial metabolism; and chemical reactions produced during the fermentation of sauce mash. The main volatile flavor components in sauces include esters, alcohols, aldehydes and ketones, phenols, organic acids, sulfur-containing compounds, furans, and nitrogen-containing heterocyclic compounds. The aroma substances of soy sauce include organic acids, alcohols, esters, phenols, aldehydes, furans, pyrazines, pyridines, and other heterocyclic substances, and their content in soy sauce is inconsistent, which together constitute the special aroma of soy sauce.
10.6 Aroma Formations Pathways in Food
There are numerous varieties of aroma substances in foods and they vary widely; formation of these aroma substances is also extremely complicated. Many reaction mechanisms and pathways are still unknown. However, the basic pathways are divided into two types; one of them is biosynthesis under direct or indirect action of enzymes, such as natural flavor substances produced during the growth, ripening, and storage of raw materials. The other type of pathway is the non-enzymatic chemical reaction pathway, such as aroma substances formed during food processing due to physical and chemical changes. For example, the aroma components of peanuts, sesame, coffee, and bread are produced during roasting and baking; meat and fish are braised and cooked; aldehydes, ketones, acids, and other aroma components formed when fat is oxidized by air.
In general, the pathways or sources of aroma substances in food are roughly as follows: biosynthesis, enzyme action, fermentation, pyrolysis, and food flavoring. This section will focus on the biosynthetic pathway of aromas.
10.6.1 Biosynthesis
The main source of aroma substances in foods is the biosynthesis of raw materials during growth, ripening, and storage; for example, the formation of aroma substances in fruits, such as apples and pears, and the production of aroma substances in certain vegetables, such as onions, garlic, and cabbage, all follow this formation pathway. Different biosynthesis precursors and pathways will produce completely different aroma substances. The aroma components in foods are mainly formed by further biosynthesis using amino acids, fatty acids, hydroxyl acids, monosaccharides, glycosides, and pigments as precursors.
10.6.1.1 Biosynthesis with Amino Acids as Precursors
Amino acid metabolism can form aroma components, such as fruity aroma, ester aroma, and spicy aroma, in fruits and vegetables. The amino acids involved in the synthesis of aroma compounds are mainly branched-chain amino acids (such as leucine), aromatic amino acids (such as phenylalanine and tyrosine), and sulfur-containing amino acids. During the metabolism of these amino acids, aldehydes are formed by transamination and decarboxylation under the action of enzymes; these aldehydes further react and form branched, aromatic, or aliphatic alcohols, carbonyl compounds, acids, and esters. During amino acid metabolism, enzyme activity and substrate specificity determine the concentration and type of branched alcohols and esters that can form.
-
1.
Branched-chain amino acids
Fruits and vegetables contain many short-chain alcohols, aldehydes, acids, esters, and other aroma components. Most of the biosynthetic precursors for these substances are derived from branched-chain amino acids. The aroma components of many fruits, such as bananas and apples, are rapidly formed as they mature during the ripening process, especially at the climacteric stage. For example, one of the characteristic aroma compounds in apple, 3-methylbutyrate, is formed in the post-ripening stage. Banana peel changes from green to yellow as the fruit ripens, and its characteristic aroma substance, isoprene acetate, also rapidly increases. These characteristic aroma compounds in apples and bananas are produced by biosynthesis using branched-chain amino acid, L-leucine, as the precursor (Fig. 10.22).
During the ripening of tomato, the content of isoamyl alcohol, isoamyl acetate, and isobutyric acid or isoamyl butyrate increased, and the key substance was isovaleraldehyde. The 14C-labeled leucine was added to fresh tomato extracts, and 14C-containing isovaleraldehyde was obtained; but when the leucine was added to the boiled tomato crude extracts, there was no such phenomenon. This shows that the process of producing isovaleraldehyde from leucine in tomato has the nature of enzymatic reaction. Pyrazine compounds give characteristic flavors to certain vegetables. For example, 2-methoxy-3-isobutylpyrazine in sweet peppers and peas, 2-methoxy-3-sec-butyl-pyrazine in sugar beets, and 2-methoxy-3-isopropylpyrazine in potatoes are all pyrazine compounds. These compounds are also biosynthesized in plants using branched-chain amino acids are precursors (Fig. 10.23).
Some microorganisms, including yeasts and certain malt aroma producing Streptococcus strains, can also convert amino acids with the method described previously. Plants can also convert amino acids, except leucine, into similar derivatives using the previously discussed biosynthetic pathway to produce aroma substances. For example, rose-scented 2-phenylethanol is synthesized by the previously discussed pathway using phenylalanine.
-
2.
