Abstract
To enhance oil recovery by injection of seawater into the production well causes an increase of hydrogen sulfide concentration in the crude oil by the environmental microbes. It is known as biological souring caused by sulfate-reducing bacteria (SRB) in the reservoir. The souring causes microbiologically influenced corrosion (MIC) of the tubing material and deterioration of crude oil. In this chapter, the change of microbial consortia during the souring process of the crude oil is described. Especially, by clarifying the population of SRB in the microbial consortia, the effects of nitrate injection not only on the biological souring but also to MIC are explained. Moreover, the possibilities of alkaline addition to suppress the biological souring and MIC of carbon steel are also argued.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
To increase the productivity of crude oil from the oil well, the recovery methods have been developed. Waterflooding serves as a main oil recovery method to be applied whenever the geological pressure became inefficient, known as a secondary oil recovery (Plankaert 2005). In the secondary oil recovery process, seawater is commonly injected to enhance oil recovery; however, this method causes biological souring (i.e., sulfide production in oil reservoirs). The crude oil including more than 0.04 mol% of hydrogen sulfide is defined as “sour oil.” Seawater contains a high concentration of sulfate (up to 27 mM) that can enhance the growth of sulfate-reducing bacteria (SRB) in the reservoir. Souring causes several problems, including microbiologically influenced corrosion of the tubing material and deterioration of crude oil (Gieg et al. 2011). Microbial sulfate reduction is an important metabolic activity in many petroleum hydrocarbon (PHC)-contaminated aquifers; contamination with mono-aromatic PHCs (e.g., benzene, toluene, ethylbenzene, and xylene) is a regulatory concern due to their solubility and toxicity. Because sulfate reduction can be coupled with the bacterial metabolism of mono-aromatic PHCs, it has received increasing attention as an intrinsic remediation process.
SRBs, which mostly belong to Deltaproteobacteria or Firmicutes, are among the microorganisms present in oil fields that induce souring. Although corrosion control measures can be used to remove oxygen from the injected water, these create an environment conducive to the growth of SRBs, which are obligate anaerobes. SRBs derive energy by coupling the oxidation of electron donors to the reduction of sulfate to sulfide. Previous studies have revealed that SRBs use volatile fatty acids (VFAs) and crude-oil components (e.g., toluene) as electron donors. The most common method to prevent souring is the injection of biocide, metabolic inhibitors such as nitrite or molybdate into reservoirs to inhibit SRB growth (Jayaraman et al. 1999; Nemati et al. 2001; Tang et al. 2009), and/or air injection to prevent anaerobic condition (Ochi et al. 1998), but these methods have yielded limited success. An alternative approach is nitrate injection, which seeks to promote the growth of nitrate-reducing bacteria (NRB) as competitors of SRB for the electron donors in the reservoir, such as volatile fatty acids (VFAs) (Agrawal et al. 2012). Thus, nitrate injection might be used to prevent and treat souring. Nitrate injection is an attractive solution to souring because nitrate is cost-effective and relatively nontoxic and can distribute evenly in the reservoir (Dunsmore et al. 2006; Gieg et al. 2011). However, so far, the effect of nitrate injection on the biocorrosion of carbon steel has not been well known.
In this chapter, the biological souring mechanisms and the prevention methods for souring are introduced. Moreover, the biocorrosion of carbon steel in the environment where the souring occurs and/or the prevention of souring is applied to the souring.
2 Identification of Crude-Oil Components and Microorganisms that Cause Souring Under Anaerobic Conditions
For biological souring, three factors are strongly related. They are sulfate as an electron acceptor, organic compounds as electron donors, and sulfate-reducing bacteria as biocatalyst. In the oil production process, the sulfate plentifully exists in the injection seawater. Therefore, to understand the mechanism of souring, the other two factors should be clarified. Various kinds of organic compounds, such as aromatics, hydrocarbons, and so on, are included in the crude oil, and a small amount of them is dissolved in the injection seawater.
