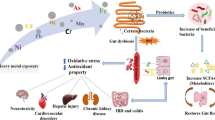
Abstract
Pollutants of environment increasing day by day and cause health hazard from past several decades. Environmental pollutants that are poorly ingested may be directly metabolized by the gut microbiota, which may lead to detrimental consequences for the host. Their exposure can alter gut microbiota that may associated with metabolic disorders and inflammatory progressions. Probiotics intervention may protect against pollutants toxicity by altering the metabolic activity and composition of gut microbiota. Probiotics have shown the lower threat of antibiotic-associated diarrhea by producing antagonistic effect against pathogenic strains. Besides, probiotics may also enhance host immune system by improving gut microbiota. This chapter summarizes the brief introduction of the classification of probiotics, gut microbiota and health along with different diseases associated with microbial community and functional mechanism of probiotics in metabolic disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Environmental pollutants are gradually increased and the term xenobiotics are commonly used in context of environmental pollution because they are synthetic compounds produced from industries and agriculture [1]. Human body has number of microorganisms commonly called as human microbiota [2, 3]. The diversity and functioning of this community depend upon body size, shape, and different environmental conditions (e.g., pH, oxygen, substrate availability, humidity, and temperature) at different sites [3]. Site-specific microbiome which associate with skin, respiratory tract, and gut are the first to encounter xenobiotics and mediate a pass to internal organ system [4]. Besides, most interaction between human microbiota and xenobiotics occurs in human gut [4, 5]. The anaerobic environment of the gut is well-suited for a hydrolytic and reductive metabolism. And this will generate low molecular weight non-polar products that can easily absorbed by host cells. In comparison, the absorbed non-polar xenobiotics are metabolized and transported in liver by a rich collection of conjugative enzymes and these hepatic metabolisms may generate high molecular weight polar metabolites. The latter reach to the gut, secreted via bile and in gut they can be re-metabolized by hydrolytic and reductive enzymes [5, 6]. Hence, xenobiotics are metabolized by gut microbiota and can exert an intense influence on the bioavailability and toxicity of xenobiotics entering in gut from different routes.
6.1.1 Probiotics and Gut Microbiota
Food and Agricultural Organization (FAO) of the United Nations and the World Health Organization (WHO) states that probiotics are supplements of feed and have so many benefits for human and affect the host by improving the microbial balance with immune system. Nobel laureate Elie Metchnikoff in 1907 introduced the concept of probiotics to the world of science. In his studies he reported that the longevity and viability of Bulgarians and lactobacilli with consumption of fermented milk products, which can be used as probiotics [7]. This study suggested that some microorganisms are beneficial for human health. From that onwards, probiotics had been widely consumed and marketed as functional food, Mechanisms of proboscis include stimulation of epithelial cells, immunomodulation, include manipulation of intestinal microbial communities, fortification of intestinal barriers, and differentiation [8]. Mostly probiotics are developed these days made from Bifidobacteria, Lactobacilli, and lactic acid bacteria, like streptococci and Lactococci. Other probiotic strains include microbial strains like Bacillus, Escherichia, and Propionibacterium and some yeast genera, mainly Saccharomyces [9].
From birth to adulthood there are many factors that may influence the gut microbiota which include diet during infancy that is the presence of antibiotics in food, exposure of antibiotics, from environmental conditions and mode of delivery [10]. The gut microbiota plays an essential role in shaping the intestinal mucus layer [11], which helps us to digest fibers and synthesize amino-acids and vitamins [12]. Such benifits help in immune system modulation, energy metabolism and storage, neurodevelopment and even regulate growth & behavior [13]. There are many diseases associated with the alteration of gut microbiota [14]. Gut microbiota dysbiosis is the major cause of obesity [15]. Although, gut microbiota is very sensitive toward the diet, drugs and environmental pollutants.
