Abstract
With an increasing number of patients reaching adulthood following surgical repair or palliation of congenital heart defects (CHDs), their regular follow-up assumes importance. For identifying complications, planning procedures and for prognostication, imaging in the form of either cardiac magnetic resonance (CMR) or cardiac computed tomography angiography (CTA) has assumed an important role. Two dimensional and Doppler echocardiography, despite being inexpensive and readily available, has its own limitations in the assessment of complicated post-operative anatomy of conduits and shunts, small residual defects and pulmonary venous drainage to name a few. The two imaging modalities (CTA and CMR) available are not only valuable for the anatomical and functional evaluation of post-operative CHDs but also identify the late post-operative sequelae and intra-/extracardiac complications of the palliative and therapeutic procedures. They also act as a guide to early intervention, prognostication and decision-making process. Though the two modalities are complementary and supplementary to echocardiography, CMR has definite advantages over cardiac CTA in the evaluation of post-operative CHDs given its superiority for the evaluation of ventricular and valvular function, assessment of the functional significance of stenosis and diagnosis and haemodynamic evaluation of small residual/recurrent septal defects. The need for multiple imaging follow-ups also tips the balance in favour of CMR, it being a radiation-free modality. However, some centres may still prefer CTA because of its availability, lower cost and faster scan times. In this chapter, common surgical procedures used for palliation and final correction of CHDs and their imaging findings are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
With an increasing number of patients reaching adulthood following surgical repair or palliation of congenital heart defects (CHDs), their regular follow-up assumes importance. For identifying complications, planning procedures and for prognostication, imaging in the form of either cardiac magnetic resonance (CMR) or cardiac computed tomography angiography (CTA) has assumed an important role. Two dimensional and Doppler echocardiography, despite being inexpensive and readily available, has its own limitations in the assessment of complicated post-operative anatomy of conduits and shunts, small residual defects and pulmonary venous drainage to name a few. Echocardiography is also limited by its poor acoustic window for evaluation of right ventricular and outflow tract anatomy and function and the operator dependence of the technique further amplifies the need for cross-sectional imaging [1]. The two imaging modalities (CTA and CMR) available are not only valuable for the anatomical and functional evaluation of post-operative CHDs but also identify the late post-operative sequelae and intra-/extracardiac complications of the palliative and therapeutic procedures. They also act as a guide to early intervention, prognostication and decision-making process. Though the two modalities are complementary and supplementary to echocardiography, CMR has definite advantages over cardiac CTA in the evaluation of post-operative CHDs given its superiority for the evaluation of ventricular and valvular function, assessment of the functional significance of stenosis and diagnosis and haemodynamic evaluation of small residual/recurrent septal defects. The need for multiple imaging follow-ups also tips the balance in favour of CMR, it being a radiation-free modality. However, some centres may still prefer CTA because of its availability, lower cost and faster scan times.
2 Suggested Imaging Protocol
2.1 CMR Protocol
May include some or all of these:
-
(i)
Localisers
-
(ii)
Spin echo sequences: T2 weighted fat saturated and T1 weighted sequences short axis and four-chamber views for evaluation of gross cardiac and extracardiac anatomy, pulmonary artery (PA) sizes, pleural, pericardial effusions, etc.
-
(iii)
Cine imaging stacks in the four-chamber view, short-axis view, vertical long-axis view and right ventricular outflow tract (RVOT) view for evaluation of ventricular function, focal dyskinesias/regional wall motion abnormalities, diagnosis of valvular/conduit regurgitation (seen as dephasing jets). Any stenosis can be evaluated in various cardiac phases to assess for its possible haemodynamic/physiological significance.
-
(iv)
Contrast-enhanced Magnetic Resonance angiography (MRA) or Time-resolved MR (TWIST: Time-resolved Angiography with Interleaved Stochastic Trajectories) Angiography for evaluation of great vessels and central/peripheral pulmonary stenosis.
-
(v)
Phase-sensitive inversion recovery (PSIR) images in similar views as cine imaging at 5 and 15 min following contrast administration for evaluation of myocardial fibrosis.
-
(vi)
Phase-contrast imaging of the main pulmonary artery and the aorta (true axials of these major arteries) to quantify residual left-to-right shunts (Qp:Qs) and assess the possible haemodynamic significance of a stenosis.