To identify the preferential substrate for souring, the mixtures of the several crude-oil components (alkanes [AL], aromatics [AR], 2,4-dimethylxylenol [XY], naphthenic acids [NA]) and crude oil [CR] diluted 1:100 with biologically inert branched alkane 2,2,4,4,6,8,8-heptamethylnonane (HMN) were overlaid on the seawater supplemented with microorganisms from oil field water (OFW) taken from oil field (Akita, Japan) (Hasegawa et al. 2014). XY and NA were investigated as the organic compounds with the intramolecular oxygen, while ALs and ARs were investigated as dominant compounds in the crude oil. All of them were incubated in high-pressure vessels under 1 MPa at 28 °C for about 3 months. The components of crude oil that decomposed under anaerobic conditions were identified. Toluene, ethylbenzene, and alkanes (C7–C17) were selectively degraded. On the other hand, no change was observed for XY and NA. It is concluded that decomposition of aromatics [AR] and alkanes [AL] was accompanied by the production of acetate as an intermediate, followed by its oxidation. In XY and NA, no biological activity was observed. It shows they are toxic to microorganisms, and the degradation of these compounds has not been well studied.
Biological conversion of crude-oil components to produce sulfide can be divided into two steps: oxidation of oil components to produce VFA (Step 1) and reduction of sulfate to sulfide coupled with oxidation of VFAs (Step 2). There are two possible degradation mechanisms of crude-oil components. One is the complete oxidation of crude-oil components to CO2 by SRB. This mechanism is supported by the detection of bssA genes that were most likely the bssA gene of Desulfobacula toluolica. In this mechanism, toluene-degrading SRB is involved in both Step 1 and Step 2. The other is syntrophic oxidation. Detection of acetate indicated that the oil field microorganisms excreted acetate as a by-product, and the subsequent decreases indicated that other microorganisms such as SRB consume acetate and produce sulfide. Production of sulfide in AL was much less than in AR, although acetate was produced in both vessels. Moreover, toluene and ethylbenzene were completely degraded, and bssA affiliated with SRB was detected in AR. The metagenomic analysis of 16S rRNA gene sequencing revealed that Desulfotignum spp. detected in AR were affiliated with the toluene-degrading SRB, D. toluenicum. Although it remains unclear whether alkanes were degraded by SRB, it seems that degradation of aromatic hydrocarbons mainly toluene contributes significantly to souring.
Community analysis revealed that abundant classes in day 49 were distributed among Deltaproteobacteria, Gammaproteobacteria, and Clostridia. Specifically, the proportions of Deltaproteobacteria and Clostridia were increased in AL, AR, and CR after 49 days. Many SRBs, including Desulfotignum spp., belong to Deltaproteobacteria. The dominant Clostridia were Fusibacter spp., a genus of anaerobic fermenting bacteria. Although the proportion of Fusibacter was lower than in AL and CR, Fusibacter spp. were also detected in AR. Fusibacter paucivorans, isolated in an oil-producing well, can transform glucose to acetate by fermentation (Ravot et al. 1999). Therefore, Fusibacter spp. detected in this experiment might be involved in acetate production by fermentation. Minor phylotypes were distributed within the Bacteroidetes. The involvement of Bacteroidetes in hydrocarbon degradation has been investigated (Zrafi-Nouira et al. 2009; Popp et al. 2006). Acinetobacter spp., which belong to Gammaproteobacteria, were also detected in AL, AR, and CR. Abboud et al. (2007) reported that some strains of Acinetobacter spp. are involved in biodegradation of crude-oil components.
3 The Effect of Nitrate Injection on the Biological Souring Under the Presence of Sulfate-Reducing Bacteria (SRB) and Nitrate-Reducing Bacteria (NRB)
As described in the introduction, to prevent souring, the nitrate addition is applied to the oil-producing process. Kamarisima et al. (2018) revealed that the nitrate addition at the beginning could suppress the biological souring by chemical analysis and by biological analysis. By chemical analysis, it was revealed that without the addition of nitrate (Nw/o) to the artificial souring environment using the 2% of crude oil in the 2,2,4,4,6,8,8-heptamethylnonane (HMN) as a substrate, the sulfide production and sulfate consumption were simultaneously observed in the seawater medium (Fig. 15.1). Moreover, after souring occurred by SRB derived from the oil field water, the nitrate addition at day 28 (N28) was also effective for the decrease of sulfide production and suppression of sulfate reduction. On the other hand, when 27 mM of nitrate at the same level of sulfate (27 mM) in the seawater was added from day 0 (N0), no sulfide production occurred for 70 days. According to the results of biological analysis based on 16S rRNA gene sequences shown in Fig. 15.2, in the conditions of Nw/o and N28, the relative abundance of Desulfotignum sp., one of the representative SRBs suspected to be the primary degrader of toluene, became dominant after 28-day incubation. It was thought that this SRB caused souring for the initial stage of incubation. Moreover, in the condition of N28, the dominant Desulfotignum sp. did not disappear till the end of incubation, even though the sulfide production was suppressed after nitrate addition at day 28. In the case of N0 condition, instead of Desulfotignum sp., Thalassospira sp. became dominant as the incubation period. Thalassospira is known as the heterotrophic nitrate-reducing bacteria (hNRB). Therefore, it is reasonable that the NRB abundance increased instead of SRB after nitrate addition to the microbial mixture. SRB and hNRB might share similar sources of electron donors, such as the hydrocarbon fraction (especially toluene) in crude oil.
