Abstract
Phytochemicals, the plant derived natural products or bioactive compounds, exhibit immense diversity regarding origin and mechanism of action. The recent two decades have witnessed renaissance in anti-cancer therapeutics stressing identification of anti-neoplastic or anti-cancer properties of different phytochemicals/plant nutraceuticals. However, the available formulations of these phytochemicals exhibit pharmacological limitations such as low water solubility and reduced bioavailability. These aspects can be possibly improved by developing nano-enabled formulations of these phytochemicals. Various nano-scale delivery vehicles which include branched globular polymeric particles (dendrimers), unilamellar micelles, double layered liposomes, and other zero-dimensional nanomaterials have been developed to address the low water solubility and poor uptake issues. The phytochemical of interest can be encapsulated, embedded, or adsorbed on these nano-scale carriers. The nano-scale dimensions of these engineered delivery vehicles could help enhance the stability of water dispersed formulations. Further, the nano-size enables easy infiltration to cancer cells at rates higher than the non-nano-formulations of the same anti-cancer phytochemical thereby reducing the dosage required to achieve effective anti-cancer action. The amenability to multiple surface functionalization of these nano-delivery vehicles can ensure decoration with ligands that can lead to targeted delivery of the phytochemical to cancer cells leading to decreased cyto-toxicity to normal tissues. This manuscript describes the various types of phytochemical payloaded nano-carriers for targeted or site-specific delivery of active anti-cancer chemical(s) to reduce undesirable side effects of chemotherapeutic agents on application in cancer subjects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
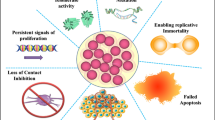
Cancer has emerged as the second most dreaded cause of human deaths claiming every one in six individuals globally. Cancer is characterized by rapid and uncontrolled growth of abnormal cells exhibiting omission of the contact inhibition phenomena followed by invasion of adjoining tissues and other visceral organs of the body leading to metastasis and ultimately death [1]. International Agency for Research on Cancer (IARC) through its World Cancer Report (2014) has predicted an upsurge in the reported cancer cases to 25 million by the year 2035. In 2018, 9.6 million deaths have been reported due to cancer with the under-developed and developing countries being the prima foci accounting for nearly 70% of the cancer related deaths. Due to expensive treatment options and post-treatment patient care costs, the economic impact of cancer is immense. In 2010, approximately US$ 1.16 trillion was the estimated gross annual economic cost of cancer [2]. Cancer Prevention and Control through an Integrated Approach (WHA70.12), a resolution passed by WHO in 2017 aims to accelerate designing strategies; conventional, alternative, and advanced, to reduce premature mortality due to cancer.
8.2 Use of Phytochemicals for Treatment of Cancer
Use of products of natural origin as remedies has been practiced since ancient time. Various ancient scriptures like Charaka Samhita from India and Wu Shi Er Bing Fang from China include illustrative documentation of more than 200 natural products of therapeutic relevance. Ben Cao Gang Mu published in sixteenth century documented more than 1000 natural bioactive agents [3]. Early nineteenth century marked the isolation of morphine from Opium plant by a German pharmacist, Friedrich Serturner, and since then it has been widely applied in the medical field as anesthesia [4]. A plethora of natural bioactive compounds have since been isolated including alkaloids, glycosides, and flavonoids. Plant based pharmaceuticals have been extensively studied as treatment options for cancer and currently over 60% of the total anti-cancer drugs in use have been derived from plants [5]. For example, curcumin, a polyphenol extracted from Curcuma longa (turmeric), possesses remarkable anti-cancer properties [6]. Similar anti-cancer potential has also been reported for secondary metabolites derived from actinobacteria [7]. However, it is earier to obtain scaled up quantities of the plant based anticancer [8]. The non-edible or even edible tissues/organs of diverse plant genera including fruits, vegetables, and medicinal plants contain anti-neoplastic phytochemicals. Relative safety of these drugs and their easy availability makes these phytochemicals a promising alternative treatment for cancer.
