Abstract
Pulmonary sequestration is a congenital respiratory malformation characterized by a cystic or solid mass of nonfunctioning primitive segmental lung tissue that does not communicate with the tracheobronchial tree and has anomalous systemic blood supply. Pulmonary sequestration is classified into two types, extralobar pulmonary sequestration and intralobar pulmonary sequestration (ILS). ILS is incorporated within the normal lung tissue and does not have a visceral pleura that separates the lesion from normal lung tissue. Here the 35 pediatric ILS cases treated in our center are summarized first and then we review several clinical reports that dealt with ILS in the past and summarize the characters and treatment of ILS.
Patients with ILS show nonspecific respiratory symptoms such as cough, expectoration, hemoptysis, and chest pain. Recently the number of the cases with fetal diagnosis is increasing. The existence of an aberrant artery to the lung lesion from systemic circulation is an important finding for the correct diagnosis of ILS. The lobectomy including the affected lung is mandatory for complete cure and the prognosis of ILS is satisfactory after the proper surgical treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Intralobar pulmonary sequestration
- Aberrant artery
- Prenatal diagnosis
- Fetal MRI
- Fetal ultrasound examination
- Lobectomy
10.1 Introduction
Pulmonary sequestration is a congenital respiratory malformation characterized by a cystic or solid mass of nonfunctioning primitive segmental lung tissue that does not communicate with the tracheobronchial tree and has anomalous systemic blood supply. Pulmonary sequestration was first reported more than 100 years ago. In 1946 Pryce [1] first used the term “sequestration” for this pathological state and classified it into two phenotypes of “intra” and “extra” pulmonary sequestration. Since then, pulmonary sequestration has come to be accepted as a distinct clinical entity.
Pulmonary sequestration is generally considered to be caused by a developmental anomaly occurring at an early gestational age and the sequestered lung tissue with a systemic arterial supply is formed independently of the normal lung. Pulmonary sequestration is not only anatomically distinct from normal lung tissue but physiologically distinct, which means that the sequestered lung is not connected to the bronchopulmonary system of the normal lung and has no gas exchange function.
Pulmonary sequestration is classified into two pathological entities: extralobar sequestration (ELS) and intralobar sequestration (ILS). Extralobar sequestration is defined as its boundary being separated from normal lung tissue by its own visceral pleura, and intralobar sequestration is incorporated within the normal lung tissue and does not have a visceral pleura that separates the lesion from normal lung tissue.
10.2 Our Cases
We present here the experience of our center in treating ILS surgically and discuss characteristics of the disease.
10.2.1 The Experience of Treatment of Pediatric ILS Cases in Our Center from 2002 to 2019
In 2015 we reported our experience of 30 pediatric ILS cases [2], and since then, we experienced another 5 cases. Here the 35 pediatric ILS cases treated in our center are summarized.
We experienced 35 pediatric cases of ILS from April 2002 to March 2019 that were treated by surgical removal of a pulmonary lobe including the sequestered lung tissue (Table 10.1).
There were 14 male patients and 21 female patients. The age at the time of the operation ranged from 5 months to 124 months (mean age, 32 months). All sequestered lungs were situated in the lower lung field; the sequestered lung was situated in the left lower lung in 17 cases and in the right lower lung in 18 cases. Fetal diagnosis was attained in 13 cases (37.1%), and its percentage has been increasing recently. Since 2014 we experienced five cases of ILS and all of them were diagnosed prenatally. The age at the time of the operation was younger in the cases with fetal diagnosis (mean age was 13 months, compared with a mean age of 42 months in the postnatally diagnosed cases). Twenty cases of ILS experienced pneumonia before the operation but no case showed respiratory distress in their daily life.
The characteristics of the aberrant artery that supplied the sequestered lung in the 35 ILS cases are summarized. The aberrant artery branched from the thoracic aorta in 13 cases, from the abdominal aorta in 17 cases, and from the celiac artery in 5 cases (Fig. 10.1). Another important factor is the number of aberrant arteries. In our series, 31 cases had one aberrant artery, and 4 cases had two aberrant arteries (Fig. 10.1). Venous drainage from the sequestered lung is another important point (Table 10.2). In 32 cases, venous drainage was through the pulmonary vein on the affected side. In one case, venous drainage was through the inferior vena cava. The remaining two cases each had two drainage veins, i.e., through the pulmonary vein and the azygos vein.