6.1.2 Classification of Probiotics
Most of the microorganisms can be used as probiotics [16]. Genus name (for example, Lactobacillus) is the first name given to the bacterial strains based on physical characteristics, metabolic needs, similarity of qualities and metabolic end products. Species is the second name of bacteria like acidophilus, based on the common characteristics and that will distinguish them from other species. Strain is the much more specific classification of bacterium which divide members of same species into subgroups and it is based on the properties that these bacteria have in common and distinct it from other species (e.g., strain LA5) [16, 17] (Table 6.1).
6.1.2.1 Lactobacillus
It involves various Gram-positive facultative anoxic or microaerophilic bacteria. These are the essential part of the lactic acid bacteria group (including Enterococcus, Pediococcus, Lactobacillus, Lactococcus, Gonococcus, Streptococcus, and Leuconostoc species) that can convert hexose sugars to lactic acid and produce an acid in the environment which can inhibit the growth of harmful species [18]. In humans, Lactobacilli are present in the GIT and vagina with Bifidobacterium which is one of the first bacteria colonized the infant gut after delivery [19].
6.1.2.2 Bifidobacterium
Bifidobacterium includes Gram-positive non-motile anoxic bacteria. They are endosymbiotic inhabitants of the vagina and gastrointestinal tract of humans [20]. Strains of the genus Bifidobacterium are also used as probiotics because they have resistance mechanism to bile salt and many beneficial effects on other probiotic bacteria, which are generated in the presence of biological fluid [21].
6.1.2.3 Saccharomyces
Saccharomyces contains several yeasts including: Saccharomyces cerevisiae used for making bread plus beer, Saccharomyces bayanus which is used for making wine, and Saccharomyces boulardii used in medicine as a probiotic [22].
6.1.2.4 Bacillus
Bacillus sp. are Gram positive, aerobes or facultative aerobes capable of spore formation. Various species of Bacillus have been reported to have potential such as B. subtilis, B. cereus, and B. coagulans [23]. The use of B. coagulans as a therapeutic like other probiotics strains such as lactobacillus and Bifidobacterium sp. has been reported, whereas presence of B. coagulans in the composition of normal gut microbes has not been reported [24].
6.1.2.5 Escherichia
Escherichia sp. comprises of Gram-negative bacteria belonging to Enterobacteriaceae family, mostly reported with virulent serotypes (E. coli O157:H7). Escherichia coli is commonly found in lower intestine as a normal microbe of gut microflora with a known probiotic strain: Escherichia coli Nissle 1917 (EcN). A study revealed the effect of Escherichia coli Nissle 1917 amalgamated with other probiotics strains on the treatment of constipation [25]. The effects of this strain on gastrointestinal disorder, Crohn’s disease [26], ulcerative colitis, IBD, and colon cancer have been studied [27].
6.1.2.6 Streptococcus and Enterococcus
Streptococcus and Enterococcus genera belong to the category of lactic acid producing bacteria and are reported to have various species that can cause heath implications such as Streptococcus pneumoniae, Streptococcus pyogenes, and vancomycin-resistant Enterococcus faecium [28]. Some species of Enterococcus like Enterococcus faecium PC4.1 show commensal relationship with skin, mouth, and intestine [29]. The potential probiotic strains are Streptococcus thermophilus, Enterococcus durans, and Lactobacillus delbrueckii subsp. bulgaricus [30, 31]. The use of Enterococcus faecium as probiotics has a long history, and proved its effectivness against antibiotic-associated diarrhea [32], the opportunistic strains of the genus serve as a reservoir of virulence and antibiotic resistance in animal study models (animal study). The use of opportunistic strains of these genera is not categorized under (GRAS) for humans consumption, but can be used as probiotics for animals [33, 34].
