Abstract
Hydrocolloid-based coatings and films, produced from polysaccharides, proteins, and their blends, have emerged as alternatives to synthetic polymers for food and packaging applications, because they are edible, versatile, renewable, and biodegradable. Coatings are formed as a thin layer directly onto food surfaces by dipping, spraying, brushing, fluidized bed, or panning method. By contrast, films are standalone pre-formed materials either placed between food components or sealed into pouches, and they are manufactured by wet- or dry-casting method. Overall, hydrocolloid-based coatings and films possess excellent barrier properties to CO2, O2, and oil under certain conditions, but moderate water vapor barrier properties. Their formation mechanisms are closely correlated with conformation of biopolymers, their aggregation and crystalline state, as well as their interactions with additives and water. This chapter discusses the existing and potential applications of coatings and films, focusing on the developments and trends of hydrocolloid-based coatings and films for the food industry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Petrochemical-based plastics, such as polyethylene (PE), poly(ethylene terephthalate) (PET), polypropylene (PP), polyvinylchloride (PVC), have dominated the food packaging market for their functionality, lightweight, ease of processing, and low cost (Siracusa et al. 2008). Despite these advantages, increased use of plastic packaging materials has led to serious ecological problems, since they are neither fully recyclable nor biodegradable. While the materials can be incinerated to reclaim the energy, this end-of-life approach can produce toxic compounds, including furans and dioxins, such as those produced from burning PVC (Marsh and Bugusu 2007). To address these issues, edible coatings and films have emerged as an alternative to synthetic petroleum-based polymers for food packaging because they are versatile, renewable, and biodegradable (Siew et al. 1999). They have the potential to delay the deterioration of food products and to prolong their shelf life due to their selective barrier properties against oxygen, carbon dioxide, water vapor, and flavor compounds (Giancone et al. 2008). Global edible packaging market is expected to reach USD 1097 million by 2023, from USD 697 million in 2016, growing at a compound average growth rate (CAGR) of 6.81% (Edible packaging-global market outlook from 2017 to 2023 2017).
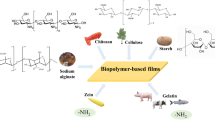
Polysaccharides, proteins, lipids, and composites derived from these materials, can be used as base materials to prepare edible coatings and films (Gennadios et al. 1996). Hydrocolloids based on polysaccharides and proteins are used extensively for the formation of coatings and films for food preservation, because of their desirable mechanical and gas barrier properties. Besides providing protective function, coatings and films can act as nutritious food ingredients due to the unique nutritional and functional properties of hydrocolloids (Viebke et al. 2014). A scheme illustrating the main characteristics of hydrocolloid-based coatings and films is shown in Fig. 8.1. Generally, there are no fundamental differences in material composition between coatings and films, other than their method of manufacture. Coatings are formed as a thin layer directly onto food surfaces by dipping, spraying, brushing, fluidized bed, or panning method (Andrade et al. 2012). By contrast, films are standalone pre-formed material either placed between food components or sealed into pouches, and they are manufactured by wet- or dry-casting method (Janjarasskul and Krochta 2010). The performance and functionality of hydrocolloid-based coatings and films are evaluated by their mechanical properties, barrier effects against oxygen (O2), carbon oxygen (CO2) and water vapor, and thermal stability. These characteristics are strongly correlated with material compositions, manufacture methods, and the end-use conditions (e.g., relative humidity, temperature, and pH) (Rojas-Graü et al. 2009).
This chapter provides an overview on different categories of hydrocolloids for coating and film formation. Methods of preparation, forming mechanisms, and the physicochemical properties for coatings and films are also discussed. Finally, recent developments and trends for packaging applications involving hydrocolloids are summarized.
2 Components of Coatings and Films
Hydrocolloid-based coatings and films are produced from polysaccharides, proteins, their blends, and/or food-grade additives. Their functional, organoleptic, nutritional, and mechanical properties are modified by addition of food-grade additives, including plasticizers, antimicrobials, antioxidants, anti-browning and crosslinking agents, nanofillers, colorants, and flavors (Otoni et al. 2017). The main components for formulation of hydrocolloid-based coatings and films are summarized in Fig. 8.2.
2.1 Polysaccharides
Polysaccharides are nontoxic and naturally occurring biopolymers. The polysaccharide film-forming materials include starch and starch derivatives, cellulose derivatives, alginate, carrageenan, chitosan, various plant gums (pectin, konjac, locust bean gum, and guar gum) and microbial gums (pullulan, xanthan) (Cazón et al. 2017). Although polysaccharide-based coatings and films have superlative barrier properties to CO2, O2, and oil under certain conditions, and high strength and structural integrity, they tend to present a poor barrier to water vapor due to their hydrophilic properties (Yang and Paulson 2000).
2.1.1 Starch and Starch Derivatives
Starch, an agricultural biopolymer found in a variety of plants, is a mixture of amylose and amylopectin whose content varies depending on its botanic origin (LeCorre et al. 2011). Amylose, a nearly linear biopolymer of α-1,4 anhydroglucose units, is known to form a coherent and relatively strong films (Campos et al. 2011). In contrast, amylopectin is a highly branched biopolymer of short α-1,4 chains linked by α-1,6 glucosidic branching points occurring every 25–30 glucose units (Durrani and Donald 1995). Its branched structure leads to form brittle and non-continuous films (De Azeredo et al. 2014). In comparison with native starch, modified starches, like acetylated starch, hydroxypropyl starch, and oxidized-starch, have been reported to form stronger and more flexible films (López et al. 2008, 2010; Hu et al. 2009).
2.1.2 Cellulose Derivatives
Cellulose, the main component of plant fibers, is essentially a linear high-molecular weight biopolymer of D-glucose units linked through β-1,4 glycosidic bonds. The close packing of cellulose chains makes it highly crystalline, fibrous, and insoluble in water (Wang et al. 2016). Water-soluble cellulose derivatives, such as methylcellulose (MC), hydroxypropyl methylcellulose (HPMC), carboxymethylcellulose (CMC), and hydroxypropyl cellulose (HPC), possess good film-forming characteristics (Dhall 2013). Among them, MC films show the lowest hydrophilic properties, whereas the water vapor permeability of HPMC and CMC films is relatively high (Sánchez-González et al. 2009; Kester and Fennema 1989). In addition, the substitution type and degree in cellulose derivatives are critical factors determining the performance of cellulose-based films (Espinoza-Herrera et al. 2011).
2.1.3 Chitosan
Chitosan is a functional biopolymer derived from chitin by deacetylation in alkaline media. It consists of randomly distributed β-(1,4)-2-acetamido-D-glucose and β-(1,4)-2-amino-D-glucose units, with the latter usually exceeding 60% (Kim et al. 2006). Chitosan has a wide spectrum of activity and high killing rate against Gram-positive and Gram-negative bacteria (Chung and Chen 2008). The antimicrobial activity and film-forming ability of chitosan are correlated to its degree of acetylation or deacetylation, and molecular weight (Hosseinnejad and Jafari 2016). Owing to its outstanding characteristics, chitosan could be potentially utilized as the antimicrobial packaging materials to improve food quality and shelf life.
2.1.4 Polysaccharides Extracted from Seaweed
Alginate, a linear polysaccharide extracted from brown seaweed, is composed of variable proportions of β-D-mannuronic acid (M block) and α-L-guluronic acid (G block) linked by 1,4 glycosidic bonds. The block copolymer consists of homopolymeric regions of M- and G-blocks, separated by regions that contain M and G units (Fu et al. 2011). The proportion and distribution of these blocks determine the physicochemical properties of the biopolymer (Lacroix and Le Tien 2005). Alginate dissolves readily in water to form homogeneous film-forming solutions, which upon drying can yield coherent, and transparent films that have a wide range of food applications (Xiao et al. 2012).
Carrageenan is an anionic linear polysaccharide, extracted from edible red seaweeds of Rhodophycea class. It is formed by alternate units of D-galactose and 3,6-anhydrogalactose linked by α-1,3 and β-1,4 glycosidic linkage (Cosenza et al. 2014). There are three types (κ, ι, and λ) of carrageenan with varying number and position of sulfate groups on the galactose dimer (Liu et al. 2015). In comparison with ι-carrageenan films, κ-carrageenan films showed the higher moisture barrier and mechanical properties, except for its flexibility (Paula et al. 2015).
2.1.5 Pectin
Pectin consists of linear homo-galacturonan (α-1,4-galacturonic acids) chains interspersed with branched rhamnogalacturonan (α-1,4-galacturonic acid to α-1,2-rhamnose) chains (Jolie et al. 2010). According to its degree of esterification (DE), pectin can be classified as high-methoxyl pectin (HMP, DE > 50%) and low-methoxyl pectin (LMP, DE ˂ 50%) (Espitia et al. 2014). The mechanical, water barrier properties and thermal stability of HMP films are better than that of LMP films (Lorevice et al. 2016).
2.1.6 Pullulan
Pullulan is an extracellular and water-soluble microbial polysaccharide produced by Aureobasidium pullulans. The linear polymer mainly consists of maltotriose units interconnected to each other by α-(1,6) glycosidic bonds, which are responsible for the flexible conformation and the ensued amorphous character of this polysaccharide in the solid state (Sutherland 1998). This unique linkage pattern endows pullulan with distinctive physical properties to form film that is strong, transparent, and with low permeability to oil and oxygen (Xiao et al. 2012, 2015).
2.2 Proteins
Proteins used for film-forming materials can be categorized into two groups based on their origin of sources: plant-derived proteins, such as corn zein, soy protein, and wheat gluten, or animal-derived proteins like casein, whey protein, gelatin, and collagen proteins (Han 2014). Depending on amino acid composition and sequence, the structure of protein can be random coil, fibrous, or globular. For globular proteins (i.e., soy protein, wheat gluten), they must be denatured by heat, acid, and/or solvent to shape extra extended structures that are required for film formation (Dhall 2013). Overall, protein-based coatings and films display considerably lower O2 and CO2 permeability and CO2/O2 permeability ratio, and moderate mechanical and water vapor barrier properties (Song and Zheng 2014).
2.2.1 Corn Zein
Corn zein, a prolamin protein, has a molecular weight ranging from 18 to 45 kDa. As a relatively hydrophobic protein, the hydrophobicity of zein is related to its high content of non-polar amino acids residues including leucine, alanine, and proline (Shukla and Cheryan 2001). Corn zein dissolves in aqueous ethanol solution to form the glossy, greaseproof, and brittle films through the hydrophobic, hydrogen, and limited disulfide (SS) bonds between zein chains (Ghanbarzadeh et al. 2007).
2.2.2 Wheat Gluten
Wheat gluten, an ethanol-soluble protein in wheat flour, is composed of gliadin and glutenin. Gliadin is monomeric protein with molecular weight of 28–55 kDa, while glutenin is aggregated protein linked by interchain SS bonds with molecular weight of about 500 to 10,000 kDa (Wieser 2007). Glutenin films presented higher mechanical strength and lower water vapor permeability than gliadin films (Hernández-Muñoz et al. 2003). Moreover, the purity of wheat gluten has positive effect on the appearance and mechanical attributes of wheat gluten films (Gennadios et al. 1993).