Though almost all sutures, clips and plugs are MR compatible, metallic artefacts can hinder optimal anatomic and functional evaluation. In such cases, using spin-echo sequences, lower echo times, small voxel sizes, thinner slices and signal averaging over multiple breath-holds can, to some extent, mitigate the problem.
For post-contrast scans, intravenous gadolinium is administered in the dose of 0.2 mmol/kg body weight.
With free-breathing acquisition techniques, imaging times have considerably reduced and general anaesthesia (GA) is no longer an absolute requirement. For neonates, infants and small children, a short GA or sedation usually suffices. Acquisition times are short and acquisition is generally smooth in slightly older and co-operative children after adequate counselling.
2.2 CTA Protocol
Non-ionic iodinated contrast (1.0–1.5 ml/kg) is administered via a peripheral intravenous line using a power injector at rates varying from 3.5 to 4.5 ml/s. Manual triggering is advised after assessment of optimal contrast enhancement in view of the complexity and multiplicity of residual shunts and likely presence of stenosis and obstructions. The data set is acquired at a lower tube potential (80 kV), using automatic tube current modulation (Care Dose). For calculation of ventricular function on CTA, acquisition could be retrospectively gated and this gives a multiphasic evaluation of an entire cardiac cycle.
For post-processing of images, optimal images are obtained in both systole and diastole with a thickness of 0.75 mm and soft tissue reconstruction. Advanced dose reduction techniques such as anatomy-based tube current modulation (Care Dose), low kV, high pitch and use of ECG-guided current modulation where the tube current is reduced to 4% of its nominal value during the non-diagnostic phases of the cardiac cycle, allow for performance of very low dose scans in paediatric patients [2].
For optimal evaluation of Glenn shunt, it is advisable to inject contrast in a vein contralateral to the side of the cavopulmonary anastomosis so as to avoid streak artefacts potentially obscuring the circuit [3]. Additional delayed acquisition is required to optimally opacify the circuit for correct evaluation and for ruling out partial thrombosis. For bilateral Glenn, a lower limb intravenous injection and delayed acquisition avoids both streak artefacts and artefacts from contrast mixing with unopacified blood simulating the appearance of a thrombus. Similarly, efforts should be made to correctly image the inferior cavopulmonary anastomosis in the Fontan circuit.
3 Common Surgical Procedures Used for Palliation and Correction of CHDs and Their Imaging Findings
For correct evaluation post-operative CHDs, a thorough understanding of the surgical procedures, their expected anatomy and function is imperative.
4 Palliative Procedures
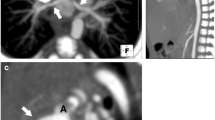
Systemic arterial-to-pulmonary artery shunts are common palliative surgical procedures performed. Their rationale is to increase the delivery of systemic blood to the lungs, thereby increasing blood oxygenation and decreasing cyanosis. They also help in enlarging the pulmonary arteries for a later stage repair. These arterial shunts are done for patients of cyanotic CHD with pulmonary stenosis/atresia or functionally single ventricles. The most commonly performed arterial shunt is the modified Blalock–Taussig (BT) shunt in which a graft is used to connect the subclavian and ipsilateral pulmonary arteries (side-to-side anastomosis), causing minimal distortion of the arterial anatomy (Fig. 18.1). Potts and Waterston-Cooley shunts between the pulmonary artery and descending and ascending aorta, respectively, have now become obsolete and are rarely seen [4]. Another shunt which is also sometimes seen during CHD follow-ups is the central shunt in which a prosthetic graft is placed to connect the ascending aorta and main pulmonary artery (Fig. 18.2). This is done for hypoplastic central pulmonary arteries (Table 18.1).
Cardiac CTA volume-rendered image (a) shows a patent left-sided Blalock–Taussig (BT) shunt (white solid arrows) connecting the left subclavian artery and left pulmonary artery (LPA). Cardiac CTA maximum intensity projection image in the coronal plane (b) shows a patent right-sided BT shunt (white solid arrows)
In some CHD patients, there is only one functional ventricle. These CHDs are characterised by atresia of an atrioventricular or semilunar valve (tricuspid atresia, mitral atresia, pulmonary atresia with intact ventricular septum, right or left ventricular hypoplasia, selected cases of Ebstein anomaly). These single ventricle type CHDs result in the mixing of the systemic and pulmonary venous blood causing cyanosis. Also, the single ventricle is chronically overloaded as it has to support both the systemic and pulmonary circulations. The ventricular function and valvular regurgitation in such cases deteriorate with time.
The rationale of palliation in such cases is to separate the systemic and pulmonary circuits by passively channelling the systemic venous return to pulmonary arteries (PAs). This avoids mixing and reduces the volume load on the single ventricle. Such palliation however comes at the cost of higher central venous pressure.
Glenn originally described a unidirectional anastomosis between the transected distal end of the right pulmonary artery and the side of the superior vena cava (SVC) [5]. The SVC was ligated distal to the anastomotic site. This procedure is currently used in its modified form and is a bidirectional end-to-side anastomosis between SVC and the right pulmonary artery and provides bidirectional non-pulsatile flow to both the lungs (Figs. 18.3 and 18.4). The azygous vein is also ligated to prevent blood egressing from the SVC. The shunt diverts upper body systemic venous blood directly to the lungs improving oxygenation, cyanosis and the workload of the single ventricle. Improvement in cyanosis is partial since the deoxygenated inferior vena cava (IVC) blood continues to reach the systemic circulation. However, it provides excellent long-term palliation in complex CHDs with low pulmonary flow and resistance (mean PA pressure less than 15 mm of Hg and pulmonary vascular resistance (PVR) less than 2 units/m2). In many cases (high-risk Fontan candidates), Glenn shunt is considered final palliation.
Coronal maximum intensity projection MRA TWIST images with peripheral intravenous injection of contrast via the right upper limb in a patient of Glenn shunt show early contrast opacification of the right subclavian vein, brachiocephalic vein and superior vena cava (a), progressive opacification of the superior vena cava and superior vena cava to right pulmonary artery anastomosis (*) (b)
In many cases, surgeons may leave the additional antegrade pulmonary flow to improve branch PA growth, to further improve oxygenation and reduce the chances of pulmonary arteriovenous malformations [6,7,8]. This, however, comes at the cost of persistent effusions and chylothorax due to higher central venous pressure [9, 10]. With various studies associating reduced survival to additional antegrade flow, the current trend is to occlude antegrade ventricle to PA flow.
The surgery prior to Fontan can be a restrictive synthetic conduit (3–4 mm internal diameter) between a systemic central vessel and a proximal PA (arterial shunts described initially) or a conduit between the right ventricle and the left PA. Both these allow the pulmonary arteries to grow to allow a low pulmonary resistance. This is followed later by the bidirectional Glenn (BDG) to the proximal right PA usually after ligating the systemic to pulmonary shunt. This staging prior to Fontan allows for adaptation.
The selected patients for Fontan should be in sinus rhythm, have adequately sized PAs, low PVR and good ventricular function. The absence of any one of these selection criteria increases the risk of a poor outcome [11].
A Fontan circulation usually performed at 1–5 years of age is a surgically created total cavopulmonary connection (TCPC) to divert the entire systemic deoxygenated blood in the SVC and IVC to the PAs circumventing the heart, thus saving it from volume overload and functional deterioration (Fig. 18.5). The connection can be either an intraatrial tunnel or an extracardiac conduit. A fenestration may be intentionally made to decompress the systemic venous pressure and increase the preload in the post-operative period. This is important in patients with suboptimal PVR.
4.1 Other Palliative Procedures
In the Rastelli procedure, performed for double outlet right ventricles (DORV) and transposition of great arteries (TGA) with pulmonary outflow obstruction, an extracardiac conduit is used to establish continuity between the right ventricle and main pulmonary artery and the VSD is enlarged, if necessary, to prevent left ventricular outflow tract obstruction (Fig. 18.6). A prosthetic tunnel is created from the left ventricle to aorta through the ventricular septal defect (VSD) [12].
Another palliative procedure, pulmonary artery banding may be done to train the left ventricle (LV) in neonates with TGA without a VSD who are not repaired in the neonatal period. It can also be used to train the LV in patients with l-TGA who may be candidates for an arterial switch procedure. It involves encircling the main PA with a ring of prosthetic material. PA banding is also performed in patients with congenital heart diseases and excessive pulmonary blood flow to protect the pulmonary vasculature from hypertrophy and irreversible pulmonary hypertension.