6.1.2.7 Lactococcus
Lactococcus genus consists of Gram-positive, lactic acid producing bacteria used to produce fermented products in the dairy industry. The acidification property of these bacteria is helpful in preventing the spoilage of milk by inhibiting the growth of spoilage microorganisms. The other properties of some species like Lactococcus lactis subsp. lactis as a probiotic of niacin production and adhesion to vaginal epithelial cells have been studied. A study on the use of Lactococcus lactis subsp. lactis CV56 in combination with other probiotics to treat antibiotic-associated diarrhea has been given [35,36,37].
6.2 Function Mechanism of Probiotics
6.2.1 Gut Barrier Function
The gut barrier defense system consists of the secretory IgA, antimicrobial peptides, mucous layer, and the epithelial junctional adhesion complex [38]. The location of epithelial cells in the center stage of the barrier effect has been reported, these cells receive molecular signals from the lumen of gut and exchange them with the underlying cells of immune system. These cells can communicate with the whole organism by the circulation of signaling molecules. Gut barrier defense plays an eminent function in the pathogenesis of various diseases associated with the GI tract like irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), infectious enterocolitis plus coeliac disease [39].
Studies conducted on the use of L. rhamnosus GG (LGG) and probiotic mix VSL#3 on mice and Caco-2 intestinal cells have shown the influence of the strain on epithelial cells of intestine to maintain the coherence of the epithelial barrier. The persistence of LGG in the GI tract was connected with its in vivo expression of pili containing a mucus-binding domain [40]. An in vitro study on LGG and its soluble factors (p75 and p40) has revealed the prevention of apoptosis in epithelial cells by activating anti-apoptotic Akt and suppressing NF-kB. In addition, an increase in the secretion of mucin by epithelial cells was observed [41].
The effect of L. plantarum, L. casei, L. rhamnosus, and L. acidophilus, on the stimulation of distinct pathways of gene-regulatory networks in the human mucosa has been reported. These regulations involve upregulation of an activator of NF-kB signaling cascade known as IL-1b, involved in the transcription of genes responsible for the maturation of B-cell and lymphogenesis, thus supporting the barrier function [42].
The effect of Lactobacillus, Bifidobacterium, and Streptococcus as probiotics on post-infectious irritable bowel syndrome (PI-IBS) caused by Trichinella spiralis showed positive results in a mouse model. Bifidobacterium or Lactobacillus treatment on PI-IBS mice showed reduction in the abdominal contractile response and withdrawal reflex score, D-lactate level, and reduced plasma diamine oxidase (DAO) concentration. The suppression of proinflammatory cytokine IL-17 and IL-6 has been reported after probiotic administration and enhancement in the expression of occludin and claudin proteins of tight junction of cells [43].
6.2.2 Production of Inhibitory Compounds by Probiotics
The antibacterial property of probiotics against Gram-negative and Gram-positive bacterial pathogens involves the production of various antibacterial substances. These substances include production of organic acids, bacteriocins, diacetyl, ethanol, hydrogen peroxide, and carbon dioxide [44, 45]. The mechanisms of action of bacteriocins to inhibit the growth of pathogens include the pore formation in the cell walls of targeted cells and inhibition of synthesis of cell wall. Nisin an antimicrobial compound associated with the formation of a complexes with the precursors of cell wall and lipid II, to inhibit the synthesis of cell walls, and also prevent pore formation in the membranes by removing complex aggregates and incorporates peptides. Bacteriocin production potential offers various advantages to the strains in complex microbial environments as they have antimicrobial properties and can inhibit the pathogens of GI tract [46, 47].
Lactobacillus acidophilus can produce various antimicrobial compounds such as acidolin. acidophillin, and lactocidin and Lactobacillus planatarum can produce another antimicrobial compound “lactolin” [48]. The effect of bacteriocin producing Lactobacillus salivarius UCC118 strain on Listeria monocytogenes infected mice have shown protective results. The effect of bacteriocin Abp118 on stimulating antimicrobial response was confirmed by this study, where Lb. salivarius showed antagonistic relationship with the pathogen [49]. The inhibition of Helicobacter pylori, E. coli, Listeria monocytogenes, Rotavirus, and Salmonella by Lactobacilli and bifidobacteria have been reported [50].
