Abstract
A 35-year-old man sustained a saw injury to the left thumb. There was an articular defect at the distal articular surface. Preoperative X-rays showed an articular defect at the base of the proximal phalanx on anteroposterior and lateral views.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Case Reports
A 35-year-old man sustained a saw injury to the left thumb. There was an articular defect at the distal articular surface. Preoperative X-rays showed an articular defect at the base of the proximal phalanx on anteroposterior and lateral views.
2 Treatment Options
The metacarpophalangeal (MCP) joints are important for finger function because their average active range of motion accounts for 36% of total finger motion [1]. Intra-articular injuries to the MCP joints can lead to posttraumatic arthritis and functional disability as a result of joint pain and loss of motion [2, 3]. Although patients may benefit from the painless stability provided by thumb MCP joint arthrodesis, preservation of motion remains a laudable goal, even at the thumb MCP joint.
Several salvage procedures have been described for MCP joint reconstruction. Boulas et al. [4] reconstructed 5 MCP joint defects using a free metatarsophalangeal osteochondral autograft and observed a mean motion arc of 74°. However, those authors also noted joint space narrowing resulting from avascular cartilage necrosis in all cases. Menon [5] obtained excellent short-term results with the use of an autogenous osteoarticular graft taken from the metatarsal, but the author observed graft degeneration after 2 years. Malinin and Ouellette [6] indicated that nutrition for the articular cartilage is supplied by the subchondral bone and that interruption of the vascularized subchondral bone could lead to cartilage degeneration, although these changes would not be apparent in the short term and would be detected only after long periods of time. Seradge et al. [7] reported that the use of autogenous perichondrium produced an average MCP joint motion arc of 22° and a treatment failure rate of 19%. Engkvist et al. [8] reported a high incidence of joint pain and no functional improvement after perichondrial arthroplasty. Zappaterra et al. [9] reconstructed MCP joints using an autograft of costal cartilage and found that patients obtained a mean arch of motion of 37°. Jung et al. [10] used an autologous bone graft from the iliac crest for MCP joint reconstruction after tumor resection and found that this technique resulted in limited joint movement and the rapid development of osteoarthritis. Prosthetic arthroplasty can be an alternative, but it has a risk of implant failure [11]. Metacarpophalangeal joint arthrodesis represents an alternative procedure but results in loss of joint motion and risks excessive bone shortening and nonunion [12]. The outcomes of studies have been shown to vary according to the patient’s age, occupation, original injuries, and daily activities; the medical examiners assigned to the case; and the length of the follow-up period [13, 14].
3 Anatomy
James et al. [13] found that nutrient arteries penetrate into the dorsum of the base of the third metacarpal. Those nutrient arteries arise from the distal and middle dorsal carpal arches, which run distally to the second to fourth DMAs (Fig. 15.1a, b). The first DMA originates from the radial artery (in 93% of cases), which connects to the dorsal carpal arches, although the first DMA has also been shown to originate from the distal dorsal carpal arch [14]. This anatomical organization is the basis for the development of a reverse DMA-based vascularized osteoarticular graft. Moreover, this flap technique can be modified according to anatomic variations. For example, the middle dorsal carpal arch is routinely present in all cases, whereas the distal dorsal arch is present in only 73% of the population. Thus, in a patient with no distal dorsal arch, the flap can be designed to obtain its blood supply from the DMAs of the middle dorsal carpal arch.
(a) Anatomy of the dorsal metacarpal arteries (DMAs), dorsal arches, and penetrating point of the nutrient arteries at the base of the third metacarpal. (b) The third metacarpal and the arteries in the sagittal plane. (c) Volar view of the base of the third metacarpal. (d) Basal view. (e) Ulnar view. We performed an osteotomy through the blue line to obtain a convex articular surface and through the red line to obtain a concave articular surface
The third metacarpal has a widened proximal base, and the surface that articulates with the capitate is convex anteriorly and concave dorsally and extends to the styloid process on the dorsolateral aspect of the metacarpal base4 (Fig. 15.1c–e). This unique contour is the basis for investigating the feasibility of using a portion of this articular surface for MCP joint reconstruction. The convex and concave surfaces are useful for reconstructing the metacarpal head and the base of the proximal phalanx, respectively.
