Abstract
The discovery of ortho-topolin, meta-topolin and its conjugates in the early 1990s prompted further research on the biological activity of these aromatic cytokinins. Structure–activity relationship studies (SAR) revealed that hydroxylation at the meta-position on the benzyl side chain of BAP and its riboside significantly improved the compound performance in standardized cytokinin bioassays compared to BAP. Elucidation of the molecular mechanism of the cytokinin signal transduction and identification of membrane-bound receptor histidine kinase (HK) led to deeper understanding of the function of topolins in planta. While aromatic cytokinins generally show weak interaction with Arabidopsis and other cytokinin-sensitive HKs, meta-topolin is able to provoke a strong response comparable to that observed with the most active isoprenoid free bases. In contrast, ortho-topolin and para-topolin are much less active, and it is possible that hydroxylation at these positions serves as a deactivation step. In contrast, derivatives of meta-topolin, although not directly interacting with cytokinin-sensitive HKs, have proven to be very useful in plant propagation techniques, and slow gradual release of the free active base allows for the potentiation of cytokinin effects. This chapter summarizes basic facts about cytokinin signalling and adds to our knowledge of signalling aspects and metabolism of aromatic cytokinins in various plant models.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Cytokinin Signal Transduction Via Multistep Phosphorelay
Cytokinins are a major class of plant hormones responsible for regulating various developmental processes, including coordination of cell division and differentiation, activity of shoot and root apical meristems, proportional organ formation and others (Kieber and Schaller 2018). CKs are perceived by a subfamily of sensor histidine kinases (HKs), which transmit the signal via a two-component phosphorelay cascade, a signalling platform sharing its origin with bacterial and yeast two-component signal transduction system (TCS) (To and Kieber 2008; Hwang et al. 2012).
Characterization of cytokinin-insensitive cre1 (cytokinin response 1) mutants and overexpression of CRE1 in a yeast mutant background led to identification of the CRE1 cytokinin receptor in Arabidopsis (Inoue et al. 2001). Other groups independently confirmed these observations and established the role of CRE1/AHK4 receptor in CK sensing (Suzuki et al. 2001; Ueguchi et al. 2001a; Yamada et al. 2001). The CK perception is initiated by ligand interaction with a sensory CHASE domain, resulting in subsequent autophosphorylation on a conserved histidine residue (His) in the transmitter domain and further intramolecular transfer to a conserved aspartate residue (Asp) in the receiver domain. The signal is then transmitted via AHP proteins (AHP1–AHP5) through the His-Asp phosphorelay signal transduction pathway to the type-B response regulators (RRs), which are localized in the nucleus. Their activation then leads to promoter binding and transcription of target genes (Hwang and Sheen 2001; Lohrmann et al. 2001; Hosoda et al. 2002; Yamada et al. 2004).
AHP proteins which act downstream of cytokinin HKs are partially redundant positive regulators of cytokinin signalling (Hutchison et al. 2006). Analyses of protein–protein interactions revealed that AHPs can interact with several upstream HKs as well as downstream RRs (Dortay et al. 2006). On the other hand, some HKs show preference for specific AHPs. The receiver domain of HK CKI1 has been shown to specifically interact with AHP2, AHP3 and AHP5 with different affinities (Pekárová et al. 2011). This particular HK preferentially interacts with AHP2 and AHP3, but only weakly with AHP5. Interestingly, the receptor domain of another hybrid HK, ETR1 receptor of the ethylene pathway, interacts with AHP1, AHP2, AHP3 and AHP5 providing evidence of a possible crosstalk mechanism between cytokinin- and ethylene-signalling pathways, presumably via ETR1 pathway-related phosphorylation of ARR10, a positive regulator of cytokinin signalling (Zdarska et al. 2019).
There are two types of RRs involved in the cytokinin pathway: type-A RRs and type-B RRs. Type-B RRs possess an N-terminal receiver domain and C-terminal output domain, which contains a DNA-binding GARP motif (named after GOLDEN2, Arabidopsis ARR type-B proteins and Chlamydomonas Psrl) defining these transcription factors as members of the MYB superfamily (Riechmann et al. 2000). In addition, the C-terminal portion of these proteins also contains nuclear localization sequences (NLSs), a prerequisite for playing a role of a transcription factor (Lohrmann et al. 2001).