Effect of nitrate addition on sulfide production and sulfate reduction. Arrows indicate the nitrate addition (Kamarisima et al. 2018)
Bacterial community profile in 70 days of incubation. The symbols of diamond and arrows indicate 16S rRNA gene copy number and time of nitrate addition, respectively (Kamarisima et al. 2018)
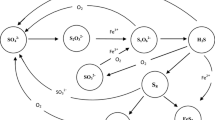
However, in the condition of N28, Thalassospira sp. did not become dominant, although a small relative abundance of Marinobacter sp., also one of the NRB, appeared at the final stage. In all conditions, Arcobacter, considered as nitrate-reducing and sulfide-oxidizing bacteria (NR-SOB) (De Gusseme et al. 2009), were the most dominant species. NR-SOB are chemoautotrophic bacteria that can oxidize sulfide coupled to reduction of nitrate. Oxidation of sulfide under denitrifying condition could lead to the formation of sulfur or sulfate. This is the reason why under the N28 condition the sulfide was not observed at the later culture period even though hNRB was not dominated after nitrate addition. The bacterial community could be divided into four groups (Fig. 15.3): (1) fermentative bacteria, (2) hNRB, (3) NR-SOB, and (4) SRB. Each group was thought to play a unique role in biological souring under each condition. Considering these relationships, at the limiting nitrate concentration to suppress SRB activity, 1 mM, SRB could coexist with NRB and promote a more diverse bacterial community (Kamarisima et al. 2019).
Possible microbial interactions based on possibility of their preference electron donor and electron acceptor under microbial souring following nitrate injection (Kamarisima et al. 2018)
4 The Effect of Nitrate Addition on Microbiologically Influenced Corrosion (MIC)
In oil and gas industrial appliances, corrosion contributes to an increase in the cost due to corrosion control and mitigation. Microbiologically influenced corrosion (MIC) has been reported to accelerate the corrosion process more than 50-fold compared to sterile conditions as reported elsewhere (Koch et al. 2001; Kruger 2011). Several groups of bacteria have been reported and proved to play a role in MIC, such as SRB (Enning and Garrelfs 2014), NRB (Iino et al. 2015), acid-producing bacteria (Gu 2012), methanogen (Uchiyama et al. 2010), and iron-oxidizing bacteria (Emerson 2018). Among them, SRB was proposed as the primary player not only of microbial souring in the crude oil but also of MIC in oil and gas industry appliances.
MIC in the oil and gas industry often occurs during or after water injection to increase the yield of oil production. The treatment for MIC is varied among the places, which includes chemical and physical treatment. The most known chemical treatment for MIC control is the application of biocide, which then turns up that it has a high cost and toxic to the environment (Skovhus et al. 2017). Since one of the leading groups of bacteria in MIC is SRB, then a similar approach for SRB control was applied to MIC as well, which is nitrate treatment. Hydrogen sulfide was known as a corrosive agent which is produced by SRB. Various studies of nitrate treatment show successful results of controlling the production of hydrogen sulfide (Gieg et al. 2011; Hubert et al. 2005; Kamarisima et al. 2018; Voordouw et al. 2009). To date, however, the application of nitrate treatment for MIC control was limited. Only several studies are available in the monitoring of nitrate treatment for MIC in the past 10 years. In this section, one example of nitrate-treatment effect on MIC is introduced.
Nitrate addition was proved to inhibit souring caused by SRB. However, the addition of nitrate can have contributed to cause severe corrosion. Based on bacterial community analysis, the bacterial community was different in the condition without and with nitrate addition as well as in the planktonic and biofilm sample of both conditions. In general, nitrate addition has increased the diversity of bacterial community in both planktonic and biofilm zone. There was no domination of specific bacteria in the planktonic zone of condition with nitrate addition until 90 days, and then Arcobacter became dominant for the later time. The common bacteria found in planktonic and biofilm site on condition with nitrate addition were identified as Arcobacter, Marinobacterium, Acetobacterium, Marinobacter, Rhodospirillaceae (f), Tindallia, Halomonas, Fusibacter, and Bacteriodales. Most of these bacteria were classified as NRB. Thus, it proved the enhancement of NRB in the condition of nitrate addition. In the biofilm attached to the surface of carbon steel coupon, these bacteria may produce various kinds of metabolites, especially volatile fatty acids, and provoke the pitting corrosion. The corrosion behavior of condition amended with nitrate was characterized by the formation of pitting corrosion as the localization of acid-bacteria and extracellular polymeric substance (EPS)-forming bacteria. Moreover, surface roughness was also contributed for more extensive pitting corrosion. The rougher the surface, the more pit was formed.
