Abstract
Plant-based polyphenolic compounds are present in various dietary sources and considered to possess antioxidant activity. Gallic acid (GA) is one particularly important polyphenol. Studies have reported that GA is cytotoxic to cancer cells while normal cells remain unaffected by such action. We suggested a mechanism that suggests the preferential killing of GA against cancer cell. Using Comet assay (single cell gel electrophoresis) and Fox assay (ferrous oxidation xylenol), it has been shown that GA behaves as prooxidant and causes DNA damage in human lymphocytes. Moreover, such DNA damage is stopped in the presence of copper chelator in cell validating the role of copper in the prooxidant DNA breakage by GA. Also, human breast cancer cell line (MDA-MB-231) growth is interrupted by GA resulting killing of cell in prooxidant manner. It is an established fact; copper levels are well elevated in different types of cancers. Consequently, cancer cells are subjected to transfer of electron between GA to produce ROS. Thus, we explain the cytotoxicity of GA towards malignant cells is because of elevated copper levels. In addition, our studies identify that nuclear copper can be responsible as a completely new target for cytotoxic behavior of GA as well as other polyphenolic compounds, which have strong potential against cancer as therapeutic agent.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Cancer is a serious health problem around the globe. In recent years, the idea of preventing cancer has been attributed to the consumption of diet-based substances. It is believed that more than 70% of human cancers can be delayed or prevented by following proper lifestyle and eating habits. Epidemiological evidence suggests that increased utilization of fruits, vegetables, and beverages may curb the harmful risk of cancer induction (Adlercreutz et al. 1995; Park and Surh 2004; Barnes et al. 1994). This has been associated with human diet found to be rich in various biologically potent polyphenolic compounds (Surh 2003). Gallic acid (3, 4, 5-trihydroxybenzoic acid) is one such naturally available phenolic acid present in food stuff such as gall-nuts, green tea, apple, grapes and acts as potent antioxidant. The known pharmacological properties of GA include antitumor, antifungal, antibacterial, and antiviral activities. The anticancer effects of GA have been well demonstrated against cancer models, both in vitro and in vivo (Liang et al. 2012; Ji et al. 2009). GA has also been reported to induce apoptosis in human HSC-2 glioma cells, HL-60 cervical cancer cells, and human DU-145 promyelocytic leukemia cells while sparing normal cells (Alyssa et al. 2013; Madlener et al. 2007; Agarwal et al. 2006). The mechanism by which polyphenolic compounds prevent proliferation of cells and show apoptosis in malignant cells has always been a remarkably interesting field of research. Many mechanisms have been suggested. A clear and definitive mechanism to explain the anticancer properties of plant polyphenols is yet to be found.
Previous studies have shown that polyphenols belonging to various classes, for example, gallocatechins (Farhan et al. 2016a), curcumin (Ahsan and Hadi 1998), tannins (Khan and Hadi 1998), flavonoids (Said et al. 1992; Arif et al. 2015), resveratrol (Ahmad et al. 2000; Shamim et al. 2012), lead to DNA breakage either in absence or presence of copper ions. Copper is a fundamental metal ion found within chromatin and is known to be linked with DNA bases (Kagawa et al. 1991). Further, it has been reported that copper concentrations are well elevated in various malignancies (Gupte and Mumper 2009; Ebadi and Swanson 1988; Margalioth et al. 1987; Yoshida et al. 1993). Plant polyphenolics have both antioxidant and prooxidant properties (Hadi et al. 2000; Ebara et al. 2000; Inoue et al. 1994). Further, it has been earlier suggested that the prooxidant activity of plant polyphenols may be instrumental for their anticancer properties (Farhan et al. 2016a; Li and Trush 1994). Such a mechanism for the killing of cancer cells selectively of these polyphenols against cancer cells require endogenous copper, perhaps chromatin-bound copper and the subsequent prooxidant action. This can also help explain their selective cytotoxicity against cancer cells, with minimum to no effect against normal cells. The chemical structure of gallic acid is shown in Fig. 1.
DNA Damage Induced by Gallic Acid in Intact and Permeabilized Cells
It has been found in our laboratory as well as of the others that various groups of plant polyphenols are efficient of breaking DNA when treated with human lymphocytes. The damage can be viewed by Comet assay technique (Pool-Zobel et al. 1993). Using increasing concentrations of GA, we analyzed the capability to induce DNA breakage in isolated lymphocytes. A dose-based rise in DNA damage was observed (Fig. 2). As per our idea, polyphenolic compounds move chromatin-associated copper which results in breakage of DNA. We also found that tail formation in Comet assay caused by GA treatment was far more in case of permeabilized cells as compared to intact cells. It can be assumed that the capability of GA is to effectively associate with nuclei in a permeabilized cellular system.
