Abstract
This chapter focuses on the recent developments in the electrochemical biosensor. On the onset historical aspects of biosensors have been discussed followed by the current state of the art, which includes the fabrication, immobilization of the receptors and the linking chemistry, sensitivity, specificity, and target detection species. The importance of the electrode platform is outlined and classified into porous and nonporous. The sensor reported on various platforms has been reviewed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Electrochemical biosensor
- Glassy carbon electrode
- Screen-printed electrode
- Plastic chip electrode
- Nanoporous anodic alumina
- Porous silicon
- Mesoporous silica
8.1 Introduction
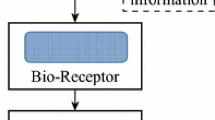
The biosensor market is growing rapidly and is expected to be valued at USD 31.5 billion by 2024 from the current value of USD 21.2 billion in 2019 (Biosensors Market Report 2019). According to the IUPAC, biosensors are defined as a self-contained integrated device, capable of providing specific quantitative or semiquantitative analytical information using a biological recognition element (bioreceptor), which is in contact with the transduction element (transducer) (McNaught and Wilkinson 1997). The biosensor is made up of two components: first, biological recognition element or bioreceptor that can be a complementary DNA, enzyme (or substrate), antigen (or antibody), or a receptor protein. Since the bioreceptors of a biosensor can bind to the target analyte very selectively, the biosensor offers very high selectivity compared to the chemical sensors. The second component of biosensor is a transducer that converts the analyte concentration into a measurable (optical, mass, electrical, or electrochemical) signal. They offer a number of distinct advantages such as high sensitivity, high specificity, reusability, label-free, short assay time, portability, and compact size in comparison to the traditional diagnostic methods (Thevenot et al. 2001). Biosensors have been developed for the range of applications from medical diagnostics, environmental monitoring, food safety, water quality to agriculture. The major application area of the biosensor is in the medical field for the diagnosis of different diseases, for example, cancer detection and blood glucose monitoring in case of diabetic patients, drug discovery, drug analysis, and whole blood analysis (Maniya 2018).
The interest in biosensors, in general, started with the introduction of the first generation glucose biosensor in 1962 (Clark and Lyons 1962). This initial electrochemical biosensor was based on the measurements of either depletion of the substrate (e.g., oxygen concentration) or generation of the product (e.g., hydrogen peroxide) in an enzyme catalyzed reaction. For example, glucose oxidase catalyzed oxidation of glucose into gluconate (Fig. 8.1). This was followed by the second-generation biosensors that were developed to overcome the drawbacks associated with the first generation biosensors. These biosensors used artificial mediators or nanomaterials to transport the signal to and from the enzyme. However many times the mediators are partially toxic. This improvement reduces the required potential window of the system, minimizing effects from interferents and helped in improving the selectivity (Ronkainen et al. 2010). The third-generation biosensors were then developed where the signal is transferred directly from the enzyme to the electrode without any intermediate stages or use of nanoparticles. These biosensors allow the continuous measurement of target species, for example, glucose in case of diabetes via subcutaneous biosensor. Electrochemical biosensors are popular due to low-cost fabrication of the microelectronic circuits and simple sensor setup with an easy interface and electronic read-out and processing. Further, the electrochemical biosensors offer several advantages such as miniaturization, low detection limits, small analyte volumes, and robustness (Das et al. 2016; Piro and Reisberg 2017).
Generally, biosensing can be performed in two detection protocols, viz., label-free and label-based detection. In label-free detection, the target species are not labeled or altered and are sensed in their natural forms. This detection method allows easy as well as convenient quantitative and kinetic measurement of molecular interaction in cost-effective manner. On the other hand, label-based detection involves the labeling of either target species or biorecognition molecules with suitable tags, such as dyes, enzymes (horseradish peroxidase, alkaline phosphatase), or nanoparticles (quantum dots) (Krismastuti et al. 2014; Medintz et al. 2005; Szili et al. 2011). The intensity of the signal is representative of the presence of the target species and also the interaction strength between target and biorecognition species. The label-based detection helps in increasing the sensitivity of the measurements; however, at the same time it suffers from the costly and laborious labeling processes. The labeling may also denature the biomolecule and interfere with the function of a biomolecule. Although label-free and label-based detection have their own positives and negatives, both are widely incorporated in the biosensors to get vital and complementary information regarding interactions among biomolecules.
The choice of electrode platform is also crucial for the performance of biosensors. The electrode not only provides the surface for the attachment of the receptor biomolecule but also in tailoring the electrochemical parameters like overpotential and electrode kinetics. The current progress in nanotechnology and advanced fabrication methods lead to the development of highly sensitive micro and nanostructured platforms for biosensor applications. Different porous and nonporous platforms have been designed for various biosensing. Among them, porous platforms based on the nanoporous anodic alumina (NAA), porous silicon (PSi), mesoporous silica (MPS), and porous polymers whereas nonporous platforms such as glassy carbon electrode (GCE), screen-printed electrode (SPE), and plastic chip electrode (PCE) has been developed (Paul et al. 2019; Reta et al. 2018; Taleat et al. 2014; Zhao et al. 2016). Porous platforms offer distinct advantages such as the high surface area to volume ratio, size exclusion of interfering molecules, and theoretically high sensitivity due to high surface area compared to the nonporous platforms. On the other hand, nonporous platforms do not require tuning of pore size, porosity, etc. In the following sections, we furnish representative examples from the two classes of electrode platforms and biosensors reported on them in the literature.
8.2 Nonporous Material-Based Biosensor
8.2.1 Glassy Carbon Electrode (GCE)
Carbonaceous electrodes are important in biomedical applications as they are biocompatible and can be used as implantable electrodes (Kadefors et al. 1970). The implantable carbon electrodes have been used as stimulus electrode in cardiac pacemakers, cuff electrodes for peripheral nerve stimulation and retinal implants (Vomero et al. 2018). Among various carbonaceous materials, graphite is not apt for electrode fabrication due to its porous nature leading to inconsistent results. Glassy carbon is controlled pyrolyzed, highly pure, and compact form of carbon. GCE is electrically conductive, gas impermeable material of highly resistive to the chemical attack (Zittel and Miller 1965). GCE has been utilized for a number of biosensing applications based on electrochemical detection method. Some of the prominent work on GCE electrode are outlined below.
Xing and Ma (2016) prepared the amperometric sensor using hybrid nanocomposite of MoS2 and reduced graphene oxide. The analysis of nanocomposite displayed porous and flower-like structure and a large specific surface. The electrochemical detection of ascorbic acid, dopamine, and uric acid was performed using the nanocomposite biosensor by cyclic voltammetry (CV) and differential pulse voltammetry (DPV). The recovery of these three species were more than 99% as detected from the spiked human serum samples.
The electrochemical determination of caffeic acid was performed on the nitrogen-doped carbon-modified GCE (Karikalan et al. 2017). The carbon nanomaterials were prepared by the flame synthesis method. The fabricated biosensor displayed the superior electrocatalytic performance for the detection of the caffeic acid in a wide linear range of 0.01–350 μM with a limit of detection (LOD) of 0.0024 μM and the limit of quantification (LOQ) of 0.004 μM. Also, the good selectivity, stability, and reproducibility with detection of caffeic acid from red wine were also demonstrated. An electrochemical biosensor was developed for the detection of the Salmonella bacteria by immobilizing the nanocomposite of reduced graphene oxide (rGO) and carboxy-modified multi-walled carbon nanotubes (MWCNTs) on the GCE surface (Jia et al. 2016). The specific detection of Salmonella was obtained by covalently binding amino-modified aptamer to the rGO-MWCNT composite. The binding of the Salmonella to the anti-Salmonella aptamer on the sensor surface blocked the electron transfer and caused the increase in impedance as measured by electrochemical impedance spectroscopy (EIS). The detection of Salmonella was obtained at a working voltage of 0.2 V in the range of 75–7.5 × 105 cfu/mL and detection limit of 25 cfu/mL.