In contrast, type-A RRs contain a receiver domain but lack the output domain necessary for transcription function. They respond rapidly to exogenous cytokinin and their expression is controlled by type-B RRs (Brandstatter and Kieber 1998; D’Agostino et al. 2000; Taniguchi et al. 2007). Transcript abundance of type-A RRs in response to CK is thus, at least in part, regulated on the transcriptional level. Analysis of ARR5::GUS transgenic plants revealed high expression in the root and shoot apical meristems, consistent with the idea that cytokinins are active in dividing cells (D’Agostino et al. 2000). The response to exogenous cytokinin is fast, and ARR5 transcript levels together with those of other RR (ARR4, ARR6, ARR7, ARR15 and ARR16) transcripts reach maximal induction 10 min following CK treatment. Generally, many type-A RRs are expressed particularly strongly in the root in response to changing CK levels providing a negative mechanism for attenuation of the cytokinin signalling output (To et al. 2004). The mechanism by which they exert their negative regulatory function is not well characterized, but likely involves phosphorylation-dependent interactions and a phosphocompetition relationship between type-A and type-B ARRs (To et al. 2007). Hence, it seems that the orchestrated action of type-B and type-A RRs mediates cell type-specific response in dividing meristematic cells and, in synergy with other signalling pathways, coordinates various aspects of root and shoot development. For example, for early phases of lateral root organogenesis (such as priming and initiation), the ectopic production of CK in the root basal meristem is required to secure development of lateral root primordia in root zones with a repressed cytokinin response (Bielach et al. 2012).
5.2 Aromatic Cytokinins Show Different Responses in Cytokinin Bioassays
Although aromatic cytokinins (ARCKs) were known for a relatively long time and initially assumed to occur as rare metabolic products in a limited number of plant species (Letham and Palni 1983), it was not until the 1990s that mT and its riboside (mTR) were detected in poplar leaves and their biological activity scrutinized (Strnad et al. 1992, 1997; Werbrouck et al. 1996; Holub et al. 1998). The surprising discovery that position isomers of topolin, oT and mT as well as their ribosides showed different biological properties stirred up a new interest in ARCKs and more detailed SAR studies. These results were centred around mT, the biologically active endogenous cytokinin form (Holub et al. 1998). While meta-position appeared to have a generally positive impact on the cytokinin signalling mediated by this ARCK, the corresponding ortho-derivative produced mostly negative effects.
Identification of cytokinin receptors and characterization of the cytokinin signalling pathway paved the way for the preparation of a new generation of synthetic CK derivatives and allowed us to generate new tools for precise quantification of the cytokinin signalling output. The ARR5::GUS was among the first reporter-based assays to allow for transcriptional output measurements elevated by CK (D’Agostino et al. 2000). Another assay based on the CRE1/AHK4 His-to-Asp phosphorelay system and assying its His-kinase activity in a mutant E. coli strain (ΔrcsC, cps::lacZ) was reported shortly afterwards (Suzuki et al. 2001; Yamada et al. 2001). This receptor together with Arabidopsis AHK2 and AHK3 are among the best characterized cytokinin receptors in the plant kingdom (Ueguchi et al. 2001b). In 2004, the bacterial assay previously used for the characterization of CRE1/AHK4 (Suzuki et al. 2001; Yamada et al. 2001) was utilized in the screening of ligand specificities of both CRE1/AHK4 and AHK3 receptors with a broad range of CKs and CK ribosides/ribotides (Spíchal et al. 2004). Consistent with the previous results, both receptors showed preference for the free bases of isoprenoid-type cytokinins (tZ and iP) but had different sensitivity to some ARCKs and CK ribosides and ribotides. Interestingly, both CRE1/AHK4 and AHK3 were considerably activated by mT, which produced responses comparable to those achieved by isoprenoid cytokinins. However, both receptors showed low sensitivity to oT. In addition, mT, but not oT, elicited high response in ARR5::GUS assay in Arabidopsis (Spíchal et al. 2004). These results have provided the first mechanistic evidence that mT interaction with Arabidopsis HKs, notably with AHK3, is most likely responsible for the high activity of this ARCK in the cytokinin bioassays.