2.2.3 Soy Protein
Soy protein is comprised of two major components, 7S (β-conglycinin) and 11S (glycinin), representing 37% and 31% of soy protein, respectively. 7S is rich in asparagine, glutamine, leucine, and arginine residues with a molecular weight of 180 kDa. 11S has a molecular weight of 320–360 kDa and contains 20 intramolecular SS bonds (Kumar et al. 2002). Films made from 11S fraction are smooth and opaque, whereas 7S films exhibit transparent and creased appearance (Kunte et al. 1997). At low relative humidity (RH), O2 permeability of soy protein isolate (SPI) films was lower than that of films based on low-density polyethylene (LDPE), methylcellulose, starch and pectin, respectively (Song et al. 2011a).
2.2.4 Casein and Caseinate
Casein mainly consists of five fractions including αs1, αs2, β, κ, and δ-casein, and their sizes vary from 11.5 to 25 kDa. Among them, β-casein is the most interesting one, as it produces films with lower permeability to water vapor than other milk protein (Mauer et al. 2000). Caseinate is a mixture of casein monomers and small aggregates formed after removing of colloidal calcium phosphate from casein micelles. Compared to casein, caseinate, particularly for sodium caseinate, is more soluble and has better film-forming capacity. Films produced from sodium caseinate possess excellent barriers to O2, CO2, and aromas, and thermal resistance (Khwaldia et al. 2004a).
2.2.5 Whey Protein
Whey protein includes β-lactoglobulin, α-lactalbumin, bovine serum albumin, immunoglobulins, lactoferrin, and proteose-peptones (Mulvihill and Ennis 2003). Films prepared from whey protein isolates (WPI) exhibited promising mechanical features, as well as moderate moisture permeability and good oxygen barrier properties, compared to the synthetic polymer films, e.g., low-density polyethylene (LDPE), high density polyethylene (HDPE), PVDC, cellophane, and polyester (Khwaldia et al. 2004b).
2.2.6 Gelatin
Gelatin is an animal protein obtained by hydrolysis of collagen. It is a combination of many fractions varying in size, including the whole α-chain of tropocollagen molecule (a trimer of around 330 kDa that aggregates to form the larger collagen structures) and hydrolytic fragments of parts of the α-chains (Boran and Regenstein 2010). Gelatin films display effective barriers against O2 and aromas at low or intermediate RH, but weak water resistance due to its hydrophilic nature. Furthermore, their mechanical properties are closely related to the renaturation level of gelatin (Bigi et al. 2004).
2.3 Food-Grade Additives
2.3.1 Plasticizers
Plasticizers are low molecular weight compounds with non-volatile compounds. Their primary role is to enhance the flexibility and processability of hydrocolloid-based coatings and films. However, their barrier properties are impaired as result of the increased free volume and molecular mobility after plasticizers addition (Sothornvit and Krochta 2005; Vieira et al. 2011). Food-grade plasticizers mainly include glycerol, sorbitol, polyethylene glycol, sucrose, glucose, fructose, mannitol, xylitol, fatty acids, and monoglycerides (Vieira et al. 2011).
2.3.2 Polysaccharide Nanofillers
Nanofillers (at least one dimension smaller than 100 nm) provide reinforcement effects due to their high aspect ratio and surface-to-volume ratios (Crosby and Lee 2007). Considering the application and safety for hydrocolloid-based coatings and films in food packaging, the polysaccharide nanofillers, e.g., cellulose nanoparticles, cellulose nanocrystals, starch nanoparticles, starch nanocrystals, chitin nanowhiskers, and chitin nanofibers, have been used as excellent candidates for improvement of their mechanical, barrier, and thermal properties (Otoni et al. 2017).
2.3.3 Antimicrobial Additives
Incorporation of antimicrobial compounds into packaging materials provides inhibitory effects against spoilage and pathogenic bacteria by maintaining active compounds on food surface (Gennadios et al. 1997). There are several categories of antimicrobial compounds that have been employed in hydrocolloid-based coatings and films, including organic acids (sorbic and its potassium salt, acetic acid, and malic acid), polypeptides and bacteriocins (lysozyme and nisin), plant essential oils (cinnamon, oregano, rosemary, and lemongrass), and polyphenols (flavonoids and phenolic derivatives) (Franssen and Krochta 2003).
3 Preparation Methods
3.1 Preparation of Hydrocolloid-Based Coatings
3.1.1 Spray Coating
Spray coating is a commonly used technique for food coatings, especially for fruits and vegetables. In this process, food products are placed on a rotating platform, then the coating-forming solution forms droplets and distributes them over the food surface by means of a set of spraying nozzles (Debeaufort and Voilley 2009). The main advantages of this technique offer uniform coating, thickness control, and the possibility of multilayer applications, such as using alternating sodium alginate and chitosan solutions (Ustunol 2009).
3.1.2 Dip Coating
Dip coating involves submerging food products into a vat containing coating solution. After dipping the products and draining away excess coating, it is dried either at room temperature or with the aid of a dryer (Andrade et al. 2012). The advantage of this method is to obtain good uniformity around the irregularly-shaped and rough food surface. Several problems may occur by using this method, such as coating dilution, build-up of trash or dirt, and microorganism growth in the dipping tank (Andrade et al. 2012).
3.1.3 Fluidized-Bed Coating
Fluidized beds are categorized by three different configurations: top spray, bottom spray, and rotating-fluidized bed. The conventional top-spray method has a greater possibility of success in the food industry compared to other methods (Andrade et al. 2012). As presented in Fig. 8.3, the coating solution is sprayed through a set of nozzles onto the surface of fluidized particles to form a shell-type structure. Its application focuses on the functional ingredients and food additives, i.e., leavening agents, enzymes, vitamins, minerals, and spices (Chen et al. 2009).
Schematic of top-spray fluidized-bed coating process, adapted from (Dewettinck and Huyghebaert 1999) with permission
3.1.4 Pan Coating
The schematic of pan coating process is displayed in Fig. 8.4. As shown, the coating solution is sprayed into a rotating bowl (referred to as pan), and the food particles are tumbled within the pan to distribute the coating solution over their surface. Forced air, either ambient or elevated temperature, is utilized to dry the coating (Agrawal and Pandey 2015). Pan coating is mainly used for the confectionery and chocolate industries or particularly small food items like nuts and raisins (Andrade et al. 2012).
Schematic of pan coating process, adapted from (Agrawal and Pandey 2015) with permission
3.2 Preparation of Hydrocolloid-Based Films
3.2.1 Wet Method
The wet method, also known as solvent casting, can be sub-classified to bench casting and continuous casting, respectively. The bench casting is commonly utilized to fabricate films at laboratory scale as it is simple and cost effective. In this method, the film-forming solution is deposited over a rimmed plate, and then followed by drying to produce a cohesive and free-standing film.
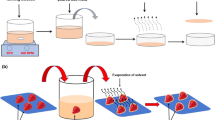
Continuous casting is more suitable for industrial applications, because it requires less space and labor. As shown in Fig. 8.5, film-forming solution is uniformly spread on a continuous steel belt that passes through a drying chamber. The dried film is then stripped from the steel belt and wound into film roller. The advantage of this method is optimizing uniformity, heat transfer, and drying efficiency, while avoiding expense of a separate substrate (Rossman 2009).
Schematic of continuous casting technique to prepare hydrocolloid-based films, adapted from (Borges et al. 2015) with permission
3.2.2 Dry Method
Dry method, i.e., compression molding and extrusion processing, is based on the thermoplastic properties of polysaccharides and proteins. In the presence of plasticizers, at low moisture levels and high temperatures and with pressure, biopolymers acquire a viscoelastic behavior that allows them to be shaped for the production of films (Gómez-Estaca et al. 2016). In general, compression molding is studied at laboratory scale as a precursor to extrusion with the aim of determining the suitable processing conditions (Hernandez-Izquierdo and Krochta 2008).
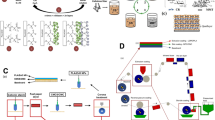
Extrusion processing is a highly efficient manufacturing method with commercial potential for large-scale production of biopolymer films (Fishman et al. 2000). The configuration of one-screw extruder is presented in Fig. 8.6. The extruder basically consists of an endless screw inside a barrel with a double casing that permits control of temperature. The biopolymer is fed from a hopper and pushed by the screw towards a die (Nur Hanani et al. 2012). To date, dry method has been successfully used in preparation of starch, alginate, wheat gluten, soy protein, and whey protein films (Mendes et al. 2016; Hernandez-Izquierdo and Krochta 2008; Azevedo et al. 2017; Ciannamea et al. 2014).
The configuration of one-screw extruder, adapted from (Borges et al. 2015) with permission
4 Microstructural and Physicochemical Characterization
The microstructural characteristics (such as chemical, crystalline structure, and morphology) of hydrocolloid-based coatings and films are closely correlated with their packaging performance (e.g., mechanical, barrier, and thermal properties).
4.1 Structural Analysis
Microscopy and spectroscopic techniques have been utilized to study the architecture and structure of hydrocolloid-based films at micro and nanometric scales. Ultrastructural and internal structure in films have been characterized by confocal laser scanning microscopy (CSLM), while scanning electron microscopy (SEM) and atomic force microscopy (AFM) are more used to study their surface and cross-section morphology (Arzate-Vázquez et al. 2012; Andreuccetti et al. 2009). Fourier transform infrared spectroscopy (FTIR) analyzes the possible functional chemical groups, conformational transitions, and molecular interactions (Yadav et al. 2014). Nuclear magnetic resonance (NMR) spectroscopy provides information about the chemical and physical properties of atoms or their related molecules, as well as reaction state, dynamics, structure and chemical environment (Karbowiak et al. 2008). For instance, for hsian-tsao gum (HG)-casein films, the hydrogen bonding interactions and Maillard reactions between HG and casein were revealed by FTIR data. Meanwhile, NMR analysis indicated that HG addition significantly changed the mobility of water molecule in casein films (Yang et al. 2015). Other complementary techniques are also utilized for structural analysis of hydrocolloid-based coatings and films, such as X-ray diffraction (XRD) to identify the information about crystalline/amorphous structures, and small-angle X-ray scattering (SAXS) to monitor crystalline and aggregate structures of membrane materials (Bodnár et al. 2007).
4.2 Mechanical Properties
Favorable mechanical properties are essential for packaging materials to perform their protective functions efficiently. Mechanical properties of selected hydrocolloid-based films are listed in Table 8.1. A standard method, ASTM-D882–91, originally developed to evaluate mechanical properties such as tensile strength (TS), elongation at break (EAB), elastic modulus (EM), and toughness of commercial plastic, is also applied to hydrocolloid-based films (ASTM-D882-91 1991). As shown in Fig. 8.7, the mechanical parameters are calculated by determining the relationship between stress and strain, when film is stretched at a set rate (distance/time). EM, a measure of intrinsic film stiffness, is the slope of the linear range of the stress–strain curve (Mauer et al. 2000). Toughness refers to the ability of a material to absorb energy during deformation up to fracture, determined as the area under the stress–strain curves (Fig. 8.7b). TS is the maximum strength measuring the resistance of the film, whereas the percentage of EAB is a measure of the stretching capacity of flexibility of the film prior to breaking. They are calculated by using Eqs. 8.1 and 8.2:
where TS is the tensile strength (MPa), F is the force (N) at maximum load, and A is the initial cross-sectional area (m2) of the film specimen.
where EAB is the elongation at break (%), l1 is the initial length, and l is the length of the film at breaking point.
(a) Schematic of tension test setup, adapted from (Pham et al. 2008) with permission, (b) mechanical properties determined from the typical stress-strain curve
4.3 Barrier Properties
The basic function of packaging materials is to control mass transfer between food and the ambient atmosphere. Water vapor in environment transferring to packaged food results in problematic microbial growth, and undesirable textural changes. Oxygen can cause deterioration of food due to oxidation of lipids and other oxygen-sensitive components. Thus, water vapor and gas permeability is a vital property for selecting or tailoring the hydrocolloid-based films.