The simultaneous detection of ascorbic acid, dopamine, and uric acid was performed on the GCE decorated with fullerene (C60) and modified with platinum nanosheets by potentiostatic deposition method (Zhang et al. 2015). The DPV measurements of the electrode showed the three well-resolved voltammetric peaks for these biomolecules. The prepared biosensor showed detection from both real plasma and urine samples with good storage stability and reproducibility. The detection of ascorbic acid, dopamine, and uric acid was in the range of 10–1800, 0.5–211.5, and 9.5–1187 μM with LOD of 0.43, 0.07, and 0.63 μM, respectively. These values of detection range and LOD were almost comparable to the values obtained by Xing and Ma (2016). A similar biosensor for the simultaneous detection of paracetamol, tramadol, and caffeine was fabricated based on GCE and modified with poly(Nile blue) by electropolymerization of Nile blue monomer using CV (Chitravathi and Munichandraiah 2016). The coating of the poly(Nile blue) film helped in increasing the electroactive surface area and peak current and decreasing the overpotential of paracetamol, tramadol, and caffeine in comparison to the bare GCE. The biosensor displayed good selectivity and sensitivity for paracetamol, tramadol, and caffeine detections in a linear range of 2.0 × 10−7–1.62 × 10−5 M, 1.0 × 10−6–3.1 × 10−4 M, and 8.0 × 10−7–2.0 × 10−5 M, with LODs of 0.08, 0.5, and 0.1 μM, respectively. Further, the biosensor also demonstrated to detect these molecules in pharmaceutical dosage forms.
The detection of the tryptophan was carried out on the metal-organic framework MIL-101(Fe) and silver nanoparticles composite (AgNPs/MIL-101) modified GCE (Peng et al. 2016). The composite increased the oxidation current of tryptophan compared to the bare electrode. The CV and DPV were used to measure the electrochemical behaviors of tryptophan on the sensor surface. The oxidation peak currents were found to be proportional to the tryptophan concentrations in the ranges of 1–50 and 50–150 μM, respectively, with LOD of 0.14 μM. The detection of tryptophan from urine samples was also performed.
The simultaneous determination of hydroquinone and catechol was performed on the GCE by pre-electrolyzing GCE in ammonium carbamate aqueous solution (Wang et al. 2016). This modification improved the electrocatalytic properties of GCE for the electro-oxidation of hydroquinone and catechol due to the incorporation of the nitrogen to the electrode surface. The simultaneous detection of both hydroquinone and catechol was in the linear range of 5 to 260 μM and LOD of 0.2 μM. The biosensor also displayed detection from real river water samples with recoveries of more than 95%.
Recently, the determination of the uric acid was performed on the GCE modified with a nanocomposite consisting of Fe3O4 onto graphene sheets and SiO2 layer (Movlaee et al. 2017). The electrochemical behavior of the uric acid on the electrode was studied by CV, DPV, and chronoamperometry. The uric acid detection displayed the sharp oxidation peak current at 330 mV due to high electrocatalytic activity. The linear increase in peak current with an increase in acid concentration was seen in the range of 0.5–250 μM with the LOD of 0.07 μM. Further, the detection of uric acid from the urine samples was demonstrated with high reproducibility (RSD: 1.7–3.4%) and excellent recoveries. In order to perform modification of GCE and electro-oxidation of doxorubicin (DOX) at a low potential, graphene QD of low toxicity was prepared by pyrolyzing citric acid in alkaline solution (Hashemzadeh et al. 2016). The coating of the graphene QDs on the GCE resulted in a decrease in the overvoltage (−0.56 V) of the DOX oxidation reaction in comparison to the bare electrodes. This was due to the good electrocatalytic activity for the redox reaction of DOX and an increase in the rate of electron transfer of DOX due to the coating. The biosensor showed a determination of the DOX from the human plasma samples in a wide linear range of 0.018–3.6 μM and LOD of 0.016 μM with good stability.
8.2.2 Screen-Printed Electrode (SPE)
Although the GCEs are excellent at the laboratory level and for proof-of-concept experiments, for scale-up research and development they are not so practical. Therefore an ancient printing technique, the screen printing, has been adapted for easy and mass fabrication of electrodes. Now, screen printing on the electrode is one of the most promising methods for simple, rapid, and cost-effective production of biosensors (Windmiller and Wang 2013). The surface modification of SPEs can be easily performed with different linking chemistries to attach bioreceptors and to achieve a variety of improvements. The point-of-care applications with SPEs are possible due to its miniaturized size, low sample requirements, and compatibility with portable reading devices. Also, SPEs do not require tedious cleaning processes and avoid memory effects problems that are present in conventional solid electrodes (Taleat et al. 2014).
SPEs are prepared by layer-by-layer depositions of ink upon a solid substrate by using a mold, stencil, screen, or mesh. This screen-printing technology offers several important advantages, such as design flexibility, process automation, good reproducibility, and a wide choice of materials (Hernandez-Vargas et al. 2018). SPEs provide the opportunity to bring working, counter, and reference electrodes in a single structural unit. Printed on various substrates like plastic, paper, ceramic, or skin substrates, they can be conveniently modified using various inks. Silver and carbon inks are most frequently used in printing while gold, platinum, noble metals, and other inks are also used (Yan et al. 2012; Neves et al. 2012). A schematic diagram of screen printing of electrode is given in Fig. 8.2. The reference electrode track of the electrode is printed using silver ink while working electrodes are printed using graphite and other inks. The selectivity and sensitivity of the electrode are tuned by varying the composition of various inks used for printing. Carbon ink used for dispersion, printing, and adhesion tasks is prepared by graphite particles, polymeric binder, and other additives. It is most commonly used ink due to low-cost, easy surface modifications, and chemical inertness. The performance of the biosensor and electron transfer reactivity of the electrode is strongly affected by the change in ink compositions such as type, size, or loading of graphite particles and printing and curing conditions. The screen-printed gold electrodes are now gaining increasing importance for the development of enzymatic, immuno, and geno-sensors due to the formation of the self-assembled monolayers (SAMs) through strong Au-S bonds on the electrode surface; however higher cost of gold restricts its application than carbon ink. In addition, the surface of SPEs can be easily modified with different bioreceptors, including DNA, enzymes, antibodies, synthetic recognition elements, and others (Renedo et al. 2007). Some significant work done on SPE has been discussed in the following paragraphs.
The detection of the 3-hydroxybutyrate was performed by using electrochemical biosensor based on the SPEs modified by reduced graphene oxide and thionine (Martínez-García et al. 2017). The biosensor was immobilized with an enzyme 3-hydroxybutyrate dehydrogenase. The amperometric detection due to the generation of NADH from the NAD+ in the presence of 3-hydroxybutyrate was observed in the linear range of 0.01–0.4 mM and with LOD of 1 μM. The detection from the spiked human serum samples was also demonstrated with two concentration levels of 0.033 and 0.29 mM with mean recoveries more than 98%. Recently, an immunosensor was developed using SPE for the detection of cancer biomarker human epidermal growth factor receptor-2 (HER2) antigen (Tallapragada et al. 2017). The anti-HER2 capture antibody was immobilized on the sensor surface without any prior surface treatment and then HER2 antigen was added. The detection was then performed in a sandwich format like ELISA by using biotinylated goat anti-human ErbB2 attached to the streptavidin conjugated horseradish peroxidase (HRP) as the detection antibody. The presence of HER2 antigen resulted in enzymatic degradation of the substrate 3,3′,5,5′-tetramethylbenzidine (TMB). The linear increase in amperometric signal was observed with increase in antigen concentration with twofold linear range of 5–20 and 20–200 ng/mL, respectively. The values of 4 and 5 ng/mL were reported as the LOD and the LOQ for biosensor. The detection of antigen from real patient samples was also demonstrated.
The electrochemical biosensors have been developed by immobilizing the DNA probe specific to the certain genetic mutations, pathogens (for example, E. coli and Salmonella), or specific diseases (for example, herpes simplex virus, Epstein-Barr virus, and cytomegalovirus). DNA detection is done based on the DNA hybridization, which is measured by the electrochemical method. The SPE surface is generally fabricated using a gold-based ink to form well-ordered gold-thiol SAMs using DNA oligos. The different signal amplification strategies involving labeled-probes have been incorporated for DNA detection on the SPE biosensor. There are many studies where direct detection of DNA hybridization does not give the required sensitivity and LOD. Thus, amplification steps are therefore required to achieve very high sensitivity (nano to pico-gram per mL) and low LOD (pico to femto-gram per mL) (Taleat et al. 2014).
