Abstract
In this era of shortages of non-renewable energy resources and rapid price hike, biomass gasification is re-emerged as an efficient option for energy production which can convert 60–90% of biomass energy into product gas which can be used either in heat generation or electricity production. Pyrolysis and char conversion are two major processes that finally lead to overall biomass conversion to fuel gas. Extensive work is available for volatile combustion model and char reduction model; however, very limited work has been proposed for pyrolysis model. Also, very few literature is available for thermally thick particles in modeling work. The existing pyrolysis models are adopted from inert pyrolysis or flaming pyrolysis in the presence of air. Therefore, research needs to be conducted using various reactants apart from air like N2, O2, CO2, oxy-steam, etc. The present study focuses on the experimental evaluation of pyrolysis rate and char conversion rate of single biomass particle under similar conditions in packed bed gasification. The pyrolysis and char conversion rates were then analyzed from the view of various factors such as varying gas flows, shape and density of biomass at fixed mass flux of inlet stream. The mass loss characteristics for each case were used as the tool for analysis, and it was studied by fabricating the single-particle reactor. NASA SP 273 code was used for getting equilibrium concentration of various species in gas stream and the adiabatic temperature.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Industrialization in recent decades has caused tremendous exploitation of fossil fuels at alarming rate. The impact of this is emerged in greenhouse gas emissions in atmosphere causing environmental change issues. These unfortunate impacts brought about by the use of non-renewable and unsustainable sources of energy have prompted increment in research for the advancement of renewable and sustainable choices. Biomass, which is frequently, alludes to plants or plant-based materials that are not utilized for sustenance or feed (explicitly called as lignocellulosic biomass); is the main sustainable source of organic carbon and it can be efficiently utilized for the production of hydrocarbons in eco-friendly way. Lignocellulosic biomass, which is made for the most part out of cellulose, hemicellulose and lignin, has a generic composition \({\text{C}}_{1} {\text{H}}_{1.4} {\text{O}}_{0.6}\) with small amount of inorganic matter and extractives and can be utilized as feed for production of the second era biofuels. Biomass gasification is sub-stoichiometric combustion process with pyrolysis, oxidation and reduction occurring in the reactor called gasifier. Oxidation of pyrolysis products (volatile matter) further reacts with char and is reduced to H2, CO, CO2, CH4 and higher hydrocarbons. In practice, gasification can convert 60% to 90% of biomass energy into heat and power.
The three major thermochemical conversion pathways that govern the procedure of conversion of biomass into energy are combustion, pyrolysis and gasification among which pyrolysis is the primary step in any thermal treatment of biomass coupled with complex reactions and heat and mass transfer phenomenon, be it gasification or combustion. Therefore, it is very necessary to understand the basic fundamentals of pyrolysis before modeling gasification and combustion processes as it is a very complex phenomenon, and the chemical mechanisms occurring during pyrolysis are not clearly understood. The pyrolysis is further classified into slow and fast pyrolysis based on the heating rate. As the name suggests, slow pyrolysis corresponds to the heating rate of 10 K/s and fast pyrolysis in the range of 150–250 K/s [1].
According to numerous researchers, there are two general pathways in which pyrolysis may be recognized: One comprises drying and charring reactions leading to the formation of charcoal, CO2 and H2O; the other comprises depolymerization and volatilization and results to the formation of combustible volatiles. These competitive schemes are helpful to describe the extreme sensitivity of pyrolysis yields distribution on the variety of feedstock and process conditions [2].
Sandeep and Dasappa [3] developed a one-dimensional spherically symmetric model for single-particle analysis using the governing equations of mass, momentum and energy conservation combined with thorough pyrolysis phenomenon, water–gas shift reactions and char–gas heterogeneous reactions. It was a lumped model of the function of temperature alone and was adopted from an inert pyrolysis model. The model was further used for the simulation of inert and flaming pyrolysis, and the pyrolysis and char conversion processes were validated independently with the results available in the literature. The effects of thermophysical properties like density, thermal conductivity and temperature on Biot number were studied to understand how the thermophysical properties affect the competing internal and external heat transfer phenomenon within the biomass particle.