4.3.1 Water Vapor Permeability (WVP)
Table 8.1 shows WVP values of selected hydrocolloid-based films. These data are obtained gravimetrically following the ASTM Standard Test Method E96, known as the “cup method” (ASTM-E96-92 1990). According to this method, a cup with an open mouth is filled with distilled water or desiccant. The film is sealed on the open mouth of the cup, the assembly is weighed, and placed under controlled temperature and RH conditions (Cazón et al. 2017). WVP is calculated according to the combined Fick–Henry laws for gas diffusion through films (Eq. 8.3).
where Δw/Δt is the rate of water gain (g/h), A is the exposed area of the film (m2), L is the mean thickness of film specimens (m), and Δp is the difference in partial water vapor pressure between the two sides of film specimens.
4.3.2 Gas Permeability
Oxygen permeability (O2P) and carbon dioxide permeability (CO2P) are evaluated on the basis of the ASTM D 3985–02 method (ASTM-D3985-02 2002). The films are sealed between two chambers with each having two channels to the exterior. In the lower chamber, O2 or CO2 is supplied at a controlled flow rate to maintain the pressure constant in that compartment. The other chamber is purged by a stream of nitrogen, also at a controlled flow. In the case of O2P measurement, the nitrogen flow leaving this chamber is connected to an O2 sensor installed on-line which measures the O2 concentration. For CO2P measurement, the nitrogen flow leaving this chamber is collected in a syringe for CO2 quantification by a gas chromatograph (Cerqueira et al. 2009). The O2P and CO2P of selected hydrocolloid-based films are listed in Table 8.1.
4.4 Thermal Properties
One key factor that influences the processing and operating temperatures of hydrocolloid-based coatings and films is their thermal properties. The properties are investigated by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and dynamic mechanical analysis (DMTA). DSC technique is used to determine the glass transition temperature (Tg), melting temperature (Tm), crystallization temperature, heat capacity difference at Tg of hydrocolloid-based coatings and films (Cheng 2002). TGA is widely employed to examine their decomposition temperature, weight loss, and activation energy of decomposition (Cheng 2002). Furthermore, the structural and viscoelastic properties of films are investigated by DMTA. Dynamic modulus, dynamic loss modulus, temperature of main chain relaxation, and temperature of local mode relaxation are measured as functions of temperature and frequency by forced oscillation method (Brown and Gallagher 2011).
5 Film-Forming Mechanism
Understanding the film-forming mechanism is important to predict material properties of hydrocolloid-based films, which is essential for the optimization of drying and processing condition. As previously mentioned, both processing methods (wet and dry) have been widely used to prepare the films. The wet method requires solubilizing the hydrocolloids in a solvent, spreading the solution onto a flat surface, and then followed by drying to produce a film. The film-forming mechanism involves conformational change of the biopolymer, as well as solvent-biopolymer and biopolymer-biopolymer interactions that continue to evolve as the solvent evaporates under different drying conditions (Watanabe et al. 2006; Xiao et al. 2014b). However, a number of polysaccharides and proteins have capacity to form gel during film-forming process, and their film-forming mechanism is related to the gelation mechanism. Although a few researchers proposed that the transition from wet gel (biopolymer-in-water) to dry film (water-in-biopolymer) is a critical stage during film-forming process, the complete transition mechanism after gelation have not yet been fully explained (Szabó et al. 2012).
By contrast, dry method involves heating and mixing biopolymers and plasticizers by extrusion and/or compression molding techniques. Over the course of extrusion, biopolymer chains denature, dissociate, unravel and align, and then recombine, crosslink, and aggregate via specific linkages with heat and pressure, which result in film formation through complete restructuring of biopolymer molecules. Thus, the film-forming mechanism is correlated with conformation changes of biopolymers, their aggregation and crystalline state, as well as the interactions among biopolymer, plasticizer, and water.
5.1 Polysaccharide-Based Films
Polysaccharides (with the exception of glycogen, etc.) are long-chain biopolymers formed from mono- or disaccharide repeating units joined together by glycosidic bonds. Owing to the presence of a large number of hydroxyl and other polar groups in their structure, hydrogen bonds and/or electrostatic interactions have a crucial function in film formation (Han 2014). Polysaccharide films are fabricated by disrupting interactions among polysaccharide segments and forming new intermolecular hydrophilic interactions and hydrogen bonding (Rhim and Ng 2007).
5.1.1 Formation Mechanism of Solvent Casting Films
For starch films, their formation mechanism depends on the starch concentration and amylose content. At relatively high concentration, aggregation and packing of swollen granules dominated the film formation, whereas both coil-to-helix transition and aggregation of double helices were operative during the film formation from dilute starch solutions (Liu 2005). Xiao et al. (2014a, b) elaborated the formation mechanism of pullulan and alginate films by monitoring the conformational change of polysaccharides, water-polysaccharide, and polysaccharide-polysaccharide interactions during drying. As pullulan drying process progressed, the oxygen atoms at the C5 and C6 carbons of the D-glucopyranose ring might preferentially form hydrogen bond with water or pullulan molecules, resulting in more-ordered structure with increased interchain interactions in pullulan films. Moreover, the less-ordered structure domain of the pullulan was first affected during drying, followed by pullulan skeleton segments. Finally, conformational changes in pullulan chains occurred as the drying process completion (Xiao et al. 2014b). In the course of the formation of alginate film, the oxygen atoms at the C2 and C3 carbons of the pyranose ring preferentially formed hydrogen bond with water or alginate molecules, while the skeletal vibrations of pyranose ring (e.g., C-C and C-O-C groups) were less perturbed than the stretching vibrations of COO− group and O-H bending vibration of alginate with drying (Xiao et al. 2014a). The film-forming mechanism of Flammulina velutipes polysaccharide might be associated with the intermolecular and intramolecular hydrogen bonds between polysaccharide chains and the formation of β-glycosidic bonds upon drying (Du et al. 2016). Li et al. (2019) proved that the electrostatic interactions and hydrogen bonds are crucial in fabricating the multilayer films based on chitosan and alginate by layer-by-layer (LbL) technique. Strong intermolecular interactions occurred among the amino, carboxyl, and hydroxyl groups of the chitosan and alginate.
5.1.2 Formation Mechanism of Extruded and Compression-Molded Films
Pushpadass et al. (2009) reported that glycerol and/or water destroyed the crystallinity of native starch, then the starch fragmentation converted into thermoplastic starch with heat and shear. During extrusion process, the inter- and intra-hydrogen bonds of starch would be unraveled when the glycerol was added into starch, and the new hydrogen bonds between starch and glycerol were formed simultaneously (Pushpadass et al. 2009). Afterwards, the starch recrystallization induction process among the helical amylose molecule occurred during cooling, which led to the Vh-type crystalline arrangement (Azevedo et al. 2017). According to Gao et al. (2017), neat alginate granules were largely de-structured by glycerol and water, and glycerol increased the mobility of alginate chains while promoting the crystallization of alginate chains with structural reorganization during compression molding.
5.2 Protein-Based Films
The main formation mechanism of protein films involves denaturation of the protein initiated by heat, solvent, or change in pH, followed by association of extended peptide chains through new intermolecular interactions, such as covalent (SS bond or crosslinking) and electrostatic, hydrophobic, or ionic interactions between protein chains (Janjarasskul and Krochta 2010).
5.2.1 Formation Mechanism of Solvent Casting Films
The formation of intact and water-insoluble WPI films was realized by heat denaturation of aqueous protein solution (Pérez-Gago and Krochta 2002). Heat denaturation unfolded whey protein and promoted the exposure of SH and hydrophobic groups. The unfolded protein might then undergo intermolecular interactions (hydrogen bonds, hydrophobic, covalent and electrostatic interactions). It is noteworthy that the cohesion of WPI films relied principally on the intermolecular SS bonds via sulphydryl/disulphide (SH/SS) exchange reactions (Guckian et al. 2006). On the other hand, WPI had the ability to form water-soluble films without heat denaturation. Since most of the hydrophobic and SH groups are buried in the interior of WPI molecule, their film-forming mechanism involves the intermolecular hydrogen bonding between protein molecules, rather than the hydrophobic and covalent interactions (Guckian et al. 2006; Pérez-Gago et al. 1999). Ciannamea et al. (2014) also proved that hydrogen bonds and hydrophobic interactions played a more important role in the formation of soy protein films. During the film formation of 11S, along with disappearance of its α-helices and disordered structures, the intermolecular hydrogen bonds between β-sheet segments predominated the aggregation of 11S (Robert et al. 2001; Subirade et al. 1998). Similar to the 11S films, the high density of intermolecular hydrogen-bonded β-sheets were conducive to the formation of gliadin network during drying (Mangavel et al. 2001). According to Pankaj et al. (2014), the film-forming mechanism of caseinate was attributed to their random coil structure which allowed them to form extensive intermolecular hydrogen, electrostatic, and hydrophobic bonds, resulting in increased interchain cohesion.
In comparison, the formation mechanism of gelatin films is related to the temperature during drying due to thermo-reversible gelation behavior of gelatin. When the gelatin films were prepared below the helix-coil transition temperature, partial renaturation of collagen in gelatin took place, which resulted in the formation of a collagen-like triple-helix structure. Moreover, the partial renaturation only took place during the advanced stage of drying (Ghoshal et al. 2014). On the contrary, a helix structure was rarely formed in gelatin films when they were dried above the helix-coil transition temperature.
5.2.2 Formation Mechanism of Extruded and Compression-Molded Films
For compression-molded soybean protein films, the high temperature promoted the crosslinking between soybean proteins through intermolecular SS bonds, either from free sulfhydryl (SH) groups or through SH/SS exchange reactions, which predominated the formation of film matrix (Ciannamea et al. 2014). During extrusion process, the aggregation and reorganization of wheat gluten molecules were principally related to the formation of intermolecular SS crosslinking bonds via oxidation of SH groups and SH/SS exchange reactions between glutenin and gliadin (Lagrain et al. 2010). The formation schematic of intermolecular SS bonds between glutenin and gliadin during heat processing is illustrated in Fig. 8.8.
Schematic of the formation of intermolecular SS crosslinking bonds between glutenin and gliadin during heat processing. Adopted and modified from (Lagrain et al. 2010) with permission
6 Applications and Recent Developments
Oxidation, microbial spoilage, and metabolism are the main causes of deterioration of food products. Thus, the primary function of packaging materials based on hydrocolloids is to maintain the quality and safety of food products during storage and conveyance. Normally, fruits and vegetables have short shelf life due to its perishable nature. Hydrocolloid-based coatings and films may act as a semipermeable barrier to selectively control the exchange of CO2, O2, and ethylene, resulting in the reduction in ethylene levels, ripening, respiration rate, and water loss on fruits and vegetables (Valencia-Chamorro et al. 2011). Several studies shown in Table 8.2. have demonstrated the ability of hydrocolloid-based coatings and films carrying bioactive compounds to retard browning reactions and microbial growth in fruits and vegetables, especially the minimally processed (MP) fruits and vegetables. Ramos-García et al. (2012) reported that lime essential oil incorporated into chitosan-beeswax blend coatings on tomato showed strong inhibitory effect against Rhizopus stolonifer and Escherichia coli DH5α during storage at 12 and 23 °C. Sarengaowa et al. (2018) coated the fresh-cut “Red Fuji” apples with alginate coatings containing thyme oil, cinnamon oil, and/or oregano oil, and observed that reduction of total coliform, yeast and mold counts in comparison with control and alginate-coated samples. Meanwhile, the respiration rate, weight loss, firmness, and browning reactions in fresh-cut apples stored at 4 °C were significantly decreased.
