Abstract
The present article focuses on experimentally investigating the saturated pool boiling performance of isopropanol and its solution with surfactant sodium lauryl sulphate (SLS) over modified surfaces intended towards application in small heat transfer equipment. A comprehensive review on enhancement of pool boiling heat transfer using different surfactants is also presented. Three types of surfaces such as plain surface, a surface having macro-channels, surface with mini-channels are tested for pool boiling. The best performing surface, the surfaces with mini-channel is tested with three solutions of isopropanol with the above-mentioned surfactant with 200, 600 and 1000 PPM concentration. The highest heat transfer coefficient is observed for the 600 PPM solution with the surface with mini-channels.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Surfactant is a chemical compound which when mixes with the solvents changes its property. In case of pool boiling, when it is added, it decreases the surface tension of the solution [1]. There are four types of the surfactant. These are (1) Non-ionic (2) Anionic, (3) Cationic, (4) Amphoteric. When the surfactant is added to the solution, the surfactant decreases surface tension until the CMC. After CMC is achieved, the extra surfactant is added, it goes to the CMC [1]. When the surface tension decreases, it enhances the vapour formation from the nucleation cavity which leads to the increase in the rate of the heat transfer. Kumar et al. [2] performed the experiment and suggested on different forces acting on the bubble in pool boiling. They discussed the implication of all these forces on the departure frequency, bubble departure diameter and heat transfer on upward, downward and vertical facing heater. It is observed that on the downward facing of the heater, there was no bubble departure, but at the same time, bubble departure was present with all orientation of heater [2]. When the surfactant is adsorbed in the liquid–vapour interface, the force of repulsion between the bubbles is seen to be critical in avoiding bubble coalescence and influences the departure away from the heater surface [2]. This is the reason for which departure frequency is more in upward facing heater and less in downward facing heater [2].
Hetsroni et al. [3] conducted an experiment by taking environmentally accepted surfactant alkyl glycosides. They compared the boiling behaviour of the surfactant added solution with water and found that the boiling behaviour of the water is very lower than the surfactant added solution [4]. Bubble generation rates become very faster in case of the surfactant added solution, but in case of pure water, immense coalescence is seen. Adding of surfactant solution initiates activation of nucleation site in the cluster mode. Dikici and Sukaini [5] conducted the experiment by taking three surfactants sodium laurel sulphate (SLS), ECOSURF™ (EH-14) and ECOSURF™ (SA-9). It has been observed that the boiling heat transfer coefficient is much higher in SLS than EH-14 and SA-9. It is observed that boiling heat transfer enhancement is higher 46% for SLS, 30% for EH-14 and 21% for SA-9 as compared to water [6]. In order to extend the research, we have to search for some environmentally safe surfactant like SA-9 and EH-14.
Wang et al. [7] used the two surfactant solutions ethanol and silicone oil which has lower surfactant solution than water and choose three surfactants. These surfactants are cationic surfactant CTAC, anionic surfactant dodecyl benzene sulphonate and non-ionic surfactant of alkyl poly glycoside. Besides the reduction of surface tension, bubble jet and bubble explosion are the phenomena which enhance the heat transfer rate. They also described the bubble jet phenomena in the paper. Due to Marangoni convection, the surfactant molecule transfers from the bottom of the bubble to the top of the bubble which divides the larger bubble into two smaller bubbles with the change of surface tension. It was found that the surfactant solution and ethanol had the best heat transfer performance [7]. Elghanam et al. [8] conducted the experiment of boiling heat transfer by using three surfactants. These are anionic sodium dodecyl sulphate, anionic sodium laurel sulphate and non-ionic Triton-X-100. By adding surfactant to the cooling water improves the heat transfer with the amount of 241% in case of SDS, 185% in case of SLES and 133% in case of Triton-X-100. In a given aqueous solution concentration, when the temperature increases, it increases the pool boiling heat transfer coefficient and active nucleation site density. Heat transfer increases for the SDS and SLS with increasing the concentration, but for the Triton-X-100, it increases up to 500 ppm of concentration and beyond that value, insignificant heat transfer observed [8].
