Abstract
Lynch syndrome (LS) is caused by pathogenic variants of one of DNA mismatch repair genes (MLH1, MSH2, MSH6, PMS2) or 3′ deletion of EPCAM in the germline. Colorectal cancer (CRC) is a hallmark of the syndrome, and the cumulative risk of CRC ranges from 10 to 46% by 70 years old, depending on the gene affected. Moreover, despite the high cumulative risk of subsequent CRC (metachronous CRC) after the first CRC is managed with surgery, the optimal surgical treatment is controversial. Prophylactic colorectal resection prior to CRC development is not recommended regardless of the type of surgery. Recent studies and our analysis show that the risk of metachronous CRC is considerably higher following segmental colectomy as compared to extended colectomy among patients with genetically proven LS. However, overall survival did not differ between patients with two different surgical procedures. The choice of surgical procedures for the first rectal cancer is complicated because of insufficient data available and thus needs further investigations. Surgical procedures for first CRC in patients with LS may depend on various factors that may vary among individuals, including the timing of genetic diagnosis (before or after the first CRC development), age at CRC diagnosis, site of CRC, genes affected, and expected quality of life. Individuals with genetically proven LS must be counselled prior to any surgical intervention for first CRC. In summary, an extended colectomy may be currently the most effective surgical technique to reduce the risk of metachronous colon cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
Colorectal cancer (CRC) is the fourth most frequently diagnosed malignancy and the second leading cause of cancer-related death globally [1]. Lynch syndrome (LS) is an autosomal dominant condition caused by pathogenic variants in one of DNA mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2) or 3′ deletion of EPCAM in the germline line [2]. Carriers of the LS pathogenic variant are at increased risk for various cancers at a young age including CRC and endometrial cancer. The European “Mallorca group” and the International Society for Gastrointestinal Hereditary Tumours recently reported that the lifetime risk of CRC by age 70 years is approximately 10–46%, depending on the gene affected [3, 4]. LS-associated CRC is characterized by right-sided predominance, low incidence in the rectum, and high incidence of synchronous and metachronous occurrence [2, 5]. These characteristics might influence the choice of surgical procedures, the timing of genetic diagnosis (before or after surgical interventions), and long-term quality of life that is potentially altered by different surgical procedures.
Segmental colectomy (SC), which comprises right hemicolectomy, left colectomy, sigmoid colectomy, or other segmental resection of the colon segment, is a standard surgical procedure for LS-associated CRC regardless of the timing of genetic diagnosis. Considering the increased risk of metachronous CRC in individuals with genetically proven LS that develop CRC, more extended colectomy (EC), which comprises subtotal colectomy with ileosigmoidal anastomosis or total colectomy with ileorectal anastomosis, should be considered for the first CRC. The optimal surgical approach for CRC patients with LS remains unclear to date because of the lack of randomized controlled trials comparing SC and EC. In addition, the oncological outcomes of LS patients with rectal cancer diagnosed with first CRC have not been well-examined because of its low prevalence. In this chapter, we reviewed the surgical approaches and outcomes in patients with genetically proven LS who underwent surgical resection for first CRC to guide decision-making for the optimal surgical approach in these patients.
10.2 Baseline Characteristics of LS-Associated CRC Updated
Baseline characteristics of LS-associated CRC have been previously reported; however, the majority of previous studies enrolled patients who fulfilled clinical criteria such as the revised Amsterdam criteria and/or those characterized with high penetrance of CRC, that is, those with proven pathogenic variant in MLH1 or MSH2. Recent advances in genetic diagnosis of LS and the widespread use of universal LS-associated tumor screening have led to the updating of characteristics of LS-associated CRC, which is an important factor in surgical treatment.
The “Mallorca group” [3] has recently conducted a prospective study of 1942 individuals with germline pathogenic variants of MLH1, MSH2, MSH6, and PMS2 and reported the revised estimates of the different penetrance of CRC. These 1942 individuals who did not develop previously cancer had colonoscopic surveillance for a total of 13782 observation years. Among those with first cancer, the cumulative incidences of CRC at 70 years by gene were 46%, 35%, 20%, and 10% for MLH1, MSH2, MSH6, and PMS2 carriers, respectively.