Recently, the development of multilayer and nanomultilayer coatings based on hydrocolloids, formed by LbL deposition technique, gained much attention for the preservation of fruits and vegetables. For instance, the multilayer coatings based on gelatin and chitosan predominantly enhanced physiological quality and reduced the bacteria, yeast, and fungi counts of fresh-cut melons (Poverenov et al. 2014). Souza et al. (2015) reported that the nanomultilayer coatings, made of alginate and chitosan, considerably inhibited putrefaction of fresh-cut mangoes during 14 days at 8 °C. At the end of the storage period, the lower values of mass loss, pH, malondialdehyde content, and browning rate were observed in the coated mangoes. Furthermore, nanoemulsion-based sodium alginate coatings with lemongrass essential oil at 0.5% or 1% (v/v) were created to completely inhibit the natural microflora of fresh-cut Fuji apples during 2 weeks at 23 °C. The application of this coating on fresh-cut apples exhibited a faster and greater inactivation of Escherichia coli during storage time compared with conventional emulsions (Salvia-Trujillo et al. 2015). Rossi Marquez et al. (2017) reported that transglutaminase crosslinked coatings prepared from whey protein and pectin were able to totally prevent the weight loss of fresh-cut potato and carrot at least until the sixth day of storage, which also maintained the phenolic and carotenoid content of fresh-cut carrot during storage.
Meat, poultry, and seafood products are common sources of proteins, yet susceptible to the spoilage microorganisms and food-borne pathogens. Thus, the hydrocolloid-based coatings and films with antimicrobial and/or antioxidant compounds are produced to prolong their shelf life (Table 8.3). The incorporation of grape seed extract and tea polyphenols into chitosan coatings predominantly delayed the degradation of ATP and lipid oxidation of red drum during refrigerated storage (Li et al. 2013). Song et al. (2011b) reported the efficacy of alginate coatings enriched with Vc and tea polyphenols in inhibiting the growth of total viable counts, reducing chemical spoilage, and improving sensory quality of refrigerated bream compared to uncoated samples. According to Kim et al. (2018b), the multilayer coatings, based on alginate, chitosan, and grapefruit seed extract, were fabricated to reduce the bacterial counts and off-flavor of shrimp stored at 4 °C.
As shown in Table 8.3, the hydrocolloid-based coatings and films with nanoemulsion, nanoencapsulation, and nanocellulose have been created to extend shelf life of meat and seafood products. Dehnad et al. (2014) proved that the application of nanocomposite films based on chitosan and nanocellulose on ground meat decreased lactic acid bacteria population up to 3.1 logarithmic cycles (compared with nylon packaged sample) at 25 °C during 6 days of storage. Noori et al. (2018) showed that the addition of ginger essential oil nanoemulsion into sodium caseinate coatings caused significant decrease of total aerobic psychrophilic bacteria of refrigerated chicken fillets during 12 days. The chitosan coatings included with nanoencapsulated Satureja plant essential oil were developed by Pabast et al. (2018) to improve the microbiological safety and prolong shelf life of lamb meat during chilled storage. Additionally, new plant extracts, as well as hydrocolloids based on non-conventional sources have been developed as potential ingredients of coatings and films (Shahbazi 2018; Jouki et al. 2014; Lee et al. 2016a; Ruiz-Navajas et al. 2015).
Cheese is nutritious food derived from milk. The shelf life of cheese is limited due to the uncontrolled and extensive fungal and bacterial proliferation on its surface. Table 8.4 shows some recent applications of antimicrobial coatings and films based on hydrocolloids in cheese. WPI coatings included with thyme and clove essential oils were produced by Kavas et al. (2015) to prolong the shelf life of semi-hard kashar cheese. The application of this coating on cheese retarded the growth of Listeria monocytogenes, Staphylococcus aureus, and Escherichia coli O157:H7 during 60 days of storage. Nanolaminate coatings based on alginate and lysozyme by LbL technique were fabricated to preserve “Coalho” cheese (Medeiros et al. 2014). After 20 days, coated cheese showed lower values of mass loss, pH, lipidic peroxidation and higher titratable acidity in comparison with uncoated cheese (Medeiros et al. 2014). Kim et al. (2018a, b) wrapped the Mozzarella cheese with chicken bone gelatine films containing cinnamon bark oil (1% w/v) and observed the reduction in the population of Listeria monocytogenes on mozzarella cheese during 20 days storage. In the current market, the commercialized hydrocolloid-based coatings, RIOCOBERT and RIOCOBERT PLUS (Becor Barbanza Ltd., A Coruña, Spain) effectively inhibited the growth of fungi on cheese (Fuciños et al. 2017).
For bakery and nuts products, most applications are hydrocolloid-based coatings rather than films. The coatings made from potato starch with potassium sorbate and citric acid were applied to extend shelf life of mini panettone (Ferreira Saraiva et al. 2016). Pinto et al. (2015) coated the cashew nuts with starch-cashew tree gum blend coatings to reduce moisture absorption, lipid oxidation, and the loss of crisp texture of nuts. Apart from that, hydrocolloid-based coatings are an additional method to improve unit operation efficiencies in the food industry. For example, they were applied in frying pre-treatments to reduce oil content in deep-fat fried products, such as chicken breasts (Dragich and Krochta 2010), potato chips (Hua et al. 2015), and fish cake (He et al. 2015). In osmotic dehydration processes of fruits and vegetables, hydrocolloid-based coatings can prevent large solute uptake without noticeably affecting water loss (Rodriguez et al. 2016; Azam et al. 2013).
7 Future Perspectives
Although hydrocolloid-based coatings and films have been utilized in food products, their mechanical and water barrier attributes are still weaker compared to those of synthetic plastic materials. Several approaches (e.g., bilayer, multilayer, crosslinking, and bio-nanocomposite films, etc.) are employed to ameliorate properties of hydrocolloid-based coatings and films. Among them, incorporation of polysaccharide nanofillers into hydrocolloids to produce bio-nanocomposites has gained increasing attention in recent years, due to their edibility, remarkable physical performance, and functional properties (Otoni et al. 2017). Thus, this type of bio-nanocomposites is expected to be a promising area of research in the future.
On the other hand, the nanodelivery systems, such as nanoencapsulation, nanoliposomes, nanoemulsion, and nanolaminate, have emerged to enhance the performance of bioactive agents and improve their effectiveness in preserving food products. Currently, they are developed as the effective tools to augment the functionality of hydrocolloid-based coatings and films (Aloui and Khwaldia 2016). Future research should focus on the development of hydrocolloid coatings and films based on nanodelivery systems as well as their interactions with food products.
As a bottom-up approach, the structure-properties of hydrocolloid coatings and films should be studied further. Practically important properties such as WVP, TS, and EAB must be correlated with molecular structure and mobility in the solid state to further develop the utilization of polysaccharides. For instance, dextran, consisting of α-1,6 glycosidic linkages, shows a poor film-forming capacity in comparison with pullulan or amylose. In addition, dextran shows the largest molecular mobility in the solid state, followed by pullulan and amylose. The physicochemical properties and molecular mobility of dextran, pullulan, and amylose in the solid state are quite different from each other because of the different modes of glucosidic linkages (Nishinari et al. 1985, 1992). Overall, hydrocolloids as packaging materials still need scientific research to improve their properties, quality and marketability. Further studies include (1) embracing big data and artificial intelligence (AI) in research and development, e.g., for process simulation, classification, pattern recognition, and transfer learning; (2) developing new techniques, equipment, machines for large-scale industrial implementation and applications.
References
Agrawal AM, Pandey P (2015) Scale up of pan coating process using quality by design principles. J Pharm Sci 104(11):3589–3611. https://doi.org/10.1002/jps.24582
Ahmad M, Benjakul S, Sumpavapol P, Nirmal NP (2012) Quality changes of sea bass slices wrapped with gelatin film incorporated with lemongrass essential oil. Int J Food Microbiol 155(3):171–178. https://doi.org/10.1016/j.ijfoodmicro.2012.01.027
Aloui H, Khwaldia K (2016) Natural antimicrobial edible coatings for microbial safety and food quality enhancement. Compr Rev Food Sci Food Saf 15(6):1080–1103. https://doi.org/10.1111/1541-4337.12226
Alves MM, Gonçalves MP, Rocha CMR (2017) Effect of ferulic acid on the performance of soy protein isolate-based edible coatings applied to fresh-cut apples. LWT Food Sci Technol 80:409–415. https://doi.org/10.1016/j.lwt.2017.03.013
Andrade RD, Skurtys O, Osorio FA (2012) Atomizing spray systems for application of edible coatings. Compr Rev Food Sci Food Saf 11(3):323–337. https://doi.org/10.1111/j.1541-4337.2012.00186.x
Andreuccetti C, Carvalho RA, Grosso CRF (2009) Effect of hydrophobic plasticizers on functional properties of gelatin-based films. Food Res Int 42(8):1113–1121. https://doi.org/10.1016/j.foodres.2009.05.010
Arnon H, Zaitsev Y, Porat R, Poverenov E (2014) Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol Technol 87:21–26. https://doi.org/10.1016/j.postharvbio.2013.08.007
Arzate-Vázquez I, Chanona-Pérez JJ, Calderón-Domínguez G, Terres-Rojas E, Garibay-Febles V, Martínez-Rivas A, Gutiérrez-López GF (2012) Microstructural characterization of chitosan and alginate films by microscopy techniques and texture image analysis. Carbohydr Polym 87(1):289–299. https://doi.org/10.1016/j.carbpol.2011.07.044
ASTM-D3985-02 (2002) Standard test method for oxygen gas transmission rate through plastic film and sheeting using a coulometric sensor. In: Annual book of American standard testing methods. American Society for Testing & Materials, Philadelphia, PA, pp 472–477