4 Surgical Technique
Perioperative X-rays are obtained for assessing the articular defects. Perioperative CT scan is performed for old injuries, to more accurately assess the extent of the defects. (Fig. 15.2a–d) Nevertheless, intraoperative assessment is critical in determining whether to perform the tissue transfer.
The first, second, and third DMAs are selected for the vascular pedicle, mainly because of their reach, reliable position, and size, as they display less anatomical variation and a greater vascular caliber than the fourth and fifth DMAs. The DMAs are located preoperatively using a Doppler probe, and the surgery is performed under brachial plexus anesthesia with tourniquet control.
A lazy S- or L-shaped incision is made over the DMA that is selected as the vascular pedicle. At the wrist, the incision is curved transversely over the capitatemetacarpal joint (Fig. 15.3a). The DMA is identified and its accompanying two veins according to where they course along the dorsal interosseous fascia. In some cases, to ensure sufficient exposure, the insertion of the extensor carpi radialis brevis tendon is partially released and retracted laterally (Fig. 15.3b). If a large bone flap is required to simultaneously reconstruct a condylar defect, the extensor carpi radialis brevis insertion is completely released and sutured to the extensor carpi radialis longus. The periosteum of the third metacarpal is incised transversely at the level of the previous epiphysis, distal to the nutrient arteries. The metacarpal is cut at an angle using a sagittal saw (Fig. 15.3c). The angle is typically between 30° and 70°, which is determined according to the recipient site defect (Fig. 15.1e). During the osteotomy and elevating the osteoarticular graft, great care is taken not to injure the nutrient arteries (Fig. 15.3d). The DMA is included in the fascia over the full width of the muscle, involving the segment of the distal carpal arch in addition to 5–7 mm of the surrounding soft tissues, and the accompanying veins in the pedicle and they are dissected proximal to the graft. If the graft is pedicled on the first DMA, the involved segment of the radial artery is also harvested to maintain continuity of the pedicle. Then, the pedicle is dissected distally to a pivot point until the required length is achieved. The pivot point is chosen at a point along the portion of the DMA that is proximal to the communicating artery of the palmar metacarpal artery (Fig. 15.4). Thereafter, the tourniquet is released to check for graft bleeding. The graft is transferred to the defective joint through a subcutaneous tunnel. The graft and the defect are fashioned to fit together using a rongeur. The graft is fixed using 0.8- to 1.2-mm K-wires buried beneath the skin (Fig. 15.5a, b).
(a) Incision design at the dorsum of the left hand. (b) 1, 2, and 3 show the first, second, and third DMAs and the accompanying veins. The arrow shows the point at which the nutrient arteries penetrate into the base of the third metacarpal. The arrowhead shows the extensor carpi radialis brevis and its insertion. (c) Osteotomy is complete. (d) Vascularized osteoarticular graft harvesting is completed
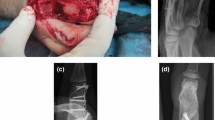
(a) The radiograph shows flap fixation (anteroposterior view). (b) Lateral view. (c) The lateral image shows the thumb metacarpophalangeal (MCP) joint 41 months after surgery, with solid bone consolidation. (d) A sagittal computed tomography image shows bone healing at the donor joint. (e) Coronal imaging
After surgery, the MCP joints and wrist are immobilized with a splint. The involved MCP joint of the finger or thumb is placed in 70° and 0° flexion, respectively. Two days after surgery, motion exercises of the interphalangeal joints are performed to decrease tendon adhesion. Two to 3 weeks after surgery, the splint and K-wire immobilizing the MCP joint are removed. Joint motion exercises are initiated. (Fig. 15.5c–e). Good thumb motion is observed 41 months after surgery (Fig. 15.6).