Kim et al. [5] reported the tumor location of first CRC and synchronous CRC in 114 LS patients with a mean age of 43 years (range, 24–82 years) who were referred to genetic testing through universal tumor screening. The tumor was located in the right colon, left colon, and the rectum in 74 (69.8%), 21 (19.8%), and 11 (10.4%) patients. Synchronous CRC developed in 12 (11.3%), while metachronous CRC developed in 13 (11.4%). Approximately half (54%) of the metachronous CRCs were located in the right colon, while four (31%) were in the rectum.
Hiatt et al. [6] analyzed 64 LS patients who underwent SC for proximal colon cancer and reported that 6 (46%) of the 13 patients who developed metachronous CRC, developed it again in the remaining proximal colon.
Win et al. [7] analyzed 79 LS patients with first rectal cancer treated with abdominal perineal resection or anterior resection and reported that 21 metachronous CRCs developed, 16 (76%) of which were in the proximal colon (cecum, ascending colon, hepatic flexure, or transverse colon).
10.3 Surgical Treatment for CRC in LS
10.3.1 Prophylactic Surgery
In the past, prophylactic colorectal resection such as total proctocolectomy with ileorectal anastomosis or total proctocolectomy with ileal pouch-anal anastomosis (IPAA) prior to first CRC development was performed in selected individuals with LS or hereditary nonpolyposis CRC (HNPCC) [8] without genetic diagnosis. However, because of the lower penetrance of CRC in carriers of the LS pathogenic variant than patients with familial adenomatous polyposis, prophylactic colorectal resection should not be performed regardless of the type of resection.
10.3.2 Metachronous Risk of CRC Following SC vs. EC for First Colon Cancer
Heneghan et al. [9], Anele et al. [10], and Malik et al. [11] conducted systematic reviews and meta-analyses to investigate the risk of metachronous CRC following SC for first CRC compared to EC (Table 10.1). Of these studies, Malik et al. [11] evaluated the largest cases and reported the genetically proven cases separately from those who were not. They demonstrated that among patients with genetically proven, the risk of metachronous CRC development was 8.56-fold higher following SC as compared with EC. Similarly, the risk was also higher (3.04-fold) following SC among patients meeting the Amsterdam criteria only. This result seems reasonable because the cohort comprised patients meeting the Amsterdam criteria, which potentially included patients with familial CRC type X [12] who are known to have a lower risk of metachronous CRC than LS patients. The risk of metachronous CRC following SC as compared with EC reported by Heneghan et al. [9] and Anele et al. [10] seems to concur with that reported by Malik et al. [11].
However, the findings of these studies should be interpreted with caution as they included a significant proportion of patients with first rectal cancer who were regarded to have undergone “colectomy.” In addition, these studies included both patients with genetically proven LS and those who fulfilled the Amsterdam criteria only. Despite the useful information they provided, these three systematic reviews and meta-analyses contain inappropriate studies to strictly evaluate the optimal surgical procedures for patients with CRC in LS. Specifically, the study by Natarajan et al. [13], which was included in all the three systematic reviews and meta-analyses, included patients who underwent EC prior to the development of first CRC as a prophylactic treatment. In the study by Win et al. [7], which was included in the systematic review and meta-analysis by Malik et al. [11], all the patients had rectal cancer and were classified into segmental resection group only.
We retrieved five studies comparing SC and EC among patients with genetically proven LS only that were reported between January 2011 and September 2018 (Table 10.2). Similar with findings in previous studies, metachronous CRC was found to occur more frequently in patients who underwent SC (144/639, 22.5%) than in patients who underwent EC (7/215, 3.3%) during long-term follow-up. Collectively, these findings indicate that compared with SC, EC can reduce metachronous CRC in LS patients with first colon cancer. However, we should note that there may be substantial selection bias because patients selected for EC were more likely to have been genetically diagnosed with LS preoperatively and were likely to have stage I cancer than those who underwent SC.
