Abstract
Nanomedicine contributes exceptional possibilities for novel therapeutic management. Nanomedicine moderately interlocks, exceeds, and overlaps other medical curriculum. As various approaches in Nanomedicine had progressed with enormous promise toward theranostic interventions, ethical queries also arise in conjunction. Nanomedicine is a unique niche in various facets, but it comes along with indications of dangers and uncertainties not confronted in other fields of medical practice or analysis. Some intellectuals agree that progress in the field of nanotechnology may present several ethical challenges, while others dispute that these challenges are not recent and that nanotechnology generally echoes frequent bioethical impasses. The objective of this article is to analyze some of the ethical concerns associated with Nanomedicine application and to reveal the queries and recent developments on whether Nanomedicine yields further ethical challenges keeping in view the principle of medical ethics. Such a conclusion should have significance on professional strategy and regulatory approaches and designing the policies for application in the upcoming days.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Currently, Nanomedicine has been considered as advancing field in scientific analyses. Nanomedicine is an expansive field that includes the development of sensors for the detection and identification of biomarkers, nanocarriers, and nanoparticles for the detection/imaging of cancers and for the delivery of therapeutic molecules. Most of the ongoing arguments surrounding the ethical problems in the field of nanomedicine are subsequent of the former technologies, as for example information technology and biotechnology (Jamison 2009). Arguments also arise over either nanomedicine has any particular ethical problem, or the ethical problems of former technologies relate to nanoscience (Sechi et al. 2014). Scientists have broadly described the possible scenarios of the ethical issues that might coincide with the advancement of Nanoscience technology. Various analyses conducted in the field of Nano-toxicology have recommended the threats comprising the ill effects of nanoparticles on the environment, humans, and fish (Patra et al. 2010; Sandler 2009). Various biotechnology and pharmaceutical associations and government firms have begun analyzing and inspecting several Nanotechnology utilization in areas of Nanomedicine. As reported, many of the Nanomedicines have been accepted for cancer therapies or are recently being tested on human trials (Resnik and Tinkle 2007). It is supposed that the intended therapeutic potential for Nanomedicine can produce capable ill effects if the accuracy becomes improperly applied or if it has higher than just the required concentration. Bottom-up synthesis from the molecular level, based either on imitating biology or on integrating components from a living structure, enhances problems about hybrid mechanisms that integrate machine and human and also reduces the preference towards Nanomedicine (Bruce 2006). Since science and technology are dealing with nanoparticles synthesis and large-scale manufacturing for drug discovery, the field of Nanomedicine is rapidly expanding. Several developing nations have assigned a considerable quantity of funding for nanotechnological analyses. In this context, the ethical and law issues involving Nanomedicine should be taken into consideration to address the effects on the environment and humans and the public reactions.
This paper, therefore, represents the ethical issues relevant to the field of Nanomedicine. Till now, however, there have been few formal analyses, arguments, or thinking regarding the ethical and social problems associated with Nanomedicine all around the globe. Therefore, it implies that we need to consider evaluation for ethical training in nanomedicine as well as initial recommendations for medical ethics and research ethics preparations for clinicians and researchers as well as for experimental analyses in nano ethics.
9.2 Nanomedicine
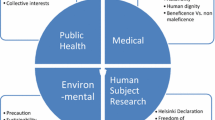
In recent years, the field of nanomedical research has shown promising growth. Nanomedicine may be defined as the application of Nanotechnology to the area of medicine. The area of Nanomedicine is in its budding phase, as several products are in the developmental stage. Development of sensors for single molecular observation, the use of nanoparticles and nanocarriers for identifying and imaging cancer, biomarkers, and ultrafast DNA sequencing are some of the many contributions of nanomedicine (Ferrari et al. 2009; Życiński 2006). Drugs like Doxil (Liposomal DOX), Lipusu (Liposomal PTX), and Abraxane (Nanoparticulate albumin/PTX) are centered out of Nanomedicine. Several such drugs have been approved by the FDA and European Medicines Agency (EMA), while the regulatory framework for these nano-enabled pharmaceutical products is being developed (Liu et al. 2016; Sharma et al. 2017). There are various kinds of organic and inorganic nanoparticles and nanomaterials like nanoshells, micelles, quantum dots, carbon nanotubes, tecto dendrimers, gold nanoparticles, etc., that are used in imaging diagnostic and several other medical applications. Nanomedicine has led to the integration of effective molecules that could not be used earlier because of being highly toxic (e.g., Mepact), exploitation of multiple mechanisms of action (e.g., Nanomag), increase in efficacy and reduction in dose and toxicity, controlled and site-specific drug targeting causing an even distribution within the body, and improvement in transport across the biological barriers. Nanomedicine thus has the potential to improve the bioavailability, dose-response, personalization, and targeting ability of conventional medicines (Ventola 2012b, 2017; Życiński 2006) and has been associated with diverse fields for application (Fig. 9.1). It can help in the advancement of detection and diagnosis of diseases, significantly improving human health.
