Abstract
Recently, graphene-based nanocomposites were reported by several researchers in various fields such as gas sensors, lithium-ion batteries, and photodetectors. The gas sensor is useful in detecting the harmful chemicals and toxic gases for the protection of human health and the environment. A recent, some researchers have reported that the gas sensors made of GO or rGO with the addition of metal oxide enhance the sensing properties of the sensor. In this context, attempts are made in reviewing various gas sensors based on graphene and its derivatives based metal-oxide nanocomposite exhibiting different sensing properties for different gases like NO2, NH3, H2, LPG gas. Different synthesis methods and the results obtained from different characterization techniques for various gases were also discussed. Characterization techniques such as SEM, TEM, XRD, FTIR, UV, and CV were depicted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
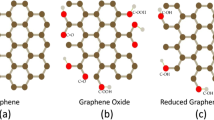
Graphene, a two-dimensional (2D) carbon nanostructure having a crystal structure of single-atom-thick sheet of sp2 hybridized carbon atoms that are packed in a hexagonal honeycomb, has become a new approach for the application of gas sensor due to its unique properties such as its electric, mechanical, and optical properties along with large surface area [1]. To design a gas sensor achieving high sensing performance like highly reproduction stability, sensitivity, and selectivity and fast sensing response and recovery time, graphene having large surface area combining with the sensitive mass device may be considered as the best option [2]. Graphene is considered as a promising candidate for gas sensors because of the absorption and desorption of the gas molecules which may lead to affect its electronic properties in a strong way regarding produce crystal lattice exhibiting high quality which creates quite low electronic noise along with good sensing area per unit volume forming the structure of 2D. There are various techniques to synthesized graphene. Mechanical exfoliation is the method in which single-layer graphene sheets are obtained but in this method, the production of layers cannot be controlled. Graphene synthesized by epitaxial growth and chemical vapor deposition (CVD) techniques are considered as the effective techniques for bottom-up synthesis of high-quality graphene sheets [3, 4] chemically synthesis of graphene oxide from graphite and the reduction of GO using chemical or thermal method are considered to be top-down approach which can produce a gas sensor to detect harmful or toxic gases [5, 6]. The graphene-based gas sensor was first reported by Schedin et al. [7] for the detection of gas molecules individually. It was observed that the sensor responses when the gas molecule attached or detached from the surface of the graphene. When there is adsorption of gas molecule, the local carrier concentration of graphene changes leading to a slight change in resistance. The author suggests that graphene as a low-noise material. Further, it was found that its resistance changes depending on the carrier concentration in graphene due to gas molecule's adsorption and further under vacuum, the sensor was annealed at 150 °C. They found the main drawback of having long recovery time which was further explained that it might have occurred due to the strong interaction of graphene and gas molecules at room temperature. To achieve fast response recovery time, external heat treatment should be given or UV illumination is needed. Yavari et al. [8] synthesized graphene films by using the CVD method for the utilization of the sensor to detect NH3 and NO2 gasses at room temperature. Excellent performance of sensing was observed against NO2 and NH3 at 500 ppb–100 ppb, respectively. Further, the joule heating treatment is given to desorb the gas molecules to achieve the whole recovery.