ASTM-D882-91 (1991) Standard test method for tensile properties of thin plastic sheeting. In: Annual book of American standard testing methods. American Society for Testing and Materials, Philadelphia, PA, pp 161–170
ASTM-E96-92 (1990) Standard test methods for water vapor transmission of materials. In: Annual book of American standard testing methods. American Society for Testing & Materials, Philadelphia, PA, pp 745–754
Avena-Bustillos RJ, Chiou B, Olsen CW, Bechtel PJ, Olson DA, McHugh TH (2011) Gelation, oxygen permeability, and mechanical properties of mammalian and fish gelatin films. J Food Sci 76(7):E519–E524. https://doi.org/10.1111/j.1750-3841.2011.02312.x
Azam M, Haq MA, Hasnain A (2013) Osmotic dehydration of mango cubes: effect of novel gluten-based coating. Dry Technol 31(1):120–127. https://doi.org/10.1080/07373937.2012.727055
Azevedo VM, Borges SV, Marconcini JM, Yoshida MI, Neto ARS, Pereira TC, Pereira CFG (2017) Effect of replacement of corn starch by whey protein isolate in biodegradable film blends obtained by extrusion. Carbohydr Polym 157:971–980. https://doi.org/10.1016/j.carbpol.2016.10.046
Bigi A, Panzavolta S, Rubini K (2004) Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials 25(25):5675–5680. https://doi.org/10.1016/j.biomaterials.2004.01.033
Bill M, Sivakumar D, Korsten L, Thompson AK (2014) The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot 64:159–167. https://doi.org/10.1016/j.cropro.2014.06.015
Bodnár I, Alting AC, Verschueren M (2007) Structural effects on the permeability of whey protein films in an aqueous environment. Food Hydrocoll 21(5):889–895. https://doi.org/10.1016/j.foodhyd.2006.11.017
Boran G, Regenstein JM (2010) Fish gelatin. In: Taylor SL (ed) Advances in food and nutrition research, vol vol 60. Academic Press, Cambridge, pp 119–143. https://doi.org/10.1016/S1043-4526(10)60005-8
Borges AF, Silva C, Coelho JFJ, Simões S (2015) Oral films: current status and future perspectives: I — Galenical development and quality attributes. J Control Release 206:1–19. https://doi.org/10.1016/j.jconrel.2015.03.006
Brown ME, Gallagher PK (2011) Handbook of thermal analysis and calorimetry: recent advances, techniques and applications, vol vol 5. Elsevier, New York
Campos CA, Gerschenson LN, Flores SK (2011) Development of edible films and coatings with antimicrobial activity. Food Bioprocess Technol 4(6):849–875. https://doi.org/10.1007/s11947-010-0434-1
Cao-Hoang L, Chaine A, Grégoire L, Waché Y (2010) Potential of nisin-incorporated sodium caseinate films to control Listeria in artificially contaminated cheese. Food Microbiol 27(7):940–944. https://doi.org/10.1016/j.fm.2010.05.025
Cazón P, Velazquez G, Ramírez JA, Vázquez M (2017) Polysaccharide-based films and coatings for food packaging: a review. Food Hydrocoll 68:136–148. https://doi.org/10.1016/j.foodhyd.2016.09.009
Cerqueira MA, Lima ÁM, Teixeira JA, Moreira RA, Vicente AA (2009) Suitability of novel galactomannans as edible coatings for tropical fruits. J Food Eng 94(3):372–378. https://doi.org/10.1016/j.jfoodeng.2009.04.003
Chen Y, Yang J, Dave RN, Pfeffer R (2009) Granulation of cohesive Geldart group C powders in a Mini-Glatt fluidized bed by pre-coating with nanoparticles. Powder Technol 191(1):206–217. https://doi.org/10.1016/j.powtec.2008.10.010
Cheng SZ (2002) Handbook of thermal analysis and calorimetry: applications to polymers and plastics, vol vol 3. Elsevier, New York
Choi WS, Singh S, Lee YS (2016) Characterization of edible film containing essential oils in hydroxypropyl methylcellulose and its effect on quality attributes of ‘Formosa’ plum (Prunus salicina L.). LWT Food Sci Technol 70:213–222. https://doi.org/10.1016/j.lwt.2016.02.036
Chung Y-C, Chen C-Y (2008) Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour Technol 99(8):2806–2814. https://doi.org/10.1016/j.biortech.2007.06.044
Ciannamea EM, Stefani PM, Ruseckaite RA (2014) Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll 38:193–204. https://doi.org/10.1016/j.foodhyd.2013.12.013
Cosenza VA, Navarro DA, Fissore EN, Rojas AM, Stortz CA (2014) Chemical and rheological characterization of the carrageenans from Hypnea musciformis (Wulfen) Lamoroux. Carbohydr Polym 102:780–789. https://doi.org/10.1016/j.carbpol.2013.10.090
Crosby AJ, Lee JY (2007) Polymer nanocomposites: the “nano” effect on mechanical properties. Polym Rev (Philadelphia, PA) 47(2):217–229. https://doi.org/10.1080/15583720701271278
Cui H, Yuan L, Li W, Lin L (2017) Edible film incorporated with chitosan and Artemisia annua oil nanoliposomes for inactivation of Escherichia coli O157:H7 on cherry tomato. Int J Food Sci Technol 52(3):687–698. https://doi.org/10.1111/ijfs.13322
de Aquino AB, Blank AF, de Aquino Santana LCL (2015) Impact of edible chitosan–cassava starch coatings enriched with Lippia gracilis Schauer genotype mixtures on the shelf life of guavas (Psidium guajava L.) during storage at room temperature. Food Chem 171:108–116. https://doi.org/10.1016/j.foodchem.2014.08.077
De Azeredo HMC, Rosa MF, De Sá M, Souza Filho M, Waldron KW (2014) The use of biomass for packaging films and coatings. In: Waldron K (ed) Advances in biorefineries. Woodhead Publishing, Sawston, pp 819–874. https://doi.org/10.1533/9780857097385.2.819
Debeaufort F, Voilley A (2009) Lipid-based edible films and coatings. In: Huber KC, Embuscado ME (eds) Edible films and coatings for food applications. Springer, New York, pp 135–168. https://doi.org/10.1007/978-0-387-92824-1_5
Dehnad D, Mirzaei H, Emam-Djomeh Z, Jafari S-M, Dadashi S (2014) Thermal and antimicrobial properties of chitosan–nanocellulose films for extending shelf life of ground meat. Carbohydr Polym 109:148–154. https://doi.org/10.1016/j.carbpol.2014.03.063
Dewettinck K, Huyghebaert A (1999) Fluidized bed coating in food technology. Trends Food Sci Technol 10(4):163–168. https://doi.org/10.1016/S0924-2244(99)00041-2
Dhall RK (2013) Advances in edible coatings for fresh fruits and vegetables: a review. Crit Rev Food Sci Nutr 53(5):435–450. https://doi.org/10.1080/10408398.2010.541568
Dragich AM, Krochta JM (2010) Whey protein solution coating for fat-uptake reduction in deep-fried chicken breast strips. J Food Sci 75(1):S43–S47. https://doi.org/10.1111/j.1750-3841.2009.01408.x
Du H, Hu Q, Yang W, Pei F, Kimatu BM, Ma N, Fang Y, Cao C, Zhao L (2016) Development, physiochemical characterization and forming mechanism of Flammulina velutipes polysaccharide-based edible films. Carbohydr Polym 152:214–221. https://doi.org/10.1016/j.carbpol.2016.07.035
Durrani CM, Donald AM (1995) Physical characterisation of amylopectin gels. Polym Gels Netw 3(1):1–27. https://doi.org/10.1016/0966-7822(94)00005-R
Edible packaging-global market outlook from 2017 to 2023 (2017). https://www.strategymrc.com/report/edible-packaging-market. Accessed July 2017
Emam-Djomeh Z, Moghaddam A, Yasini Ardakani SA (2015) Antimicrobial activity of pomegranate (Punica granatum L.) peel extract, physical, mechanical, barrier and antimicrobial properties of pomegranate peel extract-incorporated sodium caseinate film and application in packaging for ground beef. Packag Technol Sci 28(10):869–881. https://doi.org/10.1002/pts.2145
Espinoza-Herrera N, Pedroza-Islas R, San Martín-Martinez E, Cruz-Orea A, Tomás SA (2011) Thermal, mechanical and microstructures properties of cellulose derivatives films: a comparative study. Food Biophys 6(1):106–114. https://doi.org/10.1007/s11483-010-9181-0
Espitia PJP, Du W-X, Avena-Bustillos RJ, Soares NFF, McHugh TH (2014) Edible films from pectin: physical-mechanical and antimicrobial properties - a review. Food Hydrocoll 35:287–296. https://doi.org/10.1016/j.foodhyd.2013.06.005
Fakhouri FM, Martelli SM, Caon T, Velasco JI, Mei LHI (2015) Edible films and coatings based on starch/gelatin: film properties and effect of coatings on quality of refrigerated red crimson grapes. Postharvest Biol Technol 109:57–64. https://doi.org/10.1016/j.postharvbio.2015.05.015
Fernández-Pan I, Carrión-Granda X, Maté JI (2014) Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 36(1):69–75. https://doi.org/10.1016/j.foodcont.2013.07.032
Ferreira Saraiva LE, Naponucena LOM, da Silva Santos V, Silva RPD, de Souza CO, Evelyn Gomes Lima Souza I, de Oliveira Mamede ME, Druzian JI (2016) Development and application of edible film of active potato starch to extend mini panettone shelf life. LWT Food Sci Technol 73:311–319. https://doi.org/10.1016/j.lwt.2016.05.047
Fishman ML, Coffin DR, Konstance RP, Onwulata CI (2000) Extrusion of pectin/starch blends plasticized with glycerol. Carbohydr Polym 41(4):317–325. https://doi.org/10.1016/S0144-8617(99)00117-4
Franssen L, Krochta J (2003) Edible coatings containing natural antimicrobials for processed foods. In: Natural antimicrobials for the minimal processing of foods. Elsevier, New York, pp 250–262
Fu S, Thacker A, Sperger DM, Boni RL, Buckner IS, Velankar S, Munson EJ, Block LH (2011) Relevance of rheological properties of sodium alginate in solution to calcium alginate gel properties. AAPS PharmSciTech 12(2):453–460. https://doi.org/10.1208/s12249-011-9587-0
Fuciños C, Amado IR, Fuciños P, Fajardo P, Rúa ML, Pastrana LM (2017) Evaluation of antimicrobial effectiveness of pimaricin-loaded thermosensitive nanohydrogel coating on Arzúa-Ulloa DOP cheeses. Food Control 73:1095–1104. https://doi.org/10.1016/j.foodcont.2016.10.028
Gao C, Pollet E, Avérous L (2017) Properties of glycerol-plasticized alginate films obtained by thermo-mechanical mixing. Food Hydrocoll 63:414–420. https://doi.org/10.1016/j.foodhyd.2016.09.023
Garcia LC, Pereira LM, de Luca Sarantópoulos CIG, Hubinger MD (2010) Selection of an edible starch coating for minimally processed strawberry. Food Bioprocess Technol 3(6):834–842. https://doi.org/10.1007/s11947-009-0313-9
Gennadios A, Brandenburg AH, Weller CL, Testin RF (1993) Effect of pH on properties of wheat gluten and soy protein isolate films. J Agric Food Chem 41(11):1835–1839. https://doi.org/10.1021/jf00035a006
Gennadios A, Weller CL, Hanna MA, Froming GW (1996) Mechanical and barrier properties of egg albumen films. J Food Sci 61(3):585–589. https://doi.org/10.1111/j.1365-2621.1996.tb13164.x