Several strains of Bifidobacterium (B. bifidum NCFB 1454) have shown the production of a unique bacteriocin (bifidocin B), effective against Gram-positive bacteria. A high inhibition rate of E. coli C1845 and Salmonella enterica ser. Typhimurium SL1344 by two Bifidobacterium strains has been studied [50]. Inhibition of Yersinia enterocolitica an entero pathogen by twenty strains of Lactobacillus has been reported in addition with the inhibition of Listeria monocytogenes by Lactobacillus plantarum C4 and Salmonella enterica serovar Typhimurium by Lactobacillus casei. The main mechanism of inhibition involves the elevation of pH mainly from dextrose fermentation by Lactobacillus [51] (Table 6.2).
6.2.3 Adhesion Mechanism of Probiotics
Attachment to intestinal mucosa, an important characteristic for probiotics, is required for its colonization in intestine along with antagonism towards pathogens and variation of immune system. Various Lactobacillus proteins accompanied by saccharide moieties and lipoteichoic acids can improve the adhesion to mucous and bacterial surface adhesions that facilitate adhesion to the mucous layer [50, 65]. Bacterial adhesins, mucus-binding protein (MUB), from Lactobacillus reuteri are reported [66]. Probiotics, such as L. plantarum, can prevent the attachment of enteropathogenic E. coli by induction of MUC2 and MUC3 mucins. Therefore, protection against pathogens is provided by glycocalyx overlying and increased mucous layers. Moreover, due to the attachment of probiotic organisms gut epithelial surfaces, the adhesion sites are blocked for pathogen colonization [67]. Upon the ingestion of lactobacilli, it competes for the binding sites due to which few sites are available for pathogenic bacteria. Attachment is facilitated by Mannose specific adhesion proteins, that also attaches to cell surface and are important for pathogens binding in gut, facilitates the attachment of L. plantarum Lp6 onto rat mucus preventing pathogen colonization [68]. Acid resistant strains from Bifidobacterium longum and B. catenulatum are reported to have effective attachment properties to human intestinal mucus in comparison to acid-sensitive [69]. In Bifidobacteria, acid resistance improves functionality through enhancing stability plus improving surface properties.
Combination of probiotics with VSL#3 improves the mucins synthesis and facilitate expression of mucin gene, therefore, enhancing the bacterial attachment to the epithelium of intestine [70]. Keratinocyte cell death, due to Staphylococcus aureus, in undifferentiated and differentiated keratinocytes is reduced by potential probiotics, Lactobacillus reuteri ATCC 55730 and Lactobacillus rhamnosus AC413. Probiotic efficiency was higher for Keratinocyte survival when they were applied before or simultaneously with S. aureus infection. S. aureus needs α5β1 integrin for attachment to keratinocytes, protective effect like probiotic was observed by blocking of α5β1 integrin. The competition for the binding site between pathogens and L. reuteri might be the protection mechanism for keratinocytes. Therefore, inhibition of S. aureus colonization and infection prevention can be achieved by application of topical probiotic prophylactically [71].
6.3 Probiotics and Nutrients Competition
One of the mechanisms for inhibiting pathogens form colonization in human gut might be the nutrient competition. There are two different ways for such competition; firstly, preventing the nutrient and energy source uptake by pathogen which is required for growth and proliferation in human gut. Secondly, production of metabolites like short chain fatty acids (SCFAs) and organic acids through fermentation and metabolism which lowers the gut pH making it unfavorable for most of the pathogens, e.g. E. coli and Salmonella [50]. Bifidobacterium adolescentis S2-1 prevents the growth of Porphyromonas gingivalis by outcompeting it for vitamin K and other growth factors [72]. After the exposure to probiotic (Lactobacillus paracasei or Lactobacillus rhamnosus), changes in pathways such as short chain fatty acids (SCFA), amino acid, and methylamines metabolism were observed in mice (germ free) colonized with microbiota of human baby [73].