The detection of cancer biomarker prostate-specific antigen (PSA) was done on SPE printed by vegetable parchment (Yan et al. 2012). The graphene and HRP-labeled antibody (Ab2) functionalized with gold nanoparticles (GNPs) were used to increase conductivity, stability, surface area, and amplify the electrochemical signal on the SPE biosensor. By using this approach, LOD of 0.46 pg/mL and a wide linear range over six orders of magnitude was seen. Also, the PSA detection from human serum samples displayed acceptable concordance to the commercial turbidimetric immunoassay values.
SPE was also utilized for the multiplexed detection of carcinoembryonic antigen (CEA) and α-fetoprotein (AFP) in a sandwich-type immunoassay method (Lai et al. 2012). The capture antibodies were attached to the chitosan-modified SPEs. In sandwich immunoreactions, GNPs-modified antibodies were captured on the sensor surface, which further induced the silver deposition from a silver enhancer solution. Anodic stripping analysis was performed to detect deposited silver nanoparticles. The wide linear ranges of three orders of magnitude and LOD of 3.5 and 3.9 pg/mL for CEA and AFP, respectively, were obtained using this immunoassay. The detection results from clinical serum samples were in correlation with electrochemiluminescent test.
The multiplex detection of three breast cancer markers, cancer antigens 153 and 125 (CA 153, CA 125) and CEA was performed on a carbon electrode array containing three graphite working SPEs (Ge et al. 2012). Graphene was incorporated to increase the electron transfer efficiency. The capture antibody was immobilized on the GNPs-modified electrode surface. Then secondary antibody labeled with alkaline phosphatase (ALP) was captured on the sensor surface in a sandwich-type reaction and enzymatic hydrolysis of the 3-indoxyl phosphate to an indoxyl intermediate caused the reduction of the silver ions on the electrode surface. This silver deposition was measured by linear sweep voltammetry. The ALP label and silver deposition helped in amplifying the signal with a LOD of 1.5 × 10−3 U/mL for CA 153, 3.4 × 10−4 U/mL for CA 125, and 1.2 × 10−3 ng/mL for CEA.
The multiplex detection of four cancer biomarkers, viz., AFP, CEA, CA 125, and CA 153, were performed on the microfluidic paper-based biosensor by the integration of a signal amplification strategy (Wu et al. 2013). The graphene/chitosan was used to modify eight working electrodes, which had a common counter and reference electrodes and then the modified surface was immobilized with capture antibodies. The various concentrations of the antigen solutions were kept on each working electrode. To perform sandwich immunoreactions, nanobioprobe of monodispersed silica nanoparticles co-immobilized with HRP and antibody were added. The signal amplification was obtained by the application of graphene and silica nanoparticles. A solution containing O-phenylenediamine and hydrogen peroxide (H2O2) was added on the paper and the signal was detected by DPV by this paper-based electrochemical sensor. The detection of AFP, CEA, CA 125, and CA 153 was observed in the wide dynamic ranges of 0.001–100 ng/mL (LOD: 0.001 ng/mL), 0.005–100 ng/mL (LOD: 0.005 ng/mL), 0.001–100 ng/mL (LOD: 0.001 ng/mL), and 0.005–100 ng/mL (LOD: 0.005 ng/mL), respectively.
The SPE modified with carbon nanotube (CNT) and GNPs hybrid system was developed for the voltammetric detection of human anti-gliadin antibodies (AGA) IgA and IgG in serum samples specific to celiac disease (Neves et al. 2012). The surface modifications caused the amplification of the immunoreactions. The sensing was based on the gliadin-immobilized nanostructured surface and human autoantibodies. Then anti-human IgA or anti-human IgG antibodies labeled with ALP, and a mixture of 3-indoxyl phosphate with silver ions was incubated on the electrode. The silver deposition on the sensor surface was detected by anodic stripping voltammetry. The biosensor showed a LOD of 9.1 and 9.0 U/mL for AGA IgA and AGA IgG, respectively, with ability of detecting these antibodies in human serum samples.
The detection of the anti-transglutaminase (anti-tTG) IgA and anti-tTG IgG in human serum samples was performed on the array of 8-channel SPEs biosensor modified with a tTG by adsorption (Martín-Yerga and Costa-García 2015; Martín-Yerga et al. 2014). The study used anti-human antibodies labeled with Cd/ZnS quantum dots (QDs) for the immunoassay. The anodic stripping voltammetry was used to detect the Cd2+ release from the QDs after an acid attack. This approach allowed the sensitive detection of 2.2 and 2.7 U/mL of anti-tTG IgG and anti-tTG IgA, respectively, on the sensor surface.
The label SPE-based biosensor was developed for the detection of human C-reactive protein (CRP) (De Ávila et al. 2013). The detection was performed in a sandwich format on the surface of carboxylic-modified magnetic beads. The beads were captured on the disposable gold SPE by placing a magnet under the sensor surface. The HRP enzyme (used as a label) catalyzed degradation of the substrate was determined by measuring amperometric response at −0.1 V. This magneto-immunosensor showed a wide linear range of 0.07–1000 ng/mL with excellent LOD of 0.021 ng/mL and allowed the detection of CRP from dilute blood serum in the clinically relevant range.
The detection of biologically important mitochondrial metalloprotein cytochrome c (cyt c) containing a prosthetic group (heme) was obtained using SPE immunosensor in label-free manner (Pandiaraj et al. 2014). The polypyrrole (PPy) was electropolymerized on the SPE working electrode and the resulting platform was modified with either GNPs or CNTs incorporated via Nafion on the PPy matrix to enhance the direct electron transfer between the cyt c and the electrode surface. The electrode was then immobilized with a specific monoclonal antibody. The electrochemical immunosensing showed high sensitivity on both platforms; however GNP-based immunosensor (linear range = 2 nM–150 μM; LOD = 2 nM; sensitivity = 154 nA/nM) was better than the nanotube-modified platform. The detection of cyt c from cell lysates of cardiomyocytes also gave results in excellent correlation with standard ELISA.
The detection of acute myocardial infarction biomarker cardiac troponin T (cTnT) was performed on the SPEs prepared by adding amine-functionalized CNT into the printing ink during fabrication (Silva et al. 2013). The nonrandom and stable-orientated antibody was immobilized on the surface because of the presence of the amino groups. The CNTs also offered additional advantages of electrochemical properties, which helped in rapid and sensitive detection of cTnT by DPV. The current peak in DPV was decreased with increase in concentrations with a linear range of 0.0025–0.5 ng/mL and LOD of 0.0035 ng/mL. Also, the immunosensor showed results comparable to the electrochemiluminescent immunoassay from serum samples.
The SPEs were modified with a layer of citrate-capped GNPs in a one-step electrochemical technique and then immobilized with anti-cardiac troponin I antibody by electrostatic interaction for the label-free detection of the troponin I (Bhalla et al. 2012). The coating of gold layer acted as matrix for immobilization of antibody and as increased transduction properties. The binding of the highly charged nature of antigen caused the charging of SPE surface that was sensed by the capacitive component of the impedance. The biosensor showed LOD of 0.2 ng/mL, one order of magnitude better than ELISA.
The detection of the cancer biomarker CA125 was obtained on the label-free SPEs-based immunosensor (Ravalli et al. 2013). The GNPs were deposited on the electrode and SAM was formed on it by 11-mercaptoundecanoic acid. The anti-CA125 antibody was attached by 3-(3-dimethylaminopropyl)carbodiimide/N Hydroxysuccinimide (EDC/NHS) linking chemistry. The surface modifications were characterized by CV and EIS. The sensor showed a linear response of electron transfer resistance to the CA125 concentration in the range of 0–100 U/mL, LOD of 6.7 U/mL and also in serum spiked with CA125.