5 Corrective Surgeries for Common CHDs
5.1 Tetralogy of Fallot (TOF)
The rationale for the total correction of TOF includes closure of the VSD using a pericardial or synthetic patch and augmenting the right ventricular outflow tract (RVOT). Infundibulectomy (resection of the muscle band RVOT causing moderate stenosis) is performed when the pulmonary valve annulus is adequate and can be preserved. For the pulmonary valve, a commissurotomy can be performed to increase its diameter. A pericardial/Gore-Tex patch is then sewn along the margin of the defect. This increases the RVOT diameter. Branch pulmonary stenosis (PS) can be similarly dealt with. The major disadvantage of this procedure is the obligatory pulmonary regurgitation (PR) afterward. A right ventricle to pulmonary artery extracardiac conduit is generally performed in the presence of pulmonary atresia or when a major coronary artery crosses the right ventricular outflow tract (anomalous prepulmonic course) (Fig. 18.6).
5.2 Transposition of Great Arteries (TGA)
Physiological correction of d-TGA involves atrial switch where systemic venous blood from the superior vena cava (SVC) and inferior vena cava (IVC) is redirected to the left ventricle. Pulmonary venous blood from the pulmonary veins is redirected to the right ventricle through intraatrial baffle using atrial (Senning procedure) or artificial pericardial tissue (Mustard procedure).
The Jatene arterial switch operation involves switching the main PA and the aorta and relocating the ostia of the coronary arteries to the neoaorta [13]. The Lecompte procedure is also performed, whereby the main pulmonary artery bifurcation is placed anterior to the ascending aorta [14] (Fig. 18.7).
In Damus–Kaye–Stansel (DKS) procedure performed for cases d-TGA with subaortic stenosis, the main PA is transected just proximal to bifurcation and the proximal PA is anastomosed to side of ascending aorta forming a channel from the left ventricle to the aorta via the proximal PA, thus bypassing the subaortic stenosis. An external conduit is then placed between the RV and the main PA to establish pulmonary flow [15].
The patients with congenitally corrected TGA undergo an atrial correction procedure and an arterial switch, or an atrial switch and a Rastelli-type repair. These procedures are more commonly performed in children of several months to years of age.
5.3 Double Outlet Right Ventricle (DORV)
A TOF-type DORV requires a surgery similar to corrective repair of TOF. DORV, TGA type (sub-pulmonary VSDs—Taussig–Bing)—can be managed by either of these: (1) patch tunnelling of the VSD to the pulmonary artery combined with an atrial switch; (2) tunnelling of the VSD to the pulmonary artery, DKS aortopulmonary connection with valved extra cardiac RV to PA conduit; (3) direct tunnelling of the VSD to the aorta (Kawashima repair); (4) tunnelling of the VSD to the pulmonary artery combined with an arterial switch procedure; (5) VSD closure to the aorta with the placement of an RV to PA conduit; (6) tunnelling of the VSD to the aorta with translocation of the pulmonary artery (Lecompte) or aortic translocation and biventricular outflow tract reconstruction (Nikaidoh procedure).
5.4 Coarctation of Aorta
The surgical treatment of coarctation, preferred in infants, involves resection and end-to-end anastomosis or interposition graft or subclavian patch aortoplasty or extra-anatomic bypass. Associated arch or long segment thoracic aortic hypoplasia requires long segment extra-anatomic bypass grafts. Endovascular treatment involves the use of balloon angioplasty with/without covered or uncovered stents. Uncovered stents are used in patients without pseudoaneurysm.
6 Imaging of Complications of Palliative Shunts
The imaging evaluation of systemic arterial-to-pulmonary artery shunts includes CTA or Magnetic resonance angiography (MRA). Some centres prefer CTA because of its wider availability, excellent spatial resolution and multiplanar reconstruction capability. This however comes at the cost of increased radiation doses (especially important as these patients require multiple imaging follow-ups).
Anatomical distortion, shunt and subclavian artery stenosis, obstruction, anastomotic site and shunt pseudoaneurysm can be diagnosed and characterised on both modalities, CMR being a better technique for visualising shunt patency and quantifying the increase in the size of the pulmonary arteries after the procedure. CTA is an easily available modality for the same (Figs. 18.8 and 18.9). CMR also helps in the assessment of left ventricular dysfunction secondary to volume overload seen with arterial shunts [16]. A seroma, formed due to fluid seepage across the BT shunt graft, is an uncommon complication. Its presence is suggested on follow-up radiographs and its confirmation may require further imaging (Fig. 18.10). Attenuation of the lesion on CT or its signal intensity on CMR help differentiate it from close differential diagnoses (hematoma/abscess) [17].
The disadvantages of central shunt can be excessive pulmonary flow or distortion of PAs with patient growth.