Nowadays, metal/metal oxide with graphene nanocomposites has developed a great interest in research. The composition of metal-oxide-based graphene was approved in producing gas sensors which show high sensing performance. Many researchers have reported a gas sensor based on metal-oxide in detecting toxic, explosive, and harmful gases [9]. Low-dimensional nanostructured with metal-oxide materials were considered to produce high-performance gas sensor to protect the environment and health of the human. Many factories and industries produce harmful and toxic chemicals or gases in the environment which lead to damage to our environment that causes issues of global warming. Hence, monitoring the quality of air is needed to make our environment safe. Gas sensor plays an important role in detecting toxic gases and to monitor the quality of the air to protect human health and the environment [10]. Recently, Kumar et al. [11] investigated the gas sensor based on graphene composite with different metal-oxide such as metal oxides like tin oxide, ferric oxide, zinc oxide, and indium oxide to detect toxic gases. The author observed that ZnO of 25 wt% exhibits a quick response against NH3 with a short time to recover with comparison ZnO/rGO nanocomposite. The composite of SnO2-rGO nanocomposite was also studied in which sensitivity was found to be 22 and 11 for the concentration 1.12 × 10–6 toward ethanol and H2S, respectively. Nevertheless, the gas sensor based on SnO2 found to be 15 and 7. Hence, the study reveals that the gas sensor based on SnO2-rGO nanocomposite shows better sensing performance than the bare SnO2. Sensing response was observed improving with the addition of rGO. Moreover, the author investigated the sensing properties of In2O3-rGO nanocomposite synthesized by combining hard templet and ultrasonic mixing method. The study shows that the In2O3/rGO nanocomposite shows a fast sensing response toward ethanol in comparison with In2O3 but rGO did not. The author considered gas sensors based on In2O3-rGO nanocomposite as a good material to sense ethanol. The sensing performance of iron oxide with reduced graphene oxide was also investigated. The variation of sensing response was observed as the temperature is varying as of 350–450 °C. Against various concentrations of CO gas, the gas sensor was examined. At 10–200 ppm, Fe3O4/rGO nanocomposite sensor responses and the response was found to be 1.8 and 1.9 against the CO gas concentration of 100–200 ppm, respectively, at 400 °C. Goutham et al. [12] design a gas sensor based on the composite of graphene/CdO nanocomposite prepared by a cost-effective ultrasonic-assisted process against the LPG gas for various situations. At room temperature, the sensing response was observed and was found able to detect changing LPG gas concentration as 100–800 ppm. The author observed the sensitivity at 600 ppm for 1 wt. % of the sensor toward the LPG at room temperature. Further, the author suggests that CdO/graphene nanocomposite sensor shows high selectivity, good sensitivity, and stability against the LPG of low concentration. Jyoti and her co-workers [13] investigated on rGO with Zinc oxide nanocomposites for the utilization to sense the different concentration of NO2 gas in the range 4–100 ppm. The study on the effect of gas sensing with the increase of temperature was also done. It was found that at room temperature, the rGO-ZnO nanocomposite shows the highest percentage response. rGO/ZnO nanocomposite shows the limitation to detect at 4 ppm. At room temperature, the high response was obtained, i.e., 48.4% against the NO2 of 40 ppm concentration. Further, the author suggested rGO-ZnO nanocomposite material as good material in sensing toward NO2 gas sensor for room temperature. Mao et al. [14] found that the sensing performance of shows better sensing response than that of rGO toward NO2. However, rGO/SnO2 nanocomposite exhibits a weak response toward the exposure of NH3. Song with his co-workers [15] reported SnO2 quantum wire/rGO composites for the utilization in detecting H2S showed quick response and recovery time (2/292 s) ranging from 10–100 ppm. Gas sensors based on SnO2 quantum wire/rGO nanocomposites showed a better response of about 33 than the rGO. Yang et al. [16] prepared the graphene/CeO2 composites by using a facile solvothermal reaction for gas sensing applications. CeO2 of 46.7 wt% composite with graphene nanocubes exhibits high sensing response at the limit of detection 5 ppm against the NOx gas. A higher sensitivity was observed of about 10.39% and quick response time was exhibited of 7.33 s toward NOx for concentration 100 ppm at room temperature in comparison with CeO2. Zhang et al. [17] demonstrated the property of sensor toward hydrogen gas sensed using four different composites of CuO–rGO–CuO, (CuO–rGO)2–CuO, pure rGO, and CuO. The study found that the CuO/rGO film sensor exhibits good response and recovery time and it was found to achieve the highest sensitivity among the other composites. For different conditions of exposure of hydrogen gas were examined such as for 50, 500, and 1500 ppm of hydrogen by the CuO–rGO–CuO sensor. The sensing response and recovery time were found to be less than 80 and 60 s which might be attributed as a good sensing property. Kumar et al. [18] reported the synthesis of ZnO/rGO nanocomposite for the detection of NO2 gas. ZnO/rGO nanocomposite showed excellent sensing performance and high electrical conductivity than the pure rGO against NO2 gas. The author explained that the strong interaction between ZnO and rGO composite might be responsible for exhibiting high electrical and sensing properties. At the exposure of 50 ppm concentration of NO2, the high response was observed at 50 °C with the response value ca. 32% by ZnO/rGO nanocomposite sensor. Song and co-workers [15] design chemiresistive gas sensors using SnO2 quantum wires/rGO nanocomposites by spin-coating on the substrates of ceramics materials. Sintering was not done further. The sensing performance was examined for SnO2 quantum wire/rGO nanocomposites against H2S of concentration 50 ppm and was found fully reversible when the exposure of H2S begin with achieving sensor response as 33 in 2 s at 22℃. Moreover, the author explained that due to the transfer of charge in the interface of SnO2/rGO and the excellent transport capability of rGO leads in enhancing the sensing properties of SnO2 quantum wire/rGO nanocomposites. The author also suggests that a sensor made at room temperature with easy fabrication will exhibit a highly sensing response toward the ultrasensitive H2S gas with consuming less power. Many researchers have reported on graphene-based gas sensor showing excellent sensing performance (Fig. 11.1).