9.3 Difference Between Nanotechnology and Nanomedicine
The convergence of various scientific fields like biology, chemistry, physics, mathematics, and engineering has led to the development of Nanotechnology. It mainly focuses on the investigation and manipulation of atoms, molecules, and submicroscopic particles that generally range within 1–100 nm. The National Nanotechnology Initiative (NNI) of the USA describes it as a science of matter and phenomena occurring at the nanoscale level (Schottel and Karn 2016). In nanotechnology, scientists explore the natural quantum effects occurring at nanometric level, which can be effective on various properties, including biological, optical, and mechanical properties. These special characteristic properties of nanoscale materials make them different from their counterparts (Ventola 2012b).
Nanomedicine, however, is an interdisciplinary field formed by the combination of nanotechnology and medicine. It involves genomics, proteomics, molecular and cellular biology, along with bioengineering and material science. Nanomedicine deals with physiological processes at the nanoscale level and focuses on the working of biological molecules like antibodies, proteins, enzymes, and receptors. According to the European Science Foundation (ESF), Nanomedicine is the science and technology of diagnosis, treatment, and prevention of diseases, thus improving human health using molecular tools and knowledge of the human body (Resnik and Tinkle 2007; Satalkar et al. 2016).
9.4 Hype Versus Reality of Nanomedicine
Whenever a new technology is developed, a plethora of hype is created around its application. Nanomedicine has several assumptions around it, too, regarding the outcomes that this field can offer. James F Leary, in his own version of “Gartner’s Hype Cycle” showed that ultimately reality takes over both hype and unfair criticism as it is based on a variety of real factors. According to him, the advancement in nanomedical technologies will create sophisticated and efficient drug delivery methods (Leary 2013). Some major hypes surrounding nanomedicine are that it will provide low cost, exceptional medical research equipment, the nanomachines will be programmed in a way to remove fatty deposits from our bloodstream and that the nanorobots which can provide protection to the human body against viruses will be a part of preventive medicine. Overall, Nanomedicine is expected to transform the existing medical diagnosis and drug delivery system. However, all this will only be turned into a reality with rational thinking and understanding of the nanoparticles, biological molecules of the human body and their interactions (Kuiken 2011; Życiński 2006). The ongoing research projects based around Nanomedicine can lead to a brighter future for medical diagnosis.
9.5 Nondiscrimination and Integrity of Nanomedicine
Ever since the concept came out, research on Nanotechnology has been experiencing a surprising advancement in several fields. The enormous advancement was accomplished by the pharmaceutical associations in the field of drug delivery, which is fabricating considerable aftermaths. At this period, due to the advancement in pharmacogenomics and pharmacogenetics: the drug delivery system (DDS) is under analysis, which brings into the spotlight the approaches of personalized medicine (PM) (Bawa and Johnson 2007; Bawa 2005; Patra et al. 2010). All these studies are being accomplished by the biggest pharmaceutical industries globally. In the year 2006, a budget of around 12.4 billion US Dollars was used, globally, by governments, associations, and investors, which is 13% higher than that spent in the year 2005 and the budgets are escalating each year. As the nanomedical commodities are very costly, the venture capitalists have to cover-up their investments; therefore, the nanomedical market is progressing only in developed nations. In developing nations however, the governments and their citizens cannot bear the nanomedical commodities. Thus, the consecutive query arises: how ethical is it to enforce extremely costly nanomedical therapies for medical systems and patients? This circumstance is generating a gap among the conditions of the health care structure in developed nations and that of the health care structure in developing nations. Moreover, an escalating count of specialists is migrating to developed nations to have access to novel nanotechnologies in the medical care structure (Graur et al. 2011). This will eventually cause a lack of researchers in developing nations. As a result, the recent medical sciences will be unapproachable for several people of lower socio-economic condition or for those in developing nations. However, in the future, there is a probable situation where only the rich will have permission to the new treatments, while the poor are declined even to have the insight of their diseases (Bawa 2005; Patra et al. 2010). On the other hand, intellectual property theft and biopiracy are also responsible for the inadequacy of necessary drugs to the poorly developed regions around the world (de SC and Nigel 2006; Sharma et al. 2017).