Determination of DNA Damage by Gallic Acid Along with Metal Chelators in Intact and Permeabilized Cells
Metal chelators were utilized for copper, iron, and zinc to understand the involvement of the fore mentioned metals in DNA breakage by GA in intact and permeabilized cells. In case of intact cells neocuproine quenched DNA breakage. Neocuproine is known to be permeable to cell membrane which is a Cu(I)-specific chelator. On the other hand, zero inhibition was noticed when a membrane-impermeable, Cu(I) chelator, bathocuproine was used. Desferrioxamine mesylate (iron chelator) as well as histidine (zinc chelator) were unsuccessful to interact. In contrast, copper chelators neocuproine/or bathocuproine interacted in permeabilized cell system. Specific chelators of iron and zinc failed to show effectiveness even in permeabilized cells system. These results implicated that copper associated with chromatin is mobilized by GA which leads to oxidative DNA damage.
Values represent DNA damage in cells (intact/or permeabilized) along with metal chelators as induced by GA compared as percentage to control (DNA breakage resulted by GA without chelator). Data shows mean ± SEM of three independently performed experiments. 2P < 0.03 when correlated with1. 3P < 0.02 when correlated with2 (Table 1).
H2O2 Generation by Gallic Acid Inside Incubation Medium
Plant polyphenolics have a tendency to auto-oxidize when present in cell culture media leading in production of quinone and H2O2 that enter in cell nuclei and harm various macromolecules (Long et al. 2000). This effect results in the production of ROS that also leads to DNA breakage. We examined the production of H2O2 after the GA and correlated with tannic acid (TA) which is a well-established producer of H2O2 (Farhan et al. 2016b). The amount of formation of H2O2 by TA was much higher compared to GA suggesting that H2O2 is not accountable for DNA breakage in presence of GA treatment (Fig. 3).
Determination of Cell Growth by Gallic Acid in Human Breast Cancer Cells
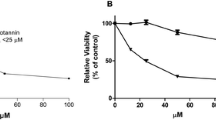
We studied the effects of GA in human breast cancer cells (MDA-MB-231) and observed growth inhibition of MDA-MB-231 cells by GA in dose-based fashion (Fig. 4a). We also noticed human normal breast epithelial cells (MCF-10A) were unaffected to GA treatment. However, when supplemented to a copper-rich medium resulted in stimulation to GA action (Fig. 4b). The results obtained are in favor of our results published earlier focusing on the cancer chemopreventive action of plant polyphenols (Arif et al. 2015; Farhan et al. 2016b; Smith et al. 1992).
(a) The impact of GA was tested on the proliferation of MDA-MB-231 as observed by MTT assay. Using mentioned concentrations of GA, the cells were subjected to treatment for the time intervals 24 h and 48 h. The results procured are mentioned when compared to the control cells. (b) MCF-10A (human normal breast epithelial cells) and MCF-10A-Cu [MCF-10A cells enriched in copper (25 μM)] were treated with GA (0 μM, 25 μM, 50 μM) for 72 h. All values shown are % control mean ± SEM of three independently performed experiments. *p < 0.03 and **p < 0.02 when correlated with control (0 μM GA)
Gallic Acid and Cancer Chemoprevention
Over the years, many scientific researchers have utilized numerous varieties of cancer cell lines to investigate the prooxidant mechanism and cytotoxicity of plant polyphenols. In an effort to explain that normal cells remain unharmed during such action (Alyssa et al. 2013; Madlener et al. 2007; Agarwal et al. 2006; Quinlan and Gutteridge 1987), our analysis showcase that the selectively killing action of GA (or plant polyphenols) against cancer cells could be attributed to the prooxidant mechanism where GA is able to mobilize endogenic copper ions.