10.3.3 Risk Factors for Metachronous CRC Following SC for First Colon Cancer
Identifying the risk factors for metachronous CRC following SC for first colon (CRC) cancer is important because most patients with LS-associated CRC underwent genetic diagnosis for MMR deficiency following SC for first CRC. The risk factors for metachronous CRC after SC have been reported previously. Kim et al. [5] reported that bowel resection ≥25 cm decreased the risk compared with less extensive resection (hazard ratio (HR): 0.10; 95% confidence interval (CI): 0.01–0.86). Parry et al. [14] also reported that the risk of metachronous CRC significantly reduced by 31% (95% CI: 12–46%, P = 0.002) for every 10 cm of bowel removed. These findings may help when surgeons select SC for patients with LS-associated first CRC. Moreover, these results may be useful for genetically proven LS patients with first CRC and their consulting surgeons to preoperatively decide the type and extent of colorectal resection.
10.3.4 Overall Survival Following SC vs. EC for First Colon Cancer and Decision Analytic Models
Two studies [5, 15] showed no significant difference in disease-free or CRC-specific survival between those who underwent SC and EC. In addition, four studies [5, 6, 15, 16] showed no significant difference in overall survival (Table 10.2). These results may be explained by the following reasons. One is that the major cause of death following surgery for first CRC is not CRC. A total of 40–61% of cancer deaths were related to extra-colonic cancers [17]. Another explanation may be the early detection of metachronous CRCs via periodic colonoscopic surveillance. Kim et al. [5] reported that 93% of patients who developed metachronous CRC presented with early-stage CRC without lymph node or systemic metastasis.
The advantage of EC may be influenced by the age at first CRC. According to the decision analysis model proposed by a Dutch study group, the overall life expectancy gain of EC (subtotal colectomy) compared with SC (hemicolectomy) at ages 27, 47, and 67 years was 2.3, 1, and 0.3 years, respectively [18]. Similarly, Syngal et al. [19] also described a decision analysis model that subtotal colectomy done at 25 years of age in patients with HNPCC led to the greatest life expectancy. Subtotal colectomy done at the time of cancer diagnosis or identification of an adenomatous polyp at an older age did not show any survival benefit compared with periodic surveillance.
Despite the low level of evidence, we should note that EC performed for first colon cancer does not lead to survival benefit compared with SC. However, EC done at young age may have significant survival benefits.
10.3.5 Risk of Metachronous Colon Cancer Following Proctectomy for First Rectal Cancer
Because of the low incidence of first rectal cancer in LS patients, we investigated the risk of metachronous CRC following rectal cancer surgery separately. Approximately 10–15% [5, 20, 21] of LS or HNPCC patients develop rectal cancer as the first CRC, and the surgical decision-making for LS patients is more complicated.
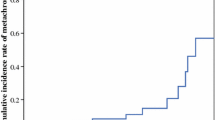
Win et al. [7] reported the results of a multinational collaborative study to evaluate the risk of metachronous CRC in 79 patients with LS (mean age, 42.8 years; range, 17–70 years) who underwent proctectomy (abdominal perineal resection in 29, anterior resection in 50) for first rectal cancer. The cumulative risk of metachronous colon cancer with a median follow-up of 9 years was 19% at 10 years, 47% at 20 years, and 69% at 30 years after proctectomy. They did not identify newly developing cancer in the remaining rectum. As described in the “Baseline characteristics of LS-associated CRC updated,” 16 (76%) of the 21 metachronous CRCs were located in the right colon, which may be due to the commonly observed shift to right colon cancers in LS. Cox proportional hazard regression analysis was performed to investigate the factors influencing the risk of metachronous colon cancer and it showed that the risk of metachronous colon cancer was not associated with sex, age at rectal cancer diagnosis, country of recruitment, cigarette smoking status, maximum tumor diameter, and histologic grade of rectal cancer. However, it was associated with a higher American Joint Committee in Cancer (AJCC) stage (HR: 6.14; 95% CI: 1.21–13.14, P = 0.03) and the presence of synchronous tumor (HR: 11.54; 95% CI: 1.06–125, P = 0.04).