Aromatic amino acid
Aromatic amino acids are synthetic precursors for volatile phenols, ethers, and certain aromatic aroma substances, such as elemicin and 5-methyl eugenol in bananas, cinnamic acid esters in strawberries and grapes, and vanillin in some fruits and vegetables. These are all aromatic amino acids with phenylalanine and tyrosine as precursors (Fig. 10.24). Since these aromatic amino acids are produced from shikimic acid in higher plants and microorganisms, this synthetic pathway is also known as the shikimate pathway. Using this pathway, aroma components associated with essential oils can also be produced.
-
3.
Sulfur-containing amino acids
The characteristic flavor substances in onion, scallion, garlic, chives, and other allium plants have a strong and penetrating aroma, and the main components are sulfur-containing flavor compounds. These flavor compounds are formed after plant tissue ruptures and the separation of enzyme from flavor precursor is destroyed; flavor precursors can then be converted into volatile substances. The flavor precursor for onion is S-(1-allyl)-L-cysteine sulfoxide; this compound is also present in chives. Alliinase can hydrolyze S-(1-allyl)-L-cysteine sulfoxide to form unstable sulfenic acid intermediates, ammonia, and pyruvic acid. Sulfenic acid is further rearranged into Syn-propanethial-S-oxide; this compound triggers tearing and is one of the characteristic components in fresh onion. Sulfonic acid can also form thiols, disulfide compounds, trisulfide compounds, and thiophene compounds; these compounds and their derivatives are flavor substances of cooked onions.
The flavor precursor of garlic is S-(2-allyl)-L-cysteine sulfoxide, and its flavor formation process is like an onion. The formation of diallyl thiosulfinate (allicin) under the action of allinase exhibits a freshly cut garlic odor. Under the action of enzyme or heat, allicin produces trisulfide, disulfide compounds, and thioethers, and these aroma also produced after garlic has been cooked or stored.
Lentinetic acid is the main flavor substance in shiitake mushrooms, and its synthetic precursor is a peptide formed by thio-L-cysteine sulfoxide and γ-glutamyl group. Lentinetic acid produces the active flavor substance lentionione in raw shiitake mushroom under the action of S-alkyl-L-cysteine sulfoxide lyase. These pathways are shown ins Figs. 10.25, 10.26, and 10.27.
10.6.1.2 Biosynthesis with Fatty Acids as Precursors
Aroma components in fruits and some squash vegetables often contain C6 and C9 alcohols, aldehydes, and esters formed by C6 and C9 fatty acids; these compounds are mostly formed by biosynthesis with fatty acids as the precursors. The catabolism of fatty acids is mainly through two pathways, lipoxygenase oxidation and β-oxidation.
-
1.
Aroma components produced by lipoxygenase
In plant tissues, oxidative cleavage of unsaturated fatty acids by enzymes is very common. The aroma substances synthesized by enzymatic reactions have unique aromas compared with compounds produced by auto-oxidation of lipid. The fatty acids used as precursors are mainly linoleic acid and linolenic acid, such as hexanal in apple, banana, grapes, pineapple, and peach, characteristic aroma substances trans-2-nonenal and cis-3-nonenol in melon and watermelon, cis-3-hexene and cis-2-hexenol in tomato, and trans-2-cis-6-nonadienal in cucumber. These characteristic aroma compounds are all produced by lipoxygenase, lyase, isomerase, and oxidase using fatty acids, linoleic acid, and linolenic acid, as precursors (Fig. 10.28). In general, C6 compounds exhibit grass aroma, C9 compounds exhibit melon-like and cucumber-like aroma, and C8 compounds have the aroma of mushroom, hoary stock, or geranium leaf.
The characteristic aroma substances in shiitake mushroom, 1-octen-3-ol, 1-octen-3-one, and 2-octenol, are also formed by enzymatic cleavage of linoleic acid. However, some studies have found that the degradation product of linoleic acid C13-hydroperoxide only found the corresponding hydroxy acid in the mushroom homogenate, and no 1-octen-3-ol was detected, and the linoleic acid C9 hydrogen Peroxide is also not a precursor of octenol.
The aroma substances of vegetables such as cucumbers and tomatoes include C6 and C9 saturated and unsaturated aldehydes and alcohols. In addition to the synthesis of linoleic acid as the precursor through the above pathways, linolenic acid can also be used as the precursor for biosynthesis. The products (3Z)-vinyl alcohol and (2Z)-hexenal are characteristic aroma substances of tomato, and (2E, 6Z)-Nadienal (alcohol) is the characteristic aroma component of cucumber.
The main component causing green-bean-like flavor in soybean products is hexanal; this compound is also formed by the action of lipoxygenase with unsaturated fatty acids (linoleic acid and linolenic acid) as precursors (Fig. 10.29).
-
2.