9.6 The Potentiality of Nanomedicine
Nanotechnology assures to benefit most industries and will have a specifically extensive effect on medicine and health care. The future effect of nanomedicine on society could be immense. Especially, Nanomedicine can enhance the quality of life of the patient, decrease socio-economic expenses correlated with healthcare, provide early recognition of pathological conditions, decrease the asperity of therapy, and lead to enhanced clinical results for the patient. Nanomedicine, in a wider sense, is the utilization of nanoscale technologies in medicinal practice, such as for detection, protection, and treatment of disease and to achieve an expanded insight of complex fundamental mechanisms of disease. Advancement in miniaturization of analytic tools, delivering nano therapies, enhanced computational and memory capacity, and enhancements in remote communications will be unified. These exertions will cross the new boundaries to the insight and practice of medicine. The fundamental aim is definitely extensive monitoring, repair, and enhancement of all biological systems of humans to improve the quality of life. Thus, Nanomedicine is not a single class of medical interference that can readily be evaluated from an ethical prospect. Nanomedicine will probably revive old questions about human improvement, justice, and dignity that have been raised several times before in the framework of pharmaceutics research, gene therapy, or cloning. Generally, Nanomedicine interference comes under two major categories: diagnostic nanomedicine and therapeutic nanomedicine. Each of these technologies and their uses is specific, and in certain cases, novel ethical significance for their advancement, use, and approachability. The two leading types of Nanomedicine products that are presently in clinical trials are related to diagnostics and drug delivery (Bawa and Johnson 2009; Bawa et al. 2005; Sandler 2009; Sechi et al. 2014). The scope of applicability of Nanomedicine is explained in Fig. 9.2.
9.7 Biocompatibility and Toxicity of Nanostructures
The most extensively improved sector of Nanomedicine is the nano drug therapy and delivery system, which utilizes a broad range of substances to deliver effective agents to various parts of the human body. The action of nanomaterials is usually uncertain, as they may act in a different way in vivo organization when compared to in vitro system: nanoparticles can break down into smaller fragments that are lethal to the human body, or they may accumulate into larger fragments as well (Oberdörster et al. 2005a). The penetration capability and absorbance of the nano drugs at various barriers of the body are also to be considered during drug designing and testing (Fig. 9.3). Thus, it is ethically acceptable to design short- and long-term analysis to decide whether Nanomedicines actually are more efficient and secure for humans in comparison to conventional drugs. However, while running those trials, there may arise some other obstacles, such as difficulty with apprehension and insight with regard to the informed assent, given the complication of nanotechnologies. Therefore, the long-term impacts of using Nanomedicines to date are still largely unexplained (Nel et al. 2006). To assure that the new drugs can be used securely by the people, Nanomedicine associations should be asked to conduct long-term analysis on nanomedical products followed by their initiation on the market. But this type of analysis is rarely practiced as it is not desired by current laws. Though it is clear that new approaches should be initiated, this should be done with much care and implementing safety guidelines, so as to avoid overregulating tendencies, as those would have an alarming impact on development, research, commercialization, exertion, and fair approach of the public towards Nanomedicines (Strom 2006; Ventola 2012b).
9.8 Demand of the Nanomedicine in Market
With the advancement in medical technologies, our world is moving closer to personalized and effective medical care. Nanomedicine can prove to be one of the major driving forces behind it. Recent statistics display that the global demand for Nanomedicine accounted for $111,912 million in 2016, which is expected to hit $261,063 million by 2023. The major parameters dividing the global Nanomedicine market are application, modality, indication, and region. Treatment and diagnostic are the two major divisions in terms of modality. Based on its several applications, it is categorized as drug delivery candidates, vaccines, regenerative medicine aid, and others. Based on further indication, their effectiveness was classified as relating to various diseases, such as immunological, oncological, neurological, urological, infectious, cardiovascular diseases, and several others. North America, Europe, and Asia-Pacific are the major regions across which the market of Nanomedicine manufacturing is based. According to the analysis conducted by Wagner et al. in 2006, there are more than 150 start-ups and small- and medium-sized enterprises based on Nanomedicine research and development projects (Siddique et al. 2019). The size of the global market for Nanomedicine was estimated to be nearly 140 billion USD in 2019. The current need for the development of early detection tools and effective diagnostic techniques can give a boost to this growth by 2025 (Chang et al. 2015; Życiński 2006). The National Science Foundation (NSF) predicts Nanomedicine being a major part of future pharmaceutical products, with the nanotechnology-based companies to be actively involved in the biomedical field (Ventola 2012b). The steps involved in the development and launching of novel nano therapeutic drugs are explained in Fig. 9.4.
