Abstract
Because of their ultrasmall core size (usually <2 nm), strong photoluminescence, facile availability, and good biocompatibility, fluorescent metal nanoclusters (MNCs) have recently emerged as a novel kind of promising fluorescent probes for biological imaging. In this chapter, we will first provide an overview on recent advances in the development of various synthesis strategies for fluorescent MNCs. Then, we will summarize researches in the utilization of fluorescent MNCs (including Au, Ag, Cu, and alloy NCs) either as single-modal imaging probes or as multimodal imaging probes in biological cells and tissues. Finally, we will give a brief outlook on the future challenges and prospects of developing fluorescent MNCs for bioimaging at both in vitro and in vivo level.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Fluorescent metal nanoclusters (MNCs), usually consisting of several to approximately a hundred metal atoms [1], have attracted extensive attention over the past few decades. MNCs have size down to less than 2 nm, which is comparable to the Fermi wavelength of electrons [2], resulting in the break up of the continuous density of states of the particles into discrete energy levels [1, 3]. MNCs exhibit distinct optical, electronic, and chemical properties, including strong photoluminescence, excellent photostability, and good biocompatibility. These unique properties make MNCs ideal probes for many applications in biological imaging and diagnosis.
Especially, near-infrared (NIR) fluorescent MNCs are promising probes for bioimaging, because biological tissues show very weak absorption and autofluorescence in the NIR spectrum window (650–900 nm wavelengths) [4, 5]. Also, NIR light can pass across several centimeters of heterogeneous living tissues [6]. Particularly, NIR-emitting MNC probes can alleviate several limitations of conventional NIR organic dyes and other nanoprobes like semiconductor quantum dots (QDs). Organic dyes show many drawbacks such as poor hydrophilicity and photostability, insufficient stability in biological systems, and weak multiplexing capability [7]. Most reported QDs display high inherent cytotoxicity and self-aggregation inside live cells, which limit their practical bio-applications [8].
Fluorescence lifetime imaging (FLIM) and two-photon imaging have been widely adopted in tissue and cell studies, and now have become powerful tools in early diseases diagnosis as well as guiding the disease treatment [9, 10]. Fluorescent MNCs possess much longer lifetime than that of cellular autofluorescence and most organic dyes, making them attractive markers for cellular FLIM applications, which is independent of fluorophore concentration and laser excitation intensity [11]. Although one-photon fluorescence imaging techniques are featured with good spatial resolution and high sensitivity, they hardly obtain anatomical or three-dimensional details of tumor tissues in vivo [12]. Compared to one-photon imaging, two-photon imaging is a powerful technique for enhanced tissue penetration depth (>500 μm), low tissue autofluorescence, and self-absorption, as well as reduced photodamage [10, 13, 14]. With relatively good biocompatibility and large two-photon absorption (TPA) cross section, MNCs are also considered as ideal probes for two-photon imaging in biological system.
Besides fluorescence (FL) imaging, several other imaging techniques have also been used in the early-stage diagnosis of cancer, such as magnetic resonance imaging (MRI), X-ray computed tomography (CT), photoacoustic imaging (PAI), positron emission tomography (PET) imaging, and single-photon emission computed tomography (SPECT) [15, 16]. Each imaging modality has its own unique advantages along with intrinsic limitations [17]. For example, CT imaging can easily differentiate various tissue densities, and allow three-dimensional visual reconstructions of tissue, which suffers from poor sensitivity in soft tissues with limited density differences [18, 19]. MR imaging is able to provide high-quality 3D information of soft tissues and possesses high spatial resolution, but has the disadvantage of relatively low sensitivity [20,21,22,23]. In contrary, FL imaging has high sensitivity and resolution for imaging at the cellular level, but it cannot provide spatial resolution and 3D tissue detail [24]. Therefore, the rational combination of different modalities, known as “multimodal imaging,” is a powerful method that can provide more reliable and accurate detection of disease sites [15, 25].
In this chapter, we mainly focus on the latest progress in fluorescent MNCs probes for biological imaging. Specifically, we summarize recent advances in the synthesis and applications of fluorescent MNCs (including Au, Ag, Cu, and alloy NCs) as novel bioimaging probes, including single-modal imaging (fluorescence intensity-based imaging, FLIM, two-photon imaging, PET imaging) probes and the combination of FL imaging with several other imaging techniques to form multimodal imaging (such as FL/CT/MRI, FL/PAI/MRI, FL/SPECT, etc.) probes. In the final section, we will give a brief outlook on the challenges and opportunities for fluorescent MNCs in bioimaging applications.
2 Synthesis of Fluorescent MNCs
Up to now, many different methods have been developed to synthesize MNCs with the photoluminescence (PL) property. Generally, these approaches can be classified into two groups, “bottom-up” and “top–down” [26]. In both strategies, surface ligands or templates play an important role in defining their final properties. Therefore, in the following, we will overview each synthetic strategy based on the type of templates or capping ligands (representative examples summarized in Table 1).
2.1 Thiols
Owing to the strong interaction between thiols and Au/Ag, small thiolate molecules are the most commonly adopted stabilizers in MNC synthesis [27]. Among them, glutathione (GSH) is the most commonly adopted one, and GSH-stabilized AuNCs with a maximum emission at 780 nm could be obtained via NaBH4 reduction. These AuNCs display strong one- and two-photon emissions, good photostability and biocompatibility [28]. By employing GSH as reducing and protecting reagent simultaneously, Zheng and coworkers [29] successfully synthesized NIR-emitting GSH-AuNCs with a core size 2.5 nm at 90 °C. Besides, water-soluble GSH-capped AuNCs were also obtained by using tetrabutylammonium borohydride (TBAB) as a mild reductant, and the yielded GSH-AuNCs showed excellent PL properties and low cytotoxicity [30]. Wu et al. [31] developed a one-pot one-cluster synthesis method to prepare mono-sized Ag14NCs capped with GSH. They found that the fluorescence quantum yield (QY) of Ag14(SG)11 NCs is strongly solvent-dependent, and the fluorescence intensity increases upon decreasing the solvent polarity or dielectric constant [32, 33]. Recently, a rapid sonochemical route to synthesize fluorescent AgNCs using hydrazine hydrate as reducing agent and GSH as capping agent was developed [34]. The as-prepared AgNCs show high photo-, time-, pH-, and ions-stability in aqueous solution, and have been exploited as probes for monitoring Pb2+ in living cells. In another work, Song and coworkers [35] reported a one-step synthetic method to prepare GSH templated CuNCs. The resultant CuNCs contain 1–3 atoms and exhibit red fluorescence (λem = 610 nm) with high QY, up to 5.0%. Interestingly, the fluorescence signal of the CuNCs is reversibly responsive to the environmental temperature in the range of 15–80 °C.
Wang and coworkers [36] proposed a different strategy to in situ biosynthesize fluorescent AuNCs inside cancer cells and tumor tissues. They found that HAuCl4 can undergo a more rapid and efficient spontaneous reduction into AuNCs inside cancerous cells than in normal ones, enabling self-bio-imaging of cancer cells and tumors by long-lasting fluorescent markers. Subsequently, they reported the intracellular biosynthesis of AgNCs by cancerous cells incubated with silver ions [37]. AgNCs were spontaneously biosynthesized in situ by HeLa cancer cells treated with a specific silver salt derivative [Ag(GSH)]+ and exogenous GSH. Recently, the same group [38] explored the preparation of an intracellular temperature nanoprobe specifically by in situ biosynthesized fluorescent CuNCs in target cancer cells upon incubation with a special copper precursor (i.e., the complex solution of GSH and copper(II)). These fluorescent CuNCs could be biosynthesized spontaneously in MDA -MB-231 cancer cells through a particular molecular process, but not in normal cells (i.e., L02 cells). In a recent study, they demonstrated that fluorescent ZnO nanoclusters and magnetic Fe3O4 nanoclusters can also be synthesized in cancer cells [39].
Using GSH as a scaffold and sulfur–hydrazine hydrate complex (S–N2H4·H2O) as the S2− source, Wang et al. [40] developed a one-step approach to prepare water-soluble fluorescent Ag2S NCs with tunable PL properties. By adjusting the amount of GSH and the ratio of Ag+ to S–N2H4·H2O, Ag2S NCs with different PL wavelengths and sizes were obtained. Subsequently, Xian group [41] successfully synthesized NIR-emitting fluorescence Ag2S QDs in aqueous solution using 3-mercaptopropionic acid (3-MPA) as sulfur source and stabilizer. Interestingly, the fluorescence intensity of Ag2S QDs was obviously enhanced upon the addition of various rare earth ions, especially in the presence of Gd3+. They speculated that the electrostatic interaction and coordination between rare earth ions and -COOH from MPA on Ag2S QDs results in QDs aggregation and displays the feature of aggregation-induced emission (AIE).
Wang and coworkers [42] used a galvanic replacement reaction to prepare AgAu alloy NCs. In the first step, the template (i.e., AgNCs) was prepared by using GSH as the stabilizing agent and N2H4·2H2O as the reducing agent. Then, when the AuCl4¯ ion and GSH were added to the aqueous solution of AgNCs, the galvanic replacement reaction occurred due to higher standard reduction potential of AuCl4¯/Au pair (0.99 V vs SHE) than that of Ag+/Ag pair (0.80 V vs SHE) [43]. The as-prepared AgAu alloy NCs displayed NIR fluorescence centered at 716 nm and showed tunable luminescence from visible red (614 nm) to NIR (716 nm) by controlling the Ag/Au ratios.
In addition to GSH, bidentate dihydrolipoic acid (DHLA) is another attractive ligand for MNCs synthesis due to its strong binding affinity to metal atoms. Shang et al. [11] synthesized NIR-emitting DHLA–AuNCs with a one-pot strategy by simply reducing a mixture of lipoic acid (LA) and gold salt with NaBH4 in aqueous solution. The obtained AuNCs possess NIR emission and long fluorescence lifetime (>100 ns), making them attractive as markers for cellular FLIM applications. Afterwards, the same group developed a microwave-assisted strategy for synthesizing DHLA–AuNCs [44]. Particularly, irradiation with microwaves during the synthesis enhanced the fluorescence QY of AuNCs by about fivefold from ∼0.6% to 2.9%, and it also shortened the reaction time from hours to several minutes. Moreover, by using microwave irradiation, the emission peak red shifts from 690 nm to 715 nm upon excitation at 580 nm. Later, via a slightly modified strategy, Nair et al. [45] reported the synthesis of NIR-emitting (Au)18(LA)14 NCs with a higher QY, 10%. Besides AuNCs, Ghosh and coworkers [46] reported the synthesis of brightly red fluorescent DHLA–CuNCs, in combination with biocompatible polymer poly(vinylpyrrolidone) (PVP) as stabilizers. The fluorescence of CuNCs was found to be pH sensitive, and the emission could be tuned reversibly according to the pH.
Besides GSH and DHLA, other thiols such as tiopronin and mercaptosuccinic acid (MSA) have also been used as stabilizers, which yielded AuNCs centered at 785 nm with QYs in the range of 3–4% [47]. Recently, Zhou et al. [48] synthesized hydrophobicity-guided self-assembled particles of AgNCs with AIE (Fig. 1). They adopted a hydrophobic ligand, thiosalicylic acid, as capping agent to prepare AgNCs which showed significant AIE behavior. This AIE property of AgNCs enables them to sensitively respond to multiple external stimuli such as solvent polarity, pH, and environmental temperature. The hydrophobic nature of thiosalicylic acid as the capping ligand of AgNCs drives the formation of self-assembled particles of AgNCs with bright luminescence.