5 Indication and Contraindication
Indication for the technique is existence of a new or old cartilage defect at either the proximal or the distal MCP joint surface. Contraindications include the existence of a small defect, an infectious condition, rheumatoid arthritis, or gout.
5.1 Case 2
A 22-year-old man had an old injury to the third MCP joint (Fig. 15.7–15.10).
References
Abboud JA, Beredjiklian PK, Bozentka DJ. Metacarpophalangeal joint arthroplasty in rheumatoid arthritis. J Am Acad Orthop Surg. 2003;11:184–91.
Peterfy CG, van Dijke CF, Lu Y, Nguyen A, Connick TJ, Kneeland JB, et al. Quantification of the volume of articular cartilage in the metacarpophalangeal joints of the hand: accuracy and precision of three-dimensional MR imaging. AJR Am J Roentgenol. 1995;165:371–5.
Boulas HJ. Autograft replacement of small joint defects in the hand. Clin Orthop Relat Res. 1996;327:63–71.
Boulas HJ, Herren A, Büchler U. Osteochondral metatarsophalangeal autografts for traumatic articular metacarpophalangeal defects: a preliminary report. J Hand Surg. 1993;18A:1086–92.
Menon J. Reconstruction of the metacarpophalangeal joint with autogenous metatarsal. J Hand Surg. 1983;8A:443–6.
Malinin T, Ouellette EA. Articular cartilage nutrition is mediated by subchondral bone: a long-term autograft study in baboons. Osteoarthritis Cartilage. 2000;8:483–91.
Seradge H, Kutz JA, Kleinert HE, Lister GD, Wolff TW, Atasoy E. Perichondrial resurfacing arthroplasty in the hand. J Hand Surg. 1984;9A:880–6.
Engkvist O, Johansson SH. Perichondrial arthroplasty. A clinical study in twenty-six patients. Scand J Plast Reconstr Surg. 1980;14:71–87.
Zappaterra T, Obert L, Pauchot J, Lepage D, Rochet S, Gallinet D, et al. Post-traumatic reconstruction of digital joints by costal cartilage grafting: a preliminary prospective study. Chir Main. 2010;29:294–300.
Jung M, Daecke W, Bernd L, Martini AK, Schroeder K. Reconstruction of phalanx and metacarpal defects by autologous iliac crest transplants after tumour resection with joint involvement. Handchir Mikrochir Plast Chir. 2007;39:381–7.
Murray PM. Current status of metacarpophalangeal arthroplasty and basilar joint arthroplastry of the thumb. Clin Plast Surg. 1996;23:395–406.
Lourie GM. The role and implementation of metacarpophalangeal joint fusion and capsulodesis: indication and treatment alternatives. Hand Clin. 2001;17;255–60.
James RD, Michael JB. Skeleton anatomy in surgical anatomy of the hand and upper extremity. Lippincott Williams & Wilkins; 2003. p. 3–72.
Sebastin SJ, Mendoza RT, Chong AK, Peng YP, Ono S, Chung KC, et al. Application of the dorsal metacarpal artery perforator flap for resurfacing soft-tissue defects proximal to the fingertip. Plast Reconstr Surg. 2011;128:166–78.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Shao, X., Zhang, X. (2021). The Use of a Third Metacarpal Base Vascularized Osteoarticular Graft for Treatment of Metacarpophalangeal Joint Traumatic Defects. In: Hou, C., Chang, S., Tang, J., Cai, Z. (eds) Practical Microsurgery Cases. Springer, Singapore. https://doi.org/10.1007/978-981-15-9716-9_15
Download citation
DOI: https://doi.org/10.1007/978-981-15-9716-9_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9715-2
Online ISBN: 978-981-15-9716-9
eBook Packages: MedicineMedicine (R0)