The SPE was modified with ZnO/Al2O3 nanocomposite for the sensitive and selective electrochemical detection of the ascorbic acid (Ganjali et al. 2017). The CV and DPV measurements were obtained for the determination of ascorbic acid on the electrode. The detection was seen in a linear range of 1.0–100 μM with LOD of 0.6 μM and from real samples. Acetylcholinesterase (AChE) biosensor was developed by step by step drop casting of dispersion of carbon black, chitosan, and AChE on the SPE (Talarico et al. 2016). This modification showed improved electrochemical response compared to the bare SPE and SPE modified only with chitosan. The enzymatic activity was measured by detecting the product thiocholine at +300 mV. The detection of pollutant paraoxon as a model compound was carried out on the biosensor as organophosphorus pesticides inhibit the AChE activity. The enzymatic inhibition for paraoxon was observed up to a concentration of 0.5 μg/L and a low detection limit of 0.05 μg/L. The detection from drinking water spiked with 0.5 μg/L was also demonstrated with a good recovery value of 97 ± 15%.
8.2.3 Plastic Chip Electrode (PCE)
The major technical bottleneck outstays in screen-printed electrodes is that its conducting layer is not the integral part of the electrode, and hence there is a possibility of its cracking or peeling. PCE is a recent development in electrode technology and is being exploited for various applications in different fields, including electrocatalysis, electrometallurgy, and anodic stripping voltammetry (Mondal et al. 2018; Perween et al. 2014; Perween and Srivastava 2017). Unlike SPE, PCE is a bulk conducting, self-standing composite electrode and hence stave off the major problem of peeling of conducting layer. PCE has also been demonstrated as a platform for ionic liquid-based solid-state reference electrode (Perween and Srivastava 2018). PCE is fabricated by the simple solution casting method at room temperature and without any additional requirement of sophisticated instruments that are generally required in the fabrication of other electrodes (Fig. 8.3). Being a self-standing, highly compact (Fig. 8.4a) composite, it can be cut in a chip of required dimension. A typical thickness of the chip is 450 microns. A random distribution of the conducting filler in the polymer matrix forms percolating channels for the passage of charge. Typically, graphite powder is used as conducting filler for the fabrication of PCE. The random distribution of the graphite into the polymer matrix makes the surface of the PCE rough (Fig. 8.4b), which relatively increases the surface area of the electrode compared to the flat electrodes. This increase in the surface area, therefore, helps in increasing the sensitivity of the biosensors. PCE is also advantageous for biosensing applications due to its low-cost, easy surface modifications, and excellent electrochemical properties.
The PCE-based electrochemical biosensor was designed for the highly sensitive and selective detection of the type (II) diabetes biomarker retinol binding protein 4 (RBP4) (Paul et al. 2019). The detection of RBP4 can be useful in the proper management of type (II) diabetes at early stage. The working area of PCE was sputter coated with a gold layer of 10 nm in order to immobilize the bioreceptor via SAMs of thiols. The surface was first modified with 4-aminothiophenol and then glutaraldehyde. The anti-RBP 4 antibody was attached to the electrode and EIS measurements were performed. The formation of Ag-Ab complex caused the increase in impedance in a wide concentration range of 100 fg/mL to 1 ng/mL and a detection limit of 100 fg/mL. The interference study using two proteins IgG and Vaspin was also conducted, which showed negligible interference on the PCE biosensor.
8.3 Porous Material-Based Biosensor
Porous platforms such as NAA, PSi, MPS, and porous polymers offer several advantages in comparison to the flat electrodes for biosensing applications (Reta et al. 2018). These platforms offer a very high surface area to volume ratio due to the porous structure and the easy surface modification ability. Due to the high surface area, a large number of bioreceptor molecules can be immobilized on the electrode, which in turn increases the sensitivity of the biosensing device. The surface chemistry of the materials can be modified by grafting different molecules for the stability and attachment of the bioreceptor. Further, the pore size of the porous materials can be optimized for specific analyte sensing and also to filter out interfering molecules from the target sample. The specific binding of bioreceptor and biomolecule can be sensed by various electrochemical techniques like potentiometry, amperometry, conductometry, or impedometry. With these advantageous properties, porous materials have shown great potential for electrochemical biosensing. Here, in this section, we will describe biosensing studies based on the nanoporous anodic alumina (NAA), porous silicon (PSi), mesoporous silica (MPS), and porous polymers (Table 8.1).
8.3.1 Nanoporous Anodic Alumina (NAA)
NAA has been exploited for a range of applications in drug delivery, biosensing, cell adhesion, cell growth, molecular separation, catalysis, solar cells, and energy storage. It is fabricated by anodization of high-purity aluminum in acidic solution by applying a specific voltage, which makes homogenously distributed and self-ordered porous structure. The pore size, interpore distance, pore length, barrier layer thickness, and wall thickness of NAA can be optimized by varying anodization conditions including type of electrolyte, electrolyte composition, temperature, anodization voltage, current, and time. NAA of different geometric properties can be designed including pore diameter of 10–400 nm, interpol distance of 50–900 nm, porosity of 5–50%, pore density of 109–1011 pores/cm2, and pore length of 10 nm to several hundreds of μm. The pretreatment of alumina is done by electropolishing, which makes a smooth surface to be used in the formation of long-range pore order (Rajeev et al. 2018).
The surface functionalization of the NAA is done to impart stability in acidic environment and to immobilize bioreceptor molecules specific to the target analyte for biosensing applications. Different modification strategies are explored for this purpose, including chemical and gas phase techniques. Among this, a self-assembling organosilane molecule with desired functional groups is most frequently used for biosensor development (Hasan et al. 2018; Pandey et al. 2013). The NAA is first treated with H2O2 to activate the hydroxyl groups, which allows the attachment of silane on the surface. The bioreceptor is then immobilized either directly to the self-assembled silane or through cross-linker such as glutaraldehyde. The second approach involves the functionalization of gold-coated NAA using alkane thiols, which form spontaneous SAMs. Another method for modification is a simple, inexpensive layer-by-layer assembly of polyelectrolyte multilayers on the surface (Krismastuti et al. 2015). Also, deposition of different metals such as gold, silver, platinum, nickel, palladium, cobalt, and titanium by thermal deposition and sputter coating are used to improve conductivity, as well as its optical, magnetic, and electrochemical properties.
NAA has several unique and interesting optical (reflectance, transmittance, chemiluminescence, and photoluminescence) and electrochemical (EIS, amperometry, voltammetry, and capacitance) properties for biosensor development. The material is stable under environmental conditions and chemically inert, which helps in detecting biomolecules in complex fluids without surface degradation and ensures long shelf life. Further, NAA can be fine-tuned to make specific pore size that filter out large biomolecules, which reduces interference effect and biofouling. Also, the biocompatibility of this platform makes it attractive for biosensor development.
DNA detection was performed on NAA biosensor using the electrochemical method. The sensing was based on DNA hybridization of target sequences with immobilized complementary ssDNA in pores of the sensor (Vlassiouk et al. 2005). The detection involved measurement techniques like CV, EIS, and direct current conductance. The DNA hybridization caused the partial blockage of the pores that resulted in limiting the diffusion of electroactive species toward the electrode. The pore size has a significant effect on sensing. Significant sensing was observed with 20 nm pores and it was completely vanished for 200 nm pores. A further, similar label-free electrochemical biosensor based on the NAA membrane having a 5′-aminated DNA probe was fabricated for the detection of 21mer DNA. The LOD of 3.1 × 10−13 M was reported with high selectivity demonstrated using sequences of single base mismatch and triple bases mismatch of a different strain of Legionella species (Rai et al. 2012).
NAA membrane-based impedance sensing was reported for rapid and sensitive DNA detection using additional amplification by GNP tags (Ye et al. 2014). The ssDNA was immobilized on the nanopores through linkers and the pore blockage caused by the DNA hybridization event was measured by EIS. The LOD of 50 pM was achieved after the amplification of detection signal by GNPs. A similar impedance biosensor for DNA detection was developed; however, the NAA membrane was fabricated at wafer-scale of well-controlled thickness, pore diameter, and overall pore density (Wu et al. 2015). The coating of silica nanoparticles (∼20 nm) on the membrane was performed to increase the total surface area. Then ssDNA was immobilized to the (3-glycidoxypropyl) trimethoxysilane functionalized surface. The selectivity was demonstrated using non-complementary ssDNA and LOD of 12.5 nM was shown in phosphate-buffered saline solution.