Schematic of the fabrication of self-assembled particles of AgNCs with AIE and their use in quantifying mercuric ion and imaging cells. Reprinted with permission from Ref. [48]
In addition to the above-mentioned strategy by direct reduction of metal ions in the presence of thiols, fluorescent MNCs can also be prepared by etching large metal nanoparticles with thiols. Au23(SG)18 NCs (SG denotes GSH) were obtained via the interfacial etching process using Au25SG18 NCs as the precursor. For interfacial etching, an interface was created by making an immiscible biphasic mixture of toluene containing octanethiol (OT) and an aqueous solution of Au25SG18. A highly fluorescent, water-soluble Au23(SG)18 cluster was obtained by etching at 25 °C [49]. Red-emitting AgNCs were produced by an interfacial etching route using GSH as a ligand etchant from MSA-protected AgNPs. These AgNCs show high photostability over time and a high stability for a wide pH range [50]. Lin and coworkers [51] developed a strategy to synthesize DHLA–AuNCs based on precursor-induced AuNPs etching in organic phase and ligand exchange with DHLA to transfer the particles into aqueous solution. Subsequently, the same group adopted a further 24 h thermal treatment at 70 °C to markedly increase the QY of AuNCs to nearly 7% [52].
2.2 Proteins, Peptides, and DNA Oligonucleotides
Biomacromolecules such as proteins and peptides have also been extensively utilized as templates for synthesizing fluorescent MNCs. Particularly, proteins possess abundant binding sites that can potentially bind and further reduce metal ions, thus offering promising scaffolds for template-driven formation of small MNCs [53, 54]. Notably, bovine serum albumin (BSA) was first reported by Xie et al. as an excellent scaffold for AuNCs due to the strong force of Au–S bonding and the steric protection (Fig. 2), where NIR-emitting AuNCs with maximum emission wavelength at about 640 nm can be obtained [55]. Recently, Yu et al. [56] reported a kind of novel hybrid membrane made with AuNC-embedded BSA (AuNCs@BSA) fibrils and activated graphene oxide (GO), which was used to remove heavy metal ions, Hg2+, from water. Later, researchers also tried many other proteins, such as transferrin-family proteins [57,58,59], trypsin [60], insulin [61], and ribonuclease A [62], as potential bioscaffolds for synthesizing MNCs. For example, transferrin (Tf)-templated CuNCs have been synthesized at room temperature via a biomineralization process, where ascorbic acid was used as the reductant. The as-prepared Tf-CuNCs exhibited intense NIR fluorescence with a QY about 6.2% [63]. Using denatured lysozyme (dLys) as the capping agent, a ratiometric fluorescent AgNCs probe was developed [64]. This probe could be utilized for ratiometric detection of H2O2 and further exploited to H2O2–generated oxidase-based biosensing, such as glucose and acetylcholine chloride. Also, dual channel fluorescence confocal images of •OH in living cells was realized using the dLys-AgNCs probe. Besides their well-known roles as capping agents, proteins such as BSA can also function as etching agents for synthesizing fluorescent AuNCs in a few cases. For example, Pradeep et al. [65] employed a core etching method to synthesize BSA-AuNCs from MSA-capped AuNPs.
Schematic of the formation of AuNCs in BSA solution under alkaline conditions. Reprinted with permission from Ref. [55]
In addition to proteins, the integration of MNCs with peptides can also combine the distinct optical properties of MNCs with the biological functions of peptides [66, 67]. For example, Gao group [68] developed a one-step biomineralization method to produce AuNCs by using a bifunctional CCYTAT peptide, which contains one domain for biomineralizing and capturing AuNCs and another domain for targeting cell nuclei. The as-prepared AuNCs showed a maximum emission at 677 nm and possessed a high fluorescence QY of about 11%. Recently, by combining biomineralization and supramolecular self-assembly of motif-designed peptide constructs, researchers reported that the emission of peptide–AuNCs can be enhanced by nearly 70-fold, which largely increases their utility for biological applications [69].
DNA have been employed in the design and fabrication of various DNA-templated metal nanostructures owing to their distinct interactions with metal cations [70]. In 2008, Dickson group [71] first reported the use of ssDNA to synthesize AgNCs with fluorescence tunable throughout the visible and NIR range. Notably, these AgNCs possessed a high QY up to 34%. Sharma et al. [72] also reported four different DNA sequences as AgNC templates with emission at different wavelengths. The resulting NIR-emitting AgNCs had QY greater than 50% and were very promising as biolabels. It has been shown in earlier reports that Ag+ has a higher binding affinity to cytosine bases than other bases [73,74,75]. Therefore, Dickson and coworkers [76, 77] reported NIR-emitting AgNCs creating in single-stranded oligo-DNA consisting of 12 or 24 cytosine bases. By using more advanced DNA structures, such as G-quadruplex, as the template, Wang group [78] reported the synthesis of fluorescent AgNCs made of 2–4 Ag atoms centered at 680 nm. Furthermore, intrinsically fluorescent AgNCs–aptamer assemblies for cell recognition were developed by Wang and coworkers [79]. They employed a cancer-targeted DNA aptamer sequence (A-strand) and cytosine-rich sequence for templated synthesis of fluorescent AgNCs (C-strand). A fluorescent sgc8c–AgNCs assembly with relatively high luminescence has been achieved and exhibited specific binding to target CCRF-CEM cells by using a six-base adenine linker. Subsequently, adopting a similar method, the same group [80] reported a label-free and turn-on aptamer strategy for cancer cell detection based on the recognition-induced conformation alteration of aptamer and hybridization-induced fluorescence enhancement effect of DNA–AgNCs in proximity of guanine-rich DNA sequences (Fig. 3). In this strategy, two tailored DNA probes were designed, namely, a recognition probe (R-Probe) and a signal probe (S-Probe). In the presence of target cancer cells, recognizing and binding of the aptamer to the protein receptors on the cancer cells surface enforces the R-Probe to undergo a conformational alteration, causing the arm segment dissociation. The hybridization between the arm segment in the R-Probe and the link sequence in the S-Probe could then be initiated. Finally, the S-Probe-templated dark AgNCs are brought close to the guanine-rich DNA sequences and changed to bright AgNCs, leading to enhanced fluorescence readout.
Schematic representation of the label-free and turn-on aptamer strategy for cancer cell detection based on DNA–AgNCs fluorescence upon recognition-induced hybridization. Reprinted with permission from Ref. [80]
2.3 Polymers
There are also efforts on using polymer as stabilizers for preparing fluorescent MNCs based on their capability of sequestering metal ions from solutions. Moreover, the terminal groups on the polymer periphery are very useful for the further bioconjugation of MNCs. For instance, Huang and coworkers [81] prepared NIR-emitting AuNCs by using multidentate polymer, thioether-terminated poly(methacrylic acid) (PTMP-PMAA), as ligands. In another report, fluorescent poly(ethylene glycol) (PEG)–AuNPs with an emission peak at 810 nm were created by thermally reducing HAuCl4 in the presence of thiolated PEG ligands with a molecular weight (MW) of 1 kDa in aqueous solution [82]. Similarly, Wang et al. [83] reported a one-pot fabrication of thiol-terminated polyethyleneimine (SH-PEI) stabilized NIR-emitting AgNCs. SH-PEI not only acts as an excellent stabilizer for AgNCs but also facilitates post-surface modification with functional biomolecules. Inouye and coworkers [84] synthesized Pt5(MAA)8 NCs with an 18% QY in water. Upon bioconjugating an antibody, they successfully labeled chemokine receptors in living HeLa cells. Afterwards, the same group investigated the formation of yellow fluorescent PEI-protected PtNCs (PtNCs@PEI) [85]. They found that PtNCs were produced in the cavities formed by coiled PEI ligands and were mostly stabilized with the amino groups (-NH2). The size and fluorescence properties of PtNCs@PEI are strongly related to the cavities formed by the coiled PEI ligands. As shown in Fig. 4, under alkaline pH conditions, PEI have the ability to coil around the surface of PtNCs to form the cavities. As for the neutral condition (all primary amines protonated), the hydrodynamic size of PtNCs is a little larger than ones produced under basic condition, resulting in the slight shift to longer emission wavelength. At acidic pH (most amines protonated), both PEI and PEI-capped NCs possess considerable positive charges, leading to an expansion of PEI chains because of the repulsion between the charged amines. The dimension of cavity in the acidic situation is much bigger than that in the basic situation, caused the larger PtNPs and no emitted fluorescence.
Schematic formation of PEI chelation with Pt ions and reduced PtNCs in PEI cavities at different pH mediums. Reprinted with permission from Ref. [85]
Recently, poly(amidoamine) (PAMAM) dendrimer-hosted Au5NCs were successfully synthesized through a two-stage growth process with a high fluorescence QY up to 25% [86]. As shown in Fig. 5, stage I presented a simultaneous self-nucleation of Au5NCs and subsequent PAMAM-hosted self-assembly with a rapid rate of fluorescence increase. At stage II, the fluorescence enhancement should be mainly dominated by the self-assembly of Au5NCs in PAMAM matrix. First, the emission from self-assembled aggregates was attributed to ligand-to-metal–metal charge transfer (LMMCT) from electron-rich-NH2 groups in PAMAM to Au atoms, which generated radiative relaxation through a metal-centered triplet state. In addition, because PAMAM endowed AuNCs with stronger inner interactions compared to those isolated species, enhanced aurophilic interactions greatly promote excited-state relaxation dynamics and enhanced rigid structures reduced the level of nonradiative relaxation of excited states, which was also responsible for enhanced emission [87].
Schematic illustration of growth process and the structure of Au5NCs self-assemblies in PAMAM matrix. Reprinted with permission from Ref. [86]
3 MNCs for Fluorescence Bioimaging
With many attractive features including ultrasmall size, good biocompatibility, brightness, and photostability, MNCs are promising fluorescence probes for biological imaging. Indeed, great progress has been achieved in recent years on employing fluorescent MNCs for biological imaging applications, as summarized in Table 2. In 2012, Shang et al. [44] demonstrated the utilization of DHLA–AuNCs for imaging intracellular Hg2+ in living HeLa cells, where they observed the intracellular fluorescence quenching effect upon addition of Hg2+ ions. Subsequently, the same group [94] systematically investigated the interactions of AgNCs with human serum albumin (HSA). They found that protein adsorption markedly changes the uptake behavior as well the cytotoxicity of AgNCs. The amount of AgNCs internalized by the cells is substantially reduced in the presence of HSA. Moreover, the fluorescence from intracellular AgNCs is stronger than that from membrane-associated particles in both cases, the fluorescence decrease in the membrane region (ca. 13-fold) is much larger than for inside the cells (ca. sevenfold). Afterwards, they systematically varied the surface charge of HSA to examine the effect of Coulomb forces in modulating the biological interactions of AuNCs [116]. By utilizing confocal fluorescence microscopy to observe the uptake and localization of AuNCs in HeLa cells, they found distinct difference in the cellular uptake of AuNCs adsorbed with differently modified HSA (Fig. 6): nHSA (native HSA) suppressed cellular uptake, aHSA (HSA with more negative surface charges) showed negligible effect, and cHSA (HSA with more positive surface charges) enhanced cellular uptake. The results provide helpful information in designing NIR AuNCs aiming to highly efficient cell labeling applications.