9.9 Nanomedicine and Ethical Concerns
As mentioned above, the confusion surrounding the behavior of the nanoparticles and the capable impacts of exposure develops an ethical issue for those unveiled to these materials (Fig. 9.5). Evaluating the assurance of nanoparticles can be crucial due to the reason that these substances are not a united group of compounds; thus every type of substance must be evaluated separately (Oberdörster et al. 2005b; Wolf and Jones 2011). Particles less than the size of 200 nm may leave the circulatory system (CS) and are also capable of invading any human cell. Dermal penetrability is also possible, as in the central nervous system (CNS) via the olfactory mucosa. Notably, following the infiltration through the blood–brain barrier (BBB), particles could persist in the CNS, leading or inducing neurodegenerative disorders in extended usage (Obermeier et al. 2013; Yeagle 2007). The nanoparticles could also impair via free radical impairment or can even act as teratogenic, perturbing the future generation. Some coated particles cannot be detached from the cells, and others may aggregate in the immune system. Usually, the mechanisms of interplay between the immune system and the nanoparticles are still not entirely evident. The exposure to nanomaterials can also be unintended via environmental sources like a contagion of underground water sources and soil penetration. These problems not only influence the scientists and workers initially unveiled to the nanoparticles but all the personnel along the complete chain of production, marketing, and distribution. Eventually, they can harm the consumers as well. To focus on these problems, a standardized approach for uncertainty evaluation is necessary (Allon et al. 2017; Turanlı and Everest 2016). The fine points related to ethical concerns of Nanomedicine are depicted in Fig. 9.5 and explained in the below sections.
9.9.1 Ethical Problems Associated with Translational Research Involving Nanomedicine
As recorded by Anderson and Kimmelman (2010) no broadly approved measures for analyses of threats, advantages, and significance in Phase I trials have been done with new drugs. Therefore, the challenge is initially to recognize and illustrate the deep-rooted pitfalls of translational research in an aspect that assembles with the fundamentals of transparency, liability, and decreasing the impact of harm to the participating individuals. Secondly, legal and ethical rules and protocol necessitate that researchers notify the subjects about the potential to intend of the analyses, approaches, advantages, significant uncertainties as well as forms of severe uncertainty, substitutes, privacy protections, and other necessary information that make a subject to make a decision whether to participate. Moreover, the challenge is whom to choose to go initially for human trials when it is futile to appropriately measure the uncertainties involved and when the impacts of failure are probably extensive (Allon et al. 2017; Solbakk and Zoloth 2011). Depicting on this prevailing knowledge relating to translational analyses, the recent condition of nanomedicine can be developed. Thus, the unsolvable ethical queries regarding translational analyses are common to nanomedicine and arise from: (a) Uncertainty regarding the initial unveiling of human trials to a specific type of nanomedical commodities in Phase I clinical trials. (b) Intrinsic inequity—in Phase I trials, individuals are enlisted into analysis designed not to profit them but instead to achieve safety knowledge for others. This ethical impasse may be usually acute in the context of nanomedicine, given the unawareness of the nanoparticles, including the experimental substances. (c) Frailty arising from ignorance and uncertainty. Certainly, forms of uncertainty are not limited entirely to measurable threats. This form comprises severe uncertainty, where possibilities cannot be accredited, as well as certain ignorance, where the sample space is not completely recognized, reign in translational analysis (Wynne 1992).
9.9.2 Ethical Problems in Personalized and Regenerative Therapy with Nanomedicine
Nanomedicine contributes to the emerging area of regenerative medicine and, as such, arises a number of ethical queries correlated to the idea that the human body can be created, remodeled, or regrown (Habets et al. 2016). The goal for improvement may pave the way in transforming medicine into a yet unknown entity. Nanomedicine impacts the elegant homeostasis between therapy and advancement by specifying the technological potentiality by regulating tissue elements, enabling the recovery of diseases and health conditions. Nanomedicine may be a mechanism in transforming personalized medicine to existence since nanotechnology provides targeted delivery and hence can contribute to the advancement of personalized medicine both for therapy and screening (Sharma et al. 2017). It facilitates the potentiality to personalize healthcare, conforming it to groups and individual patients. The transform of healthcare from disease-oriented to patient-oriented models comprises recent ethical challenges, chiefly regarding the data foundation required. Personalized nano diagnostics arise ethical queries concerning the scope and nature of genetic screening, for example, concerning the kind of screening and type of legal or social pressure to be practiced. Moreover, privacy issues and authority and ownership over data may be supplementary interests to be argued among regulatory bodies.
9.9.3 Ethical Concerns of Medical Surveillance
In the absence of a decisive cadenced to quantify environmental unveil or an analysis that firmly quantifies the impacts of nanosubstance exposure in humans, current medical inspection is the most reasonable substitute to analyze exposure safety (Hartzema et al. 2013; Oberdörster et al. 2005a). Medical inspection comprises diagnosing for the pre-clinical sign of diseases for individuals showing no symptoms, allows for early detection and more potent treatment or intervention. The norm for regulating the suitability of medical investigation involves the strain of agony, the efficiency and accuracy of the analysis method, the potency of early screening, the risk of screening, and the advantages of surveillance comparative to damage (Public Health Surveillance 2008). Currently, there is no premise for aimed “nano specific” occupational medical investigations. When threat aspects are eminent and approve statistical interpretation, and when their impacts on human health are sustained by potent evidence, it is generally not challenging to make occupational or public health decisions. In these situations, ethical concerns rarely arise. However, when handling with non-measurable forms of uncertainty, as is the matter with nanotechnology, the decision-making procedure is not sustained by an adequate quantity of information and ethical impasses may arise and take root. It is though not apparent if the applicable information on nanoparticle threat is really adequate to justify medical investigation and even what minimal level of information is necessary to support decision-making is a matter of controversy (Dresser 2012; Jamison 2009).
