Abstract
Synthetic dyes are very difficult to degrade for their complex structure. During dyeing process in industry, a huge amount of dyes are lost as effluents. Degradation of these effluents by physical and chemical processes is the most problematic issues nowadays. Enzymatic oxidation of these dyes using oxidoreductase such as laccase has received a great attention in recent years for decolorization of dyes in effluent. In the present study, an isolated fungus Aspergillus flavus PUF5 was used to produce laccase enzyme and employed for degradation of five commercially used textile dyes, phenol red, methyl orange, malachite green, bromophenol blue and Congo red. The investigations were made with the effect of redox mediators (1 mM), temperature (25–45 °C), pH (4–8) and incubation time (0–24 h) in the dye decolorization by laccase. Highest efficiency in dye decolorization was found with redox mediator 1-hydroxy-benzotriazole (HBT). The interactions between four variable factors were statistically studied using response surface methodology. Optimized states of selected variables were dye concentration (6.04 µM), enzyme concentration (78.8 U/ml), pH (5.6) and redox mediator (1.07 mM) with predicted and observed activity of laccase 85.94 and 86.3 U/ml, respectively. These results suggest that laccase is a potential enzyme for removal of dyes present in wastewater of textile industry.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
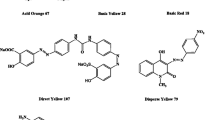
Laccases (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) is a multicopper oxidase that reduces molecular oxygen to water and simultaneously performs one-electron oxidation in a large range of inorganic and organic substrates, including mono-, di- and polyphenols, methoxyphenols, aromatic amine and aminophenols. In the recent decades, it has been reported that a number of fungal laccases could decolorize and degrade industrial dyes which are widely used in the dyeing fabric, printing paper, leather, food process industries, colour photography and as additives in petroleum-based products. Synthetic dyes are different types like azo, triarylmethane, indigoid and anthra-quinonic, used in dyeing process. The textile industry stands out, as it produces a large amount of effluents which can cause serious environmental problems like affect photosynthetic activity in aquatic life by reducing light penetration intensity and may also be toxic to some aquatic fauna and flora due to the presence of aromatics, metals, chlorides, etc. (Chacko and Subramaniam 2011). It is estimated that about 10–15% of dyes are released into processing water during dyeing and subsequent washing steps of procedure (Selvam et al. 2003). Traditional processes are usually costly, complex and produced a large number of slush but enzymatic process give an eco-friendly and capable substitute for decolorizing of dyes and also detoxify before discharging into the environment (Blanquez et al. 2004). The enzymatic approach deals mainly with ligninolytic enzymes, of which laccases are the most preferred enzymes for this purpose since they act oxidatively and less particularly on aromatic rings, thus having prospective to degrade a various range of compound. The use of mediator compounds permits an expansion of the reactivity of laccase to include such unnatural laccase substrates by generating high redox potential species. Using mediator, laccase can indirectly oxidize large molecules and even nonphenolic substrates also. 1-hydroxybenzotriazole (HBT) is one of the most important laccase mediators (Moldes and Sanroman 2006), but the prime issue of HBT using is its limited biodegradability and possible toxicity (Murugesan et al. 2007). Hence, HBT concentration optimization is much more important for efficient decolourization. In this present work, we have investigated degradation/decolourization of textile dyes, Bromophenol blue, phenol red, malachite green, methyl orange and Congo red by laccase enzyme which is produced from an isolated and identified fungus Aspergillus flavus PUF5. The aim of this study is to optimize the HBT, pH, enzyme and dyes concentrations in order to obtain the best possible results in dye decolourization by laccase.
2 Materials and Methods
2.1 Fungal Strain
The laccase producing fungi A. flavus PUF5 (GenBank accession number KX181714.1) was maintained on potato dextrose agar slants. A suspension containing about 5 × 109 spores/ml was used as an inoculum.
2.2 Laccase Enzyme Production by Solid-State Fermentation
Agricultural waste substrate ribbed gourd peel was moistened with water and autoclaved in 250 ml Erlenmeyer flasks up to 7 days with 7-day-old culture at 30 °C. After fermentation, the supernatant was collected, stored and used as enzyme source of laccase.
2.3 Enzyme Assay
Free laccase activity was determined using the process as described by Desai et al. (2011) where guaiacol was used as substrate. Laccase activity was expressed in U/ml.
2.4 Effect of Laccase and Dye Concentrations on Decolorization Efficiency
The effect of laccase concentration on decolorization efficiency was determined by incubating the synthetic dyes with varying concentrations of laccase (20–100 U/ml) for 24 h. To study the effect of dye concentrations with varying concentrations (5, 10, 15, 20, 25 µM) were used for 24 h. Immediate after incubation the dye degradation efficiency was expressed in percentage (%).