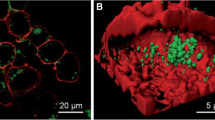
Three-dimensional fluorescence confocal images of HeLa cells upon incubation with AuNCs (2.5 μM, green) for 2 h: (a) without proteins and with 2.5 μM (b) nHSA, (c) aHSA, and (d) cHSA. Cell membranes were stained with CellMask DeepRed (red). The data are shown as sections in the x-y plane (upper left), x-z plane (lower left), and y-z plane (right). Scale bar, 10 μm. Reprinted with permission from Ref. [116]
Gao and coworkers [68] found that peptide–AuNCs with a bifunctional CCYTAT peptide could specifically target the nucleus of three different cell lines, including normal cells human gastric mucosa cells (GES-1), human embryonic lung fibroblast cells (MRC-5), and human cervical cancer cells (HeLa). Ai et al. [78] successfully employed G-quadruplex AS1411-templated AgNCs for specific bioimaging HeLa cells. Besides, Guével et al. [50] employed GSH-AgNCs as optical probes for NIR fluorescence imaging of epithelial lung cancer A549 cells. Confocal images showed that AgNCs were taken up in the cytoplasm and more specifically in the vesicles of A549 cells, but were absent in the nucleus. In contrast, Wang and coworkers [40, 42] observed that GSH-capped Ag2S NCs and AgAu alloy NCs were distributed in both cytoplasm and the cellular nucleus of MC3T3-EI cells and CAL-27 cells. These differences in the intracellular localization of MNCs upon the internalization suggest that not only the surface ligands but also the cell types can influence their intracellular fate.
A large number of reports have also focused on tumor imaging in vivo currently. For example, Wu et al. [101] reported the first example of tumor imaging with BSA-AuNCs. Their in vivo tumor targeting and ex vivo imaging studies showed that these ultrasmall AuNCs were highly accumulated in the tumor areas (Fig. 7) due to the enhanced permeability and retention (EPR) effects. Sun and coworkers [58] achieved ferritin receptor-mediated targeting and bioimaging with far-red emitting paired AuNCs. These far-red luminescent AuNCs could act as an excellent probe for targeting ferritin receptor-overexpressed human Caco-2 cells and whole female nude mice body imaging with specific targeting to the kidney. In addition, renal-clearable NIR-emitting GSH-AuNCs and PEG-AuNCs have been reported for in vivo NIR tumor targeting of MCF-7 tumor-bearing mice [29, 82]. They not only exhibited efficient renal clearance and low reticuloendothelial system (RES) accumulation but also showed a much longer tumor retention time and faster normal tissue clearance.
(a) Fluorescence images of mice bearing an MDA-MB-45 tumor. Strong signal from AuNCs was observed in the tumor (marked by the red circle). The arrowheads indicated the tumor. (b) Ex vivo fluorescence image of the tumor tissue and the muscle tissue around the tumor from the mice used in A. Reprinted with permission from Ref. [101]
Furthermore, Wang et al. [37] reported the use of biosynthesized NIR-emitting AgNCs for in situ imaging cancer cells and tumors, which did not occur in normal cells and tissues. The same group also explored the possibility of imaging cancer cells through in situ self-biosynthesized ZnNCs [117]. Particularly, in vivo imaging of subcutaneous xenografted tumors in nude mice has also established the validity of this strategy for the rapid and precise target self-bioimaging of tumors by subcutaneous injections of zinc gluconate solutions, without significant dissemination to the surrounding normal tissues. Recently, they explored a facile and green strategy to in situ biosynthesize fluorescent CuNCs in cancer cells [38]. As shown in Fig. 8, it is evident that fluorescent CuNCs could be spontaneously biosynthesized in cancer cells for intracellular fluorescence imaging, which could not be biosynthesized in normal cells. More importantly, the relevant fluorescence intensity of the in situ biosynthesized CuNCs was reversibly and sensitively responsive to physiological temperature changes in MDA-MB-231 cancer cells. Besides, Liu et al. [61] successfully synthesized fluorescent human insulin–Au nanodots (NDs) for in vivo imaging of insulin metabolism. Investigations on mice ear and ex vivo assays on human fat tissues showed that cells with rich insulin receptors had higher uptake of administrated insulin.
Schematic illustration of the process for the spontaneous biosynthesis of fluorescent CuNCs in cancer cells for intracellular fluorescence imaging and temperature measurement. Reprinted with permission from Ref. [38]
For targeted imaging of cancer cells and tumors, MNCs are modified with specific recognition units such as folic acid (FA) and streptavidin. For example, targeted imaging of folate receptor (FR) positive oral carcinoma KB cells using FA-conjugated BSA-AuNCs has been reported [65, 104]. Tumor targeting and specific affinity of FA-conjugated AuNCs for FR over-expressed tumors facilitated the accumulation of AuNCs in the tumor site, which enhanced the fluorescence signal in the tumor site, enabling in vivo targeted imaging of tumors with high specificity and also the subsequent tumor therapy [45, 60, 102]. Recently, Wang et al. [83] reported the conjugation of PEI-AgNCs with FA for both in vitro and in vivo targeted imaging. Their results indicated that the clearance rate of FA-conjugated AgNCs in the tumor-bearing mice was much slower than that in the normal mice because the high affinity of FA to target tumors inhibited FA-AgNCs from being metabolized. Moreover, Chen et al. [118] synthesized core–shell structured multifunctional nanocarriers for targeted anticancer drug delivery, where FA-conjugated amphiphilic hyperbranched block copolymer was used as shell on the surface of AuNCs. The nanocarriers specifically targeted cancer cells because of the enhanced cell uptake mediated by FA moiety. Similarly, the multifunctional anticancer drug paclitaxel (PTX)-loaded AuNCs/FA-modified poly(DBAM-co-NAS-co-HEMA) (PDNH) core-satellites nanocomposites were fabricated, which possessed simultaneous cancer imaging, targeted drug delivery, and controlled anticancer drug release [119]. In vivo studies showed the selective accumulation of FA-conjugated nanocomposites in tumor tissues, and the drug delivery process could be continuously monitored by the imaging probes, AuNCs. Similarly, streptavidin-conjugated AuNCs have been reported to specifically label endogeneous biotin within human hepatoma cells (HepG2) using the specific interactions between streptavidin and biotin [49, 51].
Apart from FA and streptavidin, other functionalized molecules have also been used to conjugate with MNCs. For instance, Kong et al. [62] developed a multifunctional nanoprobe for simultaneous targeting and imaging of human colon carcinoma Caco-2 cells by conjugating vitamin B12 to the ribonuclease A–stabilized AuNCs. Chen et al. [120] fabricated a fluorescent nanoprobe capable of specifically targeting carcinoma cells and tumors by coupling methionine (Met) and an NIR organic fluorescent dye MPA to BSA-AuNCs (Au-Met-MPA). Cui et al. [121] synthesized well-defined AuNCs nanoassembly by the self-assembly of reduced AuNCs using GSH as linkers. The as-prepared nanoassembly displayed highly effective cellular uptake and precise tumor targeting for NIRFL imaging in vivo compared to that of individual AuNCs. Wang et al. [57] reported the fabrication of Tf-AuNCs/GO nanocomposite (Tf-AuNCs/GO) for turn-on NIR fluorescence bioimaging of transferrin receptor (TfR) over-expressed HeLa cells and HeLa tumor-bearing mice.
Duan et al. [122] applied chitosan grafted with N-acetyl-L-cysteine (NAC-CS) as the template to prepare NIR fluorescent AuNCs (AuNCs@NAC-CS), which possessed many advantages in cell imaging, such as low cytotoxicity, low sensitivity to tumor cells contents (H2O2 and protease), and long-time cell imaging. During in vivo experiments, the obvious fluorescence signal of AuNCs@NAC-CS appeared in the liver and kidney of the normal mice after 6 h injection. The ultrasmall NPs were efficiently cleared which overcomes the toxicity by nonspecific accumulation in healthy tissues/organs from renal in vivo [123]. Triphenylphosphonium (TPP), a kind of delocalized lipophilic cations capable of selectively accumulating into highly negatively charged mitochondria of living cells, has been employed in functionalizing chitosan–AuNC composites (AuNCs@CS-TPP) for targeted mitochondrial imaging in living cells [124]. By functionalizing of TAT peptide on the surface of AuNCs, multifunctional TAT peptide–AuNCs are designed for simultaneous fluorescence imaging as well as NIR light activated nucleus-targeting photodynamic therapy [125]. Recently, by combining biomineralization and supramolecular self-assembly of motif-designed peptide constructs containing an RGD sequence, Su et al. [69] have demonstrated the utility of AuNC-incorporated peptide nanofibers for targeted imaging of cancer cells.
Taking advantage of their good cellular imaging properties, metal NC-based composites have been developed for real-time imaging of important physiological events in the intracellular environment. For instance, a novel nanocomposite has been developed through a crown-like assembly of dye-encapsulated silica particles decorated with satellite AuNCs for imaging of highly reactive oxygen species (hROS) in live cells [126]. This composite exhibits single-excitation and dual-emission fluorescent properties, one emission at 565 nm originating from the AuNCs, which fluorescence can be quenched substantially by hROS, and the other at 435 nm arising from the silica particles acting as an internal reference (Fig. 9a). When the composite-loaded cells were incubated with H2O2, a kind of weak ROS, strong fluorescence signals at both the blue and the red channels remained constant (Fig. 9b). However, a remarkable change was observed in the fluorescence images when the composite-loaded cells were incubated with hROS, such as HClO and ONOO−, 3-morpholinosydnon-imine (SIN-1) can slowly releases ONOO−. Chen et al. [127] reported a dual-emission BSA-templated (cerium) CeAuNCs probe for ratiometric determination of local pH values inside cells. Recently, Pan et al. [128] used viscosity-sensitive GSH-AuNCs with diffusion-dependent emission for viscosity imaging in live cells. Nystatin can induce mitochondrial malfunction by causing structural changes or swelling of mitochondria, resulting in a sharp increase of viscosity in the cells [129, 130]. A remarkable fluorescence enhancement effect can be observed for the cells successively treated with nystatin and AuNCs.