Common anti-neoplastic phytochemicals include original compounds or their derivatives namely taxanes, podophyllotoxin, vinca alkaloids, camptothecin, anthracyclines, and many others. The action mechanism of these drugs is not just limited to anti-oxidative and immune-modulatory roles, but they have been observed to actively target cancer related metabolic pathways [7]. Genistein, a plant based estrogen homolog obtained from legumes, is being tested for its therapeutic effects against cancers of pancreas, kidneys, rectum, and ovaries [9]. Likewise, lycopene (beta carotenoid), a lipophilic hydrocarbon isolated from tomatoes, papaya, watermelon, and carrots can prevent Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) mediated DNA damage. Apart from the preventative effects, lycopene also directly affects cancer related metabolic pathways in prostate cancer [10]. The phytochemicals with more targeted effect on cancer such as paclitaxel isolated from Pacific yew, Taxus brevifolia acts as a mitotic inhibitor and thus can prevent cancer cell division by stabilizing the microtubules. Both paclitaxel and its derivative compound, docetaxel, have been approved by FDA to be used as anti-cancer drugs against breast, lung, and ovarian cancers [11].
Plant based anti-cancer drugs, therefore, show a range of possibilities, from their anti-oxidative and immune-modulating activities to directly acting as anti-mitotic agents. Their low toxicity, higher tolerance, and easy availability signify their role and potential in cancer therapy. However, their use has been limited by some factors discussed below.
8.3 Phytochemical Use: Possible Limitations
Plant based drugs encounter many limitations in terms of their pharmacological activities. One of the major challenges is low solubility of phytochemicals which hinders development of drug formulation. Such poorly formulated drugs exhibit inferior blood circulation. Some of these drugs may show low gastrointestinal absorption, while others get quickly metabolized and does not remain effective for longer durations. When the drugs have faster clearance rate, higher doses have to be applied at frequent intervals, making the process very tedious (Table 8.1).
8.4 Nano-Enabled Approaches for Phytochemical Application: Can Nanotechnology Help Circumvent Possible Pharmacological Limitations of Phytochemicals?
As discussed in Sect. 8.3, the pharmacological limitations of phytochemicals need to be addressed by use of appropriate delivery systems. One of the convergent disciplines, Nanomedicine—the nanotechnological applications for biomedicine, has gained tremendous interest involving use of nanomaterials as novel drug delivery vehicles. Likewise, nano-enabled devices can ensure improved targeting, high efficacy, and decreased side effects to patient under treatment. The phytochemicals can be loaded on to nano-carriers to achieve improved solubility, better stability, bioavailability, and target specificity on application. The bio-safety of the nano-vehicles can be ensured if these carriers can be fabricated or encapsulated using biodegradable compounds [18, 19].
One of the main characters of cancerous tissue is angiogenesis. Tumors generate defective and leaky blood vessels that continuously leak fluids into interstitial spaces. From here, the fluids are drained by poorly formed lymphatic system. By targeting this phenomenon, a nano-sized drug carrier can be applied that can easily invade the tumor and accumulate there. This is called Enhanced Permeability and Retention effect or EPR effect [14]. The challenge of multi-drug resistance in tumor cells can also be averted by modifying the surface of the nano-carriers through chemical functionalization. These modifications will alter the interactions between drug carriers and cell membranes [20]. Some of the FDA approved nano-carrier based delivery systems are discussed in the following text.
8.4.1 Antibodies Drug Conjugation (ADC)
The conventional unconjugated anti-cancer drugs exhibit high cellular toxicity causing death of normal body cells besides the tumor cells due to low specificity to target the cancerous tissue alone. Therefore, one of the novel biopharmaceutical drugs, the Antibody drug conjugation (ADC), which combine the specific immunogenicity feature of monoclonal antibodies with the higher toxicity of the drug molecule can effectively ensure improved targeting of the cancer cells [21]. The monoclonal antibody(ies) is/are raised against specific surface antigens produced and secreted by the tumor cells. These antigenic moieties exist on the outer end of the cell membrane of tumor cell. Monoclonal antibodies against these specific tumor cell surface antigens are then attached to highly potent anti-cancer agents or drug molecules via a chemical linker domain. The size of the final bioconjugate remains in nano-scale dimensions; however, ADCs are considered to be non-nano anti-cancer agents. The common anti-cancer agents used include two types of cytotoxins viz., DNA damaging agents and microtubule inhibitors. Microtubule inhibitors can be further of two types: maytansinoids and auristatins. Maytansinoids are derivatives of maytansine, a phytochemical isolated from African shrub Maytenus serrata [22, 23]. Selection of suitable cytotoxin, linkers, and target antibodies are key components to be considered while designing ADCs.