Different Methods for Preparing Graphene-Oxide and rGO/metal-Oxide Nanocomposites
There are mainly three ways to form GO; they were Brodie method [19], Staudenmaier method [20], and Hummers method [21]. Hummer's method is a widely used method for synthesizing to GO. Different modified Hummer methods were reported for the preparation of GO [22]. For the reduction of GO, there are various methods such as micromechanical exfoliation, epitaxial growth, vapor deposition, and chemical reduction [23,24,25,26]. Novoselov et al. prepared single-layer graphene from graphite flakes by using scotch tapes which is deposited on a substrate of SiO2/Si to obtain highly pure graphene [1]. The exfoliation of graphene from graphite using the micromechanical method produces fewer layers of graphene having a crystalline nature with large surface area [24]. The drawbacks of this method are the less production of graphene and give a small size. Nevertheless, this method is considered a simple method where there is no need for any other facilities. Good quality graphene with a large size is obtained by using the epitaxial growth method in comparison to that of the mechanical exfoliation process. However, there is the main drawback of this method was that it cannot produce a large amount of uniformly graphene thickness. For the production of graphene, the chemical vapor deposition (CVD) method is widely used as the size and structure of graphene produced by this method can be controlled.
Stankovich and his co-workers established a new approach to synthesized graphene which is considered in the bottom-up method, known as chemical reduction of graphene oxide which gives a new way of producing a large amount of graphene that can be used in various applications and modification [27]. The synthesis of GO using the chemical reduction method is hydrophilic and by sonication, it can be dispersed in various solvents [28]. The obtained GO was used for the preparation of different composites such as rGO powders, rGO film, and rGO with other composites. It is a cost-effective method. Many rGO-based gas sensor was fabricated using different methods in which nanolithography is included. However, it is an expensive method. And there found that the rGO sensor synthesized by this method shows poor sensing performance and lengthy recovery time of about 2 h. Further, to enhance the sensing properties, it should be given a heat treatment or illumination of UV is to be done [29]. Furthermore, the fabrication of the electrode using the nanolithography method led to contaminate the surface of the sample which strongly affects the sensing performance and the transport of charge in the specimen [30, 31]. Gu et al. [32] synthesized In2O3 with rGO nanocomposites by hydrothermal method followed by the reduction of GO using hydrazine hydrate. Ghule et al. [33] prepared Fe2O3 with graphene-oxide nanocomposite film as a sensing material by a simple solid-state method to detect liquefied petroleum gas (LPG) at 27 ℃. The composite Fe2O3 with different concentrations of GO such as 0.1, 1.0, and 10 wt. % were synthesized and sintered for 2 h at 450 °C and utilize as a gas sensor material. It was found that at room temperature, the sensitivity of the composite 1.0 wt. % Fe2O3/rGO shows the response of approx. 35% for 100 ppm toward LPG as compared to GO (~15%) and Fe2O3 (~25%). Other composites exhibit a sensing response of about 20–30% and it was also explained that it can be attributed to its exhibiting large surface area and the reduction of charge transfer resistance which have surface leading to the adsorption of LPG molecules.
Preparation of GO
GO is prepared by modified Hummer’s method [34]. In this synthesis process, 5 g of graphite powder is mixed with an H2SO4 concentration of 98% and sodium nitrate of about 110 ml and 2.5 g, respectively. Stirring is to be done for about 30 min at room temperature. After that, a black slurry suspension was observed as the mixing is nicely done. The temperature of the beaker containing mixture should be controlled below 20 °C; to do this, the beaker is to be kept in a cold water bath. Potassium permanganate of 15 g is added very slowly into the dispersion when the temperature completely goes down below 20 °C under vigorously stirring condition. The temperature should be maintained below 20 °C to stay safe from the explosion and to not get overheated. Temperature is to be increased up to 35 °C after adding potassium permanganate and stirring is done for 2 h. Dark brownish color with highly dense suspension was observed for 2 h indicating the exfoliation of graphene oxide from graphite. Deionized water of 300 ml is added into the mixture. The temperature is to be raised to 98 °C and stirring is done for another 1 h. After the completion of 1 h, hydrogen peroxide having a concentration (30%) of about 15 ml is to be is added into the mixture to stop the reaction. The obtained mixture was filtered and the removal of metal ions from the resulting mixture filtration is to be done by washing the obtained product first time by HCl solution of 10% concentration and further is to be washed using deionized water for several times. After completing the washing and filtration of the resultants, it is to be kept at 60℃ for 12 h to dry under vacuum. GO is obtained.