The beneficence fundamental states that decisions should increase the profits for both the society and individuals. The approach of medical investigation of individuals unveiled to nanoparticles should maintain the advantage of appraising the employer’s health situation contrary to the ethical costs. Given the limitation of the reliable analysis, challenges in illustrating the results of both physical and verification irregularity, and the lack of specific criteria for determining the ethical expenses can be extremely high.
9.9.4 Ethical Challenges Inherent to Nanomedicine Applications
The ethical impasses related to nanomedicine are not restricted to the laboratory solely. Nanomedicine increases and whets the screening and therapeutic approaches feasible for contemporary medicine. With its commitment, however, the requirement for an ethical appraisal of the purpose and significance of nanomedical novelty in screening and therapeutics arises.
Nanomedical commodities, which could determine early screening of the disorders, incite queries concerning the implication and effect of the evidences when no therapeutic option exists (Desai 2012; Ventola 2012b). Therefore, queries concerning to an individual are right to learn, but also on the right not to learn (i.e., not to unveil to excessive information that is insignificant or incidentally approved) arise.
Ethical queries also appear with respect to therapeutics. The predicted high cost of nanomedical commodities, at least in the initial phases of marketing, increase the justice fundamental arguments with socio-economic discrimination and challenged the approachability. Arguments like enabling the rich to profit, while the poor cannot access, might broaden the social difference within and among both societies and individuals (Wolf and Jones 2011).
9.9.5 Social Ethics in Public Health Systems
The progression of nanomedicine and its potent utilization increases various socio-economic queries, specifically within the situation of public health. The economic sector has hardly any reason and also inadequate funds to generate extensive information on harmful and safety issues of nanomedicine; therefore it falls on the responsibility of governments to transport public budget towards the production of what is apparently beneficial for the public and awareness of the safety and health significance of the novel technology platform, which is transforming the world. Without specific support, the potent advantages of nanomedicine will not be illustrated. However, the impact of nanomedicine on human biology is extensive; the other societal impacts of it also account to be considered. Nanotechnological advancements also impact human interplay, economics, and politics (Keiper 2007; Solbakk and Zoloth 2011; Yeagle 2007).
9.9.5.1 Impact of Nanotechnology in Developing Nations
Due to the evidence that Nanotechnology and its utilization fields are potent of being very strong and effective, the impact of this novel technology on developing nations is a matter that should not be neglected. It is obvious to assume that the impact of Nanotechnology will vary from individuals having distinct lifestyles and convenience in a different region. In the twenty-first century, developed nations having huge industries with regard to nanotechnological capability will be an essential element for the global contest (Ebbesen et al. 2006; Ebbesen 2008; Satalkar et al. 2016).
Firstly, the distinction among the definitions of developing and developed nations should be determined. The extent of progression of a nation is depended on some factors such as equality among the people, total national product, and the political balance. According to the evaluation of these factors, advancing nations have a similar scenario. Most of the people in these nations are usually devoid of some fundamental requirements such as health services and education. Secondly, the process cycle of Nanotechnology that is manufacturing, marketing, and utilization of nano-products. In this process, there are functions for different nations such as final product manufacturer, raw material manufacture, and wastes disposer. Every function for each nation can be beneficial or disadvantageous. For example, if a nation is the manufacturer of nanomaterials, it should be benefited with some financial advantage, but on the other hand, the threat of environmental deterioration, unclear employee protection, and undefined dangers arising from nano-products arise as disadvantages for that nation. However, the raw material manufacturing and marketing of these products to technological product manufacturer nations appears advantageous (Gökçay and Berna 2015; Graur et al. 2011; Leary 2013).
As a general conclusion, nanotechnology is displaying as a beneficial field for developed nations. However, the developing nations will not be able to compete contrary to the developed nations in the area of Nanotechnology. Nanotechnology, as a consequence, will limit developed nations reliability on developing nations having raw material, and if this reason causes developing nations to fall in the economy, developing nations should work on international negotiations those promise their profits.
9.9.5.2 Impact of Nanotechnology on Laborers and Managerial Issues
It is always likely to come up with certain uncertainty in every kind of Research and Development area, which is the basic characteristics of scientific research. Generally, the unpredictability of scientific analysis outcome is also unpredictable for the safety of the scientists. When discussing Nanotechnology, the unrecognized harm of the nanoparticles could be dangerous for scientists. Unknown employee protection and autonomous dangers arising from nano-products should be considered to have an impact on laborers.