8.4.2 Nanoparticles
Nanoparticle is a term used in nanopharmaceutics to identify zero-dimensional particles falling in size range of 10–1000 nm. The drug of interest can either be embedded or coated in these nanoparticles. Chemically, the origin of nanoparticles is diverse and include metal/ non-metal oxides/sulfides/nitrides, polymers, and carbon nanomaterials. Among these nano-delivery vehicles, the polymeric nanoparticles represent a very diverse group including the nanoparticles derived or formulated from biodegradable high molecular weight polymer compounds (e.g., poly(lactic-co-glycolic acid (PLGA) nanoparticles), and natural polymers (e.g., albumin nanoparticles, gelatin cellulose nanoparticles, and chitosan nanoparticles) [24]. These nanoparticles have high storage stability. PGLA and poly lactic acid (PLA) based nanoparticles have been synthesized using formulation of ginsenoside and luteolin, and have been found to be effective against lung cancer cells [25, 26].
8.4.2.1 Liposomes
Liposome-polymeric nanoparticles were the first well-explored and prudently commercialized nano-based drug delivery system used in cancer therapeutics [27]. Structurally, liposomes are comprised of a hydrophobic shell and hydrophilic core. Therefore, the polar drugs can be loaded in the core while the non-polar drugs can be loaded in the shell. The properties of a liposome can be suitably altered by simply modifying the composition of the phospholipid bilayer. A commercial preparation under the name Lipusu® has been developed as a more stable and less toxic substitute for Taxol® using liposome based delivery. Lipusu® showed higher retention in tumor tissues in mice compared to Taxol® [28].
8.4.2.2 Micelles
Micelles are the smallest nano-vehicles (∼10 to 400 nm) that can be utilized for efficient drug delivery. These are formed when the concentration of a surfactant gets higher than critical micelle concentration (CMC). Polymeric micelles are being increasingly used for the development of several drug formulations. These micelles can be prepared by polymerization of the natural organic or synthetic compounds (monomer). Most commonly used compounds include the hydrophilic monomer PEG while the core-forming compounds include poly(propyleneoxide), poly(caprolactone), poly(D, L-lactic acid), and poly(L-aspartic acid) compounds [29]. An example of polymeric micelle formulation of a known anti-cancer drug containing Paclitaxel, Genexol-PM, has a size dimension in nano-regime (24 nm). In clinical trials for cancer, this drug has shown higher inhibition of tumor cell growth compared to Taxol [24].
8.4.2.3 Dendrimers
Dendrimers are nanoscopic, radially symmetric large molecular weight polymer molecules that exhibit extensive branched structure [30]. The peripheral groups present on the branched structure can be easily modified to help in binding of hydrophobic drug molecules [30]. The distinct physical-chemical and structural properties of dendrimers can be very useful for their role as ‘Excipients’ [31]. Dendrimers of gallic acid with polyamidoamine (PAMAM) have been formulated and found to be active against breast cancer cell lines [32].
8.4.2.4 Metal Nanoparticles
Metal nanoparticles can be synthesized using green nanotechnology approaches where solutions of desired metals are treated with suitable phytochemical solution. Phenolic compound rich extracts of plant Albizia adianthifolia were used to synthesize silver nanoparticles (AgNPs). Similarly, stem latex of Euphorbia nivulia plant have been used to generate AgNPs. Both of these nanoparticles showed anti-cancer effects against A-549 cancer cells [33]. Gold, copper, and titanium based nanoparticles have been synthesized and tried at pre-clinical levels for their effects against cancer cells.
Shape of nanoparticles is also an important aspect ensuring efficacy and uptake of the loaded anti-cancer drug. It has been demonstrated that nano-rods have 1.6-fold higher uptake by cancer cells compared to nano-spheres [34]. While larger particles are cleared quickly from the body, smaller ones are harder to filter. For EPR, 5–100 nm size has been found to be most suitable.