GA is a polyphenol that is relatively very well-absorbed (Khan et al. 2014). In a study (Wang et al. 2014) that compared the relative bioavailability of GA from acidum gallicum tablets versus brewed tea, it was found that GA from both sources was rapidly absorbed and metabolized. The mean maximum concentrations of plasma GA were 1.83 and 2.09 micromol/L from the tablets and tea, respectively. In addition, the highest concentrations of its metabolite in plasma were determined to be 2.83 and 2.64 micromol/L, respectively. Based on the available literature, it is believed that the plasma concentration of GA ranges between 2.2 and 9.9 microg/L (Manach et al. 2005). There is also evidence that the bioavailability of GA can be substantially improved by repeated doses. In a study conducted in mouse model (Shahrzad et al. 2001), it was shown that gavage administration of grape seed polyphenolic extract (GSPE) to mice could result in LC-MS detection of several key constituents of GSPE, with GA being a major one. Further, compared to a single acute dose, repeated doses significantly increased the bioavailability of GA and a 198% increase in plasma GA was observed.
A Copper-Dependent Anticancer Mechanism
The fact is that cellular, tissue, serum copper levels are increased in different types of cancers (Kaliora et al. 2013; Gupte and Mumper, 2009). Therefore, it may be safely assumed that malignant cells may be more susceptible to transfer of electron between GA (plant polyphenols) and copper ions to generate ROS. In the literature, we have sufficient data explaining the concentrations of Cu, Zn, Fe, and Se in patients suffering with cancer (Carpentieri et al. 1986; Bhadani et al. 2015). The most important point to consider is that the GA concentration needed for killing malignant cells selectively must be less than GA concentration required for killing normal cells. The same has been established that GA is cytotoxic to malignant cells rather than normal cells.
The distribution and metabolism of copper is overserved to be changed in tumor bearing humans, mice, and rats (Folkman 1972; Urso and Maffia 2015). Path-breaking studies performed by Folkman (1971; Kuo et al. 2002) showed that copper is one of the simplest angiogenic molecules. Ceruloplasmin, tripeptide glycyl-histidyl-lysine, and heparin are copper-binding proteins playing crucial role in angiogenesis. The protein is known to be non-angiogenic when not bound with copper. However, when bound to copper, they become angiogenic (Zuo et al. 2006). The change in copper metabolism is currently a hot topic and seen as a major biomarker for molecular cancer imaging in cancer patients (Kaliora et al. 2013; Apelgot et al. 1986; Semczuk and Pomykalski 1973). In the goal to exploit copper’s angiogenic potential as to evade cancer, copper chelators, for example, tetrathiomolybdate, clioquinol, etc., have shown to bring down tumor cells’ growth both in vitro and in vivo (Wachsmann and Peng 2016; Singh et al. 2016; Jain et al. 2013; Fu et al. 2014; Fu et al. 2012; Crowe et al. 2013). It is also to be noted that copper has also been recognized to possess a pivotal role in intracellular signaling and tumor metastasis by engaging itself in transcriptional regulation of E-cadherin (Schimmer 2011).
Wolfe et al. (Pushie et al. 2014; Turski and Thiele 2009) have suggested a copper-driven Fenton reaction which suggests that generation of hydroxyl radicals is indeed efficient of causing apoptosis. The hydroxyl radical is found to be electrophilic possessing higher order reactivity. For that reason, it must possess a small radius of diffusion. To achieve DNA breakage, it must be generated close to cellular DNA (Wolfe et al. 1994). The position of the redox-active metals is particularly important as hydroxyl radical is highly reactive and interacts specially in closeness to the bound metal. Normal cells possess a balance between the antioxidant defense and free radical generation (Held et al. 1996; Pryor 1988). However, it is well corroborated that cells with tumor are under constant oxidative stress and possess a changed antioxidant system (Burkitt et al. 1996). Therefore, in malignant cell, additional ROS stress reaching up to the level of threshold could be the consequence for apoptosis (Kaliora et al. 2013). All the observations imply neoplastic cells are prone to oxidative stress as they deal with enhanced levels of ROS because of higher degree of metabolism and growth (Zhou et al. 2003).
Conclusion
Plant polyphenols are generally known to oppose the effects of ROS, preventing oxidative DNA breakage and ultimately lowering risks of cancer. During the course of time, newer studies focus on the findings that polyphenolic compounds can arbitrate ROS generation when various metal ions are present. The aforementioned prooxidant action may lead to apoptosis in malignant cells. Depending on the microenvironment inside the cell-based system, polyphenols may show either antioxidant/prooxidant action in nature. Copper, when available, plant polyphenols generally show prooxidant characteristics supplementing to the transition metal-redox cycling. The prooxidant action results in ROS-generated DNA breakage.