Aroma components produced by β-oxidation of fatty acids
Many fruits, such as pears and peaches, produce a pleasant fruity aroma after ripening and many of these aroma components are medium-chain (C6-C12) compounds formed by β-oxidation of long-chain fatty acids. For example, ethyl (2E,4Z)-decadienoate is formed by linoleic acid through the β-oxidation pathway and it is a characteristic aroma component of pears (Fig. 10.30). In this pathway, C8 ~ C12 hydroxy acids are also generated at the same time. These hydroxy acids can also be cyclized under enzyme catalysis to generate γ-lactone or δ-lactone. C8 ~ C12 lactones have obvious characteristics of coconut and peach aroma. Generally, naturally mature fruits are more fragrant than artificially ripened fruits. For example, the content of lactones (especially γ-lactone) in naturally mature peaches increases rapidly, and the content of esters and benzaldehyde is 3 to 5 times more than that of artificially ripened peaches, which is related to the activity of related enzymes.
10.6.1.3 Biosynthesis with Hydroxy Acids as Precursors
Terpene compounds are important flavoring substances in foods and exhibit a special aroma in fruits, vegetables, spices, and essential oils. Terpene compounds are mostly synthesized via the isoprenoid pathway; monoterpenoids consist of 10 carbon atoms, while sesquiterpene consists of 15 carbon atoms. The precursor is mevalonate (also known as mevalonic acid) and it is enzymatically catalyzed into isoamyl pyrophosphate, then synthesized in two different pathways (Fig. 10.31). Products of these reactions mostly exhibit natural aromas, such as nerol in lemon, β-sinensal in sweet orange, and nootkatone in grapefruit. These compounds are all characteristic aroma components in different fruits.
During β-oxidation of fatty acids, C8-C12 hydroxy acids are also formed. These hydroxyl acids can undergo cyclization reaction under enzyme catalysis to form γ-lactone or δ-lactone. C8-C12 lactone has characteristic aromas of coconut and peach, and δ-octanolactone is an important aroma component in dairy products (Fig. 10.32).
10.6.1.4 Biosynthesis with Monosaccharides and Glycosides as Precursors
Monosaccharides are not just sweeteners in fruits and vegetables, but also the main synthetic precursors of many olfactory components. After monosaccharide is glycolytically converted into pyruvic acid, it is then oxidatively decarboxylated by dehydrogenase to form acetyl-CoA. Under the action of an acylase, capable of converting alcohol to esters, and a reductase, acid acetate, and acid ethyl ester are formed (Fig. 10.33).
Characteristic aroma substances are widely produced in cruciferous plants, such as cabbage, red cabbage, daikon, mustard leaf, and horseradish, are isothiocynate, thiocyanate, and nitrile compounds. Normally these spicy substances are not directly present in plants, but when plant cells are destroyed, glucosinolate, the precursor for spicy substances, is produced by myrosinase. Myrosin degrades glucosinolate into one molecule of glucose, one molecule of HSO −,4 and one molecule of an unstable intermediate, non-sugar ligand. Depending on reaction conditions, non-sugar ligand can form isothiocyanate and thiocyanate. When pH is less than 4, nitrile compounds and elemental sulfur can be easily formed (Fig. 10.34). Cabbage and red cabbage contain alyl isothiocyanate and butyronitrile; their concentrations vary as growth conditions and processing conditions change. The spicy taste in daikon is produced by 4-methylthio-3-trans-butenyl isothiocyanate; the main flavor substance in mustard leaves and horseradish is α-phenylethyl isothiocyanate and allyl Isothiocyanate.
10.6.1.5 Biosynthesis with Pigments as Precursors
Some aroma substances in foods are formed with pigments as precursors, including lycopene and carotene. For example, under the catalysis of enzymes, lycopene forms 6-methyl-2-heptene-2-oxo and farnesylacetone (Fig. 10.35). In black tea, oxidation of carotenoids produces β-ionone and β-damasone.
10.6.2 The Role of Enzymes
The effect of enzyme on food aroma mainly refers to the process of forming aroma substances under the catalysis of a series of enzymes during the processing or storage of food materials after harvest, including the direct action of enzymes and the indirect action of enzymes. The so-called direct action of enzymes refers to the action of enzymes catalyzing a certain aroma substance precursor to directly form aroma substances, while the indirect action of enzymes mainly refers to the role of oxidation products catalyzed by oxidase to oxidize the aroma substance precursors to form aroma substances. The aroma formation of onion, garlic, cabbage, and mustard greens belongs to the direct action of enzymes, while the aroma formation of black tea is a typical example of indirect enzyme action.
10.6.3 Fermentation
The aroma components of fermented food and its flavoring are mainly produced by microorganisms acting on proteins, sugars, fats, and other substances in the fermentation substrate, mainly including alcohols, aldehydes, ketones, acids, esters, and other substances. Due to the wide variety of products metabolized by microorganisms and the different proportions of various components, the aroma of fermented foods also has their own characteristics. The influence of fermentation on food aroma is mainly reflected in two aspects: on the one hand, certain substances in the raw materials are fermented by microorganisms to form aroma substances, such as the sourness of vinegar and the aroma of soy sauce; on the other hand, some non-aroma formed by microbial fermentation Substances are further transformed during the maturation and storage of the product to form aroma substances, such as the aroma components of liquor. A typical example of microbial fermentation to form aroma substances is lactic acid fermentation (Fig. 10.36). Lactic acid, diacetyl, and acetaldehyde together constitute most of the aroma of hetero-lactic fermented butter and cheese, while lactic acid, ethanol, and acetaldehyde constitute the aroma of homo-lactic fermented yogurt, of which acetaldehyde is the most important. Diacetyl is a characteristic aroma substance of draft beer and most foods with multi-strain lactic acid fermentation.
10.6.4 Food Flavoring
The flavor of food is mainly by some aroma enhancers or odor masking agents to significantly increase the aroma intensity of the original food or to mask the unpleasant smell of the original food. There are many types of aroma enhancers, but the main ones that are widely used are sodium L-glutamate, 5’-inosinic acid, 5’-guanylic acid, maltol, and ethyl maltol. Aroma enhancers themselves can also be used as odor masking agents. In addition, there are many odor masking agents used. For example, when cooking fish, adding a proper amount of vinegar can significantly reduce the fishy smell.
10.7 Aroma Formations During Heating Process
Changes in aroma components during heat treatment of food is very complex. The original aroma substances in food are lost due to volatilization by heat, and raw materials can degrade or interact with each other to form a large amount of new aroma substances. The formation of new aroma components is related not only to internal factors, such as raw material composition, but also due to external factors, such as heat treatment method and heating time.
10.7.1 Formation of Aroma Substances by Maillard Reaction
The reaction of amino compounds (such as amines, amino acids, peptides, and proteins) and carbonyl compounds (such as reducing sugars) in foods under suitable conditions is called the Maillard reaction. Maillard reaction forms a variety of flavor substances and at the same time, causes browning. This browning reaction is the main source of food color and flavor. Maillard reaction plays an important role in food flavor, such as the aroma from freshly baked bread, grilled steak, and freshly brewed coffee; these foods are appealing and are loved by consumers. However, before processing or heating, these characteristic flavors are not present in the food.
Products from the Maillard reaction are complicated, with carbonyl compounds, nitrogen-containing heterocyclic compounds, oxygen-containing heterocyclic compounds, sulfur-containing heterocyclic compounds, and oxygen-containing compounds. Products from the reaction are related to the carbonyl compounds and amino compounds involved in the reaction, and it is also related to heating temperature, time, food pH, and moisture content (Fig. 10.37). In general, when heating time is short and heating temperature is low, the main products of the reaction, in addition to Strecker aldehydes, will also include characteristic aroma substances, such as lactones and furan compounds. When the temperature is high and heat treatment time is long, aroma content will increase; baked goods aroma substances pyrazine, pyrrole, and pyridine can also be formed.
Different types of sugars and amino acids will produce different aroma substances. Maltose reacts with phenylalanine to produce a pleasant caramel aroma. Fructose reacts with phenylalanine to produce an unpleasant caramel odor, but when dihydroxyacetone is present, aroma of hoary stock can be produced. Dihydroxyacetone and methionine form a roasted potato-like aroma, while glucose and methionine react to give a burnt potato aroma. When glucose is present, proline, valine, and isoleucine can produce a pleasing baked bread aroma, but if a reduced disaccharide, such as maltose, is present, burnt cabbage odor can be produced; if a non-reducing disaccharide, such as sucrose is present, an unpleasant coke odor can be detected. When ribose is heated with other amino acids, large amount of aroma changes can occur; however, when sulfur-containing amino acids are heated under the same conditions without ribose, sulfur odor will be produced without changes to other aromas.
Different amino acids will have varied Millard reaction rates. In general, the order of degradation rate for amino acids in descending order is hydroxyl amino acids, sulfur-containing amino acids, acidic amino acid, basic amino acid, aromatic amino acid, and aliphatic amino acid. Figures 10.38, 10.39, 10.40, 10.41 and 10.42 show the formation pathways of main aroma substances imidazole, pyrroline, pyrrole, pyrazine, oxazole, and thiazole during the Maillard reaction.
10.7.2 Thermal Degradation of Sugar
When carbohydrates, especially sucrose and reducing sugars, are heated in the absence of nitrogen oxides, a series of degradation reactions can occur. Different aroma substances are formed when different heating conditions are applied, such as temperature and time; the main aroma substances are furan compounds, but lactones and cyclic diketones are also formed. Monosaccharides and disaccharides usually must be melted to go through thermal decomposition. When the heating temperature is too low or heating time is too short, a milk candy-like aroma will be produced; if continued to be heated, the carbon chain of monosaccharide will be cleaved to form low molecular substances, such as pyruvic aldehyde, glyceraldehyde, and glyoxal. If heated at a high temperature or too long, a burnt caramel smell will appear.
10.7.3 Thermal Degradation of Amino Acids
When amino acids are heated at high temperature, decarboxylation, deamination, and decarbonylation reactions generally occur first; however, the amine products formed at this step often have an unpleasant odor. If continuously heated, other products can interact with each other to form compounds with a pleasant aroma. The amino acids with the most impact on food aroma are sulfur-containing amino acids and heterocyclic amino acids. Besides hydrogen sulfide, ammonia, and acetaldehyde, the thermal decomposition products of sulfur-containing amino acids also contain thiazoles, thiophenes, and other sulfur-containing compounds; most of these compounds are highly volatile aroma substances and are important parts of cooked meat aroma. Proline and hydroxyproline in heterocyclic amino acids further react with methylglyoxal, formed from food during heating, to produce pyrrole and pyridine compounds with aromas of bread, cookie, grilled corn, and grain crop. In addition, thermal decomposition products of threonine and serine are mainly pyrazine compounds with baked goods aroma; the thermal decomposition products of lysine are mainly pyridine, pyrrole, and lactam compounds with baked goods and cooked meat aroma.
10.7.4 Thermal-Oxidative Degradation of Fat
Oxidation or decomposition of fats and oils can produce volatile substances such as aldehydes and ketones. When these compounds are present in high concentration, they will produce off-aromas like paint, fat, metal, and candle odors. When present in an adequate concentration, they will produce pleasing flavor substances.
Lipids will decompose into free fatty acids during heating; unsaturated fatty acids such as oleic acid, linoleic acid, and arachidonic acid contain double bonds and can be easily oxidized to form hydroperoxides. Hydroperoxides can easily decompose at a temperature above 150°C to produce volatile aroma substances such as carbonyl compounds, ketones, aldehydes, and acids (Fig. 10.43). Lipid thermal degradation products mainly include hydrocarbons, β-keto acids, methyl ketones, lactones, and esters. Most of the lactones are produced from linoleic acid; therefore, lipids containing linoleic acid will provide better aroma when deep fried. Thermal degradation products can continue to undergo non-enzymatic browning reactions with the small amount of proteins and amino acids present in oil forming heterocyclic compounds with a characteristic aroma. When lipids from saturated fatty acid (such as glyceryl stearate) are heated with air present, its pyrolysis products are mainly C3-C17 methyl ketones, C4-C14 lactones, C2-C12 fatty acids, and others. Low concentration of γ-lactone has peach and dairy aroma, while at high concentration, there is a deep fried food aroma. Aroma substances formed by lipid degradation in cooked meat products include aliphatic hydrocarbons, aldehydes, ketones, alcohols, carboxylic acids, and esters (Fig. 10.44).
10.7.5 Aroma Substances Formed by Degradation of Other Food Components
In addition to the three major nutrients mentioned above, other components in food will also form aroma substances during thermal degradation. The following are some of the degradation pathways of several components that have been most extensively studied and have the greatest impact on food aroma.
10.7.5.1 Thermal Degradation of Thiamine
Thiamine itself does not have any aroma, but when affected by positively charged nitrogen atoms, a typical degradation reaction occurs with a nucleophilic substitution on the methylene carbon connecting the two rings. Thermal degradation products of thiamine are very complex, mainly including furans, pyridines, thiophenes, and aliphatic sulfur-containing compounds (Fig. 10.45). Some of these compounds are flavor substances for meaty aroma.
10.7.5.2 Thermal Degradation of Ascorbic Acid
Ascorbic acid is extremely unstable and can be easily degraded under heat, oxygen, and light to form furfural and small molecule aldehydes. Furfural compounds are one of the most important components of roasted tea leaves, peanuts, and cooked beef aroma. When ascorbic acid is heated in aerobic conditions, dehydration and decarboxylation reactions occur forming furfural, glyoxal, glyceraldehyde, and other compounds. Under anaerobic conditions, the degradation products are mainly furfural. Low-molecular-weight aldehydes are aroma components on their own; they can also react with other compounds to form new aroma substances.
10.7.5.3 Oxidative Degradation of Carotenoids
Carotenoids are very unstable and are susceptible to heat or oxidative degradation during storage and processing. Products, such as cis-spirulina and β-ionone, are derived from the oxidative decomposition of β-carotene or lutein; these products can impart a rich sweet and floral aroma to tea leaves (Fig. 10.46). Even though these compounds are present in low concentrations, they are widely distributed, allowing many foods to have complete and harmonious aroma profiles.