Probiotics, for example, L. delbrueckii and L. acidophilus, prevent the availability of ferric hydroxide to pathogens by binding them to its cell surface [74]. Probiotic strains and exert inhibitory effects on Biofilm formation of pathogenic Listeria monocytogenes and Salmonella typhimurium are inhibited by L. rhamnosus and L. paracasei probiotic through different mechanisms including competition, displacement, and exclusion. A decrease of more than three log cycles biofilm cells was observed for L. monocytogenes [75].
6.4 Probiotics and Immune System
Immune system is affected by various reported pathways due to potential application of probiotics [76, 77]. Stimulating specific and nonspecific immunity is one of the possible mechanisms through which probiotics helps to prevent the intestinal disease in host. LAB products have immunomodulatory action through Toll like Receptors (TLRs) expression regulation, inflammatory responses inhibition, Dendritic cells (DCs) activation, and Natural Killer (NK) cells, among innate immunity; lymphocytes propagation, balancing the response of T-helper (Th1/Th2) cells, specific IgA secretion, in further ways [78]. Bacillus subtilis B10 and Saccharomyces boulardii targets specific TLRs and associated factors, hence, having a major role in controlling immunological functions of chicken bone marrow DCs. Probiotics get attached to surface of DCs. Upregulation in expression level of MHC-II, CD40, CD80, and CD86 genes was observed. Additionally, the expression of TLR1, TLR2, TLR4, and TLR15 (chicken specific) was enhanced and increased in levels of downstream related factors TRAF6, MyD88, NFκ- B mRNA, and TAB1was observed [79].
Accumulation and growth of healthy microorganisms in gut result in maturation of the several immune mechanisms, especially, for the IgA and IgM secreting cells circulation. After preparing, Memory B besides T cells move towards the effector sites, actively proliferate, then local stimulation of various cytokines and secretory IgA generation. Probiotic stimulates the IgA production upon entering the gut. Studies in mice (kept germ free) evidenced the IgA production in immune system [80]. Several studies suggested that improvement of innate and adaptive immunity along with alleviate allergies, prevention of gastric mucosal lesion development, and put up defense against intestinal pathogen infection was observed due to lactic acid bacteria (LAB) such as Bifidobacterium and Lactobacillus and also due to their fermented products [78].
Feeding to 1.4 years old rats resulted in enhanced immunosenescence associated Th1/Th2 imbalance, higher resistance to E. coli infection of aged mice, and increased antioxidant capacity were observed as a result of feeding Lactobacillus rhamnosus to mice (16 months old). Increase in levels of IFN-γ and decrease in levels of IL-4 and IL-10 production, increase in phagocytosis and neutrophil respiratory burst enzymes with no aggravation in plasma levels of MCP-1 and TNF-α was observed in the mice feed with probiotic. IgE levels and IgG1/IgG2a ratio decreased along with increase in activities of antioxidant enzymes were found in the probiotic fed mice, E. coli translocation to the organs of the mice were also reduced significantly [81].
6.4.1 Degradation of Toxins Receptors through Probiotics
Enzymatic modification of toxin receptor is done by probiotics; host is protected from intestinal disease of Clostridium difficile due to modification in toxin receptor in intestinal mucosa by Saccharomyces boulardii. Various other reported mechanisms are decreasing toxin production, lowering gut pH and decrease of virulence [50]. Probiotics could change receptors for toxins as well as prevent against pathology caused by toxins. Saccharomyces boulardii have the ability to degrade toxin receptors for Clostridium difficile in ileum of rabbit and by polyamines production, it can prevent cholera-prompted secretion in jejunum of rat. Impact of a multi-strain probiotic plus synbiotic formulation (Lactobacillus paracasei F8, L. plantarum F44, Bifidobacterium lactis 8:8, B. breve 46, resistant starch, isomaltooligosaccharides, and galacto-oligosaccharides) was studied in Clostridium difficile NAP1/027 infected C57BL/6 mice. Upon the formulation feeding, lactobacilli and bifidobacteria counts increased without detecting any caecal toxins. C. difficile DNA copies were found in significantly decreased after the qPCR of caecal [82].