Future Direction
Plant polyphenols compounds are certainly adept of mobilizing as well as redox cycling nuclear copper leading ROS generation. This causes DNA breakage finally leading to cell death. The malignant cells are under constant ROS exposure produced by the redox activity of endogenous copper. The same can result into antioxidant behavior of cells promoting apoptosis. It is improbable to find one-shot therapy for diverse cancers. In recent times, studies on cancer relocated to the metabolism of cancer cells. In this scenario, our recommendations hold important value as they give strong ground for designing new cytotoxic agents which thereby target the cancer cells based on increased copper levels using gallic acid or plant polyphenols in general.
References
Adlercreutz CH, Goldin BR, Gorbach SL, Hockerstedt KA, Watanabe S, Hamalainen EK, Markkanen MH, Makela TH, Wahala KT, Adlercreutz T (1995) Soybean phytoestrogen intake and cancer risk. J Nutr 125:757S–770S
Agarwal C, Tyagi A, Agarwal R (2006) Gallic acid causes inactivating phosphorylation of cdc25A/cdc25C-cdc2 via ATM-Chk2 activation; leading to cell cycle arrest; and induces apoptosis in human prostate carcinoma DU145 cells. Mol Cancer Ther 5:3294–3302
Ahmad A, Farhan A, Singh S, Hadi SM (2000) DNA breakage by resveratrol and Cu(II): reaction mechanism and bacteriophage inactivation. Cancer Lett 154:29–37
Ahsan H, Hadi SM (1998) Strand scission in DNA induced by curcumin in the presence of Cu(II). Cancer Lett 124:23–30
Alyssa GS, Jeffrey HW, Hannah E, Esther FR, Ayelet RB, Jordana RW, Tova L, Harriet LZ, Harvey B (2013) Cytotoxic and proapoptotic activities of gallic acid to human oral cancer HSC-2 cells. Oxidants and Antioxidants Med Sci 2:265–274
Apelgot S, Coppey J, Fromentin A, Guille E, Poupon MF, Roussel A (1986) Altered distribution of copper (64 Cu) in tumor-bearing mice and rats. Anticancer Res 6:159–164
Arif H, Rehmani N, Farhan M, Ahmad A, Hadi SM (2015) Mobilization of copper ions by flavonoids in human peripheral lymphocytes leads to oxidative DNA breakage: a structure activity study. Int J Mol Sci 16:26754–26769
Barnes S, Peterson G, Grubbs C, Setchell K (1994) Potential role of dietary isoflavones in the prevention of cancer. Adv Exp Med Biol 354:135–147
Bhadani B, Sharma N, Kakkar R (2015) Gallic acid: a versatile antioxidant with promising therapeutic and industrial application. RSC Adv 35:1–18
Burkitt MJ, Milne L, Nicotera P, Orrenius S (1996) 1;10-Phenanthroline stimulates internucleosomal DNA fragmentation in isolated rat-liver nuclei by promoting the redox activity of endogenous copper ions. Biochem J 313:163–169
Carpentieri U, Myers J, Thorpe L, Daeschner CW, Haggard ME (1986) Copper; zinc; and iron in normal and leukemic lymphocytes from children. Cancer Res 46:981–984
Crowe A, Jackaman C, Beddoes KM, Ricciardo B, Nelson DJ (2013) Rapid copper acquisition by developing murine mesothelioma: decreasing bioavailable copper slows tumor growth; normalizes vessels and promotes T cell infiltration. PLoS One 8:e73684
Ebadi M, Swanson S (1988) The status of zinc; copper; and metallothionein in cancer patients. Prog Clin Biol Res 259:161–175
Ebara M, Fukuda H, Hatano R, Saisho H, Nagato Y, Suzuki K, Nakajima K, Yukawa M, Kondo F, Nakayama A, Sakurai H (2000) Relationship between copper; zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J Hepatol 33:415–422
Farhan M, Khan HY, Oves M, Al-Harrasi A, Rehmani N, Arif H, Hadi SM, Ahmad A (2016a) Cancer therapy by catechins involves redox cycling of copper ions and generation of reactive oxygen species. Toxins 8:37
Farhan M, Oves M, Chibber S, Hadi SM, Ahmad A (2016b) Mobilization of nuclear copper by green tea polyphenol epicatechin-3-gallate and subsequent prooxidant breakage of cellular DNA: implications for cancer chemotherapy. Int J Mol Sci 18:34