(a) Schematic illustration of hROS detection using dye-encapsulated silica particles decorated with satellite AuNCs. (b) Confocal fluorescence microscopy images of HeLa cells after incubation with silica-AuNC composites for 1 h. Cells were (a) untreated or treated with (b) 1 mM H2O2 for 10 min, (c) 200 μM HClO for 5 min, and (d) 3 mM SIN-1 for 40 min. Reprinted with permission from Ref. [126]
Gao et al. [131] first developed ultrasmall chelator-free radioactive [64Cu]CuNCs using BSA as a scaffold for PET imaging in an orthotopic lung cancer model. By preconjugating tumor target peptide luteinizing hormone releasing hormone (LHRH) to the BSA shell, the prepared [64Cu]CuNC@BSA-LHRH showed high uptake in A549 human lung tumor, high radiolabeling stability, and rapid renal clearance characteristics. After injecting via tail vein into mice bearing orthotopic A549 lung tumors, the orthotopic A549 tumors of the left lung were clearly delineated with very little local background in the whole-body PET imaging of mice injected with [64Cu]CuNC@BSA-LHRH (Fig. 10b). It is noticeable, however, that a significant difference in [64Cu]CuNCs uptake between [64Cu]CuNC@BSA and [64Cu]CuNC@BSA-LHRH is observable after 0.5–4 h post-injection. The [64Cu]CuNC@BSA-LHRH was retained preferentially in the orthotopic lung tumor by combined active targeting and passive targeting after injection [132]. Although [64Cu]CuNC@BSA also showed partial tumor localization due to passive targeting by the effective EPR effect [132], most of the [64Cu]CuNC@BSA distributed in the kidney and bladder (Fig. 10a). In another study, Liu and coworkers [133] prepared 64Cu doped AuNCs (64CuAuNCs) functionalized with AMD3100 (or Plerixafor) for targeted PET imaging of CXCR4, an up-regulated receptor on primary tumor and lung metastasis in a mouse 4 T1 orthotopic breast cancer model. In contrast to the ligand tracer alone (64Cu–AMD3100) and NCs (64CuAuNCs) without the conjugation of AMD3100, the targeted 64CuAuNCs–AMD3100 exhibited higher sensitivity, better accuracy, and much earlier detection of CXCR4 expression in lung metastasis. Radionuclide 64Cu-doped alloy 64CuAuNCs have also be used as targeted probes for PET imaging in U87MG glioblastoma xenografted mice [134] and prostate cancer bearing mice [135].
In vivo PET imaging and biodistribution. Representative PET images of coronal single slices on orthotopic A549 lung tumor-bearing mice after intravenous injection of 6.7 MBq of [64Cu]CuNC@BSA (a) and [64Cu]CuNC@BSA-LHRH (b). Images were acquired at 0.5, 1, 2, and 4 h. White arrows indicate the lung tumor. Reprinted with permission from Ref. [131]
FLIM is a powerful technique for cell imaging, which can take advantage of MNCs that typically possess longer fluorescence lifetime than the life time of the autofluorescence from cellular organelles, and thus they can easily be imaged by using lifetime gating. Upon FLIM imaging, the researchers observed that AuNCs located near the cell membrane displayed longer lifetimes than those internalized inside the cells [11], indicating that FLIM imaging not only reveals the cellular uptake of AuNCs but also provides information on their different local environment. Later, based on the fact that the fluorescence intensity as well as the lifetime of DHLA–AuNCs is highly dependent on the temperature, Shang et al. [136] demonstrated the utilization of AuNC-based FLIM imaging for temperature sensing in live cells. As shown in Fig. 11, with increasing the temperature, the fluorescence lifetime decreased markedly from 970 ns at 14 °C to 670 ns at 43 °C, suggesting the potential of AuNC-based system for thermal sensing at the subcellular level via FLIM. In another report, Zhang and coworkers [47] demonstrated FLIM-based cellular imaging by using MSA- and tiopronin-capped AuNCs and further covalently bound PEG moieties to improve their capability of staining HeLa cells. Particularly, they observed that these PEGlyated AuNCs widely distribute throughout the cells and especially accumulate in the areas close to the cell nucleus. Irudayaraj et al. [137] reported the use of Herceptin-conjugated BSA-AuNCs (AuNCs-Her) for simultaneous imaging and enhanced cancer therapy because of its ability to induce nuclear DNA damage and apoptosis. Importantly, they found that the endocytosed AuNCs-Her could escape the endolysosomal pathway and enter the nucleus of cancer cells to enhance the therapeutic efficacy of Herceptin. FLIM indicated that almost all of the cells cultured with AuNCs-Her had specific fluorescence staining, representing DNA damage (Fig. 12d). In contrast, only a small amount of cells treated with Herceptin alone shows DNA damage under the same condition (Fig. 12e). Quantification of apoptosis positive cells as a percentage of the total number of cells revealed that only 35% of the cells treated with Herceptin underwent apoptosis due to DNA damage compared to 95% of the AuNCs-Her (Fig. 12f) treated cells.
Typical FLIM images of HeLa cells with internalized AuNCs at four different temperatures. Reprinted with permission from Ref. [136]
Fluorescence images show the apoptosis induced by AuNCs alone (a), AuNCs-Her (b), and Herceptin (c) by staining the nucleus with Hoechst 33258 (excited by UV light and the emission is 460 nm). FLIM shows DNA damage of SK-BR3 cells induced by AuNCs-Her (d) and Herceptin alone (e) indicated by the bright yellow dots. Quantitative evaluation of DNA damage of cells as a percentage of the total number of cells for different treatments (f). Reprinted with permission from Ref. [137]
The outstanding TPA cross sections of MNCs make them good candidates for application in two-photon cellular imaging, which is another attractive imaging technique because of its ability of imaging depth inside tissues and low phototoxicity of NIR light. Polavarapu and coworkers [28] investigated the two-photon excitation fluorescence imaging of SH-SY5Y human neuroblastoma cells incubated with GSH-AuNCs under excitation of femtoseond laser pulses at 800 nm. The two-photon imaging and z-stack sectioning results clearly confirmed that AuNCs were internalized inside the cells. Khandelia et al. [138] reported the use of anticancer drug doxorubicin (DOX) loaded BSA-AuNCs for imaging HeLa cells by two-photon excitation at 730 nm. Their results demonstrated that DOX-loaded AuNCs not only helped in tracking the delivery but also released drugs to the cancer cells, leading to apoptotic cell death (Fig. 13). In a recent work, Gu et al. [139] prepared RGD conjugated BSA-AuNC nano-capsules for two-photon fluorescence imaging of U87-MG cancer cells. The Z-stack sectioning of two-photon images revealed that hybrid nano-capsules were mainly resided in the cytoplasm nearby the nucleus.
A schematic illustration of the formation of DOX-loaded AuNC-embedded BSA nanoparticles, followed by uptake and release of DOX inside HeLa cells, leading to apoptotic cell death, as visualized by two-photon imaging. Reprinted with permission from Ref. [138]
4 Fluorescent MNCs as Multimodal Bioimaging Probes
At present, multimodal imaging probes based on fluorescent AuNCs for tumor imaging have also attracted plenty of attention (see the summary in Table 3). In an early work, Zhou et al. [146] reported multimodal imaging of NIR-emitting radioactive GSH-AuNPs, which were incorporated with a gold radioisotope 198Au. The 198Au in GSH-[198Au] AuNPs not only helps to quantify the pharmacokinetics of these NIR-emitting AuNPs rapidly but also allows their utility for in vivo SPECT imaging by emitting gamma rays. Thus these NIR-emitting radioactive AuNPs can serve as dual-modality imaging probes with both SPECT and FL imaging capabilities (Fig. 14). Chen and coworkers [147] recently fabricated a dual-modality FL/CT iodinated BSA-AuNCs for early accurate diagnosis of thyroid cancer. They accomplished in vivo FL and CT imaging via an orthotopic human thyroid cancer patient tissue derived xenograft (PDX) mouse model. Adopting the similar FL and CT dual-modal imaging techniques, insulin–AuNCs were used to distinguish the differentiated C2C12 myoblasts from undifferentiated ones [148]. Also, FA-conjugated GSH-AuNCs and lysozyme-AuNCs have been used for in vivo targeted dual-modal FL/CT imaging of MGC-803 tumor-bearing mice and HeLa tumor-bearing nude mice, respectively [30, 149]. Sarkar et al. [107] synthesized protein-capped AgNCs impregnated onto GO sheets for FLIM. Furthermore, AgNCs/GO assembly have a great potential as CT imaging contrasting agents, and CT images show significant contrast enhancement of bone tissues in mice models.
Representative SPECT images (top row) of BALB/c mice injected with GSH-[198Au] AuNPs. (a) 10 min, (b) 1 h, (c) 4 h, and (d) 24 h p.i.. In vivo FL imaging (bottom row) of a live mouse (e) pre-injection, and (f) 5 min, (g) 20 min, (h) 1 h, (i) 24 h after IV injection of GSH-[198Au] AuNPs. Reprinted with permission from Ref. [146]
NIRFL and MR dual-modal imaging have been reported through coupling AuNCs with magnetic agents such as Gd2O3 and Fe3O4 NPs. For example, Sun et al. [150] employed Gd2O3 functionalized BSA-AuNCs as probes for dual-modal NIRFL and MR blood pool imaging in vivo. By further bioconjugation of BSA-Gd2O3/AuNCs with arginine–glycine–aspartic acid peptide (RGD), they can be used for in vivo targeted tumor imaging of U87-MG tumor-bearing mice. Liang and coworkers [151] constructed Gd3+-functionalized AuNCs for dual-model NIRFL/MR imaging by using a cyclodecapeptide as the template. Recently, Wang et al. [152] demonstrated a facile strategy of fabricating GSH-AuNC probes decorated with magnetic Fe3O4 NPs for bimodal NIRFL/MR cell imaging. Alternatively, dual-modal bioimaging probes can be fabricated by conjugating biotinylated NIR fluorescent BSA-AuNCs to streptavidin functionalized Fe3O4 NPs [153].
At present, multifunctional theranostic systems with strong clinical imaging-guided capability, phototherapy function, and target specificity have been developed for cancer therapy. Yang et al. [154] fabricated a new imaging-guided and multifunctional cancer therapy platform with multimodal imaging and dual phototherapy function by assembling the captopril-protected Au25NCs (Au25(Capt)18−) into mesoporous silica-coated Nd3+-sensitized upconversion nanoparticles (UCNPs@SiO2). Under 808 nm NIR irradiation, the UCNPs@SiO2-Au25(Capt)18− nanocomposite can simultaneously exhibit tri-modal upconversion luminescence, photothermal, and photoacoustic imaging features in vivo. Besides, the composite can also present the MR and CT imaging effects due to the Gd3+ and Yb3+ ions in the UCNPs. Subsequently, the same group designed Fe3O4@ZIF-8-Au25(Capt)18− nanocomposites for multimodal imaging and synergistic cancer therapy [155]. Under 808 nm NIR irradiation, the attached photosensitizer agent Au25(Capt)18− clusters can produce highly reactive singlet oxygen (1O2) for photodynamic therapy (PDT). In addition, the magnetic properties of encapsulated Fe3O4 nanocrystals can simultaneously produce hyperthermal effects for photothermal therapy (PTT) and present targeting and MR imaging capability. Protein-based multifunctional nanocarriers (MFNCs) were successfully constructed by assembling gold nanorods, superparamagnetic iron oxide NPs, and AuNCs within BSA (Fig. 15) [156], without affecting their individual properties. The MFNCs showed simultaneous integration of corresponding plasmonic, magnetic, and luminescence properties, which can be used for plasmonic photothermal therapy (PPTT), two-photon and MR imaging in vitro. Moreover, the MFNCs demonstrated efficient loading and delivery of DOX to HeLa cells, resulting in efficient killing of cancer cells and tracking the delivery and release of the drug through confocal fluorescence microscopy.