6.4.2 Probiotics Roles in Anti-Proliferative
Due to the reduction in putrefactive bacteria including Bacteroides, Clostridium, and coliforms species and increase in lactobacilli and bifidobacteria that facilitate in reducing risk for colorectal cancer, probiotics are supposed to have anti-cancer activity. Probiotic, Lactobacillus salivarius ssp. Salivarius, reduced prevalence of adenocarcinoma in colon of IL-10 knockout rats [83]. Probiotic, Streptococcus thermophilus strain TH-4 have an anti-inflammatory activity along with the ability of high folate production which is important in epithelial cells for DNA repair [84, 85].
6.5 Gut Microbiota Modulation
Human gut microbes always have been immersed in the regulation of various biological functions, varying from cognitive processes and energy regulation to improving host immunity against harmful microorganisms and also neutralization of toxins. The potential application of probiotics and prebiotics always involves in the maintaining of host ideal gut health, treating/preventing host recurring inflammatory, and immune system linked diseases [86]. Probiotics have a wide range of application in prevention and treatment of several diseases which are induced or associated with the dysbiosis of gut microbiota such as acute infectious diarrhea and antibiotic-associated diarrhea, and also other GI tract diseases like colic’s or irritable bowel syndrome. At the time of treatment the gut microbial community makeup stays more steady and that it positively relates with recovery of disease symptoms [87].
6.6 Probiotics and Health
Probiotics enhance the nutritive and microbial balance of host gastrointestinal tract. Probiotics work as a carrier that transport their beneficial functional components to different target locations in the gastrointestinal tract. Ingestion of live probiotic strains has more effective results which varies from strain to strain [88]. Whereas, it is not always essential to accomplish profits [89].
6.6.1 Probiotics Role in the Treatment of Gastrointestinal Disorders
6.6.1.1 Antibiotic-Associated Diarrhea (AAD)
A systemic review study on treating of antibiotic-associated diarrhea (AAD) by usage of probiotics in aged patients (more than 65 years) and in adults (18 to 64 years) evaluated 30 random managed tests that fit in the previously developed inclusion measures. The clinical studies proposed that probiotic act as an adjuvant for antibodies which lower down the chances of antibiotic-associated diarrhea (AAD) in adults, but not in aged persons [90]. PROSPERO study proved that a number of probiotic strains such as S.boulardii and lactobacillus rhamnosus GG have involved in the prevention of antibiotic-associated diarrhea but other strains such as Lactobacillus bulgaricus, L. delbrueckii, and S.salivarius are not capable of preventing ADD [91,92,93].
6.6.1.2 Irritable Bowel Syndrome (IBS)
Several physiological, epidemiological, and clinical studied data have indicated that gut microbiota involves in the pathogenesis of irritable bowel syndrome, however, IBS pathophysiology still undiscovered [94, 95].
A functional study showed that altering the host gut microbes in conjugation with probiotics can influence some host intestinal functions, like sensitivity and motility, which seems to be related to the irritable bowel syndrome pathogenesis I [96]. A clinical experiment showed that the group of patients (35,624) that have intake of B.infantis significantly improved their disease symptoms in comparison to placebo. Moreover, the serum IL-10/IL12 ratio normalized, indicating that probiotic can helps in remission of proinflammatory state associated with irritable bowel syndrome [97, 98]. In addition, L. plantarum is better than placebo in remission of few symptoms in IBS patients. Specifically, the DSM 9843 strain radically decreased flatulence, and the 299 V and LPO1 strains appreciably lowered the intestinal pain [99,100,101].