Currently, there is a developing focus toward the field of Nanotechnology research. While the funds for Nanotechnology analysis in the USA was 2.1 billion USD in 2012, 16.5 billion USD has been used since 2001 (2012’s budget is included). From the year 2005, nanotechnological research fund that has been used in the defense industry, environmental, and health fields was 575 billion US dollar and the fund used for the analysis for legal, ethical, and social impacts of nanotechnology was higher than 390 billion US dollars.
For the regulation of research on Nanotechnology around the world in relation to the ethical dimensionality of science and technology transformation, UNESCO initiated an “International Bioethics Committee” comprising of thirty-six experts of a distinct area from various regions in the world. The goal of this committee is to share various novel ideas and knowledge in the area of life sciences, to make suggestions in the decision taking powers and to make a platform between those public and powers. It is also crucial to increase the general awareness of individuals having distinct educational qualifications. As Nanotechnology is a promptly developing area, it is immensely necessary to consider the ethical impacts for the future (Gelfert 2012; Kearnes 2006; Wolf and Jones 2011).
9.10 Future Perspectives
In the historical advancement of medicine, there has always been an innovative dialogue and mental stress between the more idealistic scientist and the patient-oriented clinician. Similarly, in the present context, Nanomedicine is at risk of being available only to privileged societies, at least initially, but it proposes uncultivated opportunities for medical advances that might benefit underprivileged populations. When the queries rise in the framework of more traditional clinical contexts and the issues are addressed in a specific, gradual way, is called type 1 research practice. On the other hand, when there are key new fields of fundamental scientific study, the leading scientists might conceive how the medicine might be basically altered. In this case, the sense of arising science regulates, and its improvement includes a deep and basic rearrangement of medicine. In such type 2 research, the primary spotlight is often on the improvement of the basic insight and tools required for this future vision to be realized. In practice, these two aspects are in constant dialogue and the dissimilarity in the approached proposal might be unseen in certain collaborative projects. Occasionally, the mental stress is clear in the same person, who is both a scientist and a clinician. In the case of medicine, the two advances together, repetitively refining one another, till a novel, more mature kind of assimilation arises, which is referred to as type 3. It associates a basic reshaping of the logics of the resulting science and engineering so that they are reactive to the realities of advancing clinical practice settings.
The stress among the Nanomedicine impacts reflects on the ethical issues. Thus, these ethical issues have been observed as beyond the science—as the issues of integrative or human-subject preservation were one-time objections. Such an accession to ethics reflects a long-standing complication between the realms of value and facts. Yet, in Nanomedicine, this older complication is no longer justifiable. The new science calls for a fundamental reconsidering of the relationship between ethical reflection and medical research. Even though it might be too early to say what a mature type 3 nanomedicine requires, but one thing is clear: it has to include a far more nuanced, crucial consideration to the institutional, cultural, and policy circumstances that allow its own practice. Without this, the full capacity of Nanomedicine will remain suppressed.
9.11 Conclusion
Concept and misconception of risks by researchers and representatives of the public similarly play an important function in decision taking to approve or reject the technologies, and also to decide how best to reduce any specific issues. Valuable decision-making arises at each step of the process cycle. Notably, the queries may not be about how “real” the threats are, but instead, how they approach to introduce in the society, which in turn defines a favorable agreement about a society and its institutions of administrations. Some researchers and ethicists have been immensely vocal about the requirement to review the threats arising from nanotechnologies and their products, recounting multiple reasons for concern. Others recommend that present systems are adequate and insist that the present approach already identifies and deals with the threats, so adding a new committee of review will only delay the development of potentially valuable products (Jotterand 2008). Nanotechnology is also located in historical importance in which wider problems of evidence, expertise, and potentiality are being called into queries. This is when the suggestion to establish an inter-agency working committee is most significant: where crucial data may be available but is not evaluated equally or properly across managerial agencies and other significant organizations. An inter-agency working committee could reduce the gaps in awareness and types of expertise, be better potent to organize more extensive and integrative study, and more importantly, deteriorate potential issues. Novel nano-products also come in review with regard to evidence-based medicine approaches, which have a greater limit for illustrating the effectiveness and may influence the threat and advantage of study accordingly. Analysis into distinct ways of organizing pre-clinical trials, including the recommendation of introducing predictive algorithms and bioinformatics or cell-based, in vitro pre-clinical analysis in addition to the visualization approaches during and after the administration of nanomedicines may transform the complete process of analyses. Medical threat evaluation performed by quantitative risk experts, regulatory authorities, and bioethicists has not apparently considered the wider scenario of the threats, comprising the market and business threat evaluations made by the translating percepts into products. Decisions taken about the novel nano-products from this perspective from clinical trial to market introduction are core on distinct inference and priorities than those utilized by regulators and bioethicists, yet there is a specific interplay between the two decision taking approaches (Hogle 2012). One way to negotiate with threats might be to encourage trial sponsors themselves to become more deliberate about the threat and the threat practices. Triggered by financial downfall as much as natural and technological disasters, many agencies have become conscious of how immensely disasters might influence the progress of the agencies for the near and long period (Hall et al. 2012; Schottel and Karn 2016; Ventola 2012b). Regulation and communication among all entities are crucial in order to avert assumptions from being concretized into exercise and to promise an interspersed, consistent reflection on review practices as a whole. If we agree that values are ingrained in threats, threat analysis, and improvement, then adding to the supervision of Nanomedicine human trial analysis by establishing an inter-agency working committee and International ethical committee with different experts representing distinct kinds of expertise and function in society will be beneficial in acknowledging and understanding the moral, social, and scientific elements of decision taking about which is a better way to proceed. They are an essential element towards responsible novelty in Nanomedicine (Ventola 2012a). Focusing attention on the promise that certain Nanomedicine drugs behold in terms of personalized medicine and targeted drug delivery, and also the associated safety and risk factors that need to be regulated in terms of benefits to the subjects, society, and environment, the formation of global negotiations should be strengthened to illuminate the ethical and legal aspects of Nanomedicine.