5 The Effect of Alkaline Addition on Souring and Microbiologically Influenced Corrosion (MIC)
Seawater injection into oil reservoirs for secondary oil recovery is frequently accompanied by souring (increased sulfide concentrations) in crude oil. The hydrogen sulfide produced by microbiological sulfate reduction in the seawater causes various problems, including corrosion of tubing materials and deterioration of crude oil. Sulfate-reducing bacteria (SRBs) play major roles in souring. However, under high pH (>9), most microbes (including SRBs) cannot grow. Moreover, it is known that iron corrosion is theoretically negligible under the alkaline condition. To investigate new approaches to simultaneously control souring and metal corrosion, Miyanaga et al. (2017) analyzed souring and metal corrosion under high-pH conditions (Fig. 15.4). NaOH was added to adjust the pH clean seawater (ca. pH 8) to 11 or 13. Then, a carbon steel test coupon was incubated for 123 days and supplemented with microbes separated from oil field water (OFW) and crude oil. At pH 11 and pH 13, the corrosion rate of the test coupon was decreased. Additionally, souring did not occur at pH 11 and 13, although it took place at pH 8 with microbes. Next-generation sequencing analysis of the 16S rRNA gene revealed drastic changes in the microbial consortia for pH 8 after incubating for 111 days. Desulfotignum, which shows a high identity compared to that of toluene-utilizing SRB, became dominant. It is thought to contribute a biological souring by utilizing toluene in the crude oil at pH 8. On the other hand, at pH 11, the microbial consortia did not change significantly after 111 days of incubation. At pH 13, the microbial consortia drastically changed compared with that of initial condition (OFW) due to cell lysis. That is, even under strict conditions (e.g., pH 13), some bacteria are not lysed, increasing their relative ratio without growth. Alkaline addition could inhibit not only metal corrosion but also biological souring.
Corrosion rate of the carbon steel coupons. Each number indicates pH value. M and P indicate with microbes and precipitate, respectively (Miyanaga et al. 2017)
References
Abboud MM, Khieifat KM, Batarseh M, Taraeneh KA, Al-Mustafa A, Al-Madadhah M (2007) Different optimization conditions required for enhancing the biodegradation of linear alkylbenzosulfonate and sodium dodecyl sulfate surfactant by novel consortium of Acinetobacter calcoaceticus and Pantoea agglomerans. Enzym Microb Technol 41:432–439
Agrawal A, Park HS, Nathoo S, Gieg LM, Jack TR, Miner K, Ertmoed R, Benko A, Voordouw G (2012) Toluene depletion in produced oil contributes to souring control in a field subjected to nitrate injection. Environ Sci Technol 46:1285–1292
De Gusseme B, De Schryver P, De Cooman M, Verbeken K, Boeckx P, Verstraete W, Boon N (2009) Nitrate-reducing, sulfide-oxidizing bacteria as microbial oxidants for rapid biological sulfide removal. FEMS Microbiol Ecol 67:151–161
Dunsmore B, Youldon J, Thrasher D, Vance I (2006) Effects of nitrate treatment on a mixed species, oil field microbial biofilm. J Ind Microbiol Biotechnol 33:454–462
Emerson D (2018) The role of iron-oxidizing bacteria in biocorrosion: a review. Biofouling 34:989–1000
Enning D, Garrelfs J (2014) Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl Environ Microbiol 80:1226–1236
Gieg LM, Jack TR, Foght JM (2011) Biological souring and mitigation in oil reservoirs. Appl Microbiol Biotechnol 92:263–282
Gu T (2012) Can acid producing bacteria be responsible for very fast MIC pitting? Paper no. C2012-0001214, CORROSION, Salt Lake City, UT, 11–15 Mar 2012
Hasegawa R, Toyama K, Miyanaga K, Tanji Y (2014) Identification of crude-oil components and microorganisms that cause souring under anaerobic conditions. Appl Microbiol Biotechnol 98:1853–1861