Similarly, cytokinin-responsive HKs from maize (ZmHK1, ZmHK2 and ZmHK3a) were expressed in the above E. coli strain that conferred cytokinin-inducibility of lacZ expression on the transformed bacteria (Yonekura-Sakakibara et al. 2004). iP triggered strong response specifically in ZmHK1, while ZmHK2 showed higher sensitivity to tZ and tZR. ZmHK3a had similar preference for tZ, cZ, iP and BAP in the bacterial assay. Direct comparison of CRE1/AHK4 and ZmHK1 in the bacterial receptor assay further confirmed that only the mT isomer interacted with Arabidopsis CRE1/AHK4 receptor, while both mT and oT were found to be equally good ligands for maize ZmHK1 (Mok et al. 2005). However, the most striking difference between HKs of monocots and dicots seems to be their different affinity for cZ—only maize receptors recognized this ligand with significant affinity (Yonekura-Sakakibara et al. 2004; Mok et al. 2005). These findings clearly show that maize cytokinin HKs differ in ligand preference and that cZ is an active form of CK at least in monocot species.
An improved version of a live cell-based assay using transgenic bacteria expressing CK receptors was developed (Romanov et al. 2005, 2006). In this assay, radiolabelled tZ was used to quantitatively assess various kinetic parameters of cytokinin binding to CRE1/AHK4 and AHK3 receptors (Romanov et al. 2006). Using intact bacteria expressing a functional receptor, it was possible to characterize important parameters of ligand–receptor interaction, including affinity and ligand specificity of the individual CK interaction partners. Both CRE1/AHK4 and AHK3 shared high affinity for tZ, but AHK3 had an approximately tenfold lower affinity to iP (Romanov et al. 2006). On the other hand, the AHK3 receptor showed genuine affinity for CK ribosides, tZR and iPR, which are considered to represent cytokinin transport forms in the long-distance acropetal and basipetal cytokinin translocations between plant organs (Kudo et al. 2010). The receptor also recognized cZ and the non-adenine compound thidiazuron as its natural ligands. Affinities of both receptors to BAP were rather low, inconsistent with the view that this ARCK is one of the most potent cytokinins for in planta use; however, this was later put into a broader perspective by the same group using bacterially expressed CHASE domain of CRE1/AHK4 and AHK2 receptors (Stolz et al. 2011). These results together with known localization patterns of both receptors (CRE1/AHK4 expression being predominantly detected in roots and AHK3 in shoots) strongly suggested that AHK3 plays a specific function in root-to-shoot communications and that both time- and space-controlled gene expression and ligand preference are the key factors to specify the receptor activity.
5.3 Metabolism and Activity of Topolins and their Derivatives in Various Plant Models
A broad range of ARCK derivatives has been utilized in the past decades, and various substitutions on the purine backbone as well as on the benzyl side chain were designed and synthesized to test their specific modus operandi in both plants and human cells. In the first of these systematic structure–activity relationship studies, the authors prepared a chemical library of BAP derivatives with various substituents attached to the phenyl ring and tested their activity in standardized assays (Doležal et al. 2006, 2007). The high biological activity of many prepared compounds, notably their ability to delay the onset of senescence measured in detached wheat leaf senescence bioassay, contrasted with their relatively low responses in CRE1/AHK4 and AHK3 bacterial receptor assays. Several of these compounds also showed strong cytotoxic activity against various cancer cell lines while having negligible cytotoxicity to a normal murine fibroblast cell line (Doležal et al. 2007). Another SAR study firmly established the role of meta-substitution at the N6-benzyl side chain of BAP. 6-Benzylamino-9-tetrahydropyran-2-ylpurine (THP) and 6-benzylamino-9-tetrahydrofuran-2-ylpurine (THF) derivatives, with variously positioned hydroxy and methoxy functional groups on the aromatic side chain, were prepared and tested (Szüčová et al. 2009). The biological activity of THP derivatives was shown to be correlated to these substitutions, as follows: meta > ortho> > para.
6-(3-Methoxybenzylamino)purine (meta-methoxy topolin, MemT) derivatives with meta-methoxy substitution on the benzyl ring and tetrahydropyranyl or 4-chlorobutyl groups at the N9-position of the cytokinin purine backbone were further used in a detailed study on two model plants, Arabidopsis and maize (Podlešáková et al. 2012; Plíhal et al. 2013). These studies brought together data from model plants after exogenous treatments with detailed metabolic profiling and distribution of various CK forms throughout the plant body. It was found that THP substitution significantly enhanced acropetal transport of the parental CK and prevented unwanted N-glucosylation (Podlešáková et al. 2012). It is well-known that cytokinins including BAP actively regulate root meristem activity via modulation of cytokinin–auxin interactions by affecting auxin transport (Růžička et al. 2009) and that the cytokinin–auxin balance is also important for lateral root formation (Chang et al. 2013). More specifically, auxin influx carrier AUX1 is required for cytokinin-dependent changes in auxin levels in the lateral root cap associated with the control of cell elongation in concert with another plant hormone, ethylene (Street et al. 2016). It is possible that substitutions of BAP at N6- and N9-positions may prevent some negative effects of glucosylation and different metabolic pathways then lead to the accumulation of metabolites with different CK activities. In the case of mT exogenous treatment in the in vitro propagation of Spathiphyllum floribundum, (OG)[9R]mT was shown to be the main metabolic product that was degraded rapidly during acclimatization (Werbrouck et al. 1996). In contrast, when BAP was used in place of mT in the plant acclimatization, the predominant metabolic product was non-active [9G]BAP, which may be responsible for affecting cytokinin–auxin balance and eventually some negative effects on the primary root development, inhibition of lateral root branching, etc. Similarly, in the case of MemT and MemTTHP treatments in maize, the authors observed fast metabolic turnover of these ARCKs leading to a noticeable accumulation of (OG)mT and deprotected mT as early as 1 h post treatment (Podlešáková et al. 2012). The elevated endogenous concentrations of the active base mT corresponded well with slow gradual increase of the transcript levels of type-A RRs that was significant already 30 min following the application of MemT and a little delayed after MemTTHP treatment.
A deeper understanding of the specific role played by ARCKs can be obtained in comparative studies with various plant models, bearing in mind that CK levels vary significantly in a time- and place-dependent manner (diurnal or seasonal cycles, etc.). Only recently, the poplar species Populus × canadensis, cv. Robusta, the first organism found to contain ARCKs, was confirmed to have five CHASE-containing histidine kinase isoforms: PcHK2, PcHK3a, PcHK3b, PcHK4a and PcHK4b (Jaworek et al. 2019). While all these receptor histidine kinases showed strong affinity for tZ—the most abundant cytokinin form in poplar—and some also to iP, the kinase activity in the presence of the CK ligand differed significantly. PcHK4a displayed over 400-fold higher kinase activity compared to other HKs, which suggests a major role in responses to changing CK levels (Jaworek et al. 2019). Interestingly, another recent study on cytokinin-specific multistep phosphorelays in poplar also showed involvement of the above cytokinin-related HKs together with 10 HPts in the suggested cytokinin signalling pathway (Héricourt et al. 2019). A yeast complementation study with the above HKs again confirmed the functionality of all these, but only HK4a, was shown to have kinase activity dependent on the presence of CK.
Both studies also showed that all poplar HKs had strong expression in all tested plant organs, but HK4a and HK4b, however, seem to show different distribution suggesting possible functional diversification of these two paralogous genes. Live-cell bacterial assay was employed for cytokinin-binding studies with radiolabelled tZ (Jaworek et al. 2019). The cytokinin-binding strengths of all cytokinin-responsive HKs declined in the following order: tZ > iP > cZ. PcHK3a and PcHK3b exhibited weaker binding to iP-type cytokinins, cZ, BAP and oT than the other HKs. PcHK2 showed relatively good affinity to mT, iPR and tZR, which was significantly better than the affinity towards BAP. It seems that mT shows relatively good affinities to PcHK2, PcHK3a and PcHK3b isoforms, having Ki values in the range of approximately 20–60 nM, whereas oT represents a relatively poor ligand for all PcHK isoforms (Ki > 1000 nM). Additionally, PcHK4 receptors showed strong binding at pH 5.5, while PcHK3 receptors proved to be more active at pH 7.5, in line with their putative membrane localization at the plasma membrane and in the membrane of the endoplasmic reticulum, respectively.
Abbreviations
- [9G]:
-
9-β-d-glucopyranosyl
- [9R]:
-
9-β-d-ribofuranosyl
- 3MeOBA9THPP:
-
3-methoxy(6-benzylamino-9-tetrahydropyran-2-yl)purine
- ARCK:
-
Aromatic cytokinin
- BAP:
-
6-benzylaminopurine
- CK:
-
Cytokinin
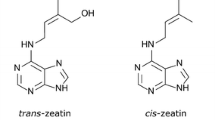
- cZ:
-
cis-zeatin
- HK:
-
Receptor histidine kinase
- iP:
-
N6-isopentenyladenine
- mT:
-
meta-topolin, 6-(3-hydroxybenzylamino)purine
- mTR:
-
meta-topolin riboside, 6-(3-hydroxybenzylamino)purine-9-riboside
- oT:
-
ortho-topolin, 6-(2-hydroxybenzylamino)purine
- oTR:
-
ortho-topolin riboside, 6-(2-hydroxybenzylamino)purine-9-riboside
- SAR:
-
Structure–activity relationship
- THF:
-
Tetrahydrofuran-2-yl
- THP:
-
Tetrahydropyran-2-yl
- tZ:
-
trans-zeatin
- tZR:
-
trans-zeatin-9-riboside
References
Bielach A, Podlešáková K, Marhavý P, Duclercq J, Cuesta C, Müller B, Grunewald W, Tarkowski P, Benková E (2012) Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell 24:3967–3981
Brandstatter I, Kieber JJ (1998) Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in Arabidopsis. Plant Cell 10:1009–1019
Chang L, Ramireddy E, Schmülling T (2013) Lateral root formation and growth of Arabidopsis is redundantly regulated by cytokinin metabolism and signalling genes. J Exp Bot 64:5021–5032
D’Agostino IB, Deruère J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol 124:1706–1717
Doležal K, Popa I, Kryštof V, Spíchal L, Fojtíková M, Holub J, Lenobel R, Schmülling T, Strnad M (2006) Preparation and biological activity of 6-benzylaminopurine derivatives in plants and human cancer cells. Bioorg Med Chem 14:875–884
Doležal K, Popa I, Hauserová E, Spíchal L, Chakrabarty K, Novák O, Kryštof V, Voller J, Holub J, Strnad M (2007) Preparation, biological activity and endogenous occurrence of N6-benzyladenosines. Bioorg Med Chem 15:3737–3747
Dortay H, Mehnert N, Bürkle L, Schmülling T, Heyl A (2006) Analysis of protein interactions within the cytokinin-signaling pathway of Arabidopsis thaliana. FEBS J 273:4631–4644
Héricourt F, Larcher M, Chefdor F, Koudounas K, Carqueijeiro I, Lemos Cruz P, Courdavault V, Tanigawa M, Maeda T, Depierreux C, Lamblin F, Glévarec G, Carpin S (2019) New insight into HPts as hubs in poplar cytokinin and osmosensing multistep phosphorelays: cytokinin pathway uses specific HPts. Plants (Basel) 8:591
Holub J, Hanuš J, Hanke DE, Strnad M (1998) Biological activity of cytokinins derived from ortho- and meta-hydroxybenzyladenine. Plant Growth Regul 26:109–115
Hosoda K, Imamura A, Katoh E, Hatta T, Tachiki M, Yamada H, Mizuno T, Yamazaki T (2002) Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. Plant Cell 14:2015–2029
Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, Maxwell BB, Perdue TD, Schaller GE, Alonso JM, Ecker JR, Kieber JJ (2006) The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 18:3073–3087
Hwang I, Sheen J (2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413:383–389
Hwang I, Sheen J, Müller B (2012) Cytokinin signaling networks. Annu Rev Plant Biol 63:353–380
Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409:1060–1063
Jaworek P, Tarkowski P, Hluska T, Kouřil S, Vrobel O, Nisler J, Kopečný D (2019) Characterization of five CHASE-containing histidine kinase receptors from Populus x canadensis cv. Robusta sensing isoprenoid and aromatic cytokinins. Planta 251:1
Kieber JJ, Schaller GE (2018) Cytokinin signaling in plant development. Development 145:1–7
Kudo T, Kiba T, Sakakibara H (2010) Metabolism and long-distance translocation of cytokinins. J Integr Plant Biol 52:53–60
Letham DS, Palni LMS (1983) The biosynthesis and metabolism of cytokinins. Annu Rev Plant Physiol 34:163–197
Lohrmann J, Sweere U, Zabaleta E, Bäurle I, Keitel C, Kozma-Bognar L, Brennicke A, Schäfer E, Kudla J, Harter K (2001) The response regulator ARR2: a pollen-specific transcription factor involved in the expression of nuclear genes for components of mitochondrial complex I in Arabidopsis. Mol Genet Genomics 265:2–13
Mok MC, Martin RC, Dobrev PI, Vaňková R, Ho PS, Yonekura-Sakakibara K, Sakakibara H, Mok DW (2005) Topolins and hydroxylated thidiazuron derivatives are substrates of cytokinin O-glucosyltransferase with position specificity related to receptor recognition. Plant Physiol 137:1057–1066
Pekárová B, Klumpler T, Třísková O, Horák J, Jansen S, Dopitová R, Borkovcová P, Papoušková V, Nejedlá E, Sklenář V, Marek J, Zídek L, Hejátko J, Janda L (2011) Structure and binding specificity of the receiver domain of sensor histidine kinase CKI1 from Arabidopsis thaliana. Plant J 67:827–839
Plíhal O, Szüčová L, Galuszka P (2013) N9-substituted aromatic cytokinins with negligible side effects on root development are an emerging tool for in vitro culturing. Plant Signal Behav 8:e24392
Podlešáková K, Zalabák D, Čudejková M, Plíhal O, Szüčová L, Doležal K, Spíchal L, Strnad M, Galuszka P (2012) Novel cytokinin derivatives do not show negative effects on root growth and proliferation in submicromolar range. PLoS One 7:e39293
Riechmann JL, Heard J, Martin G, Reuber L, Jiang C, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, Creelman R, Pilgrim M, Broun P, Zhang JZ, Ghandehari D, Sherman BK, Yu G (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290:2105–2110
Romanov GA, Spíchal L, Lomin SN, Strnad M, Schmülling T (2005) A live cell hormone-binding assay on transgenic bacteria expressing a eukaryotic receptor protein. Anal Biochem 347:129–134
Romanov GA, Lomin SN, Schmülling T (2006) Biochemical characteristics and ligand-binding properties of Arabidopsis cytokinin receptor AHK3 compared to CRE1/AHK4 as revealed by a direct binding assay. J Exp Bot 57:4051–4058
Růžička K, Šimášková M, Duclercq J, Petrášek J, Zažímalová E, Simon S, Friml J, Van Montagu MC, Benková E (2009) Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci U S A 106:4284–4289
Spíchal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmülling T (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol 45:1299–1305
Stolz A, Riefler M, Lomin SN, Achazi K, Romanov GA, Schmülling T (2011) The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors. Plant J 67:157–168
Street IH, Mathews DE, Yamburkenko MV, Sorooshzadeh A, John RT, Swarup R, Bennett MJ, Kieber JJ, Schaller GE (2016) Cytokinin acts through the auxin influx carrier AUX1 to regulate cell elongation in the root. Development 143:3982–3993
Strnad M, Peters W, Beck E, Kamínek M (1992) Immunodetection and identification of N6-(o-hydroxybenzylamino) purine as a naturally occurring cytokinin in Populus x canadensis Moench cv. Robusta leaves. Plant Physiol 99:74–80
Strnad M, Hanuš J, Vaněk T, Kamínek M, Ballantine JA, Fussell B, Hanke DE (1997) Meta-topolin, a highly active aromatic cytokinin from poplar leaves (Populus x canadensis Moench, cv. Robusta). Phytochemistry 45:213–218
Suzuki T, Miwa K, Ishikawa K, Yamada H, Aiba H, Mizuno T (2001) The Arabidopsis sensor His-kinase, AHK4, can respond to cytokinins. Plant Cell Physiol 42:107–113
Szüčová L, Spíchal L, Doležal K, Zatloukal M, Greplová J, Galuszka P, Kryštof V, Voller J, Popa I, Massino FJ, Jørgensen JE, Strnad M (2009) Synthesis, characterization and biological activity of ring-substituted 6-benzylamino-9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-ylpurine derivatives. Bioorg Med Chem 17:1938–1947
Taniguchi M, Sasaki N, Tsuge T, Aoyama T, Oka A (2007) ARR1 directly activates cytokinin response genes that encode proteins with diverse regulatory functions. Plant Cell Physiol 48:263–277
To JP, Kieber JJ (2008) Cytokinin signaling: two-components and more. Trends Plant Sci 13:85–92
To JP, Haberer G, Ferreira FJ, Deruère J, Mason MG, Schaller GE, Alonso JM, Ecker JR, Kieber JJ (2004) Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16:658–671
To JP, Deruère J, Maxwell BB, Morris VF, Hutchison CE, Ferreira FJ, Schaller GE, Kieber JJ (2007) Cytokinin regulates type-A Arabidopsis response regulator activity and protein stability via two-component phosphorelay. Plant Cell 19:3901–3914
Ueguchi C, Sato S, Kato T, Tabata S (2001a) The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant Cell Physiol 42:751–755
Ueguchi C, Koizumi H, Suzuki T, Mizuno T (2001b) Novel family of sensor histidine kinase genes in Arabidopsis thaliana. Plant Cell Physiol 42:231–235
Werbrouck SPO, Strnad M, Van Onckelen H, Debergh PC (1996) Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol Plant 98:291–297
Yamada H, Suzuki T, Terada K, Takei K, Ishikawa K, Miwa K, Yamashino T, Mizuno T (2001) The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol 42:1017–1023
Yamada H, Koizumi N, Nakamichi N, Kiba T, Yamashino T, Mizuno T (2004) Rapid response of Arabidopsis T87 cultured cells to cytokinin through His-to-Asp phosphorelay signal transduction. Biosci Biotechnol Biochem 68:1966–1976
Yonekura-Sakakibara K, Kojima M, Yamaya T, Sakakibara H (2004) Molecular characterization of cytokinin-responsive histidine kinases in maize. Differential ligand preferences and response to cis-zeatin. Plant Physiol 134:1654–1661
Zdarska M, Cuyacot AR, Tarr PT, Yamoune A, Szmitkowska A, Hrdinová V, Gelová Z, Meyerowitz EM, Hejátko J (2019) ETR1 integrates response to ethylene and cytokinins into a single multistep phosphorelay pathway to control root growth. Mol Plant 12:1338–1352
Acknowledgement
The work was supported by an ERDF project entitled “Development of Pre-Applied Research in Nanotechnology and Biotechnology” (No. CZ.02.1.01/0.0/0.0/17_048/0007323). The author of this review is grateful to Alexander Oulton, Ph.D., for his critical reading of the chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Plíhal, O. (2021). Cytokinin Signalling and Mechanism of Action of Meta-Topolin and Its Derivatives. In: Ahmad, N., Strnad, M. (eds) Meta-topolin: A Growth Regulator for Plant Biotechnology and Agriculture. Springer, Singapore. https://doi.org/10.1007/978-981-15-9046-7_5
Download citation
DOI: https://doi.org/10.1007/978-981-15-9046-7_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9045-0
Online ISBN: 978-981-15-9046-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)