The detection of single nucleotide polymorphisms (SNPs) is highly important for disease diagnosis and prevention. The NAA-based electrochemical biosensor was designed for the easy, label-free, and highly selective detection of SNPs. The nanochannels of the sensor were functionalized with a probe DNA/morpholino duplex and the diffusion flux of ferricyanide probe was monitored electrochemically. The sensing mechanism was based on the competitive binding of matched DNA and morpholino to the probe DNA, which causes a change of the surface charges. This approach helped in the detection of not only a single base or two base mismatched sequences but also the specific location of the mismatched base. Also, the detection of SNPs was demonstrated in the acute promyelocytic leukemia biomarker PML/RARα fusion gene (Gao et al. 2015).
The NAA biosensor has been prepared for different virus detection, including West Nile Virus (WNV) and dengue. The detection of whole WNV particles was performed on the NAA membrane over a platinum disk electrode (Nguyen et al. 2009). The nanochannels of sensor were immobilized with an anti-WNV protein domain III (DIII) IgM antibody capture probe via physical adsorption. The detection signal was monitored based on the electrode’s Faradaic current response toward ferrocenemethanol during the formation of immunocomplex in the nanopores. The sensor was studied for detection of both WNV-DIII and the inactivated West Nile viral particle. The biosensor displayed sensitivities comparable to the polymerase chain reaction (PCR) techniques with low detection limit of 4 pg/mL and detection time of 30 min. The detection of WNV particles from whole blood was also demonstrated with relative standard deviation (RSD) of 6.9 %.
Several studies were performed for the detection of dengue virus on the NAA biosensor. The detection of dengue type 2 virus (DENV-2) was performed on the NAA-modified platinum electrode (Cheng et al. 2012). A sensing mechanism of monitoring of electrode’s Faradaic current response toward redox probe was incorporated in this study as well. The immunocomplex formation between immobilized anti-DENV-2 monoclonal antibody and DENV-2 was characterized by DPV. The NAA biosensor showed detection in the range of 1–103 pfu/mL and LOD of 1 pfu/mL with insignificant binding to the nonspecific viruses including Chikungunya virus, WNV, and dengue type 3 virus (DENV-3). In a different approach, detection of dengue-specific DNA sequence was performed by immobilizing the DNA probe sequence on the NAA (Deng and Toh 2013). The membrane was sputter-coated on both the sides by platinum electrode. The DNA hybridization in the nanochannels caused the partial blockage of ferrocyanide redox species to the platinum electrode, which was measured by EIS. A linear increase in pore resistance was observed with increase in target DNA concentration in the range of 1 × 10−12 to 1 × 10−6 M. Also, the biosensor showed excellent selectivity being able to differentiate the complementary sequence from single base mismatched and non-complementary sequence with LOD of 2.7 × 10−12 M. EIS method was also used in another study for the detection of dengue on NAA membrane of 60 μm thick and 13 mm in diameter. The membrane was coated with a submicron layer of platinum to use as both the working and the counter electrode. The pore resistance was changed according to the concentration of the DENV-2 and DENV-3. The detection time of 40 min and LOD of 0.23 and 0.71 pfu/mL was observed for DENV-2 and DENV-3, respectively (Peh and Li 2013).
The detection of bacteria E. coli, S. aureus, and Legionella pneumophila has been done on NAA biosensor. The impedimetric immunosensor was fabricated for the label-free detection of harmful food-borne pathogenic bacteria E. coli O157:H7 from whole milk (Joung et al. 2013). The hyaluronic acid was used to reduce the nonspecific binding of biomolecules and other cells on the sensor. The detection was observed in the range of 10–105 cfu/mL with LOD of 10 cfu/mL based on the antibody-bacteria interactions. Further study on detection of bacteria from whole milk showed LOD of 83.7 cfu/mL. The nontarget bacteria such as S. aureus, B. cereus, and nonpathogenic E. coli DH5α were used to display high specificity of the sensor.
The ultrasensitive detection of E. coli O157:H7 was also investigated on the NAA membrane by using specific antibody-modified magnetic beads to enhance sensitivity. The membrane was immobilized with antibody via assembled polyethylene glycol (PEG)-silane linker and then the beads attached with bacterial cells were captured on the membrane. The binding of pure cells and magnetic bead-attached cells to the antibody-immobilized membrane with or without the magnetic field was monitored by EIS. The magnetic bead-based cell concentration approach displayed ultrasensitive detection of 10 cfu/mL (Chan et al. 2013). The simultaneous detection of E. coli O157:H7 and S. aureus was reported based on the NAA membranes integrated with a microfluidic device. The non-biofouling PEG was used to make microfluidic chip. The biosensor showed a linear detection range of 102–105 cfu/mL with LOD around 102 cfu/mL and low cross-binding of nontarget bacteria (Tian et al. 2016). NAA biosensors with varied pore size and thickness were prepared depending on the size of the target species such as virus or bacteria.
The NAA biosensor was developed for the detection of cancer biomarker CA15-3 (De la Escosura-Muñiz and Merkoçi 2011). The nanochannel allowed the filtering of the interfering molecules and detection of proteins in buffer as well as in whole blood. For this, conductivity of antibodies attached membrane toward redox indicator was tuned by primary and secondary immunoreactions with proteins and GNPs. Further, silver deposition was used to enhance the nanopore blockage by GNPs, which decreased the diffusion of the signaling indicator through the nanochannel. The detection of CA15-3 up to 52 U/mL was obtained using this immunoassay.
8.3.2 Porous Silicon (PSi)
PSi has been widely accepted material with demonstrated potential in diverse biomedical applications such as biosensing, bioimaging, biomolecular screening, tissue engineering, and drug delivery (Maniya et al. 2015). This is due to the several unique physicochemical and optical properties of the PSi. The key properties of PSi for electrochemical biosensing applications are simple and easy fabrication, high surface area, various surface modifications opportunities, tunability of pore size, porosity and thickness, and biocompatibility. The PSi material can be fabricated by electrochemical etching of silicon wafer in hydrofluoric acid-based solution. The surface of PSi can be easily modified by well-established modification methods including the oxidation, hydrosilylation, silanization, and thermal carbonization (Maniya et al. 2016). The bioreceptor molecules such as antibody, aptamer, enzyme, peptide, DNA, or RNA can be attached to this modified surface. The excellent biocompatibility, biodegradability, and non-toxicity of PSi has been used for the real-time, in vivo biosensing and as implantable device applications. The electrochemical biosensing using PSi is less studied in comparison to the optical sensing. The mechanism for PSi electrochemical biosensing is the change in current and resistance that occurs due to the pore blockage when analyte binds to the receptor.
The label-free electrochemical detection of MS2 bacteriophage has been performed on PSi membrane-based biosensor (Reta et al. 2016). The sensing mechanism for biosensor was based on the nanochannel blockage that occurs when the bacteriophage binds to the immobilized capture antibodies which causes the decrease in oxidation current of the electroactive species. PSi membranes of different pore sizes (85, 57, and 40 nm) were fabricated by varying current density during etching process. The detached membrane was then transferred to the gold slide and further modified with thermal hydrosilylation and EDC/NHS chemistry to immobilize anti-MS2 antibody. DPV-based measurements showed LOD of 6 pfu/mL in buffer and 17 pfu/mL in MS2 spiked in reservoir water samples for largest pore size PSi sensor.
The detection of bacterial enzyme hyaluronidase is also performed on hyaluronic acid-modified PSi electrochemical biosensor (Tucking et al. 2018). For this, hyaluronic acid methacrylate and PEG diacrylate polymers were coated on the thermally hydrocarbonized PSi surface. The presence of enzyme caused the degradation of hyaluronic acid methacrylate. The PSi biosensor showed concentration-dependent decrease in oxidation current as measured with DPV in both buffer and complex media such as bacterial supernatant and artificial wound fluid. A nonenzymatic glucose sensing using PSi electrochemical biosensor is also reported (Ensafi et al. 2017). PSi and nickel nanoparticle-based composite was prepared by in situ electroless assembly method. A sensor was fabricated based on carbon paste electrode having different amounts of PSi-Ni nanocomposite. The glucose detection in linear range of 2–5000 μmol/L and a low LOD of 0.2 μmol/L was observed with high stability, selectivity, and fast response time.