Zhang and Manglik [9] experimented by taking the surfactants sodium dodecyl sulphate, cetyltrimethyl ammonium bromide (CTAB) and octyl phenoxy olyethoxy ethanol (Triton-X-305). Molecular mobility of these surfactants at interface shows the dynamic surface tension, surface wetting which leads to the formation of vapour bubble from a heated surface and changes the heat transfer significantly. Heat transfer coefficient increases when the surfactant concentration less than equal to CMC, and the heat transfer coefficient decreases when the concentration greater than CMC. An optimum heat transfer enhancement is obtained at the CMC of the surfactant. Gajghate et al. [4] performed the boiling heat transfer experiment by using the NH4CL (ammonium chloride) surfactant. By the addition of the surfactant, the thermophysical properties of the solution are changed. It is observed that up to 2600 ppm, there is significant increase in heat transfer rate, and beyond that limit, no significant heat transfer is observed [10]. Decrement of surface tension and wettability are two phenomena which play an important role in nucleate boiling heat transfer in case of surfactant ammonium chloride [4].
Wasekar and Manglik [6] conducted his experiment by taking the anionic surfactant like (SDS, SLS) and compared the boiling performance of pure water with aqueous solution of anionic surfactant. Heat transfer is enhanced by the presence of SDS with the early onset of the nucleate boiling. Optimum level of enhancement is observed at critical micelle concentration (CMC). It has been observed that kinetics of the surfactant molecule is different for the room temperature and for the boiling temperature. This has impact on the formation of vapour bubbles along with increments of departure frequency, and it also decreases the tendency of coalescence which causes foaming. Hetsroni et al. [11] studied the bubble growth in saturated pool boiling in water and surfactant solution. They have done the analysis by taking two different heat fluxes q = 10 kW/m2 and q = 50 kW/m2. At lower heat flux, the lifetime, volume and shape of the bubble did not differ much from water. But at higher heat flux, boiling is more vigorous than the normal water. The lifetime of the bubble in the cluster is lesser than the single water bubble. They observed that for the water with increasing heat flux, detachment diameter of water bubbles increases, but in the case of surfactant solution, the detachment diameter of bubble decreases with increasing heat flux. Hetsroni et al. [12] conducted the experiment and stated that not only surface tension but also kinematic viscosity plays a major role in changing the boiling behaviour of a liquid in which surfactant is added. Literature survey [12] showed that by adding surfactant to sea water, it can enhance the boiling process. So, it can become cost effective to an acceptable level [12]. Hetsroni et al. [11] and Hetsroni et al. [3] took the cationic surfactant HABON-G and conducted the experiment and found that the bubbles found in the HABON-G surfactant are very much smaller than vapour formed in water and the heated surface remains covered with it.
Suryanarayan et al. [13] choose the surfactant ammonium dodecyl sulphate (ADS) for performing the experiment as it is human friendly and three times best soluble than SDS. With the addition of ADS, the molecule of ADS disturbs bonding due to cohesion and adhesion, and so the bubble formed at low temperature difference. This leads to formation of wetted bubbles which contain high vapour inside. Due to low adhesive force between liquid and solid surface, it detaches from solid surface so quickly, and bubble flow occurs in upward direction due to buoyancy effect. ADS can be used as best alternatives for SDS. Zicheng et al. [10] conducted the experiment by taking two surfactant SDS, Triton-X-114. The result showed that boiling is more vigorous with smaller size and fast departure frequency. Best heat transfer is achieved at CMC of surfactant. Optimum heat transfer is more in SDS than Triton-X-114 at the same heat flux compared to water. Zicheng et al. revealed that heat transfer enhancement is due to the reduction of the surface tension and change of contact angle. The contact angle of SDS solution is independent on the change of concentration, but for the Triton-X-100, contact angle is inversely proportional to the concentration [10]. Wasekar and Mangklik [14] performed the experiment by taking surfactant Triton-X-100 and the nichrome wire. Throughout the experiment, they tested the heat transfer effect on different concentration of the Triton-X-100. They concluded that up to 500 ppm concentration of Triton-X-100, heat transfer increases, but beyond that, there will be no significant heat transfer [14].
Thus, it can be observed from the literature that the effect of surfactant on boiling heat transfer having organic liquids is not studied. The characteristic of the organic liquids is similar to the liquids used for the electronics cooling. Thus, the present article focuses on the performance of the isopropanol with the concentration of surfactant sodium dodecyl sulphate.