References
Allon I, Ben-Yehudah A, Dekel R, Solbakk JH, Weltring KM, Siegal G (2017) Ethical issues in nanomedicine: tempest in a teapot? Medicine. Health Care Philos 20(1):3–1
Bawa R (2005) Will the nanomedicine “patent land grab” thwart commercialization? Nanomedicine: nanotechnology. Biol Med 1(4):346–340
Bawa R, Johnson S (2007) The ethical dimensions of nanomedicine. Med Clin N Am 91(5):881–887
Bawa R, Johnson S (2009) Emerging issues in nanomedicine and ethics. In: Nanotechnology & society. Springer, Dordrecht, pp 207–203
Bawa R, Bawa SR, Maebius SB, Flynn T, Wei C (2005) Protecting new ideas and inventions in nanomedicine with patents. Nanomedicine 1(2):150–158
Bruce D (2006) The question of ethics. Nano Today 1(1):6–7
Chang EH, Harford JB, Eaton MA, Boisseau PM, Dube A, Hayeshi R et al (2015) Nanomedicine: past, present and future–a global perspective. Biochem Biophys Res Commun 468(3):511–517
de SC, Nigel M (2006) The NELSI imperative: nano ethical, legal and social issues, and federal policy development. Nanotechnol Law Bus 3:159
Desai N (2012) Challenges in development of nanoparticle-based therapeutics. AAPS J 14(2):282–285
Dresser R (2012) Building an ethical foundation for first-in-human nanotrials. J Law Med Ethics 40(4):802–808
Ebbesen M (2008) The role of the humanities and social sciences in nanotechnology research and development. NanoEthics 2(1):1–3
Ebbesen M, Andersen S, Besenbacher F (2006) Ethics in nanotechnology: starting from scratch? Bull Sci Technol Soc 26(6):451–452
Ferrari M, Philibert MA, Sanhai WR (2009) Nanomedicine and society. Clin Pharmacol Ther 85(5):466–467
Gelfert A (2012) Nanotechnology as ideology: towards a critical theory of ‘converging technologies’. Sci Technol Soc 17(1):143–144
Gökçay B, Berna AR (2015) Nanotechnology, nanomedicine; ethical aspects. Rev Romana Bioet 13(3):423–432
Graur F, Elisei R, Szasz A, Neagos HC, Muresan A, Furcea L et al (2011) Ethical issues in nanomedicine. In: International conference on advancements of medicine and health care through technology. Springer, Cham, pp 9–12
Habets MG, van Delden JJ, Bredenoord AL (2016) Studying the lay of the land: views and experiences of professionals in the translational pluripotent stem cell field. Regen Med 11(1):63–61
Hall RM, Sun T, Ferrari M (2012) A portrait of nanomedicine and its bioethical implications. J Law Med Ethics 40(4):763–769
Hartzema AG, Reich CG, Ryan PB, Stang PE, Madigan D, Welebob E et al (2013) Managing data quality for a drug safety surveillance system. Drug Saf 36(1):49–48
Anderson JA, Kimmelman J (2010) Extending clinical equipoise to phase 1 trials involving patients: unresolved problems. Kennedy Inst Ethics J 20(1):75–98
Hogle LF (2012) Concepts of risk in nanomedicine research. J Law Med Ethics 40(4):809–802
Jamison A (2009) Can nanotechnology be just? On nanotechnology and the emerging movement for global justice. NanoEthics 3(2):129–126
Jotterand F (ed) (2008) Emerging conceptual, ethical and policy issues in bionanotechnology. Springer, Dordrecht
Kearnes M (2006) Chaos and control: nanotechnology and the politics of emergence. Paragraph 29(2):57–50
Keiper A (2007) Nanoethics as a discipline? The New Atlantis 16:55–57
Kuiken T (2011) Nanomedicine and ethics: is there anything new or unique? Wiley Interdiscip Rev Nanomed Nanobiotechnol 3(2):111–118
Leary JF (2013) Nanomedicine–reality will trump hype! J Nanomed Biother Discov 4:125
Liu D, Yang F, Xiong F, Gu N (2016) The smart drug delivery system and its clinical potential. Theranostics 6(9):1306
Nel A, Xia T, Mädler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761):622–627
Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K et al (2005a) Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol 2(1):8