The route of the aberrant arteries to the sequestered lung in 35 cases of ILS treated at our hospital. A single aberrant artery was seen in 31 cases and two aberrant arteries were seen in 4 cases. The aberrant artery branched from the thoracic aorta in 13 cases, the abdominal aorta in 17 cases, and the celiac artery in 5 cases. Among the four cases with double aberrant arteries, one case had aberrant arteries branching from the thoracic aorta and three cases had aberrant arteries branching from the abdominal aorta
The operative procedure was lower lobectomy by thoracotomy in all 35 cases. There were no complications experienced during and after the operation.
10.3 Previous Case Series
Pulmonary sequestration is a relatively rare disease, and a few case series [3,4,5,6,7,8,9,10] and review articles [11, 12] on pulmonary sequestration have been published that included adult cases only or both adult and pediatric cases. One review article that summarized Chinese cases of pulmonary sequestration [11] is the largest case review and it reported the characteristics of 2625 pulmonary sequestration cases. In their review, the male-to-female ratio was 1.58:1. The age of the patients at the time of the operation ranged from 1 month to 77 years, and the mean age at the time of the operation for ILS was younger than that for ELS (20 ± 8 years vs. 38 ± 9 years).
The symptoms of the disease included cough/expectoration (67.76%), fever (38.95%), hemoptysis (27.67%), and chest pain (11.13%).
10.4 Clinical Aspects
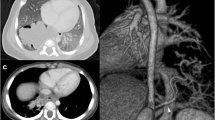
Image findings of chest computed tomography (CT) were classified as follows: mass lesion (49.01%), cystic lesion (28.57%) (Fig. 10.2a shows a typical image of a cystic lesion experienced in our center), cavity lesion (11.57%), pneumonic lesion (7.96%), and bronchiectasia (1.90%) (Fig. 10.2b shows a typical image of bronchiectasia experienced in our center). Among pulmonary sequestrations, unilateral ILS was the most common phenotype, accounting for 1873 cases (83.95%), and unilateral ELS accounted for 358 cases (16.05%). Bilateral lesions were extremely rare, with only three cases seen in the series from China. The sequestered lung was mainly localized in the left lower lobe (1457 cases; 71.53%), and the second common segment was the right lower lobe (529 cases; 25.97%). Other localized regions of sequestered lung were the left upper lobe, left lingual lobe, right upper lobe, and right middle lobe. In our experience, all lesions were situated in the lower lung segment, and the left-to-right ratio was about 1:1. ILS has a special feature compared with other congenital pulmonary cystic diseases such as congenital pulmonary airway malformation (CPAM) and bronchial atresia in that the lesion of ILS belongs to one area of the normal lung and single lobectomy is always possible for complete cure. Therefore, it is important to detect the exact site of the ILS lesion for proper surgical excision. ILS was also reported to coincide with other congenital anomalies [11] such as esophagobronchial diverticulum, diaphragmatic hernia [13], deformities of skeletal systems, and cardiac anomalies. Some case reports described the association of bronchogenic cyst with ILS [14].
Representative CT findings of lung fields and aberrant arteries in ILS. (a) Cystic lesion, which represents the sequestered lung, in the right lower lobe. (b) Hyperlucent lesion, which represents the sequestered lung, in the left lower lobe. A large aberrant artery is seen running amidst the affected lung. (c) A large aberrant artery branched from the thoracic aorta to the sequestered lung in the right lower lobe. (d) Two aberrant arteries branched from the thoracic aorta to the sequestered lung in the left lower lobe
The most important factor for safe treatment of ILS is determining the arterial supply and venous drainage of the sequestered lung. Detection of the aberrant artery is a key point for correct diagnosis of ILS [15,16,17,18], and preoperative confirmation of the blood supply and the drainage route is mandatory for carrying out a safe operative procedure.