A pan-proctocolectomy would be theoretically a choice to eliminate the risk of metachronous CRC, particularly in patients in whom the primary tumor developed in the rectum. Thus, some surgeons might recommend IPAA as a surgical intervention due to the high risk of metachronous colon cancer following first rectal cancer surgery. However, further investigations with larger series of patients with rectal cancer are needed to determine the optimal surgical treatment for first rectal cancer in LS patients. Currently, the first rectal cancer developing in LS patients should be treated based on standard oncologic principles for sporadic rectal cancer.
10.4 Assessment for Quality of Life
To date, data on postoperative quality of life along with bowel function from different surgical procedures are incomplete. Although not limited to patients with LS, You et al. [22] compared 201 patients who underwent EC (total colectomy or subtotal colectomy) and 321 patients who underwent SC and concluded that functional outcomes regarding median daily stool frequency, urgency and looseness of stool, and quality of life including sexual relations, recreation, travel, housework, and social activity were better preserved after SC than EC. Haanstra et al. [23] surveyed patients with LS who underwent surgical treatment of CRC and compared quality of life outcomes in 51 patients who underwent SC and 53 patients who underwent EC (total colectomy) with three validated instruments. After EC, there was a detrimental effect on stool frequency, social impact, and problems with defecation. However, none of the three instruments demonstrated a negative impact on the overall quality of life between the two surgical procedures.
The extent of colectomy should therefore be balanced against functional outcomes and quality of life. Prior to surgery for first CRC, patients with LS should be informed of the functional differences and outcomes but the similar overall quality of life between the two surgical procedures.
10.5 Colonoscopic Surveillance and Chemoprevention Following Resection of First CRC
Several studies on the efficacy and interval of colonoscopic surveillance before CRC diagnosis in carriers of the LS germline pathogenic variant have been conducted. A systematic review by Lindor et al. [24] concluded that colonoscopic surveillance should be done every 1–2 years starting at age 20–25 years or at 10 years younger than the youngest age diagnosed with colon cancer in patients with a family history. Meanwhile, studies on the efficacy of postoperative colonoscopic surveillance are limited. Kalady et al. [25] reported that in a colonoscopic surveillance of 253 patients meeting the Amsterdam criteria who underwent SC, 221 (88%) of whom had postoperative surveillance at a median interval of 25 months, 55 (25%) developed metachronous CRC. Parry et al. [14] reported a 16% cumulative risk of CRC following SC at 10 years postoperatively, despite an average of 1 colonoscopy every 20 months. In addition, they reported that 47% of metachronous colon cancer following SC were diagnosed as stage I, in contrast to the study by Kalady et al. [25] that reported a higher proportion of advanced-stage disease. Although evidence is limited, annual colonoscopy has been recommended after SC by some experts [26]. Because there is clearly a risk of metachronous rectal cancer, annual colonoscopic surveillance may be recommended in patients with EC (total colectomy).
Chemoprevention in combination with stringent colonoscopic surveillance could potentially reduce the risk of metachronous CRC in patients undergoing (procto)colectomy regardless of the extent of bowel resection, but evidence is also lacking. The CAPP2 randomized controlled trial [27] demonstrated that chemoprevention with high-dose aspirin (600 mg daily) reduced the risk of developing CRC in LS patients. After a mean follow-up of 55.7 months, aspirin had a protective effect against CRC development, and the effect was even higher in those taking aspirin for 2 years or longer. This finding indicates that chemoprevention using high-dose aspirin may eliminate the need for a prophylactic extended colectomy to prevent metachronous colon cancer. However, the effect of aspirin is delayed and it is not until after a latent period of approximately a decade that the risk is significantly lowered when compared to a placebo. Despite the significant beneficial effect of aspirin, the risk of metachronous CRC following SC compared to EC seems to be already increased by several fold. Therefore, the use of high-dose aspirin is not considered as an alternative approach to EC in terms of the prevention of metachronous CRC.