2 Experimental Set-up and Procedure
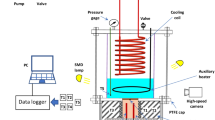
An experimental set-up is designed and developed for investigating the boiling heat transfer over small sample surfaces. The major components of the experimental facility are the test chamber, variable resistor (variac), direct current power supply, digital multi-metre and temperature indicator. The test chamber is a rectangular chamber made up of the base plate, top cover and the glass walls, and the test chamber is composed of PTFE sheet. The four side walls are made up of glass for visualization purpose. The glass and PTFE sheet can be approximated as insulators for heat loss from the saturated liquid. A copper tube carrying tap water is placed near the top wall which will act as condenser by which the vapour produced can be again be converted to liquid.
In base plate of the test chamber, the test sample made up of copper cylinder of diameter 30 mm and length 40 mm is inserted within another nylon cylinder of 42 mm diameter and 70 mm length. One 6 mm hole is drilled in the copper cylinder in parallel alignment through which the heater passes. A small hole of 2 mm size diameter is drilled at the centre of the copper through which the thermocouple passes. The tip of the T-type thermocouple is just touching the top surface of the copper block whose reading is recorded as the surface temperature. The temperature of the liquid in the test chambered is ensured to be at saturated condition before recording the temperature. The thermocouples are calibrated with a dry block type calibrator, and the error associated is within ±1 °C (Fig. 1).
At the top and the bottom of the glass window, Teflon cover is used because it is both thermal and liquid proof. The glass and Teflon layer are attached together by resin bond that prevents liquid leakage. The boiling phenomenon happening inside the chamber can be visualized through the glass chamber. The AC current supply is provided to the DC power supply through a variable resistor to control the input voltage supply. Thus, by controlling the input to the DC power supply, the required output voltage is achieved. The experiments are conducted for 6, 7, 8, 9 and 10, 11 V, measured by digital multi-metre. Thus, from the voltage and current, the power input to the heater is calculated. By dividing the heater power with the surface area of the sample copper surface, the heat flux is determined. A 12 A magnetic circuit breaker (MCB) is also used to prevent any damage to the electric circuit.
First setting up the variac at a particular voltage level is done to fix the heat flux. Then, the liquid inside the chamber starts heating up, and after reaching to saturation temperature, the boiling occurs. After that, the readings of temperature and voltage are noted down only when the steady state is achieved. Figure 2 shows the different surfaces produced for the experimental study, and the characteristics are mentioned in Table 1. The smooth surface is produced by rubbing the copper surface with a 1200 grade emery paper. The roughness average of smooth surface is 0.5 µm. The surface having macro-channels is produced by scratching the surface by a hacksaw blade several times from many directions producing a roughness average of 9–10 µm. The surface with macro-channels is produced by drawing channels through hacksaw blade. The deep channels on the surface are 0.5 mm deep and 1 mm width. The surface with very fine mini-channels is produced by using the knurling tool on the flat face of the copper cylinder. The channels are 0.5 mm width, and the height of walls of mini-channels are 0.8 mm.
The set-up is assembled and checked for any leakage. Initially, the glass chamber is cleaned with water and then with acetone. It is then kept sometime for acetone to be evaporated. Then, the test liquid is poured into the chamber. Then, the electrical power is supplied to the autotransformer. The controlling knob of the autotransformer is rotated to get the required voltage corresponding to the highest heat flux value. The voltage is checked through a digital multi-metre. Then, the liquid is heated until the steady state is achieved. Then, the final temperature readings are recorded. The heat flux values corresponding to the voltage applied are presented in Table 2.
3 Results and Discussion
The heat transfer coefficient is determined from the heat flux applied to the surface divided by the excess wall superheat, i.e. the difference between the surface temperature and the liquid temperature.
This heat transfer coefficient value shows the performance level of any surface in case of boiling heat transfer. Therefore, the HTC values are presented, compared and discussed here. Figure 3 illustrates the trend of the HTC for saturated pool boiling of isopropanol over all the three surfaces under atmospheric pressure.