Application of nanoparticles is limited due to unavailability of substantial information regarding their toxic effects. The small size that makes nanoparticles suitable for therapeutic use can also create problems inside the body. The nanoparticles can cross barriers and get accumulated in vital organs like heart, lungs, brain, and liver. This can lead to complications such as systemic failure or inactivation of immune cells, inflammation, edema, etc. [35].
8.4.2.5 Carbon Nanotubes
Carbon nanotubes are hollow, rolled cylindrical tubes derived from single C-atom sheets, graphene. These long and thin cylinders exhibit remarkable mechanical and physical properties. The walls of these nanotubes can be functionalized with desired drug [36]. Multi-walled carbon nanotubes functionalized by PTX have been shown to be effective against HeLa cell lines [37].
8.5 Conclusion
Plant based anti-cancer drugs have a unique potential for use in cancer therapy. However, the efficacy of these phytochemicals get limited due to issues regarding the stability of the formulation and its bioavailability. Therefore, among the other alternatives that have been explored for addressing these issues, use of nano-inspired or -enabled phytochemical delivery systems can possibly circumvent the pharmacological limitations of these natural products. Nano-carriers can improve the solubility due to decrease in size dimensions, bioavailability, and uptake owing to larger surface area on nano-scaling, target specificity, and kinetics of these drugs besides lowering the toxicity of these drugs [38]. Further, analysis of advanced toxicity studies can ensure the safer administration of these drugs.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
IARC (2014) World Cancer Report 2014. Stewart BW and Wild CW (Ed). https://www.who.int/cancer/publications/WRC_2014/en/. Accessed 27 February 2020
Ji HF, Li XJ, Zhang HY (2009) Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep 10(3):194–200
Ba GRH (2000) History of anesthesia: in the arms of morpheus: the development of morphine for postoperative pain relief. Can J Anesth 47(4):367–374
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79(3):629–661
Kuttan R, Bhanumathy P, Nirmala K et al (1985) Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett 29(2):197–202
Wang H, Khor TO, Shu L et al (2012) Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anti Cancer Agents Med Chem 12:1281–1305
Kaur S, Kalia A, Gangwar M (2019) Bioprospecting endophytic actinobacteria of medicinal plants as potential anticancer therapeutic agents. In: Sharma A, Kunar M (eds.) Pollutants and protectants: valuation and assessment techniques. I.K International, New Delhi, pp 196–214.
Hwang KA, Park MA, Kang NH et al (2013) Anticancer effect of genistein on BG-1 ovarian cancer growth induced by 17 beta-estradiol or bisphenol A via the suppression of the crosstalk between estrogen receptor alpha and insulin-like growth factor-1 receptor signaling pathways. Toxicol Appl Pharmacol 272:637–646
Holzapfel NP, Holzapfel BM, Champ S et al (2013) The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int J Mol Sci 14:14620–14646
Skwarczynski M, Hayashi Y, Kiso Y (2006) Paclitaxel prodrugs: toward smarter delivery of anticancer agents. J Med Chem 49(25):7253–7269
Mastropaolo D, Camerman A, Luo Y et al (1995) Crystal and molecular structure of paclitaxel (taxol). Proc Natl Acad Sci U S A 92:6920–6924
Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD et al (1994) European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol 12:2654–2666
Fang J, Nakamura H, Maeda H (2011) The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–151
Gelderblom H, Verweij J, Nooter K et al (2001) Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 37(13):1590–1598. https://doi.org/10.1016/s0959-8049(01)00171-x
Gidding CE, Meeuwsen-de Boer GJ, Koopmans P et al (1999) Vincristine pharmacokinetics after repetitive dosing in children. Cancer Chemother Pharmacol 44(3):203–209
Liu B, Staren ED, Iwamura T et al (2001) Mechanisms of taxotere-related drug resistance in pancreatic carcinoma. J Surg Res 99(2):179–186