2.5 Effect of Temperature, PH, Incubation Time and Laccase Mediator on Dye Decolorization Efficiency
During optimization of dye decolorization efficiency by laccase, the enzyme and dyes were incubated at varying temperatures (25–45 °C), pH (4.0–8.0) and incubation time (0–24 h). 1-hydroxybenzotriazole (HBT) (1 mM) was added in the reaction mixture as redox mediator and observed up to 24 h. Dye degradation efficiency was expressed in percentage (%).
2.6 Box–Behnken Response Surface Methodology
The effects of dye concentration (mM), enzyme concentration (U/ml), pH, HBT (%) on dye decolorization efficiency were evaluated. These factors were chosen as they showed influencing effects in one variable at a time (OVAT) optimization. Levels of these factors were optimized for maximum decolorization of dye using Box–Behnken statistical design (Box and Behnken 1960). Table 1 represents different selected factors where each variable was tested in three different coded levels: low (−1), middle (0) and high (+1). Table 2 represents a 29-trial of the experimental design.
2.7 Statistical Analysis
Statistical analyses were performed using statistical software Design Expert (version 8.2; STATEASE Inc., Minneapolis, MN, USA).
3 Results and Discussion
3.1 Effect of PH and Temperature on Decolorization of Dye
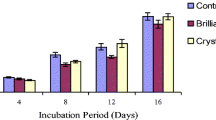
Congo red, bromophenol blue and malachite green were decolorized after 12 h of incubation (15–28%), at around pH 4.8–5.8. On the other hand, methyl orange and phenol red were most decolorized (60–75%) at pH 5, above which the decolorization process decreased remarkably. These observations are shown in (Fig. 1a) indicated that the optimum pH is mostly 5 for laccase catalyzed oxidation (Nyanhongo et al. 2002).
The effects of temperature (25–45 °C) on decolorization are shown in (Fig. 1b). Over the examined range, decolorization rate increased at 30 °C after this certain degree dye decolorization decreased as a result of the fact that the enzymatic catalysis rate becomes lower than the rate of enzyme inactivation at high temperature (Nyanhongo et al. 2002).
3.2 Effect of Laccase and Dye Concentration on Decolorization of Dye
The effect of laccase concentration on decolorization of dye found out that the optimum concentration of laccase required for maximum decolorization was 100 U/ml (Fig. 2a). The effect of dye concentration (5–25 µM) on decolorization of dyes by laccase observed that the percentage of decolorization was gradually decreased after 24 h on increasing the concentration of dye (Fig. 2b). Sathishkumar et al. (2010) had reported that when concentration of laccase increased then rate of dye decolorization also increased and concentrations of the dye when higher, then effectiveness of the enzyme gradually reduced but did not diminish completely.
3.3 Effect of Incubation Time and Mediator on Dye Decolorization
Facilitating electron transfer from O2 to laccase substrate is the main mechanism behind HBT assisted oxidation of different substrates (Forootanfar et al. 2012). Figure 3a showed that phenol red (73%), methyl orange (68%), bromophenol blue, congo red and malachite green less than 30% were decolorized after 24 h (Ado et al. 2019). In the presence of HBT as a laccase mediator Fig. 3b showed that 10–20% positive influence on decolorization percentages of all studied dyes. Maalej-Kammoun et al. 2009 reported in their study that among ten laccase mediators, HBT showed the maximum decolorization of malachite green.
3.4 Optimization of Parameters for Dye Decolorization Through Box–Behnken Response Surface Design
The optimal level of the key factors and the effect of their interactions on dye decolorization were explored by the Box–Behnken response surface methodology. By applying multiple regression analysis on the experimental data, the following second-order polynomial equation was established to explain the decolorization of dye:
where Y is the predicted yield of dye decolorization; A, B, C, D are the coded values of dye concentration (mM), enzyme concentration (U/ml), pH, HBT (%), respectively. The analysis of variance (ANOVA) was conducted to test the significance of the fit of the second-order polynomial equation for the experimental data as shown in Table 2.
The Model F-value of 1054.38 implies the model is significant. There is only a 0.01% chance that a ‘‘Model F-value” could occur due to noise. Model P-value in this study was <0.0001 which also indicates that the model was significant. The coefficient of variation (R2 = 0.999) indicates a good agreement between experimental and predicted values, which implies that the model was reliable for dye decolorization and statistically sound. The response surface plots and the contour plots are shown in Fig. 4. Shapes of response surfaces and contour plots indicate the nature and extent of the interaction between different factors (Prakash et al. 2008). Ferreiraa et al. (2009) also showed that the smallest ellipse in the contour diagram is indicating the maximum predicted value. On the basis of parameters optimization, the quadratic model predicted that the maximum decolorization of dyes was 86.30% when the dye concentration, enzyme concentration, pH and HBT were 6.04 µM, 78.8 U/ml, 5.6 and 1.07 mM, respectively.