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Folkman J (1972) Angiogenesis in psoriasis: therapeutic implications. J Invest Dermatol 59:40–43
Fu S, Naing A, Fu C, Kuo MT, Kurzrock R (2012) Overcoming platinum resistance through the use of a copper-lowering agent. Molecula Cancer Therapy 11:1221–1225
Fu S, Hou MM, Wheler J, Hong D, Naing A, Tsimberidou A, Janku F, Zinner R, Piha PS, Falchook G, Kuo MT, Kurzrock R (2014) Exploratory study of carboplatin plus the copper-lowering agent trientine in patients with advanced malignancies. Investig New Drugs 32:465–472
Gupte A, Mumper RJ (2009) Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev 35:32–46
Hadi SM, Asad SF, Singh S, Ahmad A (2000) Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life 50:167–171
Held KD, Sylvester FC, Hopcia KL, Biaglow JE (1996) Role of Fenton chemistry in thiol-induced toxicity and apoptosis. Radiat Res 145:542–553
Inoue M, Suzuki R, Koide T, Sakaguchi N, Ogihara Y, Yabu Y (1994) Antioxidant; gallic acid; induces apoptosis in HL-60RG cells. Biochem Biophys Res Commun 204:898–904
Jain S, Cohen J, Ward MM, Kornhauser N, Chuang E, Cigler T, Moore A, Donovan D, Lam C, Cobham MV, Schneider S, Hurtado SM, Benkert S, Mathijsen GC, Zelkowitz R, Warren JD, Lane ME, Mittal V, Rafii S, Vahdat LT (2013) Tetrathiomolybdate-associated copper depletion decreases circulating endothelial progenitor cells in women with breast cancer at high risk of relapse. Ann Oncol 24:1491–1498
Ji BC, Hsu WH, Yang JS, Hsia TC, Lu CC, Chiang JH, Yang JL, Lin CH, Lin JJ, Suen LJ, Gibson W, Chung JG (2009) Gallic acid induces apoptosis via caspase-3 and mitochondrion-dependent pathways in vitro and suppresses lung xenograft tumor growth in vivo. J Agric Food Chem 57:7596–7604
Kagawa TF, Geierstanger BH, Wang AH, Ho PS (1991) Covalent modification of guanine bases in double-stranded DNA. The 1.2-A Z-DNA structure of d(CGCGCG) in the presence of CuCl2. J Biol Chem 266:20175–20184
Kaliora AC, Kanellos PT, Kalogeropoulos N (2013) Gallic acid bioavailability in humans. In: Thompson M, Collins PB (eds) Handbook on gallic acid: natural occurrences; antioxidant properties and health implications. Nova Science Publishers, New York, pp 301–313
Khan NS, Hadi SM (1998) Structural features of tannic acid important for DNA degradation in the presence of Cu(II). Mutagenesis 13:271–274
Khan HY, Zubair H, Faisal M, Ullah MF, Farhan M, Sarkar FH, Ahmad A, Hadi SM (2014) Plant polyphenol induced cell death in human cancer cells involves mobilization of intracellular copper ions and reactive oxygen species generation: a mechanism for cancer chemopreventive action. Mol Nutr Food Res 58:437–446
Kuo HW, Chen SF, Wu CC, Chen DR, Lee JH (2002) Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol Trace Elem Res 89:1–11
Li Y, Trush MA (1994) Reactive oxygen-dependent DNA damage resulting from the oxidation of phenolic compounds by a copper-redox cycle mechanism. Cancer Res 54:1895s–1898s
Liang CZ, Zhang JK, Shi Z, Liu B, Shen CQ, Tao HM (2012) Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother Pharmacol 69:317–331
Long LH, Clement MV, Halliwell B (2000) Artifacts in cell culture: rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin; (−)-epigallocatechin gallate; (+)-catechin; and quercetin to commonly used cell culture media. Biochem Biophys Res Commun 273:50–53
Madlener S, Illmer C, Horvath Z, Saiko P, Losert A, Herbacek I, Grusch M, Elford HL, Krupitza G, Bernhaus A, Fritzer SM, Szekeres T (2007) Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells. Cancer Lett 245:156–162
Manach C, Williamson G, Morand C, Scalbert A, Remesy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81:230S–242S
Margalioth EJ, Udassin R, Cohen C, Maor J, Anteby SO, Schenker JG (1987) Serum copper level in gynecologic malignancies. Am J Obstet Gynecol 157:93–96
Park OJ, Surh YJ (2004) Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett 150:43–56