10.8 Aroma Control in Food Processing
Food processing is an extremely complicated procedure; series of changes occur during processing in food’s shape, structure, texture, nutrition, and flavor. Some processes can greatly enhance food aromas, such as roasting peanuts, baking bread, cooking beef, and deep-frying foods, while some processes can cause food aroma loss or off-flavor production, such as cooked-flavor from juice pasteurization, aroma deterioration of green tea stored in room temperature, over-cooked flavor of steamed beef, and burnt smell of dehydrated products. Therefore, aroma control during food processing is particularly important.
10.8.1 Control of Food Aroma
10.8.1.1 Raw Materials Selection
Food raw materials are one of the most important factors affecting food aroma. Different raw material type, origin, ripening stage, harvest time, and condition will produce distinct aromas. Different varieties of the same raw materials may cause great variances in aromas as well. Fruits harvested during respiration climax will have a much better aroma compare to fruits harvested before. Therefore, selecting the correct raw materials is one of the important factors to ensure great food aromas.
10.8.1.2 Processing Techniques
The impact of food processing techniques on food aroma is also significant. When the same raw materials are processed differently, aromas produced will vary as well, especially under heat treatment. In roasted green tea, the product made with rolling techniques often has a fresh aroma, while tea made without rolling step often exhibit a floral aroma. Fixation and drying are two key processes in the formation of roasted green tea aroma. Moderate spreading of tea leaves can increase the content of the main free-form aroma compounds in tea. Different drying methods will impose a significant difference on tea aromas.
10.8.1.3 Storage Conditions
Storage conditions also have a significant impact on the aroma. Tea will oxidize during storage, resulting in deteriorated and decreased quality. The aroma of apples stored in a controlled atmosphere is worse compare to refrigerated apples. If apples stored in a controlled atmosphere are later stored in refrigerated conditions for about 15 days, its aroma will not be significantly different from the apples that were refrigerated the entire time. An ultra-low oxygen environment is beneficial for maintaining the crunchiness of fruits, but it has an adverse effect on fruit aroma formation. Under different storage conditions, the composition of aroma components in fruits will also be different. This is mainly due to different storage conditions selectively inhibit or accelerate the pathways for the formation of certain aroma substances.
10.8.1.4 Packaging Methods
The influence of packaging methods on food aroma mainly affects two areas. Firstly, packaging changes the environmental conditions of food and this change will lead to material transformation or metabolism inside the food, eventually lead to changes in the aroma. Secondly, different packaging materials for packaged food will cause selective absorption of aroma substances. Packaging methods can selectively affect certain metabolic processes of food. For example, there is no significant difference in aldehyde, ketone, and alcohol content in apples with different bagging types; however, ester content in apples with double-layered bag is lower. Oxygen-free, vacuum, and nitrogen-filled packaging can effectively slow down quality deterioration in tea. For foods with high fat content, hermetic seal, vacuum, and nitrogen-filled packaging can significantly inhibit aroma deterioration.
10.8.1.5 Food Additives
Food ingredients or additives can interact with aroma components. There is strong binding between protein and aroma substances. Fresh milk should avoid contact with odorous substances. β-cyclodextrin has a special molecular structure and stable chemical properties; it cannot be easily decomposed by enzymes, acids, alkalis, light, and heat. It can embed aroma substances, thus reducing its volatile loss and make aroma longer lasting.
10.8.2 Food Aroma Strengthening
10.8.2.1 Aroma Recovery and Re-Addition
Aroma recovery process refers to the extraction of aroma substances initially and then adding the recovered aroma substances back into the product to maintain the original aroma profile. Main methods of aroma substance extraction include steam distillation, solvent extraction, molecular distillation, and supercritical fluid extraction. Because supercritical fluid extraction has many advantages such as high extraction rate, fast mass transfer, non-toxic, non-harmful, no residual, and no pollution, it has a broad application potential in aroma recovery.
10.8.2.2 Addition of Natural Flavors
Addition of essences is a common method of food aroma enhancement; it is also known as flavoring. Artificial essences are inexpensive, but their uses are becoming more limited due to safety reasons. The essences obtained from natural plants, microorganisms, or animals has a characteristic natural aroma and is very safe. Natural essences are becoming more popular.
10.8.2.3 Addition of Aroma Enhancer
Although aroma enhancers themselves hardly show aroma, they can significantly enhance or improve the aromatic effect in food. The mechanism of aroma enhancement is not to increase the content of aroma substances, but to improve the sensitivity of olfactory receptors to aroma substances by acting on olfactory receptors and reducing the sensory threshold of aroma substances. At present, the main applications in practice are sodium L-glutamate, 5’-inosinic acid, 5’-guanylic acid, maltol, and ethyl maltol. The most used for aroma enhancement are maltol and ethyl maltol. Maltol has a good flavor enhancement and flavor adjustment effect under acidic conditions; under alkaline conditions, its flavor adjustment effect is reduced due to the formation of salt; when it encounters iron salts, it is purple-red, so the amount of product should be appropriate to avoid affecting the color of food. Currently, the most used aroma enhancers are maltol and ethyl maltol. Ethyl maltol’s chemical properties are like maltol, but its aroma enhancement property is six times that of maltol.