Schematic depiction of preparing MFNCs, using for plasmonic photothermal therapy and two-photon/MR imaging in vitro, following successful loading and delivery of anticancer drug Dox induced cancer cells death. Reprinted with permission from Ref. [156]
In addition to the NIRFL and MRI contrasts offered by the probe, the green fluorescence of the endoperoxide triggered by 1O2 can provide additional modality for live cell imaging [153]. With the co-existence of GSH-AuNCs and Gd3+ ions, the nanoprobes can act as a multifunctional nanoplatform for triple-modal NIRFL/CT/MR imaging of A549 cancer cells and xenografted A549 tumor models [157]. Similarly, Hu and coworkers [158] prepared Au–Gd NC hybrids by using albumin as the stabilizer, which were suitable for in vivo triple-modal NIRFL/CT/MRI imaging of MCF-7 tumor-bearing mice (Fig. 16). Upon intravenously injected, the hybrid NCs were effectively accumulated in tumor tissues and quickly cleared by renal excretion, indicating their capacity of tumor targeting and low body residues. Recently, Wang and coworkers [159] developed a facile approach to construct BSA-stabilized Gd2O3-AuNCs nanoplatform for multimodal imaging and cancer therapy. The nanocomposites exhibit photoluminescent capability in NIR region, and are able to generate singlet oxygen (1O2) species under NIR laser irradiation at 808 nm for photodynamic therapy. After loading indocyanine green (ICG), the Gd2O3-AuNCs-ICG nanocomposites exhibited excellent in vivo triple-modal NIRFL/MR/CT imaging capability, as well as combined photodynamic and photothermal therapy. Wang et al. [160] reported a new method for targeted multimodal tumor bioimaging by using in situ self-biosynthesized AuNCs and iron complexes composites via simple introduction of AuCl4− (i.e., HAuCl4) and Fe2+ (i.e., FeCl2) ions to the cancer cells or xenograft tumor mice model. In a recent study, the same group [39] explored a novel in vivo multimodal NIRFL/MR/CT bioimaging method for the early detection of tumors based on in situ biosynthesized Zn&Fe oxide NCs. By introducing Zn2+ and Fe2+ ions via a single injection, fluorescent ZnO NCs and superparamagnetic Fe3O4 nanoparticles can be spontaneously self-biosynthesized in tumor cells/tissues. Xu et al. [161] synthesized AuNC-Gd2O3 integrated nanoprobe (denoted as AuGds) using BSA as the template via a biomineralization approach. After being chemically modified with FA, the FA-AuGds could specifically target FRs on KB tumor cells, and permitted in vivo NIRFL, MR, and CT imaging of xenografted KB tumor-bearing mice. Gd3+-aggregated AuNCs encapsulated by SiO2 shell (Gd3+-AuNCs@SiO2 NPs) were strategically designed and prepared. In the presence of Gd3+ ions, the GSH-capped AuNCs show aggregation-induced fluorescence (AEF) effect. The as-prepared composites can be used for in vitro and in vivo multimodal FL/MR/CT cancer imaging [162]. Hembury and coworkers [163] synthesized highly monodispersed SiO2/AuNCs by nucleating gold within hollow mesoporous silica particles in a one-phase synthetic route. These SiO2/AuNCs possessed stable NIR fluorescence and paramagnetism, thus it could be used as a promising probe for in vivo NIRFL/PAI/MR imaging of colorectal carcinoma tumor (LS174T)-bearing mice.
(a) (a and b) In vivo FL imaging of MCF-7 tumor-bearing mice after the tail-vein injection of hybrid NCs. Inset image is the FL reflectance images of urine. (b) In vivo CT images of MCF-7 tumor-bearing mice injected with the hybrid Au–Gd NCs. The arrow and red dotted circle indicate the tumor site. (c) (a and b) In vivo MRI images of MCF-7 tumor-bearing mice injected with the hybrid Au–Gd NCs. The arrow and red dotted circle indicate the tumor (a) and bladder (b) sites, respectively. Reprinted with permission from Ref. [158]
5 Conclusions and Outlooks
In this chapter, we have systematically summarized recent advances in the synthesis strategies and bioimaging applications of fluorescent MNCs. In the past few years, fluorescent MNCs have been largely explored for bioimaging due to their ultrasmall size, good biocompatibility, and easy functionalization. Although a large number of researches have been reported about MNCs currently, there are still a lot of rooms to further improve and many unclear questions to reveal.
First of all, most MNCs possess a relatively low QY (usually less than 10%) in comparison to other fluorophores such as semiconductor QDs and many organic dyes. In addition, MNCs often show size heterogeneity in the crude product, and it still remains challenging to obtain atomically precise water-soluble MNCs suitable for bioimaging applications, which markedly precludes quantitative tracking in organisms. Therefore, researchers still need to make greater effort to explore more efficient synthesis routes for size-controllable fluorescent MNCs with relatively high QY and high purity [165, 166]. Second, the present bioimaging studies mainly concentrate on fluorescent AuNCs due to their good stability and easy synthesis. Considering gold is relatively expensive compared to other metals, it would be attractive to further exploit potential bioimaging applications of other MNCs or alloy NCs. Third, up to now, relatively little is known about the behavior of these ultrasmall MNCs within the complex biological environment [167], which is actually highly important regarding the safe as well as efficient use of MNCs in bioimaging applications. Thus, further study to understand the mechanism of cellular and intravital uptake of MNCs and long-term effect after entering into biosystems would be necessary and important. Furthermore, in order to advance potential utility of MNCs as multifunctional probes for applications besides imaging, more types of MNCs-based nanocomposites should be developed by integrating other functional nanomaterials.
In the past years, significant progress has been achieved in developing fluorescent MNCs for bioimaging, but many challenges still remain to face and resolve in the future. With continuing development and more efforts within the community, we believe that more robust fluorescent MNCs will be available, which will then further advance imaging-based applications of these novel nanoprobes in medical diagnose and therapy researches.
References
Díez I, Ras RHA (2010) Few-atom silver clusters as fluorescent reporters. In: Advanced fluorescence reporters in chemistry and biology II. Springer, Dordrecht, pp 307–332
Zheng J, Nicovich PR, Dickson RM (2007) Highly fluorescent noble-metal quantum dots. Annu Rev Phys Chem 58:409–431
Díez I, Ras RHA (2011) Fluorescent silver nanoclusters. Nanoscale 3:1963–1970
He X, Wang K, Cheng Z (2010) In vivo near-infrared fluorescence imaging of cancer with nanoparticle-based probes. WIREs Nanomed Nanobiotechnol 2:349–366
Song F, Liang R, Deng J, Liu Z, Peng X (2017) Fine-tailoring the linker of near-infrared fluorescence probes for nitroreductase imaging in hypoxic tumor cells. Chin Chem Lett 28:1997–2000
Park H, Crozier KB (2013) Multispectral imaging with vertical silicon nanowires. Sci Rep 3:2460
He X, Gao J, Gambhir SS, Cheng Z (2010) Near-infrared fluorescent nanoprobes for cancer molecular imaging: status and challenges. Trends Mol Med 16:574–583
Weng J, Ren J (2006) Luminescent quantum dots: a very attractive and promising tool in biomedicine. Curr Med Chem 13:897–909
Berezin MY, Achilefu S (2010) Fluorescence lifetime measurements and biological imaging. Chem Rev 110:2641–2684
Helmchen F, Denk W (2005) Deep tissue two-photon microscopy. Nat Methods 2:932–940
Shang L, Azadfar N, Stockmar F, Send W, Trouillet V, Bruns M, Gerthsen D, Nienhaus GU (2011) One-pot synthesis of near-infrared fluorescent gold clusters for cellular fluorescence lifetime imaging. Small 7:2614–2620
Yuan L, Lin W, Zheng K, He L, Huang W (2013) Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem Soc Rev 42:622–661
Kim HM, Jung C, Kim BR, Jung SY, Hong JH, Ko YG, Lee KJ, Cho BR (2007) Environment-sensitive two-photon probe for intracellular free magnesium ions in live tissue. Angew Chem Int Ed 46:3460–3463
Zipfel WR, Williams RM, Webb WW (2003) Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol 21:1369–1377
Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC (2012) Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev 41:2656–2672
Li DZ, Chen HD, Bi F, Wang ZX (2016) Progress of multimodal molecular imaging technology in diagnosis of tumor. Chin J Anal Chem 44:1609–1618
Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS (2008) Molecular imaging in drug development. Nature Rev Drug Discov 7:591–607
Zhou B, Zheng L, Peng C, Li D, Li J, Wen S, Shen M, Zhang G, Shi X (2014) Synthesis and characterization of PEGylated polyethylenimine-entrapped gold nanoparticles for blood pool and tumor CT imaging. ACS Appl Mater Interfaces 6:17190–17199
Peng C, Qin J, Zhou B, Chen Q, Shen M, Zhu M, Lu X, Shi X (2013) Targeted tumor CT imaging using folic acid-modified PEGylated dendrimer-entrapped gold nanoparticles. Polym Chem 4:4412–4424
Zhou J, Lu Z, Shan G, Wang S, Liao Y (2014) Gadolinium complex and phosphorescent probe-modified NaDyF4 nanorods for T1- and T2-weighted MRI/CT/phosphorescence multimodality imaging. Biomaterials 35:368–377
Kim J, Piao Y, Hyeon T (2009) Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem Soc Rev 38:372–390
Tsotsalas M, Busby M, Gianolio E, Aime S, De Cola L (2008) Functionalized nanocontainers as dual magnetic and optical probes for molecular imaging applications. Chem Mater 20:5888–5893
Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J (2007) Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med 13:95–99