Gennadios A, Hanna MA, Kurth LB (1997) Application of edible coatings on meats, poultry and seafoods: a review. LWT Food Sci Technol 30(4):337–350. https://doi.org/10.1006/fstl.1996.0202
Ghanbarzadeh B, Musavi M, Oromiehie AR, Rezayi K, Razmi Rad E, Milani J (2007) Effect of plasticizing sugars on water vapor permeability, surface energy and microstructure properties of zein films. LWT Food Sci Technol 40(7):1191–1197. https://doi.org/10.1016/j.lwt.2006.07.008
Ghoshal S, Stapf S, Mattea C (2014) Protein renaturation in the gelatin film formation process. Appl Magn Reson 45(2):145–154. https://doi.org/10.1007/s00723-014-0514-x
Giancone T, Torrieri E, Pierro PD, Mariniello L, Moresi M, Porta R, Masi P (2008) Role of constituents on the network formation of hydrocolloid edible films. J Food Eng 89(2):195–203. https://doi.org/10.1016/j.jfoodeng.2008.04.017
Gómez-Estaca J, Gavara R, Catalá R, Hernández-Muñoz P (2016) The potential of proteins for producing food packaging materials: a review. Packag Technol Sci 29(4–5):203–224. https://doi.org/10.1002/pts.2198
Guckian S, Dwyer C, O'Sullivan M, O'Riordan ED, Monahan FJ (2006) Properties of and mechanisms of protein interactions in films formed from different proportions of heated and unheated whey protein solutions. Eur Food Res Technol 223(1):91–95. https://doi.org/10.1007/s00217-005-0140-9
Guo M, Jin TZ, Wang L, Scullen OJ, Sommers CH (2014) Antimicrobial films and coatings for inactivation of listeria innocua on ready-to-eat deli Turkey meat. Food Control 40:64–70. https://doi.org/10.1016/j.foodcont.2013.11.018
Han JH (2014) Edible films and coatings: a review. In: Han JH (ed) Innovations in food packaging, 2nd edn. Academic Press, San Diego, pp 213–255. https://doi.org/10.1016/B978-0-12-394601-0.00009-6
He S, Franco C, Zhang W (2015) Fish protein hydrolysates: application in deep-fried food and food safety analysis. J Food Sci 80(1):E108–E115. https://doi.org/10.1111/1750-3841.12684
Hernandez-Izquierdo VM, Krochta JM (2008) Thermoplastic processing of proteins for film formation-a review. J Food Sci 73(2):R30–R39. https://doi.org/10.1111/j.1750-3841.2007.00636.x
Hernández-Muñoz P, Kanavouras A, Ng PKW, Gavara R (2003) Development and characterization of biodegradable films made from wheat gluten protein fractions. J Agric Food Chem 51(26):7647–7654. https://doi.org/10.1021/jf034646x
Higueras L, López-Carballo G, Hernández-Muñoz P, Catalá R, Gavara R (2014) Antimicrobial packaging of chicken fillets based on the release of carvacrol from chitosan/cyclodextrin films. Int J Food Microbiol 188:53–59. https://doi.org/10.1016/j.ijfoodmicro.2014.07.018
Hosseinnejad M, Jafari SM (2016) Evaluation of different factors affecting antimicrobial properties of chitosan. Int J Biol Macromol 85:467–475. https://doi.org/10.1016/j.ijbiomac.2016.01.022
Hu G, Chen J, Gao J (2009) Preparation and characteristics of oxidized potato starch films. Carbohydr Polym 76(2):291–298. https://doi.org/10.1016/j.carbpol.2008.10.032
Hua X, Wang K, Yang R, Kang J, Yang H (2015) Edible coatings from sunflower head pectin to reduce lipid uptake in fried potato chips. LWT Food Sci Technol 62(2):1220–1225. https://doi.org/10.1016/j.lwt.2015.02.010
Janjarasskul T, Krochta JM (2010) Edible packaging materials. Annu Rev Food Sci Technol 1(1):415–448. https://doi.org/10.1146/annurev.food.080708.100836
Jolie RP, Duvetter T, Van Loey AM, Hendrickx ME (2010) Pectin methylesterase and its proteinaceous inhibitor: a review. Carbohydr Res 345(18):2583–2595. https://doi.org/10.1016/j.carres.2010.10.002
Jouki M, Mortazavi SA, Yazdi FT, Koocheki A, Khazaei N (2014) Use of quince seed mucilage edible films containing natural preservatives to enhance physico-chemical quality of rainbow trout fillets during cold storage. Food Sci Hum Wellness 3(2):65–72. https://doi.org/10.1016/j.fshw.2014.05.002
Karbowiak T, Gougeon RD, Rigolet S, Delmotte L, Debeaufort F, Voilley A (2008) Diffusion of small molecules in edible films: effect of water and interactions between diffusant and biopolymer. Food Chem 106(4):1340–1349. https://doi.org/10.1016/j.foodchem.2007.03.076
Kavas G, Kavas N, Saygili D (2015) The effects of thyme and clove essential oil fortified edible films on the physical, chemical and microbiological characteristics of Kashar cheese. J Food Qual 38(6):405–412. https://doi.org/10.1111/jfq.12157
Kavas N, Kavas G, Saygili D (2016) Use of ginger essential oil-fortified edible coatings in Kashar cheese and its effects on Escherichia coli O157:H7 and Staphylococcus aureus. CYTA J Food 14(2):317–323. https://doi.org/10.1080/19476337.2015.1109001
Kester JJ, Fennema O (1989) An edible film of lipids and cellulose ethers: barrier properties to moisture vapor transmission and structural evaluation. J Food Sci 54(6):1383–1389. https://doi.org/10.1111/j.1365-2621.1989.tb05118.x
Khwaldia K, Banon S, Perez C, Desobry S (2004a) Properties of sodium caseinate film-forming dispersions and films. J Dairy Sci 87(7):2011–2016. https://doi.org/10.3168/jds.S0022-0302(04)70018-1
Khwaldia K, Perez C, Banon S, Desobry S, Hardy J (2004b) Milk proteins for edible films and coatings. Crit Rev Food Sci Nutr 44(4):239–251. https://doi.org/10.1080/10408690490464906
Kim KM, Son JH, Kim S-K, Weller CL, Hanna MA (2006) Properties of chitosan films as a function of pH and solvent type. J Food Sci 71(3):E119–E124. https://doi.org/10.1111/j.1365-2621.2006.tb15624.x
Kim H, Beak S-E, Yang S-Y, Song KB (2018a) Application of an antimicrobial packaging material from chicken bone gelatine and cinnamon bark oil to mozzarella cheese. Int J Food Sci Technol 53(3):619–625. https://doi.org/10.1111/ijfs.13636
Kim J-H, Hong W-S, Oh S-W (2018b) Effect of layer-by-layer antimicrobial edible coating of alginate and chitosan with grapefruit seed extract for shelf-life extension of shrimp (Litopenaeus vannamei) stored at 4 °C. Int J Biol Macromol 120:1468–1473. https://doi.org/10.1016/j.ijbiomac.2018.09.160
Kumar R, Choudhary V, Mishra S, Varma IK, Mattiason B (2002) Adhesives and plastics based on soy protein products. Ind Crop Prod 16(3):155–172. https://doi.org/10.1016/S0926-6690(02)00007-9
Kunte LA, Gennadios A, Cuppett SL, Hanna MA, Weller CL (1997) Cast films from soy protein isolates and fractions. Cereal Chem 74(2):115–118. https://doi.org/10.1094/cchem.1997.74.2.115
Lacroix M, Le Tien C (2005) Edible films and coatings from nonstarch polysaccharides. In: Han JH (ed) Innovations in food packaging. Academic Press, London, pp 338–361. https://doi.org/10.1016/B978-012311632-1/50052-8
Lagrain B, Goderis B, Brijs K, Delcour JA (2010) Molecular basis of processing wheat gluten toward biobased materials. Biomacromolecules 11(3):533–541. https://doi.org/10.1021/bm100008p
Leceta I, Guerrero P, de la Caba K (2013) Functional properties of chitosan-based films. Carbohydr Polym 93(1):339–346. https://doi.org/10.1016/j.carbpol.2012.04.031
LeCorre D, Bras J, Dufresne A (2011) Influence of botanic origin and amylose content on the morphology of starch nanocrystals. J Nanopart Res 13(12):7193–7208. https://doi.org/10.1007/s11051-011-0634-2
Lee K-Y, Lee J-H, Yang H-J, Song KB (2016a) Characterization of a starfish gelatin film containing vanillin and its application in the packaging of crab stick. Food Sci Biotechnol 25(4):1023–1028. https://doi.org/10.1007/s10068-016-0165-9
Lee K-Y, Lee J-H, Yang H-J, Song KB (2016b) Production and characterisation of skate skin gelatin films incorporated with thyme essential oil and their application in chicken tenderloin packaging. Int J Food Sci Technol 51(6):1465–1472. https://doi.org/10.1111/ijfs.13119
Lee K-Y, Yang H-J, Song KB (2016c) Application of a puffer fish skin gelatin film containing Moringa oleifera Lam. leaf extract to the packaging of Gouda cheese. J Food Sci Technol 53(11):3876–3883. https://doi.org/10.1007/s13197-016-2367-9
Li T, Li J, Hu W, Li X (2013) Quality enhancement in refrigerated red drum (Sciaenops ocellatus) fillets using chitosan coatings containing natural preservatives. Food Chem 138(2):821–826. https://doi.org/10.1016/j.foodchem.2012.11.092
Li K, Zhu J, Guan G, Wu H (2019) Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: film properties, characterization, and formation mechanism. Int J Biol Macromol 122:485–492. https://doi.org/10.1016/j.ijbiomac.2018.10.188
Lin MG, Lasekan O, Saari N, Khairunniza-Bejo S (2018) Effect of chitosan and carrageenan-based edible coatings on post-harvested longan (Dimocarpus longan) fruits. CYTA J Food 16(1):490–497. https://doi.org/10.1080/19476337.2017.1414078
Liu Z (2005) Edible films and coatings from starches. In: Han JH (ed) Innovations in food packaging. Academic Press, London, pp 318–337. https://doi.org/10.1016/B978-012311632-1/50051-6
Liu J, Zhan X, Wan J, Wang Y, Wang C (2015) Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr Polym 121:27–36. https://doi.org/10.1016/j.carbpol.2014.11.063
López OV, García MA, Zaritzky NE (2008) Film forming capacity of chemically modified corn starches. Carbohydr Polym 73(4):573–581. https://doi.org/10.1016/j.carbpol.2007.12.023
López OV, Zaritzky NE, García MA (2010) Physicochemical characterization of chemically modified corn starches related to rheological behavior, retrogradation and film forming capacity. J Food Eng 100(1):160–168. https://doi.org/10.1016/j.jfoodeng.2010.03.041
Lorevice MV, Otoni CG, Moura MR, Mattoso LHC (2016) Chitosan nanoparticles on the improvement of thermal, barrier, and mechanical properties of high- and low-methyl pectin films. Food Hydrocoll 52:732–740. https://doi.org/10.1016/j.foodhyd.2015.08.003
Mangavel C, Barbot J, Popineau Y, Guéguen J (2001) Evolution of wheat gliadins conformation during film formation: a fourier transform infrared study. J Agric Food Chem 49(2):867–872. https://doi.org/10.1021/jf0009899
Mannozzi C, Cecchini JP, Tylewicz U, Siroli L, Patrignani F, Lanciotti R, Rocculi P, Dalla Rosa M, Romani S (2017) Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci Technol 85:440–444. https://doi.org/10.1016/j.lwt.2016.12.056
Maqbool M, Ali A, Alderson PG, Mohamed MTM, Siddiqui Y, Zahid N (2011) Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol Technol 62(1):71–76. https://doi.org/10.1016/j.postharvbio.2011.04.002
Marsh K, Bugusu B (2007) Food packaging-roles, materials, and environmental issues. J Food Sci 72(3):R39–R55. https://doi.org/10.1111/j.1750-3841.2007.00301.x
Martins JT, Cerqueira MA, Souza BWS, Carmo Avides MD, Vicente AA (2010) Shelf life extension of Ricotta cheese using coatings of galactomannans from nonconventional sources incorporating nisin against Listeria monocytogenes. J Agric Food Chem 58(3):1884–1891. https://doi.org/10.1021/jf902774z
Mauer LJ, Smith DE, Labuza TP (2000) Water vapor permeability, mechanical, and structural properties of edible β-casein films. Int Dairy J 10(5):353–358. https://doi.org/10.1016/S0958-6946(00)00061-3
Medeiros BGDS, Pinheiro AC, Carneiro-da-Cunha MG, Vicente AA (2012a) Development and characterization of a nanomultilayer coating of pectin and chitosan – evaluation of its gas barrier properties and application on ‘Tommy Atkins’ mangoes. J Food Eng 110(3):457–464. https://doi.org/10.1016/j.jfoodeng.2011.12.021
Medeiros BGS, Pinheiro AC, Teixeira JA, Vicente AA, Carneiro-da-Cunha MG (2012b) Polysaccharide/protein nanomultilayer coatings: construction, characterization and evaluation of their effect on ‘Rocha’ pear (Pyrus communis L.) shelf-life. Food Bioprocess Technol 5(6):2435–2445. https://doi.org/10.1007/s11947-010-0508-0
Medeiros BGDS, Souza MP, Pinheiro AC, Bourbon AI, Cerqueira MA, Vicente AA, Carneiro-da-Cunha MG (2014) Physical characterisation of an alginate/lysozyme nano-laminate coating and its evaluation on ‘Coalho’ cheese shelf life. Food Bioprocess Technol 7(4):1088–1098. https://doi.org/10.1007/s11947-013-1097-5
Mei J, Yuan Y, Wu Y, Li Y (2013) Characterization of edible starch–chitosan film and its application in the storage of Mongolian cheese. Int J Biol Macromol 57:17–21. https://doi.org/10.1016/j.ijbiomac.2013.03.003
Mendes JF, Paschoalin RT, Carmona VB, Sena Neto AR, Marques ACP, Marconcini JM, Mattoso LHC, Medeiros ES, Oliveira JE (2016) Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr Polym 137:452–458. https://doi.org/10.1016/j.carbpol.2015.10.093
Mohebbi M, Ansarifar E, Hasanpour N, Amiryousefi MR (2012) Suitability of Aloe vera and gum tragacanth as edible coatings for extending the shelf life of button mushroom. Food Bioprocess Technol 5(8):3193–3202. https://doi.org/10.1007/s11947-011-0709-1
Moreira MR, Pereda M, Marcovich NE, Roura SI (2011a) Antimicrobial effectiveness of bioactive packaging materials from edible chitosan and casein polymers: assessment on carrot, cheese, and salami. J Food Sci 76(1):M54–M63. https://doi.org/10.1111/j.1750-3841.2010.01910.x
Moreira MR, Roura SI, Ponce A (2011b) Effectiveness of chitosan edible coatings to improve microbiological and sensory quality of fresh cut broccoli. LWT Food Sci Technol 44(10):2335–2341. https://doi.org/10.1016/j.lwt.2011.04.009
Mulvihill DM, Ennis MP (2003) Functional milk proteins: production and utilization. In: Fox PF, McSweeney PLH (eds) Advanced dairy chemistry—1 proteins: part A/part B. Springer, Boston, pp 1175–1228. https://doi.org/10.1007/978-1-4419-8602-3_32
Navarro-Tarazaga ML, Massa A, Pérez-Gago MB (2011) Effect of beeswax content on hydroxypropyl methylcellulose-based edible film properties and postharvest quality of coated plums (Cv. Angeleno). LWT Food Sci Technol 44(10):2328–2334. https://doi.org/10.1016/j.lwt.2011.03.011
Nishinari K, Shibuya N, Kainuma K (1985) Dielectric relaxation in solid dextran and pullulan. Die Makromolekulare Chemie 186(2):433–438. https://doi.org/10.1002/macp.1985.021860221
Nishinari K, Kohyama K, Shibuya N, Kim KY, Kim NH, Watase M, Tsutsumi A (1992) Molecular motions in cellulose derivatives. In: Viscoelasticity of biomaterials, ACS symposium series, vol vol 489. American Chemical Society, Washington, pp 357–369. https://doi.org/10.1021/bk-1992-0489.ch024
Noori S, Zeynali F, Almasi H (2018) Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 84:312–320. https://doi.org/10.1016/j.foodcont.2017.08.015
Nur Hanani ZA, Beatty E, Roos YH, Morris MA, Kerry JP (2012) Manufacture and characterization of gelatin films derived from beef, pork and fish sources using twin screw extrusion. J Food Eng 113(4):606–614. https://doi.org/10.1016/j.jfoodeng.2012.07.002
Ollé Resa CP, Gerschenson LN, Jagus RJ (2016) Starch edible film supporting natamycin and nisin for improving microbiological stability of refrigerated argentinian Port Salut cheese. Food Control 59:737–742. https://doi.org/10.1016/j.foodcont.2015.06.056
Otoni CG, Avena-Bustillos RJ, Azeredo HMC, Lorevice MV, Moura MR, Mattoso LHC, McHugh TH (2017) Recent advances on edible films based on fruits and vegetables-a review. Compr Rev Food Sci Food Saf 16(5):1151–1169. https://doi.org/10.1111/1541-4337.12281
Pabast M, Shariatifar N, Beikzadeh S, Jahed G (2018) Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 91:185–192. https://doi.org/10.1016/j.foodcont.2018.03.047
Pankaj SK, Bueno-Ferrer C, Misra NN, O'Neill L, Tiwari BK, Bourke P, Cullen PJ (2014) Physicochemical characterization of plasma-treated sodium caseinate film. Food Res Int 66:438–444. https://doi.org/10.1016/j.foodres.2014.10.016
Paula GA, Benevides NMB, Cunha AP, de Oliveira AV, Pinto AMB, Morais JPS, Azeredo HMC (2015) Development and characterization of edible films from mixtures of κ-carrageenan, ι-carrageenan, and alginate. Food Hydrocoll 47:140–145. https://doi.org/10.1016/j.foodhyd.2015.01.004
Peretto G, Du W-X, Avena-Bustillos RJ, Berrios JDJ, Sambo P, McHugh TH (2014) Optimization of antimicrobial and physical properties of alginate coatings containing carvacrol and methyl cinnamate for strawberry application. J Agric Food Chem 62(4):984–990. https://doi.org/10.1021/jf4042886
Pérez-Gago MB, Krochta JM (2002) Formation and properties of whey protein films and coatings. In: Protein-based films and coatings. CRC Press, Boca Raton, pp 159–180
Pérez-Gago MB, Nadaud P, Krochta JM (1999) Water vapor permeability, solubility, and tensile properties of heat-denatured versus native whey protein films. J Food Sci 64(6):1034–1037. https://doi.org/10.1111/j.1365-2621.1999.tb12276.x
Pham GT, Park Y-B, Liang Z, Zhang C, Wang B (2008) Processing and modeling of conductive thermoplastic/carbon nanotube films for strain sensing. Compos Part B Eng 39(1):209–216. https://doi.org/10.1016/j.compositesb.2007.02.024
Pinto AMB, Santos TM, Caceres CA, Lima JR, Ito EN, Azeredo HMC (2015) Starch-cashew tree gum nanocomposite films and their application for coating cashew nuts. LWT Food Sci Technol 62(1, Part 2):549–554. https://doi.org/10.1016/j.lwt.2014.07.028
Pitak N, Rakshit SK (2011) Physical and antimicrobial properties of banana flour/chitosan biodegradable and self sealing films used for preserving fresh-cut vegetables. LWT Food Sci Technol 44(10):2310–2315. https://doi.org/10.1016/j.lwt.2011.05.024
Porta R, Di Pierro P, Rossi-Marquez G, Mariniello L, Kadivar M, Arabestani A (2015) Microstructure and properties of bitter vetch (Vicia ervilia) protein films reinforced by microbial transglutaminase. Food Hydrocoll 50:102–107. https://doi.org/10.1016/j.foodhyd.2015.04.008
Poverenov E, Rutenberg R, Danino S, Horev B, Rodov V (2014) Gelatin-chitosan composite films and edible coatings to enhance the quality of food products: layer-by-layer vs. blended formulations. Food Bioprocess Technol 7(11):3319–3327. https://doi.org/10.1007/s11947-014-1333-7
Prommakool A, Sajjaanantakul T, Janjarasskul T, Krochta JM (2011) Whey protein–okra polysaccharide fraction blend edible films: tensile properties, water vapor permeability and oxygen permeability. J Sci Food Agric 91(2):362–369. https://doi.org/10.1002/jsfa.4194
Pushpadass HA, Kumar A, Jackson DS, Wehling RL, Dumais JJ, Hanna MA (2009) Macromolecular changes in extruded starch-films plasticized with glycerol, water and stearic acid. Starch - Stärke 61(5):256–266. https://doi.org/10.1002/star.200800046
Qiu X, Chen S, Liu G, Yang Q (2014) Quality enhancement in the Japanese sea bass (Lateolabrax japonicas) fillets stored at 4°C by chitosan coating incorporated with citric acid or licorice extract. Food Chem 162:156–160. https://doi.org/10.1016/j.foodchem.2014.04.037
Ramos ÓL, Pereira JO, Silva SI, Fernandes JC, Franco MI, Lopes-da-Silva JA, Pintado ME, Malcata FX (2012) Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J Dairy Sci 95(11):6282–6292. https://doi.org/10.3168/jds.2012-5478
Ramos ÓL, Reinas I, Silva SI, Fernandes JC, Cerqueira MA, Pereira RN, Vicente AA, Poças MF, Pintado ME, Malcata FX (2013) Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll 30(1):110–122. https://doi.org/10.1016/j.foodhyd.2012.05.001
Ramos-García M, Bosquez-Molina E, Hernández-Romano J, Zavala-Padilla G, Terrés-Rojas E, Alia-Tejacal I, Barrera-Necha L, Hernández-López M, Bautista-Baños S (2012) Use of chitosan-based edible coatings in combination with other natural compounds, to control Rhizopus stolonifer and Escherichia coli DH5α in fresh tomatoes. Crop Prot 38:1–6. https://doi.org/10.1016/j.cropro.2012.02.016
Rhim J-W, Ng PKW (2007) Natural biopolymer-based nanocomposite films for packaging applications. Crit Rev Food Sci Nutr 47(4):411–433. https://doi.org/10.1080/10408390600846366
Robert P, Mangavel C, Renard D (2001) Infrared spectroscopy as applied to glycinin film and gel formation kinetics. Appl Spectrosc 55(6):781–787
Rodriguez A, García MA, Campañone LA (2016) Experimental study of the application of edible coatings in pumpkin sticks submitted to osmotic dehydration. Dry Technol 34(6):635–644. https://doi.org/10.1080/07373937.2015.1069325
Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O (2009) Edible coatings to incorporate active ingredients to fresh-cut fruits: a review. Trends Food Sci Technol 20(10):438–447. https://doi.org/10.1016/j.tifs.2009.05.002
Rossi Marquez G, Di Pierro P, Mariniello L, Esposito M, Giosafatto CVL, Porta R (2017) Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT Food Sci Technol 75:124–130. https://doi.org/10.1016/j.lwt.2016.08.017
Rossman JM (2009) Commercial manufacture of edible films. In: Huber KC, Embuscado ME (eds) Edible films and coatings for food applications. Springer, New York, pp 367–390. https://doi.org/10.1007/978-0-387-92824-1_14
Ruiz-Navajas Y, Viuda-Martos M, Barber X, Sendra E, Perez-Alvarez JA, Fernández-López J (2015) Effect of chitosan edible films added with Thymus moroderi and Thymus piperella essential oil on shelf-life of cooked cured ham. J Food Sci Technol 52(10):6493–6501. https://doi.org/10.1007/s13197-015-1733-3