10.6 Conclusions
Studies have shown that overall survival is not significantly different between EC and SC in CRC patients with LS, although EC may be a better surgical procedure than SC in terms of low risk of metachronous CRC. EC may be recommended for younger individuals with LS with CRC because it decreases the risk of metachronous CRC, leading to a survival benefit. However, several clinical factors, including the causative gene, the location of first CRC, the age of onset, the presence of synchronous tumor, and the AJCC stage, influence the development of metachronous CRC. Data on the location of first CRC and characteristics of metachronous CRC development are still limited. Particularly, whether the risk of metachronous CRC differs between patients undergoing a right-sided SC and those undergoing a left-sided SC for first colon cancer remains unclear. In addition, data on the characteristics of patients with MHS6, PMS2, or EPCAM variants are lacking. Thus, the optimal surgical approaches for first synchronous CRCs and metachronous CRC following SC for the first CRC are yet to be determined. The current investigation will be helpful to determine the best surgical treatment for LS patients with CRC at an individual level.
In conclusion, surgeons and patients with LS should be aware of the risk of metachronous CRC following SC despite 1–2 yearly postoperative surveillance in clinical practice. Careful preoperative counselling concerning the choice of colorectal resection for each patient is mandatory.
References
Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73.
Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18.
Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, Lindblom A, Macrae F, Blanco I, Sijmons R, Jeffries J, Vasen H, Burn J, Nakken S, Hovig E, Rødland EA, Tharmaratnam K, de Vos Tot Nederveen Cappel WH, Hill J, Wijnen J, Green K, Lalloo F, Sunde L, Mints M, Bertario L, Pineda M, Navarro M, Morak M, Renkonen-Sinisalo L, Frayling IM, Plazzer JP, Pylvanainen K, Sampson JR, Capella G, Mecklin JP, Möslein G, Mallorca Group (http://mallorca-group.eu). Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017;66:464–72.
Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, Bernstein I, Bertario L, Burn J, Capella G, Colas C, Engel C, Frayling IM, Genuardi M, Heinimann K, Hes FJ, Hodgson SV, Karagiannis JA, Lalloo F, Lindblom A, Mecklin JP, Møller P, Myrhoj T, Nagengast FM, Parc Y, Ponz de Leon M, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Sijmons RH, Tejpar S, Thomas HJ, Rahner N, Wijnen JT, Järvinen HJ, Möslein G, Mallorca group. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013;62:812–23.
Kim TJ, Kim ER, Hong SN, Kim YH, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, Lee WY, Kim K, Kim K, Chang DK. Survival outcome and risk of metachronous colorectal cancer after surgery in Lynch syndrome. Ann Surg Oncol. 2017;24:1085–92.
Hiatt MJ, Casey MJ, Lynch HT, Snyder CL, Stacey M, Walters RW. Efficacy of proximal colectomy for surgical management of right-sided first colorectal cancer in Lynch Syndrome mutation carriers. Am J Surg. 2018;216:99–105.
Win AK, Parry S, Parry B, Kalady MF, Macrae FA, Ahnen DJ, Young GP, Lipton L, Winship I, Boussioutas A, Young JP, Buchanan DD, Arnold J, Le Marchand L, Newcomb PA, Haile RW, Lindor NM, Gallinger S, Hopper JL, Jenkins MA. Risk of metachronous colon cancer following surgery for rectal cancer in mismatch repair gene mutation carriers. Ann Surg Oncol. 2013;20:1829–36.
Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–6.
Heneghan HM, Martin ST, Winter DC. Segmental vs extended colectomy in the management of hereditary nonpolyposis colorectal cancer: a systematic review and meta-analysis. Color Dis. 2015;17:382–9.
Anele CC, Adegbola SO, Askari A, Rajendran A, Clark SK, Latchford A, Faiz OD. Risk of metachronous colorectal cancer following colectomy in Lynch syndrome: a systematic review and meta-analysis. Color Dis. 2017;19:528–36.
Malik SS, Lythgoe MP, McPhail M, Monahan KJ. Metachronous colorectal cancer following segmental or extended colectomy in Lynch syndrome: a systematic review and meta-analysis. Fam Cancer. 2018;17:557–64.
Lindor NM. Familial colorectal cancer type X: the other half of hereditary nonpolyposis colon cancer syndrome. Surg Oncol Clin N Am. 2009;18:637–45.
Natarajan N, Watson P, Silva-Lopez E, Lynch HT. Comparison of extended colectomy and limited resection in patients with Lynch syndrome. Dis Colon Rectum. 2010;53:77–82.