In functionally single ventricles, the goals of Stage 1 palliation or a Glenn shunt are to provide unobstructed, but limited systemic blood flow through undistorted pulmonary arteries. The pulmonary venous return should also be unobstructed and there should be minimal atrioventricular valve regurgitation.
An elevated SVC pressure after Glenn shunt can be secondary to any aetiology which increases the PA pressure (thrombosis, distorted central PA, central/peripheral PS). Since significant elevations of central venous pressure may limit cerebral blood flow, pressures more than 18 mm Hg are an indication to investigate for such causes. Persistent pleural effusions are the result of low cardiac output due to a combination of reduced post-operative preload, systolic and diastolic dysfunction (Fig. 18.11). In such cases, it is pertinent to inquire about obstructions to systemic blood flow which increase afterload, atrioventricular (AV) regurgitation which causes post-capillary hypertension and pulmonary vein (PV) stenosis which also causes post-capillary hypertension.
Mediastinal venovenous collaterals are a manifestation of raised venous pressures [18] (Fig. 18.12). The majority of collaterals originate from the brachiocephalic vein or its junction with SVC and drain into IVC or its tributaries leading to return of systemic venous blood into the systemic circulation without oxygenation thus increasing cyanosis. Dilated azygos vein may be an indirect evidence of elevated PA pressure leading to venovenous collaterals. These collaterals can be successfully embolised when diagnosed (on contrast-enhanced cross-sectional imaging techniques) and indicated.
The development of aortopulmonary collateral vessels after the BDG shunt is well known (Fig. 18.13). These collaterals elevate the systemic venous pressure by competitive flow. Significant aortopulmonary collaterals are also a culprit for hemoptysis, increased incidence of pleural effusions and prolonged hospital stay. Triedman et al. found that the patients who underwent BDG were more likely to have aortopulmonary collaterals (65% of BDG patients) than those who underwent Fontan surgery (35% of Fontan patients) [19].
Pulmonary arteriovenous malformations (AVMs) have an incidence between 25 and 37% in patients of Glenn and likely result from endothelial dysfunction due to chronic non-pulsatile pulmonary flow or absence of unidentified factor produced by the liver [20] (Fig. 18.14). They diminish or disappear completely after Fontan completion at the cost of elevation in hepatic venous pressure. CTA and CMR are complementary and supplement invasive angiography and contrast echocardiography (Table 18.2).
A Fontan circuit is a tricky procedure as its take-up or acceptance in a patient is dependent on the delicate match between preload and afterload. Some patients develop a state of low cardiac output, pleural effusion and other signs of failure of Fontan circulation owing to a sudden reduction in preload to the hypertrophied ventricle and preload-afterload mismatch. A preoperative McGoon ratio of more than 1.8 is used to carefully select patients, as those with a ratio of less than 1.8 are at increased risk of death and Fontan dysfunction. Other complications of Fontan circulation include thromboembolic events related to atrial dilatation, arrhythmias, valvular regurgitation, stenosis of the cavopulmonary anastomosis or pulmonary arteries, ventricular dysfunction, ischemic complications due to surgical coronary artery lesions, and protein-losing enteropathy [21]. High intrathoracic lymphatic pressure or obstruction of lymphatic flow may lead to the development of lymphoalveolar fistula and bronchial casts, producing a rare complication known as plastic bronchitis.
Though CTA is routinely advised for assessment of circuit anatomy, thrombosis and coronary lesions, the haemodynamics of the circuits mandate the need for delayed acquisitions for optimal contrast opacification which come at the cost of increased radiation exposure. CMR is the modality of choice for haemodynamic evaluation, assessment of the significance of stenoses, AV regurgitation, calculation of cardiac function and identification of fibrosis.
In the Rastelli procedure, post-operative degeneration and stenosis of the extracardiac conduit (with or without regurgitation) is almost inevitable (Fig. 18.15). Left ventricular dysfunction is also common. Less common complications are: right ventricular dysfunction secondary to conduit dysfunction, obstruction of the tunnel from the left ventricle to the aortic valve and branch pulmonary artery stenosis. Extracardiac conduit patency, haemodynamic changes, ventricular function and valvular regurgitation can be effectively evaluated with CMR.