On fetal ultrasound examination, if an abnormal pulmonary lesion is detected, it should be checked whether the systemic arterial supply to the lesion exists from the aorta or from one of its branches. Fetal magnetic resonance imaging (MRI) examination may be useful to confirm the abnormal arterial supply. If such abnormal arterial branch to the lesion is detected, pulmonary sequestration should be strongly suspected. On the other hand, it is also important to determine venous drainage from the lesion because in ILS venous drainage is mainly through the pulmonary vein while in ELS venous drainage is usually through a systemic route [11]. We must carefully differentiate ILS from ELS because in some ILS cases venous drainage is through a systemic vein such as the azygos vein, semi-azygos vein, or inferior vena cava. In our series, three cases had such systemic drainage. Postnatal chest CT examination may be able to demonstrate whether the pulmonary lesion has its own visceral pleura or not, and therefore may be useful to differentiate ILS from ELS.
It is important to recognize the symptoms of ILS, especially in adults, but disease-specific symptoms do not exist and almost all symptoms such as cough, expectoration, fever, hemoptysis, and chest pain are not specific [11]. Hemoptysis is in one sense a specific symptom of ILS but in pediatric cases it is rarely seen. In our series, pneumonia was a main symptom in obtaining a correct diagnosis before the fetal diagnostic era, but recently there has been a predominance of cases with fetal diagnosis and in many cases curative operation was performed before pneumonic symptoms appeared. Recent basic research suggested that in ILS alveolar type 2 stem/progenitor cells are impaired in their proliferative potential perhaps through the interaction between endothelial thrombospondin-1 and alveolar cell surface antigen CD36, and this may be closely related to the high infection rate in patients with ILS [19]. Blood tests in patients with ILS do not show specific abnormalities, but in some reports serum CA19-9 was elevated in patients with ILS and it significantly decreased after surgery [20, 21]. The exact mechanism of this phenomenon has not yet been clarified, but serum CA19-9 might be used as a disease marker of ILS in restricted cases. Especially in adults, when patients show a high level of CA19-9, ILS should be among the differential diagnoses after ruling out malignancies of digestive organs.
10.5 Treatment
The treatment strategy for ILS is surgical removal of the pulmonary lobe containing the sequestered lung once the diagnosis is confirmed. As ILS often causes respiratory infection and hemoptysis is a nuisance complication in later life, it is recommended that patients with ILS undergo the operative treatment. In some reports, embolization of the aberrant artery was successful and this is one treatment option [22,23,24,25]. Another interesting option for surgical resection of the small lesion is indocyanine green injection and navigated partial lung resection [26]. If the lesion is very small and it is desirable to preserve as much of the normal lung as possible, this strategy may be adopted. Recently video-assisted thoracoscopic surgery (VATS) and thoracoscopic surgery have become more popular and some reports recommended such minimally invasive surgery [7, 10]. In such procedure, safe dissection of a large aberrant artery is very important and skillful technique is mandatory for preventing severe bleeding complications. Another important point for performing safe surgery is accurate preoperative diagnosis concerning the aberrant artery (Figs. 10.2c, d and 10.3a–c) and the drainage vein (Fig. 10.4). In some cases, more than one aberrant artery feeds the lesion [27,28,29,30,31,32] (Fig. 10.2d) and each artery should be ligated separately to prevent unexpected bleeding. In two large case series [11, 12], the percentage of cases with more than one aberrant artery was 20.91% and 14.8%, respectively, and in our series it was 11.4% (4/35). The aberrant artery branched from the thoracic aorta (76.55% by Wei [11] and 73.9% by Savic [12]) or the abdominal aorta (18.47% by Wei [11] and 18.7% by Savic [12]) in the two previous case series. In our series, the aberrant artery branched from the thoracic aorta in 37.1% (Fig. 10.3a), the abdominal aorta in 48.6% (Fig. 10.3b) and the celiac artery in 14.3% (5/35) (Fig. 10.3c). Regarding the route of aberrant arteries, our data do not coincide with the data of large case series published in the past, but it is not clinically important because all aberrant arteries, whether they originated from the abdomen or thorax, were ligated in the thoracic cavity. In ILS, the drainage vein is the pulmonary vein in many cases (Fig. 10.4a, b). If the drainage vein connects to the systemic venous circulation (Fig. 10.4c, d), the drainage vein runs alongside the aberrant artery and unexpected venous damage may occur during the operation in such cases. The percentage of cases with systemic venous drainage was less than 10% among all ILS cases in the two large case series [11, 12], and it was 8.6% in our series, but we must always keep in mind that this rare venous return may exist in ILS.