Synthesis of rGO-Metal-Oxide Nanocomposite
Kumar et al. [18] reported the synthesis of rGO and zinc oxide–reduced graphene-oxide (ZrGO) nanocomposite powder samples by a hydrolysis method in which 85% hydrazine hydrate was utilized as the reducing agent. In the preparation of rGO from GO by chemical, different reductants have been used like hydroquinone, hydrazine, sodium borohydride, dimethylhydrazine, etc. rGO/metal-oxide was also prepared using the hydrothermal method. The rGO-CuO nanocomposite was prepared using the hydrothermal method which is depicted. Firstly, the obtained GO product was added to 40 ml of deionized water to make its suspension very well. After that Cu(NO3)2. 2.5H2O of 0.02 g is put into the GO suspension under vigorously stirring condition and stir it for 15 min. Then, add NaOH (0.04 g) into the mixture and stir it for another 20 min. After that sealed the mixture suspension in a Teflon container of 100 ml having a steel enclosure and in the furnace, it is to be transferred. The action is to be done for 4 h at desired temperature viz., 100 °C. When the reaction was completed, the obtained product is to be kept to cool down at room temperature. Further centrifugation is done for the obtained substance and washing of the substance is required to removed metal ions using deionized water followed by ethanol several times. The drying of the obtained substance is to be done at 80 °C [35].
Structural and Composition Characterization Techniques
Scanning Electron Microscope (SEM)
SEM is a technique that gives information about the surface topography and the composition of the sample by scanning the surface with a focused beam of electrons. The image obtained of CuO/rGO nanocomposite from SEM is shown in Fig. 11.2, which reveals that confirms the CuO nanoparticles were well dispersed in the rGO sheet. The SEM image shows spherical shape morphology. The texture of the surface was observed as rough. Nevertheless, the size of the particle is found to be in the range of nano [36].

Reprinted with permission from Ref. [14]. Copyright (2012) Royal Society of Chemistry
(i) Schematic of the novel gas-sensing platform of an rGO sheet decorated with metal-0oxide nanocomposite. (ii) Diagrammatic illustration for the testing system of the sensor.

Reprinted with permission from Ref. [36]. Copyright (2018) Taylor and Francis
SEM image of CuO/rGO nanocomposite.
Transmission Electron Microscopy (TEM)
TEM is a technique in which a beam of electrons is transmitted through a specimen to form an image. It gives us information about the size and morphology of the sample. Figure 11.3 shows the images obtained from TEM and HRTEM of CuO/rGO nanocomposite. The CuO nanoparticles were found to be agglomerated from the image. It obtained a particle size in the range of 60–65 nm. The image also confirms that CuO/rGO nanocomposites are highly crystalline in nature. CuO is well in contact with the surface of rGO.

Reprinted with permission from Ref. [36]. Copyright (2018) Taylor and Francis
a–b TEM image of CuO/rGO nanocomposite c–d HRTEM image of CuO/rGO nanocomposite.
X-Ray Diffraction (XRD)
X-ray diffraction is a non-destructive analytical technique mainly used for phase identification and structural characterization of crystalline materials. Bragg's Law is expressed as:
where θ is the incident angle, λ is the wavelengths of the x-rays, and d (hkl) is spacing between two crystal lattice planes.
The XRD pattern of the CuO/rGO composite is shown in Fig. 11.4. XRD pattern of the composition confirms the diffraction peak positions with the standard CuO and rGO specimens confirming the formation of the monoclinic phase for the CuO. The peaks were appeared corresponding to the planes (110), (−111), (−111), (−202), (020), (202), (−113), (−311), and (220). Other peaks were also observed at the planes (002) and (100). It is attributed due to the presence of rGO. The diffraction peak for GO was not observed as it was well reduced from GO. But the intensity of reflection peaks of rGO become weak in the composite while comparing with as-synthesized rGO. Less agglomeration and disordered stacking of the rGO sheets inside the composite might be responsible.

Reprinted with permission from Ref. [36]. Copyright (2018) Taylor and Francis
XRD patterns for CuO/rGO nanocomposites.
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR spectra of CuO/rGO nanocomposite are depicted in Fig. 11.5. The spectrum for the CuO/rGO composite was found to be at 526 cm−1 for Cu–O bond. It can be attributed that during the synthesis process, oxidation of cuprous ions was occurring due to GO. Another peak appears at 3433 cm−1 which corresponds to the stretching vibration of OH. The results reveal that the composite was well reduced in the presence of cuprous ions.

Reprinted with permission from Ref. [36]. Copyright (2018) Taylor and Francis
FTIR spectra of CuO/rGO nanocomposite.
Raman Spectroscopy
The Raman spectra for the composite CuO/rGO are depicted in Fig. 11.6. It was found that the D and G bands for the rGO/CuO nanocomposite appeared at 1330 cm−1 and 1594 cm−1, respectively. In general, it is accepted that the Raman ID/IG ratio (where ID and IG are the D- and G-peak Raman intensities, respectively) is related to the density of defects in graphene-based materials, and the graphitization degree of carbonaceous materials.

Reprinted with permission from Ref. [36]. Copyright (2018) Taylor and Francis
Raman spectrum of CuO/rGO nanocomposite.
Cyclic Voltammetry
It is an electrochemical technique that is used to measure the developing current in an electrochemical cell under conditions where voltage is more than that predicted by the Nernst equation. The CV is measured by varying different cycles of the potential of a working electrode and the obtained current is measured. The cyclic voltammetry measurement is done for CuO/rGO nanocomposite. The results for the CV measurement for the samples G-CuO composite, G, GO, rGO-CuO composites and CuO were depicted in Fig. 11.7. The range of the potential is set from −0.3 V to +0.8 V. Majumdar et al. achieved the values of specific capacitance by the CV measurement for all the samples were found to be 3.2 Fg−1, 4.3 Fg−1, 13.1 Fg−1, and 263.6 Fg−1 with respective to graphite, GO, graphene-CuO, and rGO-CuO composites samples at a scan rate of 5 mVs−1. As compared to graphite, it was found that GO exhibits high specific capacitance. CuO/GO nanocomposite shows 4 times high specific capacitance than the pure graphite. The specific capacitance value for bare CuO was found to be 39.7 Fg−1 which is 6 times lower as compared to rGO-CuO nanocomposite for the same scan rate. Hence, as compared to other composites, CuO/rGO nanocomposite found to achieve the highest specific capacitance. In Fig. 11.7, CV curves were plotted of voltage versus current density for different scan rates for all the composites [37].

Reprinted with permission from Ref. [37]. Copyright (2017) Elsevier
a–e Cyclic voltammetry measurement of GO, G, G–CuO composite and CuO in the potential range −0.3 V to +0.8 V at 5 mVs−1. f Plots of current density versus voltage at different scan rates for the rGO/CuO composite.
X-Ray Photoelectron Spectroscopy (XPS)
The surface composition and element analysis for the resulting products were characterized by an XPS technique. Figure 11.8 shows the XPS spectra of GO, rGO, and composites. It was observed that all reveal the two peaks. One peak appears at 285.5 and another at 531.0 eV that corresponds to C1s and O1s, respectively. The peak for Cu2p and Cu3p was also observed which appears at 933.3 eV and 77.0 eV, respectively [38].

Reprinted with permission from Ref. [38]. Copyright (2014) American Chemical Society
XPS spectra of CuO/rGO, rGO and GO.

Reprinted with permission from Ref. [38]. Copyright (2014) American Chemical Society
Impedances of the sensors based on a reduced graphene oxide, b metal-oxide and c rGO/metal-oxide composite under different RHs measured at different frequencies.

Reprinted with permission from Ref. [38]. Copyright (2014) American Chemical Society
Response and recovery properties of the sensors fabricated with a metal-oxide and the composites b rGO/metal-oxide nanocomposite.
Gas Sensing of Metal-Oxide/rGO Nanocomposite Toward Different Gases.
NO2 Gas Sensor
Recently, Jyoti et al. [13] investigated the composite of metal-oxide/rGO against the various concentration of NO2 from 4–100 ppm. They also studied the sensing performance by varying the temperature and it was found that at room temperature, maximum percentage response of 48.4% at the concentration of NO2 at 40 ppm was obtained. Mao et al. [14] reported that rGO-based SnO2 gas sensor enhances the sensing properties toward NO2 while for NH3, the sensing becomes weak. SnO2/rGO nanocomposite sensors exhibit a sensitivity of 1.29–2.87 for NO2 (100 ppm) and sensitivity increases by 3.0–33.4% as compared to bare rGO. Whereas it shows 1.20–1.47 sensitivity for NH3 and the sensitivity reduces by 13.4–23.8% with comparison to rGO. Kumar [18] and his co-workers investigated the chemically synthesized rGO/ZnO nanocomposite. The author suggested that the ZnO/rGO nanocomposite gas sensor exhibits a good sensing response toward NO2 gas. As compared to pure rGO, ZnO/rGO nanocomposite shows a high sensing response of ca. 32% against the NO2 gas for 50 ppm at 50 ℃. Gu et al. [32] demonstrated the sensing performance of In2O3/rGO nanocomposite. The results reveal that In2O3/rGO nanocomposite exhibits excellent selectivity, high response, and relatively short response and recovery time while detecting the NO2 at room temperature. For the concentration 5–50 ppm of NO2, there shows a rapid increase of response. While after concentration reached 50–100 ppm, it was found that response increases slowly which might be attributed to the fact that NO2 reaches its saturation. The In2O3/rGO nanocomposite gas sensor shows a response at 8.25, response time in 4 min and recovery time in 24 min.
LPG Gas Sensor
Goutham et al. [12] designed a gas sensor using CdO/rGO nanocomposite for the detection of LPG gas. When there was an exposure of LPG, a decrease in resistivity was observed. CdO/rGO (1 wt. %) sensor shows a sensitivity of LPG gas at 27 °C for concentration 600 ppm with the increasing response of 70%. Further, the author concluded that even at room temperature, CdO/rGO nanocomposite gas sensor shows highly selective, stable, and sensitive to the low concentration of LPG. Ghule et al. [33] investigated the sensing performance of Fe2O3/graphene-oxide nanocomposite toward the LPG gas. The sensitivity against LPG for 100 ppm concentration at room temperature shows that the 1.0 wt.% GO/Fe2O3composite sensor exhibits ~35% to that of other GO/Fe2O3 composite sensors, GO sensor and Fe2O3 sensor which shows 20–30%, ~15%, and ~25%, respectively. The author attributed to that of large specific surface area and reduced charge transfer resistance, which, in general, has more surface-active surface sites for adsorbing LPG molecules than those of individual and other low-surface area sensors.
Ammonia Gas Sensor
Sakthivel et al. [35] synthesized CuO/rGO nanocomposite for the use in detecting ammonia. It was found that the rGO-CuO nanocomposite sensor showed a fast response in 12 s and a short recovery time of around 90 s for concentration 600 ppm toward NH3 at 150 °C (Figs. 11.9 and 11.10).
Conclusions
The previous work reported by the researchers on graphene-based metal-oxide nanocomposite was reviewed in this context. The sensing properties observed toward different gases like NO2, LPG gas, NH3, H2, etc., by various rGO-based metal-oxide nanocomposite were discussed. The addition of rGO with metal-oxide improved the sensing properties and enhanced the response of the sensor. The results were obtained differently for different gas sensors prepared by different synthesis processes. The characterization techniques such as SEM, TEM, XRD, FTIR, Raman, and CV measurement give information about the structural, morphology, and behavior of the metal-oxide/rGO nanocomposite that were also depicted.
References
Novoselov, K.S., Geim, A.K., Morozov, S.V., Jiang, D., Zhang, Y., Dubonos, S.V., Grigorieva, I.V., Firsov, A.A.: Electric Field Effect in Atomically Thin Carbon Films. Science 306, 666–669 (2004)
Zhang, D., Chang, H., Li, P., Liu, R., Xue, Q.: Fabrication and characterization of an ultrasensitive humidity sensor based on metal oxide/graphene hybrid nanocomposite. Sens. Actuat. B-Chem. 225, 233–240 (2016)
Srivastava, A., Galande, C., Ci, L., Song, L., Rai, C., Jariwala, D., Kelly, K.F., Ajayan, P.M.: Novel Liquid Precursor-Based Facile Synthesis of Large-Area Continuous, Single, and Few-Layer Graphene Films. Chem. Mater. 22, 3457–3461 (2010)
Gilje, S., Han, S., Wang, M., Wang, K.L., Kaner, R.B.: A Chemical Route to Graphene for Device Applications. Nano Lett. 7, 3394–3398 (2007)
Mc Allister, M.J., Li, J.L., Adamson, D.H., Schniepp, H.C., Abdala, A.A., Liu, J., Herrera-Alonso, M., Milius, D.L., Car, R., Prud’homme, R.K.: Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 19, 4396–4404 (2007)
Park, S., Ruoff, R.S.: Chemical Methods for the Production of Graphenes. Nat. Nanotechnol. 4, 217–224 (2009)
Schedin, F., Geim, A.K., Morozov, S.V., Hill, E.W., Blake, P., Katsnelson, M.I., Novoselov, K.S.: Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 6, 652–655 (2007)
Yavari, F., Koratkar, N.: Graphene-based chemical sensor. J. Phys. Chem. Lett. 3, 1746–1753 (2012)
Goutham, S., Sadasivuni, K.K., Kumar, D.S., Rao, K.V.: Flexible ultra-sensitive and resistive NO2 gas sensor based on nanostructured Zn(x) Fe(1–x)2O4. RSC Adv. 8, 3243–3249 (2018)
Dieter, K.: Function, and applications of gas sensors. J. Phys. D Appl. Phys. 34 (2017)
Naveen Kumar, J.R., Shrinivasa Mayya, D., Savitha, M.B., Prasad P.: Graphene-based metal oxide nanocomposites for gas sensing application. Int. J. Appl. Eng. Manage. Lett. (IJAEML) 2 (2018)
Goutham, S., Jayarambabu, N., Sandeep, C., Sadasivuni, K.K., Kumar, D.S., Rao, K.V.: Resistive room temperature LPG sensor based on a graphene/CdO nanocomposite Microchim. Acta. 186, 62 (2019)
Jyoti, Kanaujiya, N., Varma, G.D.: Highly selective room temperature NO2 gas sensor based on rGO–ZnO Composite. In: AIP Conference Proceedings, vol. 1953, p. 030039 (2018)
Mao, S., Cui, S., Lu, G., Yu, K., Wen, Z., Chen, J.: Tuning gas-sensing properties of reduced graphene oxide using tin oxide nanocrystals. J. Mater. Chem. 22, 11009 (2012)
Song, Z., Wei, Z., Wang, B., Luo, Z., Xu, S., Zhang, W., Yu, H., Li, M., Huang, Z., Zang, J., Yi, F., Liu, H.: Sensitive Room-Temperature H2S Gas Sensors Employing SnO2 Quantum Wire/Reduced Graphene Oxide Nanocomposites. Chem. Mater. 28, 1205–1212 (2016)
Yang, Y., Tian, C., Sun, L., Lü, R., Zhou, W., Shi, K., Kan, K., Wang, J., and Fu H.: Growth of small-sized CeO2 particles in the interlayers of expanded graphite for high-performance room temperature NOx gas sensor. J. Mater. Chem. A. 1–8 (2013)
Zhang, D., Yin, N., Jiang, C., Xia, B.: Characterization of CuO–reduced graphene oxide sandwiched nanostructure and its hydrogen sensing characteristics. J. Mater. Sci. Mater. Electron. 28, 2763–2768 (2017)
Kumar, N., Srivastava, A.K., Patel, H.S., Gupta, B.K., Varma, G.D.: Facile Synthesis of ZnO–Reduced Graphene Oxide Nanocomposites for NO2 Gas Sensing Applications. Eur. J. Inorg. Chem. 11, 1912–1923 (2015)
Brodie, B.C.: On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 149, 249–259 (1859)
Staudenmaier, L.: Verfahren Zur darstellung der graphitsäure. Ber. Dtsch. Chem. Ges. 31, 1481–1487 (1898)
Hummers, W.S., Offeman, R.E.: Preparation of graphitic oxide. JACS. 80, 1339–1339 (1958)
Roy, I., Rana, D., Sarkar, G., Bhattacharyya, A., Saha, N.R., Mondal, S., Pattanayak, S., Chattopadhyay, S., Chattopadhyay, D.: Physical and electrochemical characterization of reduced graphene oxide/silver nanocomposites synthesized by adopting a green approach. RSC Adv. 5, 25357–25364 (2015)