Pool-Zobel BL, Guigas C, Klein R, Neudecker C, Renner HW, Schmezer P (1993) Assessment of genotoxic effects by lindane. Food Chem Toxicol 31:271–283
Pryor WA (1988) Why is the hydroxyl radical the only radical that commonly adds to DNA? Hypothesis: it has a rare combination of high electrophilicity; high thermochemical reactivity; and a mode of production that can occur near DNA. Free Radic Biol Med 4:219–223
Pushie MJ, Nienaber KH, Summers KL, Cotelesage JJ, Ponomarenko O, Nichol HK, Pickering IJ, George GN (2014) The solution structure of the copper clioquinol complex. J Inorg Biochem 133:50–56
Quinlan GJ, Gutteridge JM (1987) Oxygen radical damage to DNA by rifamycin SV and copper ions. Biochem Pharmacol 36:3629–3633
Said M, Fazal F, Rahman A, Hadi SM, Parish JH (1992) Activities of flavonoids for the cleavage of DNA in the presence of Cu(II): correlation with generation of active oxygen species. Carcinogenesis 13:605–608
Schimmer AD (2011) Clioquinol - a novel copper-dependent and independent proteasome inhibitor. Curr Cancer Drug Targets 11:325s–331s
Semczuk B, Pomykalski M (1973) Serum copper level in patients with laryngeal carcinoma. Otolar Polska = Otolaryngol Pol 27:17–23
Shahrzad S, Aoyagi K, Winter A, Koyama A, Bitsch I (2001) Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J Nutr 131:1207–1210
Shamim U, Hanif S, Albanyan A, Beck FW, Bao B, Wang Z, Banerjee S, Sarkar FH, Mohammad RM, Hadi SM, Azmi AS (2012) Resveratrol-induced apoptosis is enhanced in low pH environments associated with cancer. J Cell Physiol 227:1493–1500
Singh BP, Dwivedi S, Dhakad U, Murthy RC, Choubey VK, Goel A, Sankhwar SN (2016) Status and Interrelationship of Zinc; Copper; Iron; Calcium and Selenium in Prostate Cancer. Indian J Clin Biochem 31:50–56
Smith C, Halliwell B, Aruoma OI (1992) Protection by albumin against the pro-oxidant actions of phenolic dietary components. Food Chem Toxicol 30:483–489
Surh YJ (2003) Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3:768–780
Turski ML, Thiele DJ (2009) New roles for copper metabolism in cell proliferation; signaling; and disease. J Biol Chem 284:717–721
Urso E, Maffia M (2015) Behind the link between copper and angiogenesis: Established mechanisms and an overview on the role of vascular copper transport systems. J Vasc Res 52:172–196
Wachsmann J, Peng F (2016) Molecular imaging and therapy targeting copper metabolism in hepatocellular carcinoma. World J Gastroenterol 22:221–231
Wang K, Zhu X, Zhang K, Zhu L, Zhou F (2014) Investigation of gallic acid induced anticancer effect in human breast carcinoma MCF-7 cells. J Biochem Mol Toxicol 28:387–393
Wolfe JT, Ross D, Cohen GM (1994) A role for metals and free radicals in the induction of apoptosis in thymocytes. FEBS Lett 352:58–62
Yoshida D, Ikeda Y, Nakazawa S (1993) Quantitative analysis of copper; zinc and copper/zinc ratio in selected human brain tumors. J Neuro-Oncol 16:109–115
Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P (2003) Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood 101:4098–4104
Zuo XL, Chen JM, Zhou X, Li XZ, Mei GY (2006) Levels of selenium; zinc; copper; and antioxidant enzyme activity in patients with leukemia. Biol Trace Elem Res 114:41–53
Conflict of Interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry
Farhan, M., Aatif, M., Hadi, S.M., Ahmad, A. (2022). Mechanism of Gallic Acid Anticancer Activity Through Copper-Mediated Cell Death. In: Chakraborti, S., Ray, B.K., Roychoudhury, S. (eds) Handbook of Oxidative Stress in Cancer: Mechanistic Aspects. Springer, Singapore. https://doi.org/10.1007/978-981-15-9411-3_179
Download citation
DOI: https://doi.org/10.1007/978-981-15-9411-3_179
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9410-6
Online ISBN: 978-981-15-9411-3
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences