Abstract
Steroid hormones are associated with the regulation of various processes in fish, like embryonic development, sex differentiation, metabolism, immune responses, circadian rhythms, stress response, and reproduction. Steroids in fish are generally classified into C21, C19, and C18 steroids based on their structure. These steroids like estrogens and androgens are used in fish farming to increase fish production based on sexual dimorphism. Progesterone (P4), 17,20β-dihydroxy-4-pregnen-3-one (17,20βP or MIH or DHP), 17,20β,21-trihydroxy-4-pregnen-3-one (20βS) and 11-deoxycortisol (S) are some of the major C21 steroids that are essential for gonadal maturation and production of other endogenous steroids. The C19 steroids, i.e. testosterone (T), 17α-Methyltestosterone (MT), and 11-Ketotestosterone (11-KT) classified as androgens help in fish spermatogenesis and C18 steroids, called as Estranes, are known as female hormones. Except for the role of steroids in fish reproduction they have a major role in immunity, puberty, and stress. Corticosteroids, a major C21 steroid, are associated with stress response in fish. Steroids like, 17β-estradiol (E2), 11KT, medroxyprogesterone, 17α,20β-dihydroxy-4-pregnen-3-one (DHP), are associated with fish adaptive and innate immunity response. Similarly, 11KT is a major steroid for fish puberty. At present, further insights are required in the field of synthetic steroids in fish and their impacts over various roles in fish physiology and future economic importance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
There are different reproductive strategies possessed by fishes with respect to their aquatic environment in which they adopt. But still some regulatory processes related to reproduction are conserved among fishes. In the early phase of females reproductive cycle, i.e. growth of oocytes is triggered by the estradiol hormone, whereas in the late phase, i.e. oocyte maturation prior to ovulation is influenced by the maturation inducing steroid (MIS) (Tokarz et al. 2015). In the case of males, 11 ketotestosterone (11-KT) induces spermatogenesis, whereas the maturation of spermatocytes by initiation meiotic cell divisions is done by MIS (Mananós et al. 2008). The pituitary hormones, i.e. follicle-stimulating hormone (FSH) and luteinizing hormone (LH) play an important role in reproduction by the downstream processes of gonadal steroid hormone synthesis. In the case of commercially important fish species, the ultimate goal for farmers is to produce year-round seed through induced breeding technique. So the development of induced breeding technique is always a challenge for fish breeders and researchers. Steroids like C21 steroids, C19 steroids, C18 steroids, and some endocrine disrupting chemicals (EDCs) are the key role players for gonadal steroidogenesis and ultimately ovulation/spermiation. Among these, some important EDCs are Kepone (Chlordecone), o,p-DDD, Diethylstilbestrol (DES), etc.
Synthesis of Steroid Hormones
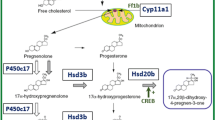
Hypothalamus–pituitary–interrenal and hypothalamus-pituitary-gonadal axis regulate steroid biosynthesis in fishes (Fig. 4.1). The cholesterol acts as the precursor for the de novo synthesis of all classes of steroid hormones. The removal of the side chain of cholesterol by Cytochrome p450 enzyme (Cyp11a1) produces pregnenolone and this process is regulated by steroidogenic acute regulatory protein (StAR). The StAR also helps in transfer of cholesterol across the barrier of the outer and inner mitochondrial membrane. Several enzymes modify the steroid nucleus including side-chain cleavage, Δ5/Δ4-isomerization, hydrogenation, aromatization, hydroxylation, reduction, or oxidation, downstream of this synthesis pathway. The three important bottlenecks in the process of steroidogenesis are cytochrome p450 enzymes cholesterol side-chain cleavage (cyp11a1), 17α-hydroxylase/lyase (cyp17a), and aromatase (cyp19a1). The Cyp11a1 is the only enzyme that converts cholesterol to pregnenolone and that is why regarded as the entrance of the steroidogenesis pathway. Similarly, the Cyp17a is the only enzyme responsible for the conversion of C21 steroids to C19 steroids and that is why regarded as the next bottleneck in the pathway. This enzyme can use a variety of substrates, but the two most important products (17α-hydroxyprogesterone and androstenedione) cannot be synthesized by other enzymes. The Cyp19a1 i.e. responsible for the production of C18 steroids, is regarded as the most important enzyme for hormonal control of sexual development in teleost fishes (Tokarz et al. 2015).
17β-hydroxysteroid dehydrogenase type 3 (hsd17b3) is an essential enzyme for the synthesis of 11-ketotestosterone, the active androgen in fish, whereas type 1 (hsd17b1) converts inactive estrone (E1) to active receptor-binding estradiol (E2). 20β-hydroxysteroid dehydrogenase (Hsd20b) converts 17α-hydroxyprogesterone and 11-deoxycortisol to maturation inducing steroids (MIS) 17α,20β-dihydroxy-4-pregnen-3-one (17,20β-P, or DHP) and 17α,20β,21-trihydroxy-4-pregnen-3-one (20β-S), respectively. The hsd20b has so far been the only gene identified in fishes among the genes responsible for MIS synthesis.
Steroid Hormone Receptors
Receptors for steroid hormones are either nuclear or membrane bound. These hormones may act in a genomic or a non-genomic manner depending on the receptor. In the case of nuclear receptors, steroid hormones bind to the respective cytosolic nuclear receptors and act in a genomic mode by binding to their respective response elements on the genomic DNA. The membrane-bound receptors on the cell surface control the non-genomic action of steroid hormones to initiate rapid intracellular responses. Some receptors with their ligand binding and mode of action have been given in Table 4.1.
C18 Steroids
C18 steroids are characterized by the presence of an aromatic ring in their structure. These are classified into the class of steroids called Estranes. Estradiol is the major C18 steroid used in the case of fish maturation studies in females (Fig. 4.1). During oocyte growth, follicular cells receive the pituitary hormone FSH by FSH receptor and secrete estradiol-17β into the circulation. The hepatic synthesis of yolk precursor, vitellogenin (Vg) is due to estradiol-17β. The estradiol-17β travels to the liver via blood and helps in the production of Vg (Fig. 4.1). The Vg is then transported to the ovary via the bloodstream and is selectively taken up into the oocyte by specific cell surface receptors. The Vg helps in yolk globules increase and gonadotropin helps in the uptake of Vg by the oocytes. The oocytes acquire yolk protein after completion of the initial growth phase and become competent due to the action of gonadotropins, activins, and other factors (Busby et al. 2010). The estradiol levels are decreased or downregulated due to the downregulation of ovarian aromatase, when the process of oocyte growth and vitellogenesis completes (De Kime 1993).
In the case of males, estradiol-17β plays an important role in spermatogonial renewal like other vertebrates but has no role in proliferation and meiosis. Sex reversal or production of the mono-sex population has been achieved for commercial benefit using 17β-estradiol orally or through the water. The fish spawn is administered with 17β-estradiol to produce 100% female population. This is beneficial for Salmon and Carps, as female is more beneficial from the economic point of view in these groups. These steroids are also easy to administer through feed and gives 100% results. However, for inducing differentiated testis to the ovary, exogenous estrogen alone is not enough. So, the testicular trans-differentiation can be induced by the administration of E2 and simultaneous blockage of androgen synthesis. Recently, genome editing technologies have revealed the disruption of Cyp19a1a in induced testicular development, which indicates this to be a key gene for estrogen synthesis and differentiation or maintenance of ovary.
C19 Steroids
The C19 steroids include androgens like testosterone (T) and 11-Ketotestosterone (11-KT). These are major male steroids and have been classified as Androstanes. These are especially male specific (Fig. 4.1). 11-KT was first identified as a major androgenic steroid in the male sockeye salmon (Oncorhynchus nerka). FSH stimulates the production of spermatogenesis-inducing steroid, 11-KT during the initiation of spermatogonial proliferation. FSH can induce the production of 11-KTin testis in vitro (Schulz et al. 2010). The 11-KT is involved in the initiation of spermatogonial proliferation towards meiosis. 11-KT initiates spermatogonial proliferation by two members of the TGFβ superfamily, anti-Müllerian hormone (AMH) (Miura et al. 2002) and activin B (Miura et al. 1995). AMH inhibits differentiation of spermatogonia and expression of AMH is suppressed by KT. Activin B induces the proliferation of spermatogonia. Synthesis of activin B is induced by 11-KT. The seasonal changes in the plasma level due to increased spawning maturation and peak levels at the onset of the spawning period is shown by 11 KT (Borg 1994).
The synthetic steroid 17α-methyltestosterone (MT) is commonly included in the fish feed to produce male populations. The administration of MT has stimulated spermatogenesis and steroidogenesis in a number of fish species. Moreover, sex reversal or production of the mono-sex population can be achieved for commercial benefit using MT orally or through the water. The fish spawn is administered with MT to produce the all-male population. This is beneficial for Tilapia and giant freshwater prawn economy.
C21 Steroids
Progesterone (P4), 17,20β-dihydroxy-4-pregnen-3-one (17,20βP or MIH or DHP), 17,20β,21-trihydroxy-4-pregnen-3-one (20βS), and 11-desoxycortisol (S) are major C21 steroids of this group which have been under research in fish reproduction. Progesterone is an endogenous steroid involved in oocyte maturation. It is also called Pregn-4-ene-3,20-dione and its chemical formula is C21H30O2. It is an important metabolic intermediate in the production of other endogenous steroids and helps in brain function as a neurosteroid and is not a biologically active steroid in the case of fish. 17,20βP or MIH has its chemical formula as C21H32O3 and is the most potent steroid for inducing final oocyte maturation in several species of fish. Similarly, 20βS and S have a chemical formula as C25H36O6and C21H30O4, respectively. S produces this 20βS with the help of 20β-hydroxysteroid dehydrogenase (20β-HSD) in ovarian follicular cells due to the action of pituitary gonadotropin (Ogino et al. 2016) and stimulates the meiotic maturation of oocytes during GVBD. So C21 steroids are of importance in both male and female (Fig. 4.1).
Due to the downregulation of aromatase estradiol concentrations drops. LH stimulates the synthesis of hsd20b in the granulosa cells resulting in the synthesis of the MIS. MIS initiates the processes of oocyte maturation (Nagahama and Yamashita 2008). The substrate for Hsd20b, 17α-hydroxyprogesterone, is provided by the thecal cells. The orders of fish, i.e. Salmoniformes, Cypriniformes, Cyprinodontiformes, Siluriformes, Beloniformes, Esociformes, Osteoglossiformes, and Clupeiformes possess DHP, whereas in many Perciformes group fishes 20β-S acts as the MIS (Tokarz et al. 2015). The intracellular signaling is triggered and in turn stimulates all the processes of oocyte maturation when the MIS binds to mPRs. These processes include germinal vesicle breakdown with respect to the first meiotic cell division, spindle formation, chromosome condensation and formation of the first polar body. The MIS regulates ovulation by genomic mechanisms, thus utilizing a nuclear PR and this was earlier hypothesized by Nagahama and Yamashita (2008).
After gametogenesis, the 11-KT level decreases and this results in increased synthesis of the MIS under the action of the pituitary hormone LH. As seen in case of females, MIS stimulates the maturation of spermatocytes by initiating meiotic cell divisions. The production of seminal fluid by the efferent ducts along with the enhancement of sperm motility by alteration of pH and fluidity of the seminal fluid is done by MIS. The milt formation occurs when the matured spermatozoa are released into the seminal fluid and this is stored until the process of spawning (Mananós et al. 2008).
Steroids in Puberty
The transition or transformation from an immature juvenile to a mature adult in the reproductive cycle, i.e. when the fish becomes capable of reproducing is called puberty. The rapid proliferation of spermatogonia in males and the entry into the lipid droplet stage of oocyte development (Sundararaj et al. 1972) are the first stages requiring pituitary input in fish. While defining the endpoint of puberty process, it can be said that the first successful reproduction or the production of the first batch of fertile gametes, i.e. spermiation and sperm hydration in males; ovulation in females (Okuzawa 2002).
11-KT is a firm candidate for the regulation of the onset of puberty in teleosts (Cavaco et al. 1998). The stimulation of Sertoli cells to produce growth factors, thus promoting spermatogonial proliferation leading to meiosis and spermatogenesis is by this 11-KT. Whereas E2 helps in the first pituitary-dependent stage of ovarian development, the lipid droplet stage and increased cortical alveoli stage leading to puberty. The start of puberty is considered as the transition to the first wave of rapid spermatogonial proliferation or to the first batch of oocytes accumulating cortical alveoli, which is regulated by Fsh. The Fsh express the fshr gene and that is why can directly stimulate Leydig cells. The up-regulation of expression of pituitary fshβ and ovarian fshr gene starts prior to vitellogenesis followed by the accumulation of cortical alveoli and continued through vitellogenesis (Guzmán et al. 2014). The Fsh signaling is important during the accumulation of cortical alveoli in oocytes in the early stages of puberty in fish (Taranger et al. 2010; Rather et al. 2016).
Steroids in Immunity
The immune system of teleost fish possesses both innate and adaptive immune responses. In the case of innate immunity, the epithelium and mucosal tissues act as the physical barriers for fish, while phagocytes, i.e. granulocytes and macrophages, non-specific cytotoxic cells, and eosinophilic cells, including mast cells, represent the cellular effectors. A variety of other molecules like the acute phase proteins, natural antibodies, cytokines, etc. act for the humoral immune response. The adaptive immune response comprises lymphocytes, i.e. B and T cells that acts as cellular components and immunoglobulins (Ig) acts as the humoral component.
Estrogens regulate the immune system of fish and several leukocyte functions. An increase in serum 17β-estradiol (E2) levels promotes the movement of acidophilic granulocytes from the head kidney after an inflammation response (Chaves-Pozo et al. 2018). In the case of head kidney phagocytes, E2 also enhances the inflammation by increasing the production of a pro-inflammatory cytokine and the interleukin-1β (IL1β). As compared to the effect of E2, the androgens on the immune system of fish have been less studied. Moreover, testosterone causes a reduction in IgM secreting cells in peripheral blood, head kidney, spleen, and skin leukocytes. Similarly, 11-KT decreases the number and capability of IgM secreting cells of the spleen, head kidney, blood, and skin. Moreover, 11-KT alone, or in combination with testosterone, downregulates immunogenic expression in macrophages. Interestingly, the innate immune system of fish is stimulated by testosterone and upregulates expression of interleukin 1β, il1b, and some tlr genes.
The production of T-cell derived factors are inhibited by the action of progestins. The E2 pro-inflammatory effects are seen in several tissues like injured vessels, endometrium, and cervix. The Progestin medroxyprogesterone (MPA) blocks this E2 pro-inflammatory effect. The activity of both DHP and MPA inhibits the nitric oxide release by activated leukocytes and downregulates the transcription of immune related-factors of pro-inflammatory type I.
Another important aspect related to fish immune response and stress indicator for fish is the release of cortisol into the bloodstream. Cortisol regulates immunity as well as reproduction. The pathways, through which, steroids and cortisol work, are interconnected. The sensitivity of leukocytes to sex steroids is controlled by cortisol and stress conditions, by the regulation of transcription and production of nuclear estrogen receptors (ESRs), G protein-coupled estrogen receptor (GPER1), and local aromatase.
Steroids in Stress
Physiological responses of fish to stressors are of two types, i.e. primary responses and secondary responses. Primary responses include the initial neuroendocrine responses, i.e. release of catecholamines from chromaffin tissue and the stimulation of the hypothalamic-pituitary-interrenal (HPI) axis for the release of corticosteroid hormone. Secondary responses are related to the changes in plasma, tissue ion and metabolite levels, and heat-shock or stress proteins (HSPs), i.e. finally related to metabolism, respiration, immune function, cellular responses, etc. Primary responses are mainly related to steroids.
When fish are exposed to a stressor, the central nervous system (CNS) perceives the response. As a result of this the chromaffin cells, i.e. located in the anterior kidney, release catecholamines via cholinergic receptors. Later cortisol releases from the HPI axis with the help of corticotropin-releasing hormone (CRH) and adrenocorticotropin (ACTH). ACTH in turn stimulates the interrenal cells in the kidney to release corticosteroids. The cortisol and 1α-hydroxycorticosterone are the major corticosteroids in fish (Ruiz-Jarabo et al. 2019). Naturally occurring corticosteroids are cortisol (C21H30O5), corticosterone (C21H30O4), cortisone (C21H28O5), and aldosterone (C21H28O5). All the corticosteroids are C21 steroids. All these corticosteroids help in two ways, i.e. a glucocorticoid function for metabolism and growth, and a mineralocorticoid function for the transport of ions and water. But fish lacks aldosterone as a mineralocorticoid and instead 11-deoxycorticosterone is present. This 11-deoxycorticosteroid is an important part of ovarian or testicular steroidogenesis. The gonadal corticosteroidogenesis process is mediated by the presence of cortisol and 11-deoxycortisol in the ovary, sperm, and seminal fluid.
At present, pollutions from pharmaceutical companies are releasing synthetic corticosteroids to the aquatic environment which can have adverse effects on fish. Many synthetic corticosteroids, i.e. betamethasone, prednisone, prednisolone, triamcinolone, etc. need to be studied in fish for their impact on stress and reproduction.
Conclusion and Future Direction
The steroidogenesis process in case of fish and the steroids like C21 steroids, C19 steroids, C18 steroids, and some Endocrine Disrupting Chemicals (EDCs) are important for ovulation / spermiation. These steroid hormones are the key for induced breeding techniques in fishes. The C18 steroids in case of female, C19 steroids in case of male, and C21 steroids in the pathway of both oocyte maturation and spermiation are important. In the near future synthetic and eco-friendly use of steroids and the researches based on them are required in case of fish. Mono-sex culture of commercially important fish species and the successful application of synthetic steroids are of concern at present. Moreover, the residual effects from industrial synthetic steroid pollutants need to be stopped for conservation of riverine fish stocks by avoiding their sterility. Role of steroids in immunity, puberty, and stress needs to be studied further for new outcomes in the field of aquaculture.
References
Borg, B. (1994). Androgens in teleost fishes. Comparative Biochemistry and Physiology, 109C, 219–245.
Busby, E. R., Roch, G. J., & Sherwood, N. M. (2010). Endocrinology of zebrafish: A small fish with a large gene pool. Fish Physiology, 29, 173–247.
Cavaco, J. E. B., Vilrokx, C., Trudeau, V. L., Schulz, R. W., & Goos, H. J. (1998). Sex steroids and the initiation of puberty in male African catfish (Clarias gariepinus). American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 275(6), R1793–R1802.
Chaves-Pozo, E., García-Ayala, A., & Cabas, I. (2018). Effects of sex steroids on fish leukocytes. Biology (Basel), 7: 9.
Guzmán, J. M., Luckenbach, J. A., Yamamoto, Y., & Swanson, P. (2014). Expression profiles of Fsh-regulated ovarian genes during oogenesis in coho salmon. PLoS One, 9(12), e114176.
Hammes, S. R., & Levin, E. R. (2007). Extranuclear steroid receptors: Nature and actions. Endocrine Reviews, 28(7), 726–741.
Kime, D. E. (1993). Classical’ and ‘non-classical’ reproductive steroids in fish. Reviews in Fish Biology and Fisheries, 3, 160–180.
Mananós, E., Duncan, N., & Mylonas, C. C. (2008). Reproduction and control of ovulation spermiation and spawning in cultured fish. In E. Cabrita, V. Robles, & P. Herráez (Eds.), Methods in reproductive aquaculture: Marine and freshwater species (pp. 3–80). Boca Raton: CRC Press.
Miura, T., Miura, C., Konda, Y., & Yamauchi, K. (2002). Spermatogenesis-preventing substance in Japanese eel. Development, 129, 2689–2697.
Miura, T., Miura, C., Yamauchi, K., & Nagahama, Y. (1995). Human recombinant activin induces proliferation of spermatogonia in vitro in the Japanese eel (Anguilla japonica). Fisheries Science, 61, 434–437.
Nagahama, Y., & Yamashita, M. (2008). Regulation of oocyte maturation in fish. Development, Growth & Differentiation., 50(Suppl. 1), S195–S219. https://doi.org/10.1111/j.1440-169X.2008.01019.x.
Ogino, Y., Miyagawa, S., & Iguchi, T. (2016). Subchapter 94C—17,20β,21-Trihydroxy-4-pregnen-3-one. In Y. Takei, H. Ando, & K. Tsutsui (Eds.), (2015), Handbook of hormones: Comparative endocrinology for basic and clinical research (pp. 511–512). Academic Press.
Okuzawa, K. (2002). Puberty in teleosts. Fish Physiology and Biochemistry, 26, 31–41.
Rather, M. A., Bhat, I. A., Sharma, N., Sharma, R., Chaudhari, A., & Sundaray, J. K. (2016). Molecular characterization, tissue distribution of Follicle-Stimulating Hormone (FSH) beta subunit and effect of kisspeptin-10 on reproductive hormonal profile of Catla catla (Hamilton, 1822). Aquaculture Research, 47(7), 2089–2100.
Ruiz-Jarabo, I., Barragán-Méndez, C., Jerez-Cepa, I., Fernández-Castro, M., Sobrino, I., Mancera, J. M., et al. (2019). Plasma 1α-hydroxycorticosterone as biomarker for acute stress in catsharks (Scyliorhinus canicula). Frontiers in Physiology, 10, 1217.
Schulz, R. W., de França, L. R., Lareyre, J. J., LeGac, F., Garcia, H. C., Nobrega, R. H., et al. (2010). Spermatogenesis in fish. General and Comparative Endocrinology, 165(3), 390–411.
Sundararaj, B. I., Anand, T. C., & Donaldson, E. M. (1972). Effects of partially purified salmon pituitary gonadotropin on ovarian maintenenace, ovulation, and vitellogenesis in the hypophysectomized catfish, Heteropneustes fossilis (Bloch). General and Comparative Endocrinology, 18, 102–114.
Taranger, G. L., Carrillo, M., Rüdiger, W., Fontaine, S. P., Zanuy, S., Felip, A., et al. (2010). Control of puberty in farmed fish. General and Comparative Endocrinology, 165(3), 483–515.
Tokarz, J., Möller, G., de Angelis, M. H., & Adamski, J. (2015). Steroids in teleost fishes: A functional point of view. Steroids, 103, 123–144.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Tripathy, P.S., Parhi, J., Mandal, S.C. (2021). Steroids and Its Receptors in Fish Reproduction. In: Sundaray, J.K., Rather, M.A., Kumar, S., Agarwal, D. (eds) Recent updates in molecular Endocrinology and Reproductive Physiology of Fish. Springer, Singapore. https://doi.org/10.1007/978-981-15-8369-8_4
Download citation
DOI: https://doi.org/10.1007/978-981-15-8369-8_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-8368-1
Online ISBN: 978-981-15-8369-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)