8.3.3 Mesoporous Silica (MPS)
MPS is another important porous material that has been studied for biosensor development due to its important characteristics. The MPS can be fabricated with very high surface area (up to 1500 m2/g) and controllable channel diameter of 2–50 nm and shape. The fabrication of porous structure is done by using either nanoparticles or surfactant self-assembly as templates and then removal of these templates. The fabricated porous structure allows easy functionalization based on its hydroxyl-terminated surface.
A label-free MPS-based biosensor was fabricated for the simultaneous multiplexed detection of two tumor markers, including CEA and AFP (Lin et al. 2011). The MPS channels were co-immobilized with anti-CEA monoclonal antibody and ferrocenecarboxylic acid for label-free CEA detection. While anti-AFP monoclonal antibody and HRP were immobilized as a capture probe for AFP. The immunoassay reaction confined the antigens and substrates into the internal pore walls as the external surface of MPS were blocked. The nonconductive immunocomplex blocked the electron transfer, which was measured by the electrochemical method. This biosensor allowed the simultaneous detection of CEA and AFP with the detection limits of 0.2 and 0.5 ng/mL, respectively.
The fabrication of single-wall CNTs inside the channels of MPS was reported for the sensitive detection of AFP (Lin et al. 2013). The internal pore walls of MPS were attached with amino groups via its silanol groups, whereas external surface groups were blocked by trimethylchlorosilane (TMCS). These surface modifications allowed the covalent binding of the CNTs and anti-AFP antibodies inside the mesopores of MPS. The sensing electrode was fabricated using a layer-by-layer assembly of graphene sheets and antibody-immobilized MPS on the GCE. The sensing based on the decrease in peak current due to the immunocomplex formation gave AFP detection in a linear range of 0.1 to 100 ng/mL with a LOD of 0.06 ng/mL. A similar strategy for AFP detection was used based on the MPS and GNPs (Lin et al. 2012). Aminopropyltriethoxysilane was used for the surface modification of the silanol groups on the internal pore walls while TMCS was used for the blocking of the silanol groups on the external surface of MPS. Then GCE was modified with MPS having anti-AFP antibody and GNPs confined inside the mesopores. This approach allowed the AFP detection in a range of 1 to 90 ng/mL with a detection limit of 0.2 ng/mL.
The MPS SBA-15 based immunoassay channeling sensor was designed for the sensitive detection of the AFP by immobilizing the ALP-labeled antibody as a biosensing element inside the mesopores. In order to increase the film adherence and prevent the leakage of antibody, ionic liquid-chitosan hybrid was used. The attachment of AFP to the anti-AFP antibody caused immunocomplex formation and the ALP-based catalysis of the substrate of 1-naphthyl phosphate. The peak current was decreased due to nanochannel blockage caused by nonconductive immunoconjugates. The detection of AFP in a range of 1–150 ng/mL and detection limit of 0.8 ng/mL was reported using this biosensor (Lin et al. 2009).
The electrochemical detection of streptomycin was performed on the MPS film coated on the gold electrode (Roushani and Ghanbari 2019). The coating of the silver nanoparticles on the MPS was used to increase the surface area, improve the electrical conductivity, and to bind a high degree of bioreceptor aptamer. The diffusion of hexacyanoferrate redox probe was hindered due to the binding of the streptomycin to the aptamer in the nanochannels of mesoporous film. The sensor showed a response in the 1 fg/mL to 6.2 ng/mL streptomycin concentration range and LOD of 0.33 fg/mL. The biosensing platform was also validated in milk and blood serum samples spiked with streptomycin.
Similar MPS thin film-based label-free aptasensor was designed for the detection of cancer marker PSA (Argoubi et al. 2018). The film was coated on the gold electrode and anti-PSA-specific DNA aptamer was covalently attached onto the outer surface of the silica nanopores. The detection of PSA in the range of 1–300 ng/mL and a detection limit of 280 pg/mL was demonstrated with high specificity, reproducibility, and storage stability. PSA detection from spiked artificial urine samples and in blood serum from a prostate cancer-free male patient was also validated. Also, depending on the surface chemistry and nanoconjugates used, the MPS can be recycled and reused by varying environment conditions such as high temperature and strong acid conditions (Hasan and Pandey 2016).
8.3.4 Porous Polymers
Porous polymers have been studied for the development of electrochemical biosensors. The electrochemical sensing was performed on nanochannel arrays of polystyrene nanospheres that were formed by the self-assembly method (De La Escosura-Muñiz et al. 2015). Porous polystyrene-based biosensor was designed for the detection of IgG by immobilizing the anti-IgG antibody on the carboxyl-terminated nanospheres by EDC/NHS chemistry. The sensor development involved the fabrication of the polystyrene on the indium tin oxide (ITO) electrode and then screen printing onto a polyethylene terephthalate substrate. The well-ordered interparticle space or nanochannels were generated due to the assembly of nanospheres. The nanosphere sizes of 200 and 500 nm having a nanochannel diameter of 24 and 65 nm, respectively, were used in the study. The blocking of the diffusion of the redox probe in nanochannels was caused due to the immunocomplex formation by the binding of the IgG to the immobilized anti-IgG antibody, which resulted in decrease in DPV signal. A high specificity and LOD of 580 ng/mL were obtained using 200 nm nanospheres. Further, the IgG detection from human urine samples was also better compared to the previous reports using a nanochannel blockage approach based on NAA substrates.
The selective and sensitive detection of AFP was performed on the macroporous polyaniline doped with poly(sodium 4-styrene sulfonate) (Liu et al. 2018). The electrochemical biosensor showed high conductivity and large surface area and allowed the attachment of anti-AFP antibodies. The biosensor showed a reagent-less AFP detection in a wide linear range of 0.01–1000 pg/mL and a detection limit of 3.7 fg/mL. The sensitivity of biosensor was two times higher in comparison to the planar polyaniline-modified electrode.
The biosensors based on the enzymes are studied tremendously. To develop enzymatic biosensors, the electrodes are required to immobilize the enzyme and fast electron transfer between the active sites of enzyme and electrode surface should occur. Specifically, porous electrodes can be highly beneficial due to their high surface area and fast electron transfer. Block copolymers allow the design of different hierarchically porous structures and tuning of the pore shapes and sizes by selectively etching the domains forming by a specific block(s). The prepared porous block copolymer showed both micropores and nanopores by macro- and meso-phase separation that was induced by spinodal decomposition. Then, the glucose oxidase enzyme was immobilized on the porous film. The detection of glucose in a range of 10–4500 μM was demonstrated with LOD of 0.05 μM and a response time of 2 s. Thus, it was reported that the block copolymer film could be used for immobilization of the enzyme and to develop novel high performance biosensor (Guo et al. 2018).