PA banding can result in dilatation of the proximal portion of the PA, with resultant valve regurgitation, pulmonary valve injury (when the band is placed too close to the pulmonary valve), erosion of the band with secondary stenosis or pseudoaneurysm, pulmonary artery stenosis (particularly right pulmonary stenosis if the band migrates distally) [22]. CTA provides anatomical characterisation, relationship to surrounding structures and appearance of the pulmonary vascular tree (Fig. 18.16). CMR is paramount in the evaluation of mass and function of the pulmonary ventricle.
7 Imaging of Complications of Repaired CHDs
7.1 Tetralogy of Fallot (TOF) and TOF-Type Double Outlet Right Ventricle (DORV)
CMR is the investigation of choice for the evaluation of repaired TOF and DORV. Residual RVOT stenosis (the commonest late complication post-infundibulectomy) is diagnosed in suspected cases by visualising the narrowing of RVOT (which persists even during diastole) [23]. CMR can help in its quantification. Right ventricular outflow patch dyskinesia/aneurysm can occur secondary to RV dysfunction. Varying degree of tricuspid regurgitation (TR) is associated with the obligatory PR in patients after transannular patch repair (Figs. 18.17 and 18.18). Recurrent/residual ventricular septal defect also requires MR quantification of Qp/Qs which is not possible on CTA. Also, small defects in distorted post-operative anatomies are better imaged on CMR. Progressive left ventricular dysfunction is a poor prognostic marker in these patients. This is assessed best on CMR, including subclinical dysfunction utilising tagging and strain imaging. A myocardial infarction secondary to vascular injury is visualised on CMR, though the anomalous course of coronary and its relationship to surrounding structures is best diagnosed on CTA.
Same case as Fig. 18.17—CMR phase-sensitive inversion recovery images show late gadolinium enhancement in the right ventricle (RV) and right ventricular outflow tract outpouching (*)
Regarding PR, there are definite CMR criteria for decision-making for pulmonary valve replacement [24] (Fig. 18.19).
Residual main pulmonary and missed branch pulmonary stenosis are culprits for RV dysfunction and PR. These require a combination of both anatomical and functional imaging. TWIST on CMR can provide both, though has a poor spatial resolution (Fig. 18.20).
7.2 Transposition of Great Arteries (TGA) and TGA-Type Double Outlet Right Ventricle (DORV)
A common complication of atrial switch procedure is baffle and vena cava stenosis or obstruction [25]. Obstruction is more common in the superior than in the inferior conduit. The patency of the venous pathway can be effectively evaluated on CMR. Small baffle leaks are more common than obstruction (Fig. 18.21). They are usually small and haemodynamically insignificant but the risk for paradoxical embolism increases. There is progressive right (systemic) ventricular hypertrophy and dilatation with secondary tricuspid valve insufficiency and ultimately right (systemic) ventricular failure. This often occurs in the second or third decades of life when transplantation is the only solution. Late gadolinium-enhanced imaging can be used to detect fibrosis of the systemic ventricle, a finding that is associated with ventricular dysfunction, poor functional tolerance, arrhythmia, and progressive deterioration (Fig. 18.22).
A Jatene arterial switch requires serial imaging for quantitative evaluation of the function of the ventricles, ischemic complications (due to coronary artery translocation) and evaluation of the outflow tracts for obstruction and insufficiency (Fig. 18.23) [26]. In patients in whom the presence of coronary ischemia is suspected, conventional catheter coronary angiography is probably the imaging modality of choice.
Cardiac CTA images in the axial (a) and oblique sagittal (b) planes in a patient of Jatene arterial switch and Le compte manoeuvre show the main pulmonary artery (MPA) anterior to the neoaorta. There is significant ostial stenosis of the left pulmonary artery (white solid arrow) and short segment mild narrowing of the ascending aorta (*)
The pulmonary arteries need assessment for central and peripheral stenosis related to inadequate growth. Dilatation of neoaorta is invariable, and despite neoaortic root dilatation, haemodynamically significant aortic valve regurgitation is uncommon. Infrequently, a neoaorta aneurysm bulges into the pulmonary bifurcation during systole. The anatomic and functional significance of such obstructions can be more accurately assessed with MR imaging.
The development of left ventricular dysfunction is a rare complication of a double-switch procedure.
Post DKS, CMR helps in the quantitative evaluation of right and left ventricular function, conduit and outflow tract obstruction and insufficiency.