3.5 Validation of the Optimized Condition
To verify the predicted result, validation experiment was performed in triplicate tests. Under the optimized condition, experimental and predicted values 86.3 and 85.94 U/ml of laccase yield were in good agreement. The predicted value and the effectiveness of the model, indicating that the optimized medium favours the dye decolorization ability of laccase.
4 Conclusion
The results implement that laccase was produced by A. flavus PUF5 through solid-state fermentation presented a highly decolorizing ability, for azo and triphenylmethane dyes. Further statistically optimized dye decolorization efficiency of laccase. All results advice that laccase and laccase-mediator systems are valuable biocatalysts for the management of effluents from printing, textile and dye industries. This will enable its prompt removal from the effluent before discharge.
References
Ado BV, Onilude AA, Oluma HOA et al (2019) Production of fungal laccase under solid state bioprocessing of agroindustrial waste and its application in decolourization of synthetic dyes. Biol Biotechnol 21:1–17. https://doi.org/10.9734/jabb/2019/v21i430100
Blanquez P, Casas N, Font X et al (2004) Mechanism of textile metal dye biotransformation by Trametes versicolor. Water Res 38:2166–2172. https://doi.org/10.1016/j.watres.2004.01.019
Box GEP, Behnken DW (1960) Simplex-sum designs: a class of second order rotatable designs derivable from those of first order. Ann Math Statist 31(4):838–864. https://doi.org/10.1214/aoms/1177705661
Chacko JT, Subramaniam K (2011) Enzymatic degradation of azo dyes—a review. Int J env sci 1:1250–1260
Desai SS, Tennali GB, Channur N et al (2011) Isolation of laccase producing fungi and partial characterization of laccase. J Biotechnol Bioinfo Bioengg 1:543–549
Ferreiraa S, Duartea AP, Ribeiro MHL et al (2009) Response surface optimization of enzymatic hydrolysis of Cistus ladanifer and Cytisus striatus for bioethanol production. Biochem Eng 45:192–200. https://doi.org/10.1016/j.bej.2009.03.012
Forootanfar H, Moezzi A, Aghaie-Khozani M et al (2012) Synthetic dye decolorization by three sources of fungal laccase. IJEHSE 9:27. https://doi.org/10.1186/1735-2746-9-27
Maalej-Kammoun M, Zouari-Mechichi H, Belbahri L et al (2009) Malachite green decolourization and detoxification by the laccase from a newly isolated strain of Trametes sp. Int Biodeter Biodegr 63:600–606. https://doi.org/10.1016/j.ibiod.2009.04.003
Moldes D, Sanroman MA (2006) Amelioration of the ability to decolorize dyes by laccase: relationship between redox mediators and laccase isoenzymes in Trametes versicolor. World J Microbiol Biotechnol 22:1197–1204. https://doi.org/10.1007/s11274-006-9161-1
Murugesan K, Dhamija A, Nam IH et al (2007) Decolourization of reactive black 5 by laccase: optimization by response surface methodology. Dyes Pigm 75:176–184. https://doi.org/10.1016/j.dyepig.2006.04.020
Nyanhongo GS, Gomesa J, Gubitz GM et al (2002) Decolorization of textile dyes by laccases froma newly isolated strain of Trametes modesta. Water Res 36:1449–1456. https://doi.org/10.1016/s0043-1354(01)00365-7
Prakash O, Talat M, Hasan SH et al (2008) Factorial design for the optimization of enzymatic detection of cadmium in aqueous solution using immobilized urease from vegetable waste. Bioresour Technol 99:7565–7572. https://doi.org/10.1016/j.biortech.2008.02.008
Sathishkumar P, Murugesan K, Palvannan T (2010) Production of laccase from Pleurotus florida using agro-wastes and efficient decolorization of reactive blue 198. J Basic Microbiol 50:360–367. https://doi.org/10.1002/jobm.200900407
Selvam K, Swaminathan K, Keo-Sang C (2003) Microbial decolourization of azo dyes and dye industry effluent by Fomes lividus. World J Microbiol Biotechnol 19:591–593. https://doi.org/10.1023/A:1025128327853
Acknowledgements
The authors are thankful to the University Grant Commission [Sanction no: F/2016-17/NFO201517OBCWES 32372/(SAIII/Website)], Government of India for the financial assistance in this study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Ghosh, P., Ghosh, U. (2021). Biodegradation of Dyes by Laccase from Isolated Strain Aspergillus flavus PUF5. In: Ramkrishna, D., Sengupta, S., Dey Bandyopadhyay, S., Ghosh, A. (eds) Advances in Bioprocess Engineering and Technology . Lecture Notes in Bioengineering. Springer, Singapore. https://doi.org/10.1007/978-981-15-7409-2_11
Download citation
DOI: https://doi.org/10.1007/978-981-15-7409-2_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-7408-5
Online ISBN: 978-981-15-7409-2
eBook Packages: EngineeringEngineering (R0)