References
Argoubi W, Sánchez A, Parrado C, Raouafi N, Villalonga R (2018) Label-free electrochemical aptasensing platform based on mesoporous silica thin film for the detection of prostate specific antigen. Sens Actuators B Chem 255:309–315
Bhalla V, Carrara S, Sharma P, Nangia Y, Suri CR (2012) Gold nanoparticles mediated label-free capacitance detection of cardiac troponin I. Sens Actuators B Chem 161:761–768
Biosensors Market Report. 2019. https://www.marketsandmarkets.com/PressReleases/biosensors.asp. Accessed 10 May 2019
Chan KY, Ye WW, Zhang Y, Xiao LD, Leung PHM, Li Y, Yang M (2013) Ultrasensitive detection of E. coli O157: H7 with biofunctional magnetic bead concentration via nanoporous membrane based electrochemical immunosensor. Biosens Bioelectron 41:532–537
Cheng MS, Ho JS, Tan CH, Wong JPS, Ng LC, Toh CS (2012) Development of an electrochemical membrane-based nanobiosensor for ultrasensitive detection of dengue virus. Anal Chim Acta 725:74–80
Chitravathi S, Munichandraiah N (2016) Voltammetric determination of paracetamol, tramadol and caffeine using poly (Nile blue) modified glassy carbon electrode. J Electroanal Chem 764:93–103
Clark LC Jr, Lyons C (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann NY Acad Sci 102:29–45
Das P, Das M, Chinnadayyala SR, Singha IM, Goswami P (2016) Recent advances on developing 3rd generation enzyme electrode for biosensor applications. Biosens Bioelectron 79:386–397
De Ávila BEF, Escamilla-Gómez V, Campuzano S, Pedrero M, Salvador JP, Marco MP, Pingarrón JM (2013) Ultrasensitive amperometric magnetoimmunosensor for human C-reactive protein quantification in serum. Sens Actuators B Chem 188:212–220
De la Escosura-Muñiz A, Merkoçi A (2011) A nanochannel/nanoparticle-based filtering and sensing platform for direct detection of a cancer biomarker in blood. Small 7:675–682
De La Escosura-Muñiz A, Espinoza-Castañeda M, Hasegawa M, Philippe L, Merkoçi A (2015) Nanoparticles-based nanochannels assembled on a plastic flexible substrate for label-free immunosensing. Nano Res 8:1180–1188
Deng J, Toh CS (2013) Impedimetric DNA biosensor based on a nanoporous alumina membrane for the detection of the specific oligonucleotide sequence of dengue virus. Sensors 13:7774–7785
Ensafi AA, Ahmadi N, Rezaei B (2017) Nickel nanoparticles supported on porous silicon flour, application as a non-enzymatic electrochemical glucose sensor. Sens Actuators B Chem 239:807–815
Ganjali MR, Nejad FG, Beitollahi H, Jahani S, Rezapour M, Larijani B (2017) Highly sensitive voltammetric sensor for determination of ascorbic acid using graphite screen printed electrode modified with ZnO/Al2O3 nanocomposite. Int J Electrochem Sci 12:3231–3240
Gao HL, Wang M, Wu ZQ, Wang C, Wang K, Xia XH (2015) Morpholino-functionalized nanochannel array for label-free single nucleotide polymorphisms detection. Anal Chem 87:3936–3941
Ge S, Yu F, Ge L, Yan M, Yu J, Chen D (2012) Disposable electrochemical immunosensor for simultaneous assay of a panel of breast cancer tumor markers. Analyst 137:4727–4733
Guo T, Gao J, Qin X, Zhang X, Xue H (2018) A novel glucose biosensor based on hierarchically porous block copolymer film. Polymers 10:723
Hasan A, Pandey LM (2016) Kinetic studies of attachment and re-orientation of octyltriethoxysilane for formation of self-assembled monolayer on a silica substrate. Mat Sci Eng C 68:423–429
Hasan A, Saxena V, Pandey LM (2018) Surface functionalization of Ti6Al4V via self-assembled monolayers for improved protein adsorption and fibroblast adhesion. Langmuir 34:3494–3506
Hashemzadeh N, Hasanzadeh M, Shadjou N, Eivazi-Ziaei J, Khoubnasabjafari M, Jouyban A (2016) Graphene quantum dot modified glassy carbon electrode for the determination of doxorubicin hydrochloride in human plasma. J Pharm Anal 6:235–241
Hernandez-Vargas G, Sosa-Hernández J, Saldarriaga-Hernandez S, Villalba-Rodríguez A, Parra-Saldivar R, Iqbal H (2018) Electrochemical biosensors: A solution to pollution detection with reference to environmental contaminants. Biosensors 8:29
Jia F, Duan N, Wu S, Dai R, Wang Z, Li X (2016) Impedimetric Salmonella aptasensor using a glassy carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim Acta 183:337–344
Joung CK, Kim HN, Lim MC, Jeon TJ, Kim HY, Kim YR (2013) A nanoporous membrane-based impedimetric immunosensor for label-free detection of pathogenic bacteria in whole milk. Biosens Bioelectron 44:210–215
Kadefors R, Reswick JB, Martin RL (1970) A percutaneous electrode for long-term monitoring of bio-electrical signals in humans. Med & Biol Eng 8:129–135
Karikalan N, Karthik R, Chen SM, Chen HA (2017) A voltammetric determination of caffeic acid in red wines based on the nitrogen doped carbon modified glassy carbon electrode. Sci Rep 7:45924
Krismastuti FSH, Pace S, Voelcker NH (2014) Porous silicon resonant microcavity biosensor for matrix metalloproteinase detection. Adv Funct Mater 24:3639–3650
Krismastuti FSH, Bayat H, Voelcker NH, Schönherr H (2015) Real time monitoring of layer-by-layer polyelectrolyte deposition and bacterial enzyme detection in nanoporous anodized aluminum oxide. Anal Chem 87:3856–3863
Lai G, Wang L, Wu J, Ju H, Yan F (2012) Electrochemical stripping analysis of nanogold label-induced silver deposition for ultrasensitive multiplexed detection of tumor markers. Anal Chim Acta 721:1–6
Lin J, He C, Zhang S (2009) Immunoassay channels for α-fetoprotein based on encapsulation of biorecognition molecules into SBA-15 mesopores. Anal Chim Acta 643:90–94
Lin J, Wei Z, Mao C (2011) A Label-free immunosensor based on modified mesoporous silica for simultaneous determination of tumor markers. Biosens Bioelectron 29:40–45
Lin J, Wei Z, Chu P (2012) A label-free immunosensor by controlled fabrication of monoclonal antibodies and gold nanoparticles inside the mesopores. Anal Biochem 421:97–102
Lin J, Wei Z, Zhang H, Shao M (2013) Sensitive Immunosensor for the label-free determination of tumor marker based on carbon nanotubes/mesoporous silica and graphene modified electrode. Biosens Bioelectron 41:342–347
Liu S, Ma Y, Cui M, Luo X (2018) Enhanced electrochemical biosensing of alpha-fetoprotein based on three-dimensional macroporous conducting polymer polyaniline. Sens Actuators B Chem 255:2568–2574
Maniya NH (2018) Recent advances in porous silicon based optical biosensors. Rev Adv Mater Sci 53:49–73
Maniya NH, Patel SR, Murthy ZVP (2015) Development and in vitro evaluation of acyclovir delivery system using nanostructured porous silicon carriers. Chem Eng Res Des 104:551–557
Maniya NH, Patel SR, Murthy ZVP (2016) Drug delivery with porous silicon films, microparticles, and nanoparticles. Rev Adv Mater Sci 44:257–272
Martínez-García G, Pérez-Julián E, Agüí L, Cabré N, Joven J, Yáñez-Sedeño P, Pingarrón JM (2017) An Electrochemical enzyme biosensor for 3-hydroxybutyrate detection using screen-printed electrodes modified by reduced graphene oxide and thionine. Biosensors 7:50
Martín-Yerga D, Costa-García A (2015) Towards a blocking-free electrochemical immunosensing strategy for anti-transglutaminase antibodies using screen printed electrodes. Bioelectrochemistry 105:88–94
Martín-Yerga D, González-García MB, Costa-García A (2014) Electrochemical immunosensor for anti-tissue transglutaminase antibodies based on the situ detection of quantum dots. Talanta 130:598–602
McNaught AD, Wilkinson A (1997) IUPAC. Compendium of chemical terminology, Gold Book. Blackwell Scientific Publications, Oxford
Medintz IL, Uyeda HT, Goldman ER, Mattoussi H (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 4:435–446
Mondal A, Paul A, Srivastava DN, Panda AB (2018) NiO hollow microspheres as efficient bifunctional electrocatalysts for overall water-splitting. Int J Hydrogen Energy 43:21665–21674