Figure 3 shows the variation of the PBHTC with heat flux for all the three surfaces taking isopropanol as the working liquid. Moreover, the variation in PBHTC for isopropanol is similar to that of the acetone, i.e. it is observed to be increasing with heat flux for all the three surfaces. Again, the PBHTC are higher for the surface with mini-channels than the other two types of surfaces.
The following points can be inferred from Fig. 3. (a) The heat transfer coefficients increase with raising the heat flux level applied to the heater surface. (b) The heat transfer coefficients are highest for the mini-channel surface at all heat flux levels and then follows the order as channelled > rough > smooth.
The reason for the first observation is very much well established from previous investigations. The number of activated nucleation sites increases due to increase in the heat input level to the surface. With the higher heat flux level, the surface temperature increases which activates more number of cavities on the surfaces leading to more number of incipience of bubble formation. Thus, due to the latent heat of evaporation dissipation from the surface, the heat transfer coefficients increase. There are two reasons for the second observation, i.e. the mini-channel surface is having highest heat transfer coefficient values. The mini-channel surface is having higher surface area due to which the rate of heat dissipation is higher. The small channels on the surface help in vapour trapping in nucleation sites along the channels like re-entrant cavities. The vapour trapping of the nucleation sites is necessary for bubble formation. It means that the nucleation sites must have little amount of vapour covered with pool of liquid to be able to incipience bubble formation.
To study the effect of surfactant, sodium lauryl sulphate (SDS) is added with isopropanol to get solutions of different concentrations. The solutions prepared are 200, 600 and 1000 PPM. Figure 4 shows the compound and the three solutions.
Figure 5 depicts the comparison between the pool boiling performances of isopropanol over the mini-channel surfaces for all the heat flux values. It has been observed that the heat transfer coefficient values are higher for isopropanol with surfactant than the pure isopropanol. The surfactant mixed with the isopropanol decreases the surface tension value which leads to more number of active nucleation sites. This increases the heat transfer rate.
It can be seen that the HTC rises with rise in heat flux value for all the three solutions of isopropanol with 200, 600 and 1000 PPM. This is due to the fact that with the increment in heat flux value, the number of active nucleation sites increases from which the bubbles generate. Thus, more number of bubble formation leads to more extraction of latent heat of vapourization from the surface. Therefore, the boiling HTC and heat flux are directly proportional for all the solutions.
However, it is also observed that the heat transfer coefficient is lower for the solution with 1000 PPM than that corresponding to 600 PPM. This happens because of the fact that as the surface tension decreased further, the bubble growth is also hindered. As the surface tension has two counteracting effects on the bubble dynamics, an optimized amount of surfactant can be the best solution. In this present case, the solution with 600 PPM performs better than the 200 and 1000 PPM solution. Figure 6 shows the variation of wall superheats obtained during the experiments.
The values of pool boiling heat transfer coefficient obtained from the experiment are correlated in terms of heat flux value and concentration of surfactant through the regression modelling approach. The correlation obtained is presented in Eq. (2). The predicted values lie within ±15% of the experimental value as observed in Fig. 7.
Thus, the above investigation showed that the pool boiling heat transfer coefficient of isopropanol on the surface having mini-channels is enhanced by addition of the surfactant. However, as the concentration reaches the critical micelles concentration, the PBHTC starts declining. This is 600 PPM for SLS with isopropanol over the surface with mini-channels. The condition may vary depending on the characteristics of the surface.
4 Conclusion
The saturated pool boiling performance of pure isopropanol is studied on three kinds of surfaces such as smooth, surface with macro-channels and surface with mini-channels. The surface with mini-channels outperformed than the smooth and macro-channel surfaces. Then, this surface is chosen for the further study on the effect of surfactant on the pool boiling performance. It is observed that the heat transfer coefficient increases with increase in concentration of surfactant up to a certain point and then decreases. Thus, in the present investigation, the solution with 600 PPM solution is found to be best among the three solutions. Addition of surfactant also decreases the surface tension which enhances the formation of vapour bubbles in the nucleation cavity. These bubbles take away heat content of the surface by the vapour and increase the heat transfer rate. After addition of the surfactant, the departure diameter of the bubbles becomes very less as compared to the normal water due to the decrease of the surface tension. It leads to the increase in heat transfer coefficient. If the PPM is less than 200 ppm, then the heat transfer coefficient is less as compared to the solution with 600 ppm concentration. Again if the concentration is more than 1000 ppm, then also, the HTC decreases due to the formation of critical micelles concentration.