Simmons and Ragland [4] conducted experiments on 10 mm sized pine wood cubes to study the pyrolysis effect and combustion phenomenon in air atmosphere at a controlled temperature range from 900 to 1200 K. The results were used to simulate the combustion of biomass particle in a reactor furnace undergoing a changing inside temperature inscribing both flaming pyrolysis and char combustion. From the results, it was found that devolatilization rate and char conversion rates are affected by the variation in flow velocity (Reynolds number), furnace temperature and oxygen concentration. They found from the experimental results that the burning time of 10 mm pine cube reduces from 81 to 68 s with increase in Reynolds number from 60 to 240. The experimental results concluded that the burning rate per unit mass increases linearly with decrease in particle size, and it is higher for the biomass with high density (oak wood cubes) in comparison to low density biomass (pine wood cubes).
1.1 Chemistry and Thermodynamics of the Gasification Process
Typical biomass formula on moisture- and ash-free basis is represented as CH1.4O0.6. Ideally, smallest amount of oxygen is to be added to get maximum amount of CO and H2 according to the following reaction
Some energy is added from external source since CO and H2 have more energy than biomass which eventually complicates the reaction. Practically, some excess amount of O2 is added for gasification, producing some CO2 and H2O according to reaction
The quality of producer gas is measured by CO/CO2 (or \({\text{H}}_{2} /{\text{H}}_{2} {\text{O}}\)) ratio. For the gasification to take place, approximately, 30% of the feedstock burns to provide the necessary energy for the process to occur.
At the temperature favorable for efficient gasification (typically 973–1273 K), there are only limited stable combinations of main elements of biomass such as C, CO, CO2, CH4, H2 and H2O. The respective amount of these species that will be reached at equilibrium can be predicted from the pressure, the amount of each element and the equilibrium constant determined from the temperature and thermodynamic properties, subject to an energy balance. Software like NASA SP 273 code uses the principle of minimization of Gibbs free energy and gives the equilibrium concentration and adiabatic temperature which is the temperature attained when biomass establishes equilibrium with O2 in system. The oxygen used in process determines the products and temperature of reaction. In case of gasification, the equivalence ratio (ɸ) (which is the actual amount of oxygen used in the process relative to that required for the complete combustion) is kept approximately equal to 0.25. At this value of equivalence ratio (ɸ), all of the char is converted to gas, and the portion of energy in the wood changed over to gas achieves maximum value. With less amount of oxygen, more or less of the char is not converted, whereas with more oxygen, temperature increases rapidly and some of the gas is burned.
2 Present Study
The present study focuses on the effect of shape and size of particles on pyrolysis and char conversion rates under reactive environment of CO2, N2, O2 at constant mass flux of inlet gas stream. This study is useful because pyrolysis is an integral part of gasification which often takes place in reactive conditions and not inert. Surface-area-to-volume ratio plays an important role in affecting pyrolysis rate and its product especially the amount of char produced. This work mainly aims at conducting various set of experiments in laboratory to obtain results particularly regarding the effect of surface-area-to-volume ratio on pyrolysis under different reactive media.
2.1 The Experiments
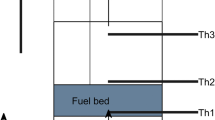
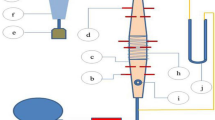
The experimental setup consists of a glass quartz reactor for sustaining high temperatures with diameter 100 mm and height 130 mm, consisting of a base metal cup reactor having orifice at the bottom with a wire mesh for the uniform gas stream distribution throughout the reactor. The flow rates of the gas streams were regulated with the variable area flowmeters for CO2, N2 and O2 with capacity of 50 LPM each. The sample was suspended from a holder into the reactor, and the gas stream after mixing in the mixer was passed into the reactor from the bottom, and the particle is subjected to flaming pyrolysis. The holder consisting biomass particle was placed over microbalance having the provision of data acquisition of the mass loss rate (dm/dt), total conversion time and flow rate of gases in liters per minute (LPM).
Experiments were conducted on biomass particles to study the effect of density, shape and reactive media on pyrolysis and char conversion rate at constant inlet mass flux of 0.1 kg/m2s. The wood specimen was finished into spheres and cubes, and the samples were dried in an oven at 373 K for approximately 30 h. The dried samples were measured to obtain the weight, surface area and volume. The effect of varying density and shape (surface area) was studied under the three combinations of reactant gases O2, N2 and CO2, respectively (Fig. 1 and Table 1).
3 Results and Discussions
3.1 Effect of Temperature on Conversion Rate
The figures above depict the mass loss rate profile of babul wood spheres and cuboids of identical weights under quiescent conditions in the reactive atmosphere of CO2, N2 and O2. The weight loss occurs as the volatiles are released during the thermochemical conversion process. The pyrolysis rate increases exponentially with temperature rise, requiring higher heat transfer rates to the kinetically limited region, thus resulting in faster conversion rates due to rapid devolatilization.