Aortic angiography to demonstrate aberrant arteries to the sequestered lung in ILS. (a) An aberrant artery branched from the thoracic aorta to the sequestered lung in the right lower lobe. (b) An aberrant artery branched from the abdominal aorta to the sequestered lung in the left lower lobe. (c) An aberrant artery branched from the celiac artery to the sequestered lung in the right lower lobe
Representative cases of drainage veins from the sequestered lung. (a) The drainage vein from the sequestered lung is the left pulmonary vein as shown by selected angiography of the aberrant artery. (b) The drainage vein is the right pulmonary vein as shown by selected angiography of the aberrant artery. (c) The drainage vein is the inferior vena cava as shown by aortography. (d) The drainage veins are the azygos vein and left pulmonary vein as shown by aortography
10.6 Prognosis
The prognosis of ILS is satisfactory if appropriate lobectomy is performed after a correct diagnosis is made. Although ILS is a rare disease and it is difficult to obtain a correct diagnosis in some cases due to the nonspecific respiratory symptoms, it is important for physicians to suspect ILS and plan the correct imaging examination at the early stage.
References
Pryce DM. Lower accessory pulmonary artery with intralobar sequestration of lung: a report of seven cases. J Pathol Bacteriol. 1946;58:457.
Takezoe T, Tahara K, Watanabe T, Ohno M, Kawasaki K, Higuchi M, Matsuo M, Nosaka S, Miyazaki O, Tsutsumi Y, Kanamori Y. Patterns of blood supply and venous drainage in pediatric intralobar pulmonary sequestration: a retrospective analysis of 30 pediatric cases from a single center. Open J Pediatr. 2016;6:274–9.
Genc O, Gurkok S, Dakak M, Gozubuyuk A, Ozakan M, Caylak H. Pulmonary sequestration and surgical treatment. Asian Cardiovasc Thorac Ann. 2006;14:3–6.
Kestenholz PB, Schneiter D, Hillinger S, Lardinois D, Weder W. Thoracoscopic treatment of pulmonary sequestration. Eur J Cardiothorac Surg. 2006;29:815–8.
Polaczek M, Baranska I, Szolkowska M, Zych J, Rudzinski P, Szopinski J, Orlowski T, Roszkowski-Sliz K. Clinical presentation and characteristics of 25 adult cases pulmonary sequestration. J Thoracic Dis. 2017;9:762–7.
Zhang N, Zeng Q, Chen C, Yu J, Zhang X. Distribution, diagnosis, and treatment of pulmonary sequestration: report of 208 cases. J Pediatr Surg. 2018;54:1286–92.
Genç O, Gürkök S, Dakak M, Gözübüyük A, Özkan M, Çaylak H. Pulmonary sequestration and surgical treatment. Asian Cardiovasc Thorac Ann. 2006;14:3–6.
Marks C, Wiener SN, Reydman M. Pulmonary sequestration. Chest. 1972;61:253–7.
Buntain WL, Wooley MM, Mahour GH, Isaacs H Jr, Payne V Jr. Pulmonary sequestration in children: a twenty-five-year experience. Surgery. 1977;81:413–20.
Tashtoush B, Memarpour R, Gonzalez J, Gleason JB, Hadeh A. Pulmonary sequestration: a 29 patient case series and review. J Clin Diagn Res. 2015;9:5–8.
Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2625 cases in China. Eur J Cardiothorac Surg. 2011;40:e39–42.
Savic B, Birtel FJ, Tholen W, Funke HD, Knoche R. Lung sequestration: report of seven cases and review of 540 published cases. Thorax. 1979;34:96–101.
Lim D, Kostin R. Intralobar pulmonary sequestration associated with Bochdalek hernia: first reported cases in an adult male and literature review. J Surg Case Rep. 2018;8:1–3.
Traibi A, Strauss C, Validire P, Gossot D. Intralobar pulmonary sequestration associated with bronchogenic cyst in adult. Asian Cardiovasc Thorac Ann. 2012;20:597–9.
Fumino S, Iwai N, Kimura O, Ono S, Higuchi K. Preoperative evaluation of the aberrant artery in intralobar pulmonary sequestration using multidetector computed tomography angiography. J Pediatr Surg. 2007;42:1776–9.