Michaelis J, Hettich C, Mlynek J, Sandoghdar V (2000) Optical microscopy using a single-molecule light source. Nature 405:325–328
Louie A (2010) Multimodality imaging probes: design and challenges. Chem Rev 110:3146–3195
Lu Y, Chen W (2012) Sub-nanometre sized metal clusters: from synthetic challenges to the unique property discoveries. Chem Soc Rev 41:3594–3623
Jin R (2010) Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2:343–362
Polavarapu L, Manna M, Xu QH (2011) Biocompatible glutathione capped gold clusters as one- and two-photon excitation fluorescence contrast agents for live cells imaging. Nanoscale 3:429–434
Liu J, Yu M, Zhou C, Yang S, Ning X, Zheng J (2013) Passive tumor targeting of renal-clearable luminescent gold nanoparticles: long tumor retention and fast normal tissue clearance. J Am Chem Soc 135:4978–4981
Zhang C, Zhou Z, Qian Q, Gao G, Li C, Feng L, Wang Q, Cui D (2013) Glutathione-capped fluorescent gold nanoclusters for dual-modal fluorescence/X-ray computed tomography imaging. J Mater Chem B 1:5045–5053
Yang J, Xia N, Wang X, Liu X, Xu A, Wu Z, Luo Z (2015) One-pot one-cluster synthesis of fluorescent and bio-compatible Ag14 nanoclusters for cancer cell imaging. Nanoscale 7:18464–18470
Pyo K, Thanthirige VD, Kwak K, Pandurangan P, Ramakrishna G, Lee D (2015) Ultrabright luminescence from gold nanoclusters: rigidifying the Au(I)-thiolate shell. J Am Chem Soc 137:8244–8250
Li Y, Wang X, Xu S, Xu W (2013) The solvent effect on the luminescence of silver nanoclusters. Phys Chem Chem Phys 15:2665–2668
Wang C, Wu J, Jiang K, Humphrey MG, Zhang C (2017) Stable Ag nanoclusters-based nano-sensors: rapid sonochemical synthesis and detecting Pb2+ in living cells. Sens Actuators B Chem 238:1136–1143
Wang C, Ling L, Yao Y, Song Q (2015) One-step synthesis of fluorescent smart thermo-responsive copper clusters: a potential nanothermometer in living cells. Nano Res 8:1975–1986
Wang J, Zhang G, Li Q, Jiang H, Liu C, Amatore C, Wang X (2013) In vivo self-bio-imaging of tumors through in situ biosynthesized fluorescent gold nanoclusters. Sci Rep 3:1157
Gao S, Chen D, Li Q, Ye J, Jiang H, Amatore C, Wang X (2014) Near-infrared fluorescence imaging of cancer cells and tumors through specific biosynthesis of silver nanoclusters. Sci Rep 4:4384
Ye J, Dong X, Jiang H, Wang X (2017) An intracellular temperature nanoprobe based on biosynthesized fluorescent copper nanoclusters. J Mater Chem B 5:691–696
Du T, Zhao C, ur Rehman F, Lai L, Li X, Sun Y, Luo S, Jiang H, Selke M, Wang X (2017) Rapid and multimodal in vivo bioimaging of cancer cells through in situ biosynthesis of Zn&Fe nanoclusters. Nano Res 10:2626–2632
Wang C, Wang Y, Xu L, Zhang D, Liu M, Li X, Sun H, Lin Q, Yang B (2012) Facile aqueous-phase synthesis of biocompatible and fluorescent Ag2S nanoclusters for bioimaging: tunable photoluminescence from red to near infrared. Small 8:3137–3142
Ding C, Cao X, Zhang C, He T, Hua N, Xian Y (2017) Rare earth ions enhanced near infrared fluorescence of Ag2S quantum dots for the detection of fluoride ions in living cells. Nanoscale 9:14031–14038
Wang C, Xu L, Xu X, Cheng H, Sun H, Lin Q, Zhang C (2014) Near infrared Ag/Au alloy nanoclusters: tunable photoluminescence and cellular imaging. J Colloid Interface Sci 416:274–279
Sun Y, Xia Y (2004) Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium. J Am Chem Soc 126:3892–3901
Shang L, Yang L, Stockmar F, Popescu R, Trouillet V, Bruns M, Gerthsen D, Nienhaus GU (2012) Microwave-assisted rapid synthesis of luminescent gold nanoclusters for sensing Hg2+ in living cells using fluorescence imaging. Nanoscale 4:4155–4160
Nair LV, Nazeer SS, Jayasree RS, Ajayaghosh A (2015) Fluorescence imaging assisted photodynamic therapy using photosensitizer-linked gold quantum clusters. ACS Nano 9:5825–5832
Ghosh R, Goswami U, Ghosh SS, Paul A, Chattopadhyay A (2015) Synergistic anticancer activity of fluorescent copper nanoclusters and cisplatin delivered through a hydrogel nanocarrier. ACS Appl Mater Interfaces 7:209–222
Zhang J, Fu Y, Conroy CV, Tang Z, Li G, Zhao RY, Wang G (2012) Fluorescence intensity and lifetime cell imaging with luminescent gold nanoclusters. J Phys Chem C 116:26561–26569
Pan S, Liu W, Tang J, Yang Y, Feng H, Qian Z, Zhou J (2018) Hydrophobicity-guided self-assembled particles of silver nanoclusters with aggregation-induced emission and their use in sensing and bioimaging. J Mater Chem B 6:3927–3933
Muhammed MAH, Verma PK, Pal SK, Kumar RCA, Paul S, Omkumar RV, Pradeep T (2009) Bright, NIR-emitting Au23 from Au25: characterization and applications including biolabeling. Chem Eur J 15:10110–10120
Le Guével X, Spies C, Daum N, Jung G, Schneider M (2012) Highly fluorescent silver nanoclusters stabilized by glutathione: a promising fluorescent label for bioimaging. Nano Res 5:379–387
Lin CAJ, Yang TY, Lee CH, Huang SH, Sperling RA, Zanella M, Li JK, Shen JL, Wang HH, Yeh HI (2009) Synthesis, characterization, and bioconjugation of fluorescent gold nanoclusters toward biological labeling applications. ACS Nano 3:395–401
Wang HH, Lin CAJ, Lee CH, Lin YC, Tseng YM, Hsieh CL, Chen CH, Tsai CH, Hsieh CT, Shen JL, Chan WH, Chang WH, Yeh HI (2011) Fluorescent gold nanoclusters as a biocompatible marker for in vitro and in vivo tracking of endothelial cells. ACS Nano 5:4337–4344
Shang L, Dong S, Nienhaus GU (2011) Ultra-small fluorescent metal nanoclusters: synthesis and biological applications. Nano Today 6:401–418
Shang L, Nienhaus GU (2015) Biomineralization: nanocrystals by design. Nat Chem 7:769–770
Xie J, Zheng Y, Ying JY (2009) Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc 131:888–889
Yu X, Liu W, Deng X, Yan S, Su Z (2018) Gold nanocluster embedded bovine serum albumin nanofibers-graphene hybrid membranes for the efficient detection and separation of mercury ion. Chem Eng J 335:176–184
Wang Y, Chen JT, Yan XP (2013) Fabrication of transferrin functionalized gold nanoclusters/graphene oxide nanocomposite for turn-on near-infrared fluorescent bioimaging of cancer cells and small animals. Anal Chem 85:2529–2535
Sun C, Yang H, Yuan Y, Tian X, Wang L, Guo Y, Xu L, Lei J, Gao N, Anderson GJ, Liang XJ, Chen C, Zhao Y, Nie G (2011) Controlling assembly of paired gold clusters within apoferritin nanoreactor for in vivo kidney targeting and biomedical imaging. J Am Chem Soc 133:8617–8624
Le Guevel X, Daum N, Schneider M (2011) Synthesis and characterization of human transferrin-stabilized gold nanoclusters. Nanotechnology 22:275103
Liu JM, Chen JT, Yan XP (2013) Near infrared fluorescent trypsin stabilized gold nanoclusters as surface plasmon enhanced energy transfer biosensor and in vivo cancer imaging bioprobe. Anal Chem 85:3238–3245
Liu CL, Liu TM, Hsieh TY, Liu HW, Chen YS, Tsai CK, Chen HC, Lin JW, Hsu RB, Wang TD, Chen CC, Sun CK, Chou PT (2013) In vivo metabolic imaging of insulin with multiphoton fluorescence of human insulin-Au nanodots. Small 9:2103–2110
Kong Y, Chen J, Gao F, Brydson R, Johnson B, Heath G, Zhang Y, Wu L, Zhou D (2013) Near-infrared fluorescent ribonuclease-A-encapsulated gold nanoclusters: preparation, characterization, cancer targeting and imaging. Nanoscale 5:1009–1017
Zhao T, He XW, Li WY, Zhang YK (2015) Transferrin-directed preparation of red-emitting copper nanoclusters for targeted imaging of transferrin receptor over-expressed cancer cells. J Mater Chem B 3:2388–2394
Liu F, Bing T, Shangguan D, Zhao M, Shao N (2016) Ratiometric fluorescent biosensing of hydrogen peroxide and hydroxyl radical in living cells with lysozyme-silver nanoclusters: lysozyme as stabilizing ligand and fluorescence signal unit. Anal Chem 88:10631–10638
Muhammed MAH, Verma PK, Pal SK, Retnakumari A, Koyakutty M, Nair S, Pradeep T (2010) Luminescent quantum clusters of gold in bulk by albumin-induced core etching of nanoparticles: metal ion sensing, metal-enhanced luminescence, and biolabeling. Chem Eur J 16:10103–10112
Yuan Q, Wang Y, Zhao L, Liu R, Gao F, Gao L, Gao X (2016) Peptide protected gold clusters: chemical synthesis and biomedical applications. Nanoscale 8:12095–12104
Yu X, Wang Z, Su Z, Wei G (2017) Design, fabrication, and biomedical applications of bioinspired peptide–inorganic nanomaterial hybrids. J Mater Chem B 5:1130–1142
Wang Y, Cui Y, Zhao Y, Liu R, Sun Z, Li W, Gao X (2012) Bifunctional peptides that precisely biomineralize Au clusters and specifically stain cell nuclei. Chem Commun 48:871–873
Zhang W, Lin D, Wang H, Li J, Nienhaus GU, Su Z, Wei G, Shang L (2017) Supramolecular self-assembly bioinspired synthesis of luminescent gold nanocluster-embedded peptide nanofibers for temperature sensing and cellular imaging. Bioconjug Chem 28:2224–2229
Pitchiaya S, Krishnan Y (2006) First blueprint, now bricks: DNA as construction material on the nanoscale. Chem Soc Rev 35:1111–1121
Richards CI, Choi S, Hsiang JC, Antoku Y, Vosch T, Bongiorno A, Tzeng YL, Dickson RM (2008) Oligonucleotide-stabilized Ag nanocluster fluorophores. J Am Chem Soc 130:5038–5039
Sharma J, Yeh HC, Yoo H, Werner JH, Martinez JS (2010) A complementary palette of fluorescent silver nanoclusters. Chem Commun 46:3280–3282
Shukla S, Sastry M (2009) Probing differential Ag+−nucleobase interactions with isothermal titration calorimetry (ITC): towards patterned DNA metallization. Nanoscale 1:122–127
Soto-Verdugo V, Metiu H, Gwinn E (2010) The properties of small Ag clusters bound to DNA bases. J Chem Phys 132:195102
Schultz D, Gwinn E (2011) Stabilization of fluorescent silver clusters by RNA homopolymers and their DNA analogs: C,G versus A,T(U) dichotomy. Chem Commun 47:4715–4717