Subrahmanyam, K.S., Panchakarla, L.S., Govindaraj, A., Rao, C.N.R.: Simple method of preparing graphene flakes by an arc-discharge method. J. Phys. Chem. C. 113, 4257–4259 (2009)
Subrahmanyam, K.S., Vivekchand, S.R.C., Govindaraj, A., Rao, C.N.R.: A study of graphenes prepared by different methods: characterization, properties, and solubilization. J. Mater. Chem. 18, 1517–1523 (2008)
Paola, R., Anming, H., Giuseppe, C.: Synthesis, properties and potential applications of porous graphene: a review. Nano-Micro Lett. 5, 260–273 (2013)
Yang, Z., Gao, R.G., Hu, N.T., Chai, J., Cheng, Y.W., Zhang, L.Y., Wei, H., Kong, E.S.W., Zhang, Y.F.: The prospective two-dimensional graphene nanosheets: preparation, functionalization, and applications. Nano-Micro Lett. 4, 1–9 (2012)
Stankovich, S., Dikin, D.A., Dommett, G.H., Kohlhaas, K.M., Zimney, E.J., Stach, E.A., Piner, R.D., Nguyen, S.T., Ruoff, R.S.: Graphene-based composite materials. Nature 442, 282–286 (2006)
Paredes, J.I., Villar-Rodil, S., Martínez-Alonso, A., Tascon, J.M.D.: Graphene oxide dispersions in organic solvents. Langmuir 24, 10560–10564 (2008)
Yuan, W., Shi, G.: Graphene-based gas sensors. J. Mater. Chem. A 1, 10078–10091 (2013)
Dan, Y.P., Lu, Y., Kybert, N.J., Luo, Z.T., Johnson, A.T.C.: Intrinsic response of graphene vapor sensors. Nano Lett. 9, 1472–1475 (2009)
Lu, G.H., Park, S., Yu, K.H., Ruoff, R.S., Ocola, L.E., Rosenmann, D., Chen, J.H.: Toward practical gas sensing with highly reduced graphene oxide: a new signal processing method to circumvent run-to-run and device-to-device variations. ACS Nano 5, 1154–1164 (2011)
Gu, F., Nie, R., Han, D., Wang, Z.: In2O3–graphene nanocomposite based gas sensor for selective detection of NO2 at room temperature. Sensors Actuat. B. 219, 94–99 (2015)
Ghule, B.G., Shaikh, F.S., Shinde, N.M., Sushil, S., Shinde, P.V., Mane, R.S.: Promoted room-temperature LPG gas sensor activity of graphene oxide@Fe2O3 composite sensor over individuals. Mater. Res. Express. 5, 125001 (2018)
Marcano, D.C., Kosynkin, D.V., Berlin, J.M., Sinitskii, A., Sun, Z.Z., Slesarev, A., Alemany, L.B., Lu, W., Tour. J.M.: Improved synthesis of graphene oxide. ACS Nano 4, 4806–4814 (2010)
Bhuvaneshwari, S., Gopalakrishnan, N.: Selective ammonia sensor based on copper oxide/reduced graphene oxide nanocomposite. J. Alloy Compd. 788, 422–428 (2019)
Sagadevan, S., Zaman Chowdhury, Z., Johan, M.R.B., Aziz, F.A., Salleh, E.M., Hawa, A., Rafique, R.F.: A one-step facile route synthesis of copper oxide/reduced graphene oxide nanocomposite for supercapacitor applications. J. Exp. Nanosci. 13, 302–313 (2018)
Majumdar, D., Baugh, N., Bhattacharya, S.K.: Ultrasound assisted the formation of reduced graphene oxide-copper (II) oxide nanocomposite for energy storage applications. Physicochem. Eng. Aspects. 512, 158–170 (2017)
Wang, Z., Xiao, Y., Cui, X., Cheng, P., Wang, B., Gao, Y., Li, X., Yang, T., Zhang, T., Lu, G.: Humidity-sensing properties of urchinlike cuo nanostructures modified by reduced graphene oxide. ACS Appl. Mater. Interfaces. 6, 3888–3895 (2014)
Acknowledgements
The author would like to thank the Department of Physics, NIT Manipur for giving the Internet service which helps in accessing many online journals.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Devi, N.A., Swain, B.P. (2021). Investigation of Metal-Oxide/Reduced Graphene-Oxide Nanocomposites for Gas Sensor Applications. In: Swain, B.P. (eds) Nanostructured Materials and their Applications. Materials Horizons: From Nature to Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-15-8307-0_11
Download citation
DOI: https://doi.org/10.1007/978-981-15-8307-0_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-8306-3
Online ISBN: 978-981-15-8307-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)