3.2 Study on Flaming Pyrolysis
The experiments suggested that the small difference in the weight loss profiles of cube and sphere diminishes as the temperature approaches to 1200 K due to almost same internal resistance offered by them regardless of external heat flux. The flaming time suggests that the process is mainly limited by conduction.
After the flaming pyrolysis, char undergoes reaction with ambient air, and residual 20% weight loss takes place owing to carbon conversion progression [3].
3.3 Influence of Density on Conversion Rate
Sandeep and Dasappa [3] depicted comparison of thermal conductivity of various biomasses with their corresponding density to find the correlation between these two thermophysical properties. The reference of data for thermal conductivity, density and specific heat was taken from Wood Handbook published by U.S Department of Agriculture [5]. From the data presented by Sandeep and Dasappa [3], it was clear that thermal conductivity is a linear function of density, and it increases with increase in the density of wood.
Ragland et al. [6] from their extensive research study on effect of thermophysical properties on variety of biomass gave a linear correlation between thermal conductivity of wood and their respective density of biomass.
where \(K_{\text{wood}}\) denotes thermal conductivity of wood and \(\rho_{w}\) is respective wood density in g/m3. The correlation formulated by Ragland et al. [6] showed a close approximation with the correlation derived from Wood Handbook [5].
Varunkumar et al. [7] in their study reported that high-density pellets cause more rise in bed temperature than low-density pellets. They considered density as the only factor accountable to this behavior. The data concluded that high-density pellet results in higher ash content, implying increased thermal conductivity causing reduction of conductive resistance. The increased penetration of heat causes fast pyrolysis including the availability of oxidizer, and in combustion process, it is prudent to assume increased temperature (Tables 2 and 3).
4 Conclusion
The present study has conducted experiments on babul wood spheres and cubes of identical weight under the reactive environment of O2, N2 and CO2, respectively. From the experiments, it was observed from the comparison of pyrolysis time for sphere and cuboid that it is more in all cases for spheres than cuboid showing the dependence of pyrolysis on surface-area-to-volume ratio. Larger surface area provides more vicinity to the heat penetration and hence favors fast pyrolysis. As the surface area of cube is larger than sphere, it provides more area for heat diffusion resulting in reduction of pyrolysis time. Also, it was observed from the mass loss rate curves of babul spheres and cubes, the observed mass loss with respect to time was found to be 0.007 gm/sec for most of the cases during flaming in the varying reactive atmosphere. This suggests that pyrolysis mainly depends on heating rate rather than the composition of reactive media.
References
Ni, M., Leung, D.Y., Leung, M.K., Sumathy, K.: An overview of hydrogen production from biomass. Fuel Process. Technol. 87(5), 461–472 (2006)
Zaror, C.A., Pyle, D.L.: The pyrolysis of biomass: a general review. Proc. Indian Acad. Sci. Sect. C: Eng. Sci. 5(4), 269 (1982)
Kumar, S., Dasappa, S.: Modeling and analysis of single particle conversion of biomass in a packed bed gasification system. Appl. Therm. Eng. 112, 1382–1395 (2017)
Simmons, W.W., Ragland, K.W.: Burning rate of millimeter sized wood particles in a furnace. Combust. Sci. Technol. 46(1–2), 1–15 (1986)
Simpson, W., TenWolde, A.: Chapter 3 - Physical properties and moisture relations of wood. U.S. Department of Agriculture. Wood Hand book - Wood as an engineering material (1999) 3.1–3.24
Ragland, K.W., Aerts, D.J., Baker, A.J.: Properties of wood for combustion analysis. Biores. Technol. 37(2), 161–168 (1991)
Varunkumar, S., Rajan, N.K.S., Mukunda, H.S.: Single particle and packed bed combustion in modern gasifier stoves—density effects. Combust. Sci. Technol. 183(11), 1147–1163 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Vikram, S., Kumar, S. (2021). Single-Particle Analysis of Thermally Thick Wood Particles in O2, N2, CO2 Atmosphere. In: Bose, M., Modi, A. (eds) Proceedings of the 7th International Conference on Advances in Energy Research. Springer Proceedings in Energy. Springer, Singapore. https://doi.org/10.1007/978-981-15-5955-6_128
Download citation
DOI: https://doi.org/10.1007/978-981-15-5955-6_128
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5954-9
Online ISBN: 978-981-15-5955-6
eBook Packages: EnergyEnergy (R0)