Vosch T, Antoku Y, Hsiang JC, Richards CI, Gonzalez JI, Dickson RM (2007) Strongly emissive individual DNA-encapsulated Ag nanoclusters as single-molecule fluorophores. Proc Natl Acad Sci U S A 104:12616–12621
Antoku Y, Hotta J, Mizuno H, Dickson RM, Hofkens J, Vosch T (2010) Transfection of living HeLa cells with fluorescent poly-cytosine encapsulated Ag nanoclusters. Photochem Photobiol Sci 9:716–721
Ai J, Guo W, Li B, Li T, Li D, Wang E (2012) DNA G-quadruplex-templated formation of the fluorescent silver nanocluster and its application to bioimaging. Talanta 88:450–455
Yin J, He X, Wang K, Qing Z, Wu X, Shi H, Yang X (2012) One-step engineering of silver nanoclusters-aptamer assemblies as luminescent labels to target tumor cells. Nanoscale 4:110–112
Yin J, He X, Wang K, Xu F, Shangguan J, He D, Shi H (2013) Label-free and turn-on aptamer strategy for cancer cells detection based on a DNA-silver nanocluster fluorescence upon recognition-induced hybridization. Anal Chem 85:12011–12019
Huang X, Luo Y, Li Z, Li B, Zhang H, Li L, Majeed I, Zou P, Tan B (2011) Biolabeling hematopoietic system cells using near-infrared fluorescent gold nanoclusters. J Phys Chem C 115:16753–16763
Liu J, Yu M, Ning X, Zhou C, Yang S, Zheng J (2013) PEGylation and zwitterionization: pros and cons in the renal clearance and tumor targeting of near-IR-emitting gold nanoparticles. Angew Chem Int Ed 52:12572–12576
Wang Y, Dai C, Yan XP (2014) Fabrication of folate bioconjugated near-infrared fluorescent silver nanoclusters for targeted in vitro and in vivo bioimaging. Chem Commun 50:14341–14344
Tanaka S, Miyazaki J, Tiwari DK, Jin T, Inouye Y (2011) Fluorescent platinum nanoclusters: synthesis, purification, characterization, and application to bioimaging. Angew Chem Int Ed 50:431–435
Huang X, Ishitobi H, Inouye Y (2016) Formation of fluorescent platinum nanoclusters using hyper-branched polyethylenimine and their conjugation to antibodies for bio-imaging. RSC Adv 6:9709–9716
Yang L, Wang H, Li D, Li L, Lou X, Liu H (2018) Self-nucleation and self-assembly of highly fluorescent Au5 nanoclusters for bioimaging. Chem Mater 30:5507–5515
Yam VWW, Cheng ECC, Zhou ZY (2000) A highly soluble luminescent decanuclear gold(I) complex with a propeller-shaped structure. Angew Chem Int Ed 39:1683–1685
Das NK, Ghosh S, Priya A, Datta S, Mukherjee S (2015) Luminescent copper nanoclusters as a specific cell-imaging probe and a selective metal ion sensor. J Phys Chem C 119:24657–24664
Kong L, Chu X, Liu W, Yao Y, Zhu P, Ling X (2016) Glutathione-directed synthesis of Cr(vi)-and temperature-responsive fluorescent copper nanoclusters and their applications in cellular imaging. New J Chem 40:4744–4750
Ge W, Zhang Y, Ye J, Chen D, Rehman FU, Li Q, Chen Y, Jiang H, Wang X (2015) Facile synthesis of fluorescent Au/Ce nanoclusters for high-sensitive bioimaging. J Nanobiotechnol 13:8
Huang H, Li H, Feng JJ, Wang AJ (2016) One-step green synthesis of fluorescent bimetallic Au/Ag nanoclusters for temperature sensing and in vitro detection of Fe3+. Sens Actuators B Chem 223:550–556
Wang P, Lin L, Guo Z, Chen J, Tian H, Chen X, Yang H (2016) Highly fluorescent gene carrier based on Ag-Au alloy nanoclusters. Macromol Biosci 16:160–167
Desai ML, Jha S, Basu H, Singhal RK, Sharma PK, Kailasa SK (2018) Chicken egg white and L-cysteine as cooperative ligands for effective encapsulation of Zn-doped silver nanoclusters for sensing and imaging applications. Colloid Surface A 559:35–42
Shang L, Dörlich RM, Trouillet V, Bruns M, Nienhaus GU (2012) Ultrasmall fluorescent silver nanoclusters: protein adsorption and its effects on cellular responses. Nano Res 5:531–542
Cao H, Chen Z, Zheng H, Huang Y (2014) Copper nanoclusters as a highly sensitive and selective fluorescence sensor for ferric ions in serum and living cells by imaging. Biosens Bioelectron 62:189–195
Liu CL, Ho ML, Chen YC, Hsieh CC, Lin YC, Wang YH, Yang MJ, Duan HS, Chen BS, Lee JF, Hsiao JK, Chou PT (2009) Thiol-functionalized gold nanodots: two-photon absorption property and imaging in vitro. J Phys Chem C 113:21082–21089
Bian P, Zhou J, Liu Y, Ma Z (2013) One-step fabrication of intense red fluorescent gold nanoclusters and their application in cancer cell imaging. Nanoscale 5:6161–6166
Shang L, Dorlich RM, Brandholt S, Schneider R, Trouillet V, Bruns M, Gerthsen D, Nienhaus GU (2011) Facile preparation of water-soluble fluorescent gold nanoclusters for cellular imaging applications. Nanoscale 3:2009–2014
Hu S, Ye B, Yi X, Cao Z, Wu D, Shen C, Wang J (2016) Dumbbell-shaped metallothionein-templated silver nanoclusters with applications in cell imaging and Hg2+ sensing. Talanta 155:272–277
Chandirasekar S, Chandrasekaran C, Muthukumarasamyvel T, Sudhandiran G, Rajendiran N (2015) Sodium cholate-templated blue light-emitting Ag subnanoclusters: in vivo toxicity and imaging in zebrafish embryos. ACS Appl Mater Interfaces 7:1422–1430
Wu X, He X, Wang K, Xie C, Zhou B, Qing Z (2010) Ultrasmall near-infrared gold nanoclusters for tumor fluorescence imaging in vivo. Nanoscale 2:2244–2249
Chen H, Li S, Li B, Ren X, Li S, Mahounga DM, Cui S, Gu Y, Achilefu S (2012) Folate-modified gold nanoclusters as near-infrared fluorescent probes for tumor imaging and therapy. Nanoscale 4:6050–6064
Le Guével X, Hötzer B, Jung G, Schneider M (2011) NIR-emitting fluorescent gold nanoclusters doped in silica nanoparticles. J Mater Chem 21:2974–2981
Retnakumari A, Setua S, Menon D, Ravindran P, Muhammed H, Pradeep T, Nair S, Koyakutty M (2010) Molecular-receptor-specific, non-toxic, near-infrared-emitting Au cluster-protein nanoconjugates for targeted cancer imaging. Nanotechnology 21:055103
Sarparast M, Noori A, Ilkhani H, Bathaie SZ, El-Kady MF, Wang LJ, Pham H, Marsh KL, Kaner RB, Mousavi MF (2016) Cadmium nanoclusters in a protein matrix: synthesis, characterization, and application in targeted drug delivery and cellular imaging. Nano Res 9:3229–3246
Pandya A, Tripathi A, Purohit R, Singh S, Nandasiri MI, Karakoti A, Singh SP, Shanker R (2015) Fluorescent magnesium nanocomplex in a protein scaffold for cell nuclei imaging applications. RSC Adv 5:94236–94240
Kundu N, Mukherjee D, Maiti TK, Sarkar N (2017) Protein-guided formation of silver nanoclusters and their assembly with graphene oxide as an improved bioimaging agent with reduced toxicity. J Phys Chem Lett 8:2291–2297
Wang Y, Cui Y, Liu R, Wei Y, Jiang X, Zhu H, Gao L, Zhao Y, Chai Z, Gao X (2013) Blue two-photon fluorescence metal cluster probe precisely marking cell nuclei of two cell lines. Chem Commun 49:10724–10726
Ghosh R, Sahoo AK, Ghosh SS, Paul A, Chattopadhyay A (2014) Blue-emitting copper nanoclusters synthesized in the presence of lysozyme as candidates for cell labeling. ACS Appl Mater Interfaces 6:3822–3828
Tian L, Li Y, Ren T, Tong Y, Yang B, Li Y (2017) Novel bimetallic gold-silver nanoclusters with “Synergy”-enhanced fluorescence for cyanide sensing, cell imaging and temperature sensing. Talanta 170:530–539
Wang X, Wang Y, He H, Ma X, Chen Q, Zhang S, Ge B, Wang S, Nau WM, Huang F (2017) Deep-red fluorescent gold nanoclusters for nucleoli staining: real-time monitoring of the nucleolar dynamics in reverse transformation of malignant cells. ACS Appl Mater Interfaces 9:17799–17806
Li J, Zhong X, Cheng F, Zhang JR, Jiang LP, Zhu JJ (2012) One-pot synthesis of aptamer-functionalized silver nanoclusters for cell-type-specific imaging. Anal Chem 84:4140–4146
Zhu X, Shi H, Shen Y, Zhang B, Zhao J, Li G (2015) A green method of staining DNA in polyacrylamide gel electrophoresis based on fluorescent copper nanoclusters synthesized in situ. Nano Res 8:2714–2720
Tian H, Guo Z, Chen J, Lin L, Xia J, Dong X, Chen X (2012) PEI conjugated gold nanoparticles: efficient gene carriers with visible fluorescence. Adv Healthc Mater 1:337–341
Li Y, Feng L, Yan W, Hussain I, Su L, Tan B (2019) PVP-templated highly luminescent copper nanoclusters for sensing trinitrophenol and living cell imaging. Nanoscale 11:1286–1294
Shang L, Yang L, Seiter J, Heinle M, Brenner-Weiss G, Gerthsen D, Nienhaus GU (2014) Nanoparticles interacting with proteins and cells: a systematic study of protein surface charge effects. Adv Mater Interfaces 1:1300079
Su M, Ye J, Li Q, Ge W, Zhang Y, Jiang H, Amatore C, Wang X (2015) In vivo accurate target bio-marking of tumors through in situ biosynthesized fluorescent zinc nanoclusters. RSC Adv 5:74844–74849
Chen T, Xu S, Zhao T, Zhu L, Wei D, Li Y, Zhang H, Zhao C (2012) Gold nanocluster-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. ACS Appl Mater Interfaces 4:5766–5774
Chen D, Luo Z, Li N, Lee JY, Xie J, Lu J (2013) Amphiphilic polymeric nanocarriers with luminescent gold nanoclusters for concurrent bioimaging and controlled drug release. Adv Funct Mater 23:4324–4331
Chen H, Li B, Ren X, Li S, Ma Y, Cui S, Gu Y (2012) Multifunctional near-infrared-emitting nano-conjugates based on gold clusters for tumor imaging and therapy. Biomaterials 33:8461–8476
Cui HD, Hu DH, Zhang JN, Gao GH, Zheng CF, Gong P, Xi XH, Sheng ZH, Cai LT (2017) Theranostic gold cluster nanoassembly for simultaneous enhanced cancer imaging and photodynamic therapy. Chin Chem Lett 28:1391–1398
Duan Y, Duan R, Liu R, Guan M, Chen W, Ma J, Chen M, Du B, Zhang Q (2018) Chitosan-stabilized self-assembled fluorescent gold nanoclusters for cell imaging and biodistribution in vivo. ACS Biomater Sci Eng 4:1055–1063
Wei Q, Chen Y, Ma X, Ji J, Qiao Y, Zhou B, Ma F, Ling D, Zhang H, Tian M, Tian J, Zhou M (2018) High-efficient clearable nanoparticles for multi-modal imaging and image-guided cancer therapy. Adv Funct Mater 28:1704634