10.8.2.4 Addition of Aroma Precursors
Addition of substances, such as carotene and ascorbic acid, to fresh tea leaves after fixing can enhance the aroma of black tea. The biggest difference between addition of aroma precursor and direct addition of food essence is that the aromas formed by precursors are more natural and harmonious. Currently, research in this area is also an important part of food flavor chemistry.
10.8.2.5 Enzyme Technology
Flavor enzymes are those enzymes that can be added to food to significantly enhance the flavor of food. The basic principle of using flavor enzymes to enhance food aroma is mainly in two aspects. On the one hand, the aroma substances in food may be free or bonded, and only free aroma substances can cause olfactory stimulation. Bonded aroma substances affect food aroma. The presentation is not contributing. Therefore, under certain conditions, the aroma substances existing in the form of bonding state in food are released to form free aroma substances, which will undoubtedly greatly improve the aroma quality of food; on the other hand, there are some precursors of aroma substances that can be transformed by enzymes in food. Under the action of specific enzymes, these precursors will be transformed into aroma substances and enhance the aroma of food. This research is also a hot spot in flavor chemistry.
The bonded aroma substances in food mainly exist in the form of glycosides. For example, many fruits and vegetables such as grapes, apples, tea, pineapple, mangoes, passionfruit, etc., have a certain amount of bonded aroma substances. Adding a certain amount of glycosidase to the wine can significantly improve the aroma, and adding a certain amount of glucosidase to the dried cabbage can make the aroma of the product stronger. In addition, some bonded aroma substances in food may also exist in the form of being embedded, adsorbed or wrapped on some macromolecular substances. For the release of this kind of bonded aroma substances, the corresponding macromolecular substances are generally hydrolyzed by hydrolytic enzymes. For example, adding pectinase in the processing of green tea beverages can release a large amount of linalool and geraniol.
There are many enzymes that catalyze the conversion of aroma precursors in foods, but more research has focused on polyphenol oxidase and peroxidase. Studies have shown that polyphenol oxidase and peroxidase can be used to improve the aroma of black tea, and the effect is obvious. Catalase and glucose oxidase can be used for terpene aroma substances in tea beverages and have a fixed aroma effect on tea beverages.
Any enzyme that can affect the flavor of food can be called a flavor enzyme. Under specific conditions, flavor enzymes can release bound aroma substances in food to form free aroma substances and can produce aroma components by acting on aroma precursors. Ripe fruits and vegetables contain free-form flavor substances; they also contain flavor precursors that can combine with sugars to form glycosides and other substances. Addition of flavor enzymes to foods can release bound aromas thus enhance and improve food flavor. By adding an amount of glycosidase to red wine can significantly increase wine aroma, while adding glucosidase to dried cabbage can intensify the product’s aroma.
10.9 Summary
Flavor is an important indicator of food quality. It does not only affect appetite, but also has the potential to impact people psychologically and physiologically. Food flavor is the combination of all sensory aspects in the food ingested, with the most important being gustatory and olfactory senses. Gustatory perception is the sense produced when water-soluble compounds in foods stimulate chemoreceptors in the mucous membranes of the tongue; while olfactory perception is mainly caused by certain volatile compounds in foods stimulating the olfactory neurons in the nasal cavity. In most cases, the taste or smell produced by food is the result of a combination of numerous flavor substances and aroma substances. Different types of substances have varied flavor mechanisms, and different gustatory senses can interact with each other. Compare to gustation, olfaction is much more complex. This is not only reflected in the complexity of olfaction production mechanisms, but also the difficulties in quantifying the compounds contributing to food aroma. The aroma substances in foods are mainly formed by biosynthesis, pyrolysis, and addition of food flavoring. Food processing has a major impact on the formation of food aromas; therefore, measures should be taken to enhance and maintain food aroma.
Questions
-
1.
What do the threshold value and aroma value of food refer to, and how does the interaction of taste substances affect the flavor?
-
2.
Briefly describe the mechanism of sweet, sour, bitter, and fresh taste substances.
-
3.
Briefly describe the formation and control methods of food aroma substances.
-
4.
Taste characteristics of common sweeteners, sour agents, and umami agents in food.
-
5.
What factors are related to the flavor of food?
-
6.
Why do people always feel sweet and spicy first, then sourness and bitterness last?
-
7.
Why does the dough emit an attractive fragrance after baking?
-
8.
Why are artificially ripened fruits not as strong as naturally ripened fruits?
-
9.
Why does the saying “If you want to be sweet, add salt first”?
-
10.