6.6.1.3 Ulcerative Colitis
A clinical experiment showed that the mesalamine treatment with strain Lactobacillus GG might be more efficient than standard treatment for preventing the relapsing time of disease [102]. E. coli strain Nissle 1917 showed similar effective results as of 5-aminosalicyclates in averting the relapsing of ulcerative colitis in adults [103].
6.6.1.4 Crohn’s Disease
Clinical experiments performed with E-coli strain Nissle 1917 and with distinct strains of Lactobacillus had not shown any higher effect than placebo in averting the occurrence of Crohn’s disease [104, 105]. A studied proved that daily intake of 3 g mesalamine alone was less effective than 2 g daily intake of mesalamine along with S. boulardii in lowering the relapsing of Crohn’s disease in patients. But later on a clinical study did not verify these results [106, 107].
6.6.1.5 Pouchitis
Pouchitis is an inflammatory condition of the ileal reservoir in patients with acute and chronic refractory ulcerative colitis experienced restorative proctocolectomy with ileal pouchanal anastomosis (IPAA) [108]. Several clinical trials with probiotics have been conducted that have shown their safety and effectiveness in sustaining the reduction of pouch inflammation, also antibiotic treatment attained subsequent, like 5-aminosalicyclic acid also helps in relapsing of chronic pouchitis and prevention of acute pouchitis [109, 110]. A systematic review from the Cochrane Collaboration showed that VSL#3 was very efficient in sustaining the reduction of chronic pouchitis and also in averting the onset of pouchitis than placebo [111].
6.6.2 Probiotics for Depression and Anxiety
Depression and anxiety are two most common human mental health conditions, with lifetime prevalence rates worldwide. Gut and brain interact with each other through a particular pathway called gut-brain axis pathway that includes immune, endocrine, and neural systems. Administration of probiotic mixture containing Bifidobacterium longum BL04, L. plantrum LP, Lactobacillus fermentum LF16, and L. rhamnosus LR06 was given to examine the effect of probiotics on depression and anxiety was reported. The study did not provide any positive effect on sleep quality and depressive mood state [112]. Thus more significant clinical trials are needed to explore the effect of probiotics on depression and anxiety.
6.6.3 Human Gut Microbial Community
Human gut microbiota is the microorganisms that live in the human gut. It is complex community of microbes—estimated to contain 200 trillion cells and containing greater than 1000 diverse microbial species Fig. 6.1. Human gut microbiota is composed of a wide range of bacteria, fungi, archaea, and viruses [113]. Gut microbiota—biome of microorganisms that live in the digestive tract of human beings whether on the intestinal mucosal surface or within the gut lumen.
Microbial density in the gut [114]
Individual has their own stable fecal microbiota for lifetime and harbors different characteristic pattern of gut microbial flora. Around 90% of human gut microbiota are made up of Bacteroidetes and Firmicutes.
6.6.3.1 Function of Gut Microbiota
Intact microbiome is essential for the development of the GIT in many ways including—immune tolerance, the mucosa associated immune system, motility and vascularity, epithelial and barrier function. The microbiota which exhibiting commensalism in host provide homeostatic functions like immunomodulation, pathogen exclusion, upregulation of cytoprotective genes, regulation prevention of apoptosis, and maintenance of barrier function.
6.6.3.2 Metabolic Functions
N-digestible dietary residue fermentation e.g. cellulose, starch by aerobic bacteria, and short chain fatty acids (SCFAs), are the source for energy of both host and resident bacteria Gut Bacteroides involves in the breakdown of complex N-glycan with the help of enzymatic apparatus which is encoded by multiple co-regulated genetic loci [115]. Putrefaction of exogenous and endogenous protein (like sloughed epithelium and lysed bacteria) has been done by anaerobic bacteria, SCFAs as well as toxic substances like ammonia and amines [116].