7.3 Coarctation of Aorta
Contrast-enhanced MRA provides anatomical characterisation of pre- or post-surgical coarctation with respect to the site, length, associated arch/thoracic/abdominal aortic abnormalities, patent ductus and collateralisation. In addition, CMR assesses biventricular and valvular function, myocardial mass, fibrosis, associated intracardiac defects (including VSD and bicuspid aortic valve). Phase-contrast images are important for the calculation of gradients across the aortic valve as well as the coarcted segment which is important for assessing the haemodynamic significance of native and recurrent/residual coarctation. The amount of flow in the aorta just below the level of diaphragm minus flow volume just distal to the stenosed segment gives the exact volume of collateral flow. Though contrast-enhanced MRA provides excellent anatomical detail of the aorta and collaterals, cine images of the aorta (both transverse and longitudinal axes) and phase-contrast imaging for calculation of gradients are sufficient for diagnosis, treatment planning and assessment of re-coarctation. Time-resolved, velocity-encoded 3D phase-contrast MRI (4D flow MRI) is another non-invasive method of assessment of pressure gradients which has shown excellent correlation with invasive catheter measurements [27, 28]. In light of these advantages, CMR with MRA has definite advantages over CTA. These patients also require multiple follow-ups and cumulative radiation doses from CTA are a major disadvantage. Gated CTA can provide a similar anatomic assessment of aortic, intracardiac, thoracic and extrathoracic abnormalities including calculation of biventricular function but fails in the detection of myocardial fibrosis and in the calculation of gradients.
8 Conclusion
CMR and MRA when available, are the modalities of choice for the appropriate radiation-free evaluation of post-operative CHDs, as these provide comprehensive morphoanatomical and functional characterisation. MRA with routine cine images are sufficient for anatomical information of native vessels as well as shunts/conduits. By quantitatively assessing the haemodynamics, they aid decision-making for re-interventions (valve replacements, angioplasties, closure of residual defects, etc.). Cardiac function and fibrosis which are prognostic markers are best evaluated on CMR.
References
Opfer E, Shah S. Advances in pediatric cardiovascular imaging. Mo Med. 2018;115(4):354–60.
Litmanovich DE, Tack DM, Shahrzad M, Bankier AA. Dose reduction in cardiothoracic CT: review of currently available methods. Radiographics. 2014 Oct;34(6):1469–89.
Sonavane SK, Milner DM, Singh SP, Abdel Aal AK, Shahir KS, Chaturvedi A. Comprehensive imaging review of the superior vena cava. Radiographics. 2015 Nov-Dec;35(7):1873–92.
Rodríguez E, Soler R, Fernández R, Raposo I. Postoperative imaging in cyanotic congenital heart diseases: part 1. Normal findings. AJR Am J Roentgenol. 2007 Dec;189(6):1353–60.
Glenn WW. Circulatory bypass of the right side of the heart. IV. Shunt between superior vena cava and distal right pulmonary artery; report of clinical application. N Engl J Med. 1958 Jul 17;259(3):117–20.
Caspi J, Pettitt TW, Ferguson TB, Stopa AR, Sandhu SK. Effects of controlled antegrade pulmonary blood flow on cardiac function after bidirectional cavopulmonary anastomosis. Ann Thorac Surg. 2003;76:1917–22.
Calvaruso DF, Rubino A, Ocello S, Salviato N, Guardì D, Petruccelli DF, Cipriani A, Fattouch K, Agati S, Mignosa C, Zannini L, Marcelletti CF. Bidirectional Glenn and antegrade pulmonary blood flow: temporary or definitive palliation? Ann Thorac Surg. 2008 Apr;85(4):1389–95.
van Slooten Y, Elzenga NJ, Waterbolk TW, van Melle JP, Berger RMF, Ebels T. The effect of additional pulmonary blood flow on timing of the total cavopulmonary connection. Ann Thorac Surg. 2012;93:2028–34.
McElhinney DB, Marianeschi SM, Reddy VM. Additional pulmonary blood flow with the bidirectional Glenn anastomosis: does it make a difference? Ann Thorac Surg. 1998;66:668–72.
Ferns SJ, El Zein C, Multani K, Sajan I, Subramanian S, Polimenakos AC, Ilbawi MN. Is additional pulsatile pulmonary blood flow beneficial to patients with bidirectional Glenn? J Thorac Cardiovasc Surg. 2013 Feb;145(2):451–4.
Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. 2016 Jul 15;102(14):1081–6.