Movlaee K, Norouzi P, Beitollahi H, Rezapour M, Larijani B (2017) Highly selective differential pulse voltammetric determination of uric acid using modified glassy carbon electrode. Int J Electrochem Sci 12:3241–3251
Neves MMPS, González-García MB, Santos-Silva A, Costa-García A (2012) Voltammetric immunosensor for the diagnosis of celiac disease on the quantification of anti-gliadin antibodies. Sens Actuators B Chem 163:253–259
Nguyen BTT, Koh G, Lim HS, Chua AJS, Ng MML, Toh CS (2009) Membrane-based electrochemical nanobiosensor for the detection of virus. Anal Chem 81:7226–7234
Pandey LM, Pattanayak SK, Delabouglise D (2013) Properties of adsorbed bovine serum albumin and fibrinogen on self-assembled monolayers. J Phys Chem C 117:6151–6160
Pandiaraj M, Sethy NK, Bhargava K, Kameswararao V, Karunakaran C (2014) Designing label-free electrochemical immunosensors for cytochrome c using nanocomposites functionalized screen-printed electrodes. Biosens Bioelectron 54:115–121
Paul A, Chiriaco MS, Primiceri E, Srivastava DN, Maruccio G (2019) Picomolar detection of retinol binding protein 4 for early management of type II diabetes. Biosens Bioelectron 128:122–128
Peh AEK, Li SFY (2013) Dengue virus detection using impedance measured across nanoporous alumina membrane. Biosens Bioelectron 42:391–396
Peng Z, Jiang Z, Huang X, Li Y (2016) A novel electrochemical sensor of tryptophan based on silver nanoparticles/metal–organic framework composite modified glassy carbon electrode. RSC Adv 6:13742–13748
Perween M, Srivastava DN (2017) A cost-effective, unmodified platform for the detection of heavy metals via anodic stripping voltammetry at nanomolar level. Chem Select 2:4428–4432
Perween M, Srivastava DN (2018) Designing ionic-liquid based practical reference electrode. Ind J Chem Tech 25:74–80
Perween M, Parmar DB, Bhadu GR, Srivastava DN (2014) Polymer-graphite composite: a versatile use and throw plastic chip electrode. Analyst 139:5919–5926
Piro B, Reisberg S (2017) Recent advances in electrochemical immunosensors. Sensors 17:794
Rai V, Deng J, Toh CS (2012) Electrochemical nanoporous alumina membrane-based label-free DNA biosensor for the detection of Legionella sp. Talanta 98:112–117
Rajeev G, Simon BP, Marsal LF, Voelcker NH (2018) Advances in nanoporous anodic alumina-based biosensors to detect biomarkers of clinical significance: a review. Adv Healthcare Mater 7:1700904
Ravalli A, Pilon dos Santos G, Ferroni M, Faglia G, Yamanaka H, Marrazza G (2013) New label free CA125 detection based on gold nanostructured screen-printed electrode. Sens Actuators B Chem 179:194–200
Renedo OD, Alonso-Lomillo MA, Martínez MA (2007) Recent developments in the field of screen-printed electrodes and their related applications. Talanta 73:202–219
Reta N, Michelmore A, Saint C, Prieto-Simon B, Voelcker NH (2016) Porous silicon membrane-modified electrodes for label-free voltammetric detection of MS2 bacteriophage. Biosens Bioelectron 80:47–53
Reta N, Saint CP, Michelmore A, Prieto-Simon B, Voelcker NH (2018) Nanostructured electrochemical biosensors for label-free detection of water- and food-borne pathogens. ACS Appl Mater Interfaces 10:6055–6072
Ronkainen NJ, Halsall HB, Heineman WR (2010) Electrochemical biosensors. Chem Soc Rev 39:1747–1763
Roushani M, Ghanbari K (2019) An electrochemical aptasensor for streptomycin based on covalent attachment of the aptamer onto a mesoporous silica thin film-coated gold electrode. Microchim Acta 186:115
Silva BVM, Cavalcanti IT, Silva MMS, Dutra RF (2013) A carbon nanotube screen-printed electrode for label-free detection of the human cardiac troponin T. Talanta 117:431–437
Szili EJ, Jane A, Low SP, Sweetman M, Macardle P, Kumar S, Smart RSC, Voelcker NH (2011) Interferometric porous silicon transducers using an enzymatically amplified optical signal. Sens Actuators B Chem 160:341–348
Talarico D, Arduini F, Amine A, Cacciotti I, Moscone D, Palleschi G (2016) Screen-printed electrode modified with carbon black and chitosan: a novel platform for acetylcholinesterase biosensor development. Anal Bioanal Chem 408:7299–7309
Taleat Z, Khoshroo A, Mazloum-Ardakani M (2014) Screen-printed electrodes for biosensing: a review (2008-2013). Microchim Acta 181:865–891
Tallapragada SD, Layek K, Mukherjee R, Mistry KK, Ghosh M (2017) Development of screen-printed electrode based immunosensor for the detection of HER2 antigen in human serum samples. Bioelectrochemistry 118:25–30
Thevenot DR, Toth K, Durst RA, Wilson GS (2001) Electrochemical biosensors: recommended definitions and classification. Biosens Bioelectron 16:121–131
Tian F, Lyu J, Shi J, Tan F, Yang M (2016) A polymeric microfluidic device integrated with nanoporous alumina membranes for simultaneous detection of multiple foodborne pathogens. Sens Actuators B Chem 225:312–318
Tucking KS, Vasani RB, Cavallaro AA, Voelcker NH, Schonherr H, Prieto-Simon B (2018) Hyaluronic acid-modified porous silicon films for the electrochemical sensing of bacterial hyaluronidase. Macromol Rapid Commun 39:1800178
Vlassiouk I, Takmakov P, Smirnov S (2005) Sensing DNA hybridization via ionic conductance through a nanoporous electrode. Langmuir 21:4776–4778
Vomero M, Oliveira A, Ashouri D, Eickenscheidt M, Stieglitz T (2018) Graphitic carbon electrodes on flexible substrate for neural applications entirely fabricated using infrared nanosecond laser technology. Sci Rep 8:14749
Wang X, Xi M, Guo M, Sheng F, Xiao G, Wu S, Uchiyama S, Matsuura H (2016) An electrochemically aminated glassy carbon electrode for simultaneous determination of hydroquinone and catechol. Analyst 141:1077–1082
Windmiller JR, Wang J (2013) Wearable electrochemical sensors and biosensors: a review. Electroanalysis 25:29–46
Wu Y, Xue P, Kang Y, Hui KM (2013) Paper based microfluidic electrochemical immunodevice integrated with nanobioprobes onto graphene film for ultrasensitive multiplexed detection of cancer biomarkers. Anal Chem 85:8661–8668
Wu S, Ye WW, Yang M, Taghipoor M, Meissner R, Brugger J, Renaud P (2015) Impedance sensing of DNA immobilization and hybridization by microfabricated alumina nanopore membranes. Sens Actuators B Chem 216:105–112
Xing L, Ma Z (2016) A glassy carbon electrode modified with a nanocomposite consisting of MoS2 and reduced graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 183:257–263
Yan M, Zang D, Ge S, Ge L, Yu J (2012) A disposable electrochemical immunosensor based on carbon screen-printed electrodes for the detection of prostate specific antigen. Biosens Bioelectron 38:355–361
Ye WW, Shi JY, Chan CY, Zhang Y, Yang M (2014) A nanoporous membrane based impedance sensing platform for DNA sensing with gold nanoparticle amplification. Sens Actuators B Chem 193:877–882
Zhang X, Ma LX, Zhang YC (2015) Electrodeposition of platinum nanosheets on C60 decorated glassy carbon electrode as a stable electrochemical biosensor for simultaneous detection of ascorbic acid, dopamine and uric acid. Electrochim Acta 177:118–127
Zhao Y, Gaur G, Retterer ST, Laibinis PE, Weiss SM (2016) Flow-through porous silicon membranes for real-time label-free biosensing. Anal Chem 88:10940–10948
Zittel HE, Miller FJ (1965) A glassy-carbon electrode for voltammetry. Anal Chem 37:200–203
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Maniya, N.H., Srivastava, D.N. (2020). Medical Diagnostics Based on Electrochemical Biosensor. In: Chandra, P., Pandey, L. (eds) Biointerface Engineering: Prospects in Medical Diagnostics and Drug Delivery . Springer, Singapore. https://doi.org/10.1007/978-981-15-4790-4_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-4790-4_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4789-8
Online ISBN: 978-981-15-4790-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)