Zhuang Q, Jia H, Du L, Li Y, Chen Z, Huang S, Liu Y (2014) Targeted surface-functionalized gold nanoclusters for mitochondrial imaging. Biosens Bioelectron 55:76–82
Vankayala R, Kuo CL, Nuthalapati K, Chiang CS, Hwang KC (2015) Nucleus-targeting gold nanoclusters for simultaneous in vivo fluorescence imaging, gene delivery, and NIR-light activated photodynamic therapy. Adv Funct Mater 25:5934–5945
Chen T, Hu Y, Cen Y, Chu X, Lu Y (2013) A dual-emission fluorescent nanocomplex of gold-cluster-decorated silica particles for live cell imaging of highly reactive oxygen species. J Am Chem Soc 135:11595–11602
Chen YN, Chen PC, Wang CW, Lin YS, Ou CM, Ho LC, Chang HT (2014) One-pot synthesis of fluorescent BSA-Ce/Au nanoclusters as ratiometric pH probes. Chem Commun 50:8571–8574
Pan S, Zhou J, Liu W, Ye Y, Chen G, Xu J, Qian Z, Chen J, Feng H (2019) Viscosity-sensitive thiolated gold nanoclusters with diffusion-controlled emission for intracellular viscosity imaging. Analyst 144:4483–4487
Ma Y, Zhao Y, Guo R, Zhu L, Lin W (2018) A near-infrared emission fluorescent probe with multi-rotatable moieties for highly sensitive detection of mitochondrial viscosity in an inflammatory cell model. J Mater Chem B 6:6212–6216
Yang Z, He Y, Lee JH, Park N, Suh M, Chae WS, Cao J, Peng X, Jung H, Kang C, Kim JS (2013) A self-calibrating bipartite viscosity sensor for mitochondria. J Am Chem Soc 135:9181–9185
Gao F, Cai P, Yang W, Xue J, Gao L, Liu R, Wang Y, Zhao Y, He X, Zhao L (2015) Ultrasmall [64Cu]Cu nanoclusters for targeting orthotopic lung tumors using accurate positron emission tomography imaging. ACS Nano 9:4976–4986
McDonald DM, Baluk P (2002) Significance of blood vessel leakiness in cancer. Cancer Res 62:5381–5385
Zhao Y, Detering L, Sultan D, Cooper ML, You M, Cho S, Meier SL, Luehmann H, Sun G, Rettig M, Dehdashti F, Wooley KL, DiPersio JF, Liu Y (2016) Gold nanoclusters doped with 64Cu for CXCR4 positron emission tomography imaging of breast cancer and metastasis. ACS Nano 10:5959–5970
Hu H, Huang P, Weiss OJ, Yan X, Yue X, Zhang MG, Tang Y, Nie L, Ma Y, Niu G, Wu K, Chen X (2014) PET and NIR optical imaging using self-illuminating 64Cu-doped chelator-free gold nanoclusters. Biomaterials 35:9868–9876
Zhao Y, Sultan D, Detering L, Luehmann H, Liu Y (2014) Facile synthesis, pharmacokinetic and systemic clearance evaluation, and positron emission tomography cancer imaging of 64Cu-Au alloy nanoclusters. Nanoscale 6:13501–13509
Shang L, Stockmar F, Azadfar N, Nienhaus GU (2013) Intracellular thermometry by using fluorescent gold nanoclusters. Angew Chem Int Ed 52:11154–11157
Wang Y, Chen J, Irudayaraj J (2011) Nuclear targeting dynamics of gold nanoclusters for enhanced therapy of HER2+ breast cancer. ACS Nano 5:9718–9725
Khandelia R, Bhandari S, Pan UN, Ghosh SS, Chattopadhyay A (2015) Gold nanocluster embedded albumin nanoparticles for two-photon imaging of cancer cells accompanying drug delivery. Small 11:4075–4081
Gu W, Zhang Q, Zhang T, Li Y, Xiang J, Peng R, Liu J (2016) Hybrid polymeric nano-capsules loaded with gold nanoclusters and indocyanine green for dual-modal imaging and photothermal therapy. J Mater Chem B 4:910–919
Hu D, Sheng Z, Fang S, Wang Y, Gao D, Zhang P, Gong P, Ma Y, Cai L (2014) Folate receptor-targeting gold nanoclusters as fluorescence enzyme mimetic nanoprobes for tumor molecular colocalization diagnosis. Theranostics 4:142–153
Qiao J, Mu X, Qi L, Deng J, Mao L (2013) Folic acid-functionalized fluorescent gold nanoclusters with polymers as linkers for cancer cell imaging. Chem Commun 49:8030–8032
Pyo K, Ly NH, Yoon SY, Shen Y, Choi SY, Lee SY, Joo SW, Lee D (2017) Highly luminescent folate-functionalized Au22 nanoclusters for bioimaging. Adv Healthc Mater 6:1700203
Jiang H, Xu G, Sun Y, Zheng W, Zhu X, Wang B, Zhang X, Wang G (2015) A “turn-on” silver nanocluster based fluorescent sensor for folate receptor detection and cancer cell imaging under visual analysis. Chem Commun 51:11810–11813
Li J, You J, Zhuang Y, Han C, Hu J, Wang A, Xu K, Zhu JJ (2014) A “light-up” and “spectrum-shift” response of aptamer-functionalized silver nanoclusters for intracellular mRNA imaging. Chem Commun 50:7107–7110
Chen F, Goel S, Hernandez R, Graves SA, Shi S, Nickles RJ, Cai W (2016) Dynamic positron emission tomography imaging of renal clearable gold nanoparticles. Small 12:2775–2782
Zhou C, Hao G, Thomas P, Liu J, Yu M, Sun S, Oz OK, Sun X, Zheng J (2012) Near-infrared emitting radioactive gold nanoparticles with molecular pharmacokinetics. Angew Chem Int Ed 51:10118–10122
Chen X, Zhu H, Huang X, Wang P, Zhang F, Li W, Chen G, Chen B (2017) Novel iodinated gold nanoclusters for precise diagnosis of thyroid cancer. Nanoscale 9:2219–2231
Liu CL, Wu HT, Hsiao YH, Lai CW, Shih CW, Peng YK, Tang KC, Chang HW, Chien YC, Hsiao JK, Cheng JT, Chou PT (2011) Insulin-directed synthesis of fluorescent gold nanoclusters: preservation of insulin bioactivity and versatility in cell imaging. Angew Chem Int Ed 50:7056–7060
Liu Y, Tian GF, He XW, Li WY, Zhang YK (2016) Microwave-assisted one-step rapid synthesis of near-infrared gold nanoclusters for NIRF/CT dual-modal bioimaging. J Mater Chem B 4:1276–1283
Sun SK, Dong LX, Cao Y, Sun HR, Yan XP (2013) Fabrication of multifunctional Gd2O3/Au hybrid nanoprobe via a one-step approach for near-infrared fluorescence and magnetic resonance multimodal imaging in vivo. Anal Chem 85:8436–8441
Liang G, Ye D, Zhang X, Dong F, Chen H, Zhang S, Li J, Shen X, Kong J (2013) One-pot synthesis of Gd3+-functionalized gold nanoclusters for dual model (fluorescence/magnetic resonance) imaging. J Mater Chem B 1:3545–3552
Wang C, Yao Y, Song Q (2015) Gold nanoclusters decorated with magnetic iron oxide nanoparticles for potential multimodal optical/magnetic resonance imaging. J Mater Chem C 3:5910–5917
Shibu ES, Sugino S, Ono K, Saito H, Nishioka A, Yamamura S, Sawada M, Nosaka Y, Biju V (2013) Singlet-oxygen-sensitizing near-infrared-fluorescent multimodal nanoparticles. Angew Chem Int Ed 52:10559–10563
He F, Yang G, Yang P, Yu Y, Lv R, Li C, Dai Y, Gai S, Lin J (2015) A new single 808 nm NIR light-induced imaging-guided multifunctional cancer therapy platform. Adv Funct Mater 25:3966–3976
Yang D, Yang G, Gai S, He F, An G, Dai Y, Lv R, Yang P (2015) Au25 cluster functionalized metal-organic nanostructures for magnetically targeted photodynamic/photothermal therapy triggered by single wavelength 808 nm near-infrared light. Nanoscale 7:19568–19578
Pan UN, Khandelia R, Sanpui P, Das S, Paul A, Chattopadhyay A (2016) Protein-based multifunctional nanocarriers for imaging, photothermal therapy, and anticancer drug delivery. ACS Appl Mater Interfaces 9:19495–19501
Hou W, Xia F, Alfranca G, Yan H, Zhi X, Liu Y, Peng C, Zhang C, de la Fuente JM, Cui D (2017) Nanoparticles for multi-modality cancer diagnosis: simple protocol for self-assembly of gold nanoclusters mediated by gadolinium ions. Biomaterials 120:103–114
Hu DH, Sheng ZH, Zhang PF, Yang DZ, Liu SH, Gong P, Gao DY, Fang ST, Ma YF, Cai LT (2013) Hybrid gold-gadolinium nanoclusters for tumor-targeted NIRF/CT/MRI triple-modal imaging in vivo. Nanoscale 5:1624–1628
Han L, Xia JM, Hai X, Shu Y, Chen XW, Wang JH (2017) Protein-stabilized gadolinium oxide-gold nanoclusters hybrid for multimodal imaging and drug delivery. ACS Appl Mater Interfaces 9:6941–6949
Zhao C, Du T, Rehman F, Lai L, Liu X, Jiang X, Li X, Chen Y, Zhang H, Sun Y, Luo S, Jiang H, Selke M, Wang X (2016) Biosynthesized gold nanoclusters and iron complexes as scaffolds for multimodal cancer bioimaging. Small 12:6255–6265
Xu C, Wang Y, Zhang C, Jia Y, Luo Y, Gao X (2017) AuGd integrated nanoprobes for optical/MRI/CT triple-modal in vivo tumor imaging. Nanoscale 9:4620–4628
Wu X, Li C, Liao S, Li L, Wang T, Su Z, Wang C, Zhao J, Sui C, Lin J (2014) Silica-encapsulated Gd3+-aggregated gold nanoclusters for in vitro and in vivo multimodal cancer imaging. Chem Eur J 20:8876–8882
Hembury M, Chiappini C, Bertazzo S, Kalber TL, Drisko GL, Ogunlade O, Walker-Samuel S, Krishna KS, Jumeaux C, Beard P, Kumar CS, Porter AE, Lythgoe MF, Boissiere C, Sanchez C, Stevens MM (2015) Gold-silica quantum rattles for multimodal imaging and therapy. Proc Natl Acad Sci U S A 112:1959–1964
Shen D, Henry M, Trouillet V, Comby-Zerbino C, Bertorelle F, Sancey L, Antoine R, Coll JL, Josserand V, Le Guével X (2017) Zwitterion functionalized gold nanoclusters for multimodal near infrared fluorescence and photoacoustic imaging. APL Mater 5:053404
Jin R, Zeng C, Zhou M, Chen Y (2016) Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem Rev 116:10346–10413
Wang S, Meng X, Das A, Li T, Song Y, Cao T, Zhu X, Zhu M, Jin R (2014) A 200-fold quantum yield boost in the photoluminescence of silver-doped AgxAu25-x nanoclusters: the 13 th silver atom matters. Angew Chem Int Ed 53:2376–2380
Shang L, Nienhaus GU (2017) Research Update: Interfacing ultrasmall metal nanoclusters with biological systems. APL Mater 5:053101
Acknowledgements
This chapter was modified from the paper published by our group in Chinese Chemical Letters (Xu and Shang 2018; 29:1436–1444). The related contents are re-used with the permission.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Xu, J., Shang, L. (2020). Fluorescent Metal Nanoclusters for Bioimaging. In: Wu, FG. (eds) Fluorescent Materials for Cell Imaging. Springer, Singapore. https://doi.org/10.1007/978-981-15-5062-1_5
Download citation
DOI: https://doi.org/10.1007/978-981-15-5062-1_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-5061-4
Online ISBN: 978-981-15-5062-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)