References
Ajinath K, Kulkarni K (2019) Review on natural and synthetic surfactant for pool boiling. In: Proceedings of second Shri Chhatrapati Shivaji Maharaj QIP conference on engineering innovations, pp 393–395
Kumar N, Raza Q, Raj R (2018) Surfactant aided bubble departure during pool boiling. Int J Therm Sci 131:105–113. https://doi.org/10.1016/j.ijthermalsci.2018.05.025
Hetsroni G, Gurevich M, Mosyak A, Rozenblit R, Segal Z (2004) Boiling enhancement with environmentally acceptable surfactants. Int J Heat Fluid Flow 25(5):841–848. https://doi.org/10.1016/j.ijheatfluidflow.2004.05.005
Gajghate S, Acharya AR, Pise AT (2014) Experimental study of aqueous ammonium chloride in pool boiling heat transfer. Exp Heat Transfer J Therm Energy Gen Transp Storage Conver 27(2):113–123. https://doi.org/10.1080/08916152.2012.757673
Dikici B, Al-Sukaini BQA (2017) Comparison of aqueous surfactant solutions. In: ASME power conference, 2016, POWER2016-59351, pp 1–7. https://doi.org/10.1115/POWER2016-59351
Wasekar VM, Manglik RM (2002) The influence of additive molecular weight and ionic nature on the pool boiling performance of aqueous surfactant solutions. Int J Heat Mass Transfer 45(3):483–493. https://doi.org/10.1016/S0017-9310(01)00174-0
Wang J, Li FC, Li XB (2016) On the mechanism of boiling heat transfer enhancement by surfactant addition. Int J Heat Mass Transfer 101:800–806. https://doi.org/10.1016/j.ijheatmasstransfer.2016.05.121
Elghanam RI, Fawal MMEL, Aziz RA, Skr MH, Khalifa AH (2011) Experimental study of nucleate boiling heat transfer enhancement by using surfactant. Ain Shams Eng J 2(3–4):195–209. https://doi.org/10.1016/j.asej.2011.09.001
Zhang J, Manglik RM (2016) Additive adsorption and interfacial characteristics of nucleate pool boiling in aqueous surfactant solutions. J Heat Transfer 127(7):684–691. https://doi.org/10.1115/1.1924626
Zicheng H, Jiaqiang G, Xinnan S, Qian W (2011) Pool boiling heat transfer of aqueous surfactant solutions. In: Proceedings—4th international conference on intelligent computation technology and automation. ICICTA 2011, vol 2, pp 841–844. https://doi.org/10.1109/ICICTA.2011.497
Hetsroni G, Mosyak A, Pogrebnyak E, Sher I, Segal Z (2006) Bubble growth in saturated pool boiling in water and surfactant solution. Int J Multiph Flow 32(2):159–182. https://doi.org/10.1016/j.ijmultiphaseflow.2005.10.002
Hetsroni G, Zakin J, Lin Z, Mosyak A, Pancallo E, Rozenblit R (2001) The effect of surfactants on bubble growth, wall thermal patterns and heat transfer in pool boiling. Int J Heat Mass Transf 44(2):485–497. https://doi.org/10.1016/s0017-9310(00)00099-5
Suryanarayana G, Rao G, Balakrishna N (2015) Experimental investigation on pool boiling heat transfer with sodium dodecyl sulfate. Int J Mech Eng Comput Appl 3(2):032–037
Wasekar VM, Manglik RM (2000) Pool boiling heat transfer in aqueous solutions of an anionic surfactant. J Heat Mass Transfer 122(4):708–715
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Swain, A., Sarangi, R.K., Kar, S.P., Sekhar, P.C., Swain, S. (2021). Enhancement of Boiling Heat Transfer Using Surfactant Over Surface with Mini-Channels. In: Revankar, S., Sen, S., Sahu, D. (eds) Proceedings of International Conference on Thermofluids. Lecture Notes in Mechanical Engineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-7831-1_24
Download citation
DOI: https://doi.org/10.1007/978-981-15-7831-1_24
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7830-4
Online ISBN: 978-981-15-7831-1
eBook Packages: EngineeringEngineering (R0)