Hubert C, Nemati M, Jenneman G, Voordouw G (2005) Corrosion risk associated with microbial souring control using nitrate or nitrite. Appl Microbiol Biotechnol 68:272–282
Iino T, Ito K, Wakai S, Tsurumaru H, Ohkuma M, Harayama S (2015) Iron corrosion induced by nonhydrogenotrophic nitrate-reducing Prolixibacter sp. strain MIC1-1. Appl Environ Microbiol 81:1839–1846
Jayaraman A, Mansfeld FB, Wood TK (1999) Inhibiting sulfate-reducing bacteria in biofilms by expressing the antimicrobial peptides indolicidin and bactenecin. J Ind Microbiol Biotechnol 22:167–175
Kamarisima, Hidaka K, Miyanaga K, Tanji Y (2018) The presence of nitrate- and sulfate-reducing bacteria contributes to ineffectiveness souring control by nitrate injection. Int Biodeterior Biodegradation 129:81–88
Kamarisima, Miyanaga K, Tanji Y (2019) The utilization of aromatic hydrocarbon by nitrate- and sulfate-reducing bacteria in single and multiple nitrate injection for souring control. Biochem Eng J 143:75–80
Koch GH, Brongers MPH, Thompson NG, Virmani YP, Payer JH (2001) Corrosion cost and preventive strategies in the United States. FHWA-RD-01-156. CC Technologies Laboratories, NACE International, Dublin, OH
Kruger J (2011) Cost of metallic corrosion. In: Revie RW (ed) Uhlig’s corrosion handbook, 3rd edn. Wiley, Hoboken, NJ, pp 15–20
Miyanaga K, Hasegawa R, Tanji Y (2017) Addition of sodium hydroxide to seawater inhibits sulfide production (souring) by microbes in oil field water. J Chem Eng Jpn 50:850–856
Nemati M, Mazutinec TJ, Jenneman GE, Voordouw G (2001) Control of biogenic H2S production with nitrite and molybdate. J Ind Microbiol Biotechnol 26:350–355
Ochi T, Kitagawa M, Tanaka S (1998) Controlling sulfide generation in force mains by air injection. Water Sci Technol 37:87–95
Plankaert M (2005) Oil reservoir and oil production. In: Ollivier B, Magot M (eds) Petroleum microbiology. ASM, Washington, DC, pp 123–142
Popp N, Schlomann M, Mau M (2006) Bacterial diversity in active stage of a bioremediation system for mineral oil hydrocarbon- contaminated soils. Microbiology 152:3291–3304
Ravot G, Magot M, Fardeau M, Patel BKC, Thomas P, Garcia J, Ollivier B (1999) Fusibacter paucivorans gen. nov., sp. nov., an anaerobic, thiosulfate-reducing bacterium from an oil-producing well. Int J Syst Bacteriol 49:1141–1147
Skovhus TL, Eckert RB, Rodrigues E (2017) Management and control of microbiologically influenced corrosion (MIC) in the oil and gas industry—overview and a North Sea case study. J Biotechnol 256:31–45
Tang K, Baskaran V, Nemati M (2009) Bacteria of the sulphur cycle: an overview of microbiology, biokinetics and their role in petroleum and mining industries. Biochem Eng J 44:73–94
Uchiyama T, Ito K, Mori K, Tsurumaru H, Harayama S (2010) Iron-corroding methanogen isolated from a crude-oil storage tank. Appl Environ Microbiol 76:1783–1788
Voordouw G, Grigoryan AA, Lambo A, Lin S, Park HS, Jack TR, Coombe D, Clay B, Zhang F, Ertmoed R, Miner K, Arensdorf JJ (2009) Sulfide remediation by pulsed injection of nitrate into a low temperature Canadian heavy oil reservoir. Environ Sci Technol 43:9512–9518
Zrafi-Nouira I, Guermazi S, Chouari R, Safi NMD, Pelletier E, Bakhrouf A, Saidane-Mosbahi D, Sghir A (2009) Molecular diversity analysis and bacterial population dynamics of adapted seawater microbiota during the degradation of Tunisian zarzatine oil. Biodegradation 20:467–486
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Miyanaga, K. (2020). Biocorrosion and Souring in the Crude-Oil Production Process. In: Ishii, M., Wakai, S. (eds) Electron-Based Bioscience and Biotechnology . Springer, Singapore. https://doi.org/10.1007/978-981-15-4763-8_15
Download citation
DOI: https://doi.org/10.1007/978-981-15-4763-8_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4762-1
Online ISBN: 978-981-15-4763-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)