Manuja A, Raguvaran R, Kumar B, Kalia A, Tripathi BN (2020) Accelerated healing of full thickness excised skin wound in rabbits using single application of alginate/acacia based nanocomposites of ZnO nanoparticles. Intl J Biol Macromol, 155:823–833
Raguvaran R, Manuja BK, Chopra M, Thakur R, Anand T, Kalia A, Manuja A (2017) Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Intl J Biol Macromol, 96:185–191
Patel NR, Pattni BS, Abouzeid AH et al (2013) Nanopreparations to overcome multidrug resistance in cancer. Adv Drug Deliv Rev 65:1748–1762
Nejadmoghaddam MR, Minai-Tehrani A, Ghahremanzadeh R et al (2019) Antibody-drug conjugates: possibilities and challenges. Avicenna J Med Biotechnol 11(1):3–23
Kupchan SM, Komoda Y, Court WA et al (1972) Maytansine, a novel antileukemic ansa macrolide from Maytenus ovatus. J Am Chem Soc 94:1354–1356
Lopus M, Oroudjev E, Wilson L et al (2010) Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther 9:2689–2699
Liang Y, Xiao L, Li Y et al (2011) Poly(ester anhydride)/mPEG amphiphilic block co-polymer nanoparticles as delivery devices for paclitaxel. J Biomater Sci Polym Ed 22:701–715
Geng L, Fan J, Gao QL et al (2016) Preliminary study for the roles and mechanisms of 20(R)-ginsenoside Rg3 and PEG-PLGA-Rg3 NPs in the Lewis lung cancer mice. Beijing Da Xue Xue Bao 48(3):496–501
Majumdar D, Jung KH, Zhang H, Nannapaneni S, Wang X, Amin ARMR et al (2014) Luteolin nanoparticle in chemoprevention: in vitro and in vivo anticancer activity. Cancer Prev Res 7(1):65–73
Bulbake U, Doppalapudi S, Kommineni N et al (2017) Liposomal formulations in clinical use: an updated review. Pharmaceutics 9(2):12
Ye L, He J, Hu Z et al (2013) Antitumor effect and toxicity of Lipusu in rat ovarian cancer xenografts. Food Chem Toxicol 52:200–206
Torchilin VP (2001) Structure and design of polymeric surfactant-based drug delivery systems. J Control Release 73:137–172
de Araújo RV, Santos SS, Ferreira EI et al (2018) New advances in general biomedical applications of PAMAM dendrimers. Molecules 23:2849. https://doi.org/10.3390/molecules23112849
Santos A, Veiga F, Figueiras A (2020) Dendrimers as pharmaceutical excipients: synthesis, properties, toxicity and biomedical applications. Materials 13:65. https://doi.org/10.3390/ma13010065
Sharma A, Gautam SP, Gupta AK (2011) Surface modified dendrimers. Bioorg Med Chem 19:3341–3346. https://doi.org/10.1016/j.bmc.2011.04.046
Valodkar M, Jadeja RN, Thounaojam MC et al (2011) In vitro toxicity study of plant latex capped silver NPs in human lung carcinoma cells. Mater Sci Eng C 31(8):1723–1728
Barua S, Yoo JW, Kolhar P et al (2013) Particle shape enhances specificity of antibody-displaying nanoparticles. Proc Natl Acad Sci U S A 110:3270–3275
Akhter S, Ahmad J, Rizwanullah M et al (2015) Nanotechnology-based inhalation treatments for lung cancer: state of the art. Nanotechnol Sci Appl 8:55–66
Jogi H, Maheshwari R, Raval N et al (2018) Carbon nanotubes in the delivery of anticancer herbal drugs. Nanomedicine 13(10):1187–1220. https://doi.org/10.2217/nnm-2017-0397
Tian Z, Shi Y, Yin M et al (2011) Functionalized multiwalled carbon nanotubes anticancer drug carriers: synthesis, targeting ability and antitumor activity. Nano Biomed Eng 3:157–162. https://doi.org/10.5101/nbe.v3i3.p157-16
Lagoa R, Silva J, Rodrigues JR et al (2020) Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2019.04.004
Acknowledgements
The authors are thankful to the Head, Department of Microbiology, Punjab Agricultural University, Ludhiana for providing the necessary infrastructural facilities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kalia, A., Kaur, G. (2020). Nano-Delivery Carriers for Enhanced Bioavailability of Antitumor Phytochemicals. In: Kumar, M., Sharma, A., Kumar, P. (eds) Pharmacotherapeutic Botanicals for Cancer Chemoprevention . Springer, Singapore. https://doi.org/10.1007/978-981-15-5999-0_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-5999-0_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5998-3
Online ISBN: 978-981-15-5999-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)