Explanation of terms: flavor, threshold, aroma value, relative sweetness, contrast effect of taste, alternation effect of taste, elimination effect of taste, multiplication effect of taste, adaptation phenomenon of taste, spiciness, astringency, Umami.
Bibliography
Belitz, H.D., Grosch, W., Schieberle, P.: Food Chemistry. Springer-Verlag Berlin, Heidelberg (2009)
Bi, S., Xu, X., Luo, D., Lao, F., Pang, X., Shen, Q., Hu, X., Wu, J., Characterization of key aroma compounds in raw and roasted peas (Pisum sativum L.) by application of instrumental and sensory techniques. J. Agricul. Food Chem. 68(9), 2718–2727 (2020)
Damodaran, S., Parkin, K.L., Fennema, O.R.: Fennema’s Food Chemistry. CRC Press/Taylor & Francis, Pieter Walstra (2008)
Ding, N.K.: Food Flavor Chemistry. China Light Industry Press, Beijing (2006)
Furusawa, R., Goto, C., Satoh, M., Nomi, Y., Murata, M.: Formation and distribution of 2,4-dihydroxy-2,5-dimethyl-3(2H)-thiophenone, a pigment, an aroma and a biologically active compound formed by the Maillard reaction, in foods and beverages. Food Funct. 4(7), 1076–1081 (2013)
Gonzalez-Barreiro, C., Rial-Otero, R., Cancho-Grande, B., Simal-Gandara, J.: Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 55(2), 202–218 (2015)
Granvogl, M., Christlbauer, M., Schieberle, P.: Quantitation of the intense aroma compound 3-mercapto-2-methylpentan-1-ol in raw and processed onions (Allium cepa) of different origins and in other Allium varieties using a stable isotope dilution assay. J. Agric. Food Chem. 52(10), 2797–2802 (2004)
Hu, K., Jin, G.J., Mei, W.C., Li, T., Tao, Y.S.: Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 239, 495–501 (2018)
Kan, J.: Food Chemistry. China Agricultural University Press, Beijing (2016)
Li, M., Yang, R., Zhang, H., Wang, S., Chen, D., Lin, S.: Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of tricholoma matsutake singer. Food Chem. 290, 32–39 (2019)
Mahmoud, M.A.A., Buettner, A.: Characterisation of aroma-active and off-odour compounds in German rainbow trout (Oncorhynchus mykiss). Part II: Case of fish meat and skin from earthen-ponds farming. Food Chem. 232, 841–849 (2017)
Nishimura, T., Egusa, A.S., Naga, A., Odahara, T., Sugise, T., Mizoguchi, N., Nosho, Y.: Phytosterols in onion contribute to a sensation of lingering of aroma, a koku attribute. Food Chem. 192, 724–728 (2016)
Renault, P., Coulon, J., de Revel, G., Barbe, J.C., Bely, M.: Increase of fruity aroma during mixed T-delbrueckii/S-cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 207, 40–48 (2015)
Smid, E.J., Kleerebezem, M.: Production of aroma compounds in lactic fermentations. In: Doyle, M.P., Klaenhammer, T.R. (eds.) Annual Review of Food Science and Technology, vol. 5, pp. 313–326 (2014)
Spaggiari, G., Di Pizio, A., Cozzini, P.: Sweet, umami and bitter taste receptors: State of the art of in silico molecular modeling approaches. Trends Food Sci. Technol. 96, 21–29 (2020)
Wang, Z., Xu, S.Y., Tang, J.: Food Chemistry. China Light Industry Press, Beijing (2007)
Xie, B.Y.: Food Chemistry. China Science Press, Beijing (2011)
Yu, A.N., Tan, Z.W., Wang, F.S.: Mechanism of formation of sulphur aroma compounds from L-ascorbic acid and L-cysteine during the Maillard reaction. Food Chem. 132(3), 1316–1323 (2012)
Zeng, L., Watanabe, N., Yang, Z.: Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 59(14), 2321–2334 (2019)
Zhang, Q., Zhang, F., Gong, C., Tan, X., Ren, Y., Yao, K., Zhang, Q., Chi, Y.: Physicochemical, microbial, and aroma characteristics of Chinese pickled red peppers (Capsicum annuum) with and without biofilm. Rsc Advances 10(11), 6609–6617 (2020)
Zhao, M.M.: Food Chemistry. China Agricultural Press, Beijing (2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ma, L., Li, J. (2021). Food Flavor Substances. In: Kan, J., Chen, K. (eds) Essentials of Food Chemistry. Springer, Singapore. https://doi.org/10.1007/978-981-16-0610-6_10
Download citation
DOI: https://doi.org/10.1007/978-981-16-0610-6_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0609-0
Online ISBN: 978-981-16-0610-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)



















