Parry S, Win AK, Parry B, Macrae FA, Gurrin LC, Church JM, Baron JA, Giles GG, Leggett BA, Winship I, Lipton L, Young GP, Young JP, Lodge CJ, Southey MC, Newcomb PA, Le Marchand L, Haile RW, Lindor NM, Gallinger S, Hopper JL, Jenkins MA. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut. 2011;60:950–7.
Renkonen-Sinisalo L, Seppälä TT, Järvinen HJ, Mecklin JP. Subtotal colectomy for colon cancer reduces the need for subsequent surgery in Lynch syndrome. Dis Colon Rectum. 2017;60:792–9.
Stupart DA, Goldberg PA, Baigrie RJ, Algar U, Ramesar R. Surgery for colonic cancer in HNPCC: total vs segmental colectomy. Color Dis. 2011;13:1395–9.
Pylvänäinen K, Lehtinen T, Kellokumpu I, Järvinen H, Mecklin JP. Causes of death of mutation carriers in Finnish Lynch syndrome families. Familial Cancer. 2012;11:467–71.
de Vos tot Nederveen Cappel WH, Buskens E, van Duijvendijk P, Cats A, Menko FH, Griffioen G, Slors JF, Nagengast FM, Kleibeuker JH, Vasen HF. Decision analysis in the surgical treatment of colorectal cancer due to a mismatch repair gene defect. Gut. 2003;52:1752–5.
Syngal S, Weeks JC, Schrag D, Garber JE, Kuntz KM. Benefits of colonoscopic surveillance and prophylactic colectomy in patients with hereditary nonpolyposis colorectal cancer mutations. Ann Intern Med. 1998;129:787–96.
Kalady MF. Surgical management of hereditary nonpolyposis colorectal cancer. Adv Surg. 2011;45:265–74.
Kalady MF, Lipman J, McGannon E, Church JM. Risk of colonic neoplasia after proctectomy for rectal cancer in hereditary nonpolyposis colorectal cancer. Ann Surg. 2012;255:1121–5.
You YN, Chua HK, Nelson H, Hassan I, Barnes SA, Harrington J. Segmental vs. extended colectomy: measurable differences in morbidity, function, and quality of life. Dis Colon Rectum. 2008;51:1036–43.
Haanstra JF, de Vos Tot Nederveen Cappel WH, Gopie JP, Vecht J, Vanhoutvin SA, Cats A, van der Zaag-Loonen HJ, Langers AM, Bergmann JH, van de Meeberg PC, Dekker E, Kleibeuker JH, Vasen HF, Nagengast FM, van Duijvendijk P. Quality of life after surgery for colon cancer in patients with Lynch syndrome: partial versus subtotal colectomy. Dis Colon Rectum. 2012;55:653–9.
Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KH, Lynch P, Burke W, Press N. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296:1507–17.
Kalady MF, McGannon E, Vogel JD, Manilich E, Fazio VW, Church JM. Risk of colorectal adenoma and carcinoma after colectomy for colorectal cancer in patients meeting Amsterdam criteria. Ann Surg. 2010;252:507–11.
Herzig DO, Buie WD, Weiser MR, You YN, Rafferty JF, Feingold D, Steele SR. Clinical practice guidelines for the surgical treatment of patients with Lynch syndrome. Dis Colon Rectum. 2017;60:137–43.
Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, Bisgaard ML, Dunlop MG, Ho JW, Hodgson SV, Lindblom A, Lubinski J, Morrison PJ, Murday V, Ramesar R, Side L, Scott RJ, Thomas HJ, Vasen HF, Barker G, Crawford G, Elliott F, Movahedi M, Pylvanainen K, Wijnen JT, Fodde R, Lynch HT, Mathers JC, Bishop DT, CAPP2 Investigators. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Ishida, H., Ishibashi, K., Kumamoto, K. (2020). Surgical Approach for Colorectal Cancer in Patients with Lynch Syndrome. In: Tomita, N. (eds) Lynch Syndrome. Springer, Singapore. https://doi.org/10.1007/978-981-15-6891-6_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-6891-6_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6890-9
Online ISBN: 978-981-15-6891-6
eBook Packages: MedicineMedicine (R0)