Wani SA, Mufti GN, Bhat NA, Baba AZ. Pulmonary sequestration: early diagnosis and management. Case Rep Pediatr. 2015;454860.
Qi W, Zhao J, Shi G, Yang F. Intralobar pulmonary sequestration displayed as localized emphysema on computed tomography image. J Cardiothorac Surg. 2017;12:83.
Mylan S, Maity S, Machlan J. Diagnosing intralobar pulmonary sequestration. Arch Dis Child. 2018;0:1.
Li K, Wu Q, Sun X, Geng Y, Leng D, Li H, Zhang S, Wang Q, Wu J, Xu L, Li X, Li Y, Zhang Q, Kurkciyan A, Liang J, Jiang D, Chen H. Tsp1 promotes alveolar stem cell proliferation and its down-regulation relates to lung inflammation in intralobar pulmonary sequestration. Oncotarget. 2017;39:64867–77.
Han P, Tian D, Yan W, Liu J, Chang Y, Xie W, Huang H. Pulmonary sequestration presenting with left upper abdominal bloating and marked elevation of serum carbohydrate antigen 19-9: a case report. Oncol Lett. 2014;7:1493–6.
Fu S, Wang H. Pulmonary sequestration associated with a synchronous elevation of carbohydrate antigen 50 and 19-9: a case report. Ann Transl Med. 2018;6:212.
Ahn SJ, Kim EY, Kim JH, Byun SS, Kim HS, Choi HY, Sun YH. Successful endovascular treatment of bilateral intralobar pulmonary sequestration with a bridging isthmus in a child. Pediatr Pulmonol. 2014;49:E126–9.
Ojha V, Samui PP, Dakshit D. Role of endovascular embolization in improving the quality of life in a patient suffering from complicated intralobar pulmonary sequestration – a case report. Respir Med Case Rep. 2015;16:24–8.
Herbert CE, Reddy SRV, Lemler MS. Use of amplatzer vascular plugs for the treatment of combined extralobar and intralobar pulmonaru sequestration in a 5-year-old child. Cardiol Young. 2016;26:1441–4.
Ellis J, Brahmbhatt S, Desmond D, Ching B, Hostler J. Coil emobolization of intralobar pulmonary sequestration – an alternative to surgery: a case report. J Med Case Rep. 2018;12:375.
Yamanashi K, Okumura N, Nakazono C, Matsuoka T. Surgery for intralobar pulmonary sequestration using indocyanine green fluorescence navigation: a case report. Semin Thoracic Surg. 2018;30:122–4.
Jotsuka T, Matsuguma H, Sawafuji M, Yokoi K, Hirose K, Mori K, Tominaga Y, Imura J. Intralobar pulmonary sequestration with three aberrant arteries: a case report and review of the Japanese literature. Kyobu Geka. 1998;51:142–6.
Tsunezuka Y, Sato H. Intralobar pulmonary sequestration with three aberrant arteries in a 75-year-old patient. Chest. 1998;114:936–8.
Kanazawa S, Miyake T, Ishida A, Ohtani H, Tsunoda T, Tanemoto K. Intralobar pulmonary sequestration supplied by multiple anomalous arteries: report of a case. Surg Today. 2001;31:701–4.
Georgoff P, Singhal S. Multivessel intralobar pulmonary sequestration. Ann Thorac Sur. 2012;93:1318.
Theodoropoulos I, Schwartz MZ. Intralobar pulmonary sequestration: an uncommon case with triple arterial supply and systemic venous drainage. Pediatr Surg Int. 2012;28:741–4.
Erden ES, Yetim TD, Balci A, Akcay AB, Hakverdi S, Demirkose M. Intralobar pulmonary sequestration with arterial supply from two different origins: a case report. Ann Thorac Cardiovasc Surg. 2012;18:560–3.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kanamori, Y. (2020). Intralobar Pulmonary Sequestration. In: Sago, H., Okuyama, H., Kanamori, Y. (eds) Congenital Cystic Lung Disease. Springer, Singapore. https://doi.org/10.1007/978-981-15-5175-8_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-5175-8_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5174-1
Online ISBN: 978-981-15-5175-8
eBook Packages: MedicineMedicine (R0)