Oberdörster G, Oberdörster E, Oberdörster J (2005b) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113(7):823–829
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19(12):1584
Patra D, Haribabu E, McComas KA (2010) Perceptions of nano ethics among practitioners in a developing country: a case of India. NanoEthics 4(1):67–65
Resnik DB, Tinkle SS (2007) Ethical issues in clinical trials involving nanomedicine. Contemp Clin Trials 28(4):433–431
Sandler R (2009) Nanomedicine and nanomedical ethics. Am J Bioeth 9(10):16–17
Satalkar P, Elger BS, Shaw DM (2016) Defining nano, nanotechnology and nanomedicine: why should it matter? Sci Eng Ethics 22(5):1255–1256
Schottel BL, Karn B (2016) The national nanotechnology initiative approach to environment, health, and safety: a model for future science investments. Fed Hist 8:48
Sechi G, Bedognetti D, Sgarrella F, Eperen LV, Marincola FM, Bianco A et al (2014) The perception of nanotechnology and nanomedicine: a worldwide social media study. Nanomedicine 9(10):1475–1476
Sharma R, Mody N, Agrawal U, Vyas SP (2017) Theranostic nanomedicine; a next generation platform for cancer diagnosis and therapy. Mini Rev Med Chem 17(18):1746–1747
Siddique S, Alexander A, Yadav P, Agrawal M, Shehata AM, Shaker MA et al (2019) Nanomedicines: challenges and perspectives for future nanotechnology in the healthcare system. Sci Res Essays 14(5):32–38
Solbakk JH, Zoloth L (2011) The tragedy of translation: the case of “first use” in human embryonic stem cell research. Cell Stem Cell 8(5):479–471
Strom BL (2006) How the US drug safety system should be changed. JAMA 295(17):2072–2075
Turanlı ET, Everest E (2016) Nanomedicine. In: Low-dimensional and nanostructured materials and devices. Springer, Cham, pp 579–587
Ventola CL (2012a) The nanomedicine revolution: part 1: emerging concepts. Pharm Ther 37(9):512
Ventola CL (2012b) The nanomedicine revolution: part 3: regulatory and safety challenges. Pharm Ther 37(11):631
Ventola CL (2017) Progress in nanomedicine: approved and investigational nanodrugs. Pharm Ther 42(12):742
Wolf SM, Jones CM (2011) Designing oversight for nanomedicine research in human subjects: systematic analysis of exceptional oversight for emerging technologies. J Nanopart Res 13(4):1449–1445
Wynne B (1992) Uncertainty and environmental learning: reconceiving science and policy in the preventive paradigm. Glob Environ Chang 2(2):111–117
Yeagle J (2007) Nanotechnology and the FDA. VA J Law Technol 12:1
Życiński J (2006) Ethics in medical technologies: the Roman Catholic viewpoint. J Clin Neurosci 13(5):518–513
Acknowledgement
The authors are thankful to Chettinad Academy of Research and Education (CARE) for providing the infrastructural support and to SERB, DST, Govt. of India, for providing the financial support to complete this piece of work.
Author Contribution
The idea and design of the study was conceived by Antara Banerjee. Alakesh Das, Dikshita Deka, Syed Sana Abrar, Surajit Pathak, and Antara Banerjee wrote the manuscript and designed the diagrammatic representations. All the authors read and approved the final version of the manuscript.
Funding
This work was supported by the grants sanctioned to Dr. Antara Banerjee (PI) from the SERB-DST, Govt. of India with the sanction file no ECR/2017/001066 and departmental grants at CARE.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Das, A., Deka, D., Abrar, S.S., Pathak, S., Banerjee, A. (2021). Ethics in Nanomedicine. In: Malik, A., Afaq, S., Tarique, M. (eds) Nanomedicine for Cancer Diagnosis and Therapy. Springer, Singapore. https://doi.org/10.1007/978-981-15-7564-8_9
Download citation
DOI: https://doi.org/10.1007/978-981-15-7564-8_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7563-1
Online ISBN: 978-981-15-7564-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)