Naito Y, Fujita T, Tomino T, Koh Y, Isobe F, Manabe H, Kamiya T. Rastelli operation as one stage anatomical correction for infants with complete transposition of the great arteries and ventricular septal defect. Jpn J Surg. 1985 Jan;15(1):36–42.
Schidlow DN, Jenkins KJ, Gauvreau K, Croti UA, Giang DTC, Konda RK, Novick WM, Sandoval NF, Castañeda A. Transposition of the great arteries in the developing world: surgery and outcomes. J Am Coll Cardiol. 2017 Jan 3;69(1):43–51.
Kim YJ, Song H, Lee JR, Rho JR, Suh KP. Lecompte procedure for complete transposition of the great arteries with ventricular septal defect and pulmonary stenosis. Ann Thorac Surg. 1994 Apr;57(4):876–9.
Rosenblum J, Anvari F, Alsoufi B. The Damus-Kaye-Stansel 0peration: management of systemic ventricular outflow tract obstruction. Multimed Man Cardiothorac Surg. 2018 Feb;13:2018.
Ntsinjana HN, Hughes ML, Taylor AM. The role of cardiovascular magnetic resonance in pediatric congenital heart disease. J Cardiovasc Magn Reson. 2011 Sep 21;13:51.
LeBlanc J, Albus R, Williams WG, Moes CA, Wilson G, Freedom RM, Trusler GA. Serous fluid leakage: a complication following the modified Blalock Taussig shunt. J Thorac Cardiovasc Surg. 1984 Aug;88(2):259–62.
Heinemann M, Breuer J, Steger V, Steil E, Sieverding L, Ziemer G. Incidence and impact of systemic venous collateral development after Glenn and Fontan procedures. Thorac Cardiovasc Surg. 2001 Jun;49(3):172–8.
Triedman JK, Bridges ND, Mayer JE Jr, Lock JE. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol. 1993 Jul;22(1):207–15.
Ashrafian H, Swan L. The mechanism of formation of pulmonary arteriovenous malformations associated with the classic Glenn shunt (superior cavopulmonary anastomosis). Heart. 2002 Dec;88(6):639.
Fredenburg TB, Johnson TR, Cohen MD. The Fontan procedure: anatomy, complications, and manifestations of failure. Radiographics. 2011 Mar-Apr;31(2):453–63.
Pinho P, Von Oppell UO, Brink J, Hewitson J. Pulmonary artery banding: adequacy and long-term outcome. Eur J Cardiothorac Surg. 1997 Jan;11(1):105–11.
Ho KW, Tan RS, Wong KY, Tan TH, Shankar S, Tan JL. Late complications following tetralogy of Fallot repair: the need for long-term follow-up. Ann Acad Med Singap. 2007 Nov;36(11):947–53.
Geva T. Indications for pulmonary valve replacement in repaired tetralogy of fallot: the quest continues. Circulation. 2013 Oct 22;128(17):1855–7.
Reddy V, Sharma S, Cobanoglu A. Atrial switch (Senning procedure) in the era of the arterial switch operation: current indications and results. Eur J Cardiothorac Surg. 1996;10(7):546–50.
Vargo P, Mavroudis C, Stewart RD, Backer CL. Late complications following the arterial switch operation. World J Pediatr Congenit Heart Surg. 2011 Jan;2(1):37–42.
Goubergrits L, Riesenkampff E, Yevtushenko P, Schaller J, Kertzscher U, Hennemuth A, Berger F, Schubert S, Kuehne T. MRI-based computational fluid dynamics for diagnosis and treatment prediction: clinical validation study in patients with coarctation of aorta. J Magn Reson Imaging. 2015 Apr;41(4):909–16.
Rengier F, Delles M, Eichhorn J, Azad YJ, von Tengg-Kobligk H, Ley-Zaporozhan J, Dillmann R, Kauczor HU, Unterhinninghofen R, Ley S. Noninvasive pressure difference mapping derived from 4D flow MRI in patients with unrepaired and repaired aortic coarctation. Cardiovasc Diagn Ther. 2014 Apr;4(2):97–103.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Jagia, P., Sinha, M. (2021). CT and MR Imaging in Post-operative CHD. In: Rajeshkannan, R., Raj, V., Viswamitra, S. (eds) CT and MRI in Congenital Heart Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-15-6755-1_18
Download citation
DOI: https://doi.org/10.1007/978-981-15-6755-1_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6754-4
Online ISBN: 978-981-15-6755-1
eBook Packages: MedicineMedicine (R0)