Saberi B, Golding JB, Marques JR, Pristijono P, Chockchaisawasdee S, Scarlett CJ, Stathopoulos CE (2018) Application of biocomposite edible coatings based on pea starch and guar gum on quality, storability and shelf life of ‘Valencia’ oranges. Postharvest Biol Technol 137:9–20. https://doi.org/10.1016/j.postharvbio.2017.11.003
Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O (2015) Use of antimicrobial nanoemulsions as edible coatings: impact on safety and quality attributes of fresh-cut Fuji apples. Postharvest Biol Technol 105:8–16. https://doi.org/10.1016/j.postharvbio.2015.03.009
Sánchez-González L, Vargas M, González-Martínez C, Chiralt A, Cháfer M (2009) Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocoll 23(8):2102–2109. https://doi.org/10.1016/j.foodhyd.2009.05.006
Santos AR, da Silva AF, Amaral VCS, Ribeiro AB, de Abreu Filho BA, Mikcha JMG (2016) Application of edible coating with starch and carvacrol in minimally processed pumpkin. J Food Sci Technol 53(4):1975–1983. https://doi.org/10.1007/s13197-016-2171-6
Sarengaowa HW, Jiang A, Xiu Z, Feng K (2018) Effect of thyme oil–alginate-based coating on quality and microbial safety of fresh-cut apples. J Sci Food Agric 98(6):2302–2311. https://doi.org/10.1002/jsfa.8720
Shahbazi Y (2018) Characterization of nanocomposite films based on chitosan and carboxymethylcellulose containing Ziziphora clinopodioides essential oil and methanolic Ficus carica extract. J Food Process Preserv 42(2):e13444. https://doi.org/10.1111/jfpp.13444
Shen XL, Wu JM, Chen Y, Zhao G (2010) Antimicrobial and physical properties of sweet potato starch films incorporated with potassium sorbate or chitosan. Food Hydrocoll 24(4):285–290. https://doi.org/10.1016/j.foodhyd.2009.10.003
Shin YJ, Song HY, Seo YB, Song KB (2012) Preparation of red algae film containing grapefruit seed extract and application for the packaging of cheese and bacon. Food Sci Biotechnol 21(1):225–231. https://doi.org/10.1007/s10068-012-0029-x
Shukla R, Cheryan M (2001) Zein: the industrial protein from corn. Ind Crop Prod 13(3):171–192. https://doi.org/10.1016/S0926-6690(00)00064-9
Siew DCW, Heilmann C, Easteal AJ, Cooney RP (1999) Solution and film properties of sodium caseinate/glycerol and sodium caseinate/polyethylene glycol edible coating systems. J Agric Food Chem 47(8):3432–3440. https://doi.org/10.1021/jf9806311
Silva KS, Mauro MA, Gonçalves MP, Rocha CMR (2016) Synergistic interactions of locust bean gum with whey proteins: effect on physicochemical and microstructural properties of whey protein-based films. Food Hydrocoll 54:179–188. https://doi.org/10.1016/j.foodhyd.2015.09.028
Siracusa V, Rocculi P, Romani S, Rosa MD (2008) Biodegradable polymers for food packaging: a review. Trends Food Sci Technol 19(12):634–643. https://doi.org/10.1016/j.tifs.2008.07.003
Soares NMF, Oliveira MSG, Vicente AA (2015) Effects of glazing and chitosan-based coating application on frozen salmon preservation during six-month storage in industrial freezing chambers. LWT Food Sci Technol 61(2):524–531. https://doi.org/10.1016/j.lwt.2014.12.009
Song Y, Zheng Q (2014) Ecomaterials based on food proteins and polysaccharides. Polym Rev (Philadelphia, PA) 54(3):514–571. https://doi.org/10.1080/15583724.2014.887097
Song F, Tang D-L, Wang X-L, Wang Y-Z (2011a) Biodegradable soy protein isolate-based materials: a review. Biomacromolecules 12(10):3369–3380. https://doi.org/10.1021/bm200904x
Song Y, Liu L, Shen H, You J, Luo Y (2011b) Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 22(3):608–615. https://doi.org/10.1016/j.foodcont.2010.10.012
Song N-B, Song H-Y, Jo W-S, Song KB (2013) Physical properties of a composite film containing sunflower seed meal protein and its application in packaging smoked duck meat. J Food Eng 116(4):789–795. https://doi.org/10.1016/j.jfoodeng.2013.02.002
Song N-B, Lee J-H, Al Mijan M, Song KB (2014) Development of a chicken feather protein film containing clove oil and its application in smoked salmon packaging. LWT Food Sci Technol 57(2):453–460. https://doi.org/10.1016/j.lwt.2014.02.009
Song N-B, Lee J-H, Song KB (2015) Preparation of perilla seed meal protein composite films containing various essential oils and their application in sausage packaging. J Korean Soc Appl Biol Chem 58(1):83–90. https://doi.org/10.1007/s13765-015-0031-0
Sothornvit R, Krochta JM (2005) Plasticizers in edible films and coatings. In: Han JH (ed) Innovations in food packaging. Academic Press, London, pp 403–433. https://doi.org/10.1016/B978-012311632-1/50055-3
Sothornvit R, Pitak N (2007) Oxygen permeability and mechanical properties of banana films. Food Res Int 40(3):365–370. https://doi.org/10.1016/j.foodres.2006.10.010
Souza MP, Vaz AFM, Cerqueira MA, Texeira JA, Vicente AA, Carneiro-da-Cunha MG (2015) Effect of an edible nanomultilayer coating by electrostatic self-assembly on the shelf life of fresh-cut mangoes. Food Bioprocess Technol 8(3):647–654. https://doi.org/10.1007/s11947-014-1436-1
Subirade M, Kelly I, Guéguen J, Pézolet M (1998) Molecular basis of film formation from a soybean protein: comparison between the conformation of glycinin in aqueous solution and in films. Int J Biol Macromol 23(4):241–249. https://doi.org/10.1016/S0141-8130(98)00052-X
Sutherland IW (1998) Novel and established applications of microbial polysaccharides. Trends Biotechnol 16(1):41–46. https://doi.org/10.1016/S0167-7799(97)01139-6
Szabó B, Süvegh K, Zelkó R (2012) Real time positron annihilation lifetime spectroscopy for the detection of the hydrocolloid gel-film transition of polymers. Polym Test 31(4):546–549. https://doi.org/10.1016/j.polymertesting.2012.02.004
Tomadoni B, Moreira MR, Pereda M, Ponce AG (2018) Gellan-based coatings incorporated with natural antimicrobials in fresh-cut strawberries: microbiological and sensory evaluation through refrigerated storage. LWT Food Sci Technol 97:384–389. https://doi.org/10.1016/j.lwt.2018.07.029
Treviño-Garza MZ, García S, del Socorro Flores-González M, Arévalo-Niño K (2015) Edible active coatings based on pectin, pullulan, and chitosan increase quality and shelf life of strawberries (Fragaria ananassa). J Food Sci 80(8):M1823–M1830. https://doi.org/10.1111/1750-3841.12938
Tulamandi S, Rangarajan V, Rizvi SSH, Singhal RS, Chattopadhyay SK, Saha NC (2016) A biodegradable and edible packaging film based on papaya puree, gelatin, and defatted soy protein. Food Packag Shelf Life 10:60–71. https://doi.org/10.1016/j.fpsl.2016.10.007
Ünalan İU, Arcan I, Korel F, Yemenicioğlu A (2013) Application of active zein-based films with controlled release properties to control Listeria monocytogenes growth and lipid oxidation in fresh Kashar cheese. Innov Food Sci Emerg Technol 20:208–214. https://doi.org/10.1016/j.ifset.2013.08.004
Ustunol Z (2009) Edible films and coatings for meat and poultry. In: Huber KC, Embuscado ME (eds) Edible films and coatings for food applications. Springer, New York, pp 245–268. https://doi.org/10.1007/978-0-387-92824-1_8
Valencia-Chamorro SA, Palou L, del Río MA, Pérez-Gago MB (2011) Antimicrobial edible films and coatings for fresh and minimally processed fruits and vegetables: a review. Crit Rev Food Sci Nutr 51(9):872–900. https://doi.org/10.1080/10408398.2010.485705
Viebke C, Al-Assaf S, Phillips GO (2014) Food hydrocolloids and health claims. Bioact Carbohydr Diet Fibre 4(2):101–114. https://doi.org/10.1016/j.bcdf.2014.06.006
Vieira MGA, da Silva MA, dos Santos LO, Beppu MM (2011) Natural-based plasticizers and biopolymer films: a review. Eur Polym J 47(3):254–263. https://doi.org/10.1016/j.eurpolymj.2010.12.011
Wang S, Lu A, Zhang L (2016) Recent advances in regenerated cellulose materials. Prog Polym Sci 53:169–206. https://doi.org/10.1016/j.progpolymsci.2015.07.003
Watanabe A, Morita S, Kokot S, Matsubara M, Fukai K, Ozaki Y (2006) Drying process of microcrystalline cellulose studied by attenuated total reflection IR spectroscopy with two-dimensional correlation spectroscopy and principal component analysis. J Mol Struct 799(1):102–110. https://doi.org/10.1016/j.molstruc.2006.03.018
Wieser H (2007) Chemistry of gluten proteins. Food Microbiol 24(2):115–119. https://doi.org/10.1016/j.fm.2006.07.004
Xiao Q, Lim L-T, Tong Q (2012) Properties of pullulan-based blend films as affected by alginate content and relative humidity. Carbohydr Polym 87(1):227–234. https://doi.org/10.1016/j.carbpol.2011.07.040
Xiao Q, Gu X, Tan S (2014a) Drying process of sodium alginate films studied by two-dimensional correlation ATR-FTIR spectroscopy. Food Chem 164:179–184. https://doi.org/10.1016/j.foodchem.2014.05.044
Xiao Q, Tong Q, Lim L-T (2014b) Drying process of pullulan edible films forming solutions studied by ATR-FTIR with two-dimensional correlation spectroscopy. Food Chem 150:267–273. https://doi.org/10.1016/j.foodchem.2013.10.122
Xiao Q, Lu K, Tong Q, Liu C (2015) Barrier properties and microstructure of pullulan–alginate-based films. J Food Process Eng 38(2):155–161. https://doi.org/10.1111/jfpe.12151
Yadav M, Rhee KY, Park SJ (2014) Synthesis and characterization of graphene oxide/carboxymethylcellulose/alginate composite blend films. Carbohydr Polym 110:18–25. https://doi.org/10.1016/j.carbpol.2014.03.037
Yang L, Paulson AT (2000) Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Res Int 33(7):571–578. https://doi.org/10.1016/S0963-9969(00)00093-4
Yang H, Wen X, Guo S, Chen M, Jiang A, Lai L-S (2015) Physical, antioxidant and structural characterization of blend films based on hsian-tsao gum (HG) and casein (CAS). Carbohydr Polym 134:222–229. https://doi.org/10.1016/j.carbpol.2015.07.021
Yang H-J, Lee J-H, Won M, Song KB (2016) Antioxidant activities of distiller dried grains with solubles as protein films containing tea extracts and their application in the packaging of pork meat. Food Chem 196:174–179. https://doi.org/10.1016/j.foodchem.2015.09.020
Acknowledgemensts
The author thanks Prof. Loong-Tak Lim of University of Guelph (Guelph, Canada), for his helpful suggestions and review of manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Xiao, Q. (2021). Coating and Film-Forming Properties. In: Fang, Y., Zhang, H., Nishinari, K. (eds) Food Hydrocolloids. Springer, Singapore. https://doi.org/10.1007/978-981-16-0320-4_8
Download citation
DOI: https://doi.org/10.1007/978-981-16-0320-4_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0319-8
Online ISBN: 978-981-16-0320-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)