6.6.3.3 Trophic Functions
Short chain fatty acids induce the differentiation and proliferation of epithelial cell. Moreover, butyrate promotes cells reversion from neoplastic to non-neoplastic phenotype (Fig. 6.2).
Microbiota derived SCFAs and atherosclerosis [117]
6.7 Development and Homeostasis of Immune System
Specialized epithelial cells (M cells), sample luminal antigens as well as the microflora transport them to the lymphoid follicles to develop tolerating anti-inflammatory response (Th2 response) through the production of IL 10 and TGFB. Due to the pertinacious interactions between the host and its bacteria the immunity of host constantly changed. Host microorganisms try to change the immune response by changing its surface antigenicity, so that organism can avoid detection by immunosurveillance and maintain predominance of ecological niche in intestinal tract. Bacteria commensalism have play an essential role in sustaining the intestinal epithelial homeostasis and these gut bacteria are recognized under normal steady-state conditions by TLRs. TLRs activation through commensal microflora is important for protection from gut injury and associated mortality [118].
Animal’s colonization with major gut microbes, Bacteroides fragilis, physical and cellular maturation during immune system development is directed by a bacterial polysaccharide (PSA). During the colonization of B. fragilis, main activities of PSA are directing lymphoid organogenesis, correcting systemic T cell deficiencies and T(H)1/T(H)2 imbalances [119]. Communication between the host immune system and symbiotic microbiota facilitate by the bacterial metabolites and also affecting the balance between pro- and anti-inflammatory mechanisms [120]. Short chain fatty acids (SCFA), microbial metabolites regulate colonic Treg cell homeostasis [121].
6.7.1 Protective Function (Barrier Effect)
In barrier protective function microorganisms compete and attach to the brush border of host intestinal epithelial layer. Beneficial microorganisms compete for accessible nutrients and secrete antimicrobial (bacteriocins) [122].
6.7.2 Colonization Mechanism
Inflammation host responses change in microbiota composition and growth suppression induced by Salmonella enterica subspecies 1 serovar Typhimurium (S. Tm). Avirulent invGsseD mutant failed to trigger the colitis which was surpass by the gut microbiota in compare to wild type S. Tm. Inflammation can cause colonization resistance. Host immune defense system can alter the equilibrium between the pathogen and defensive microbiota in favor of the harmful microorganism [123].
6.7.3 Function of Uncultured Bacteria
The human gut microbial composition is associated with diseases and health of the host environment, but the awareness of different host microbial community is still needed for identifying the vast biological roles of the gut microbiota. The whole composition of human gut microbiota remains unknown. A study reported the identification of 1952 uncultured candidate bacterial species from 11,850 human gut microbiomes via reconstructing 92,143 metagenome-assembled genomes (Fig. 6.3). The identification of these species can help in understanding the interaction between probiotics and their beneficial effects [124].
Influence of gut microbial metabolism on human health [125]
6.8 The Gut Microbiota and Cancers
Colorectal cancer increases in human beings having age less than 50 years and it is related with human diet factors and daily eating habits which eventually affect the gut microbiota and CRC is the third most widespread cancer worldwide. In vitro experiments proliferation of CRC cells promoted by F. nucleatum. in mice, it is derived from the patient cells by CRC xenografts. Enterotoxigenic Bacteroides fragilis is the most long-studied human bacterial pathogen which causes diarrhea and inflammation in gastrointestinal tract of human beings. Enterotoxigenic Bacteroides fragilis (ETBF) increases colorectal cancer formation in mice. Currently, it was found in precancerous colonic lesions and biofilms coating human CRCs called adenomas (Fig. 6.4). Escherichia coli improve tumorigenesis in preclinical CRC experimental models by expressing the genomic island polyketide synthase (pks+) and are enriched in human colorectal cancer (CRC) tissues. Pks + E. coli secrete the genotoxin colibactin which caused alkylation in DNA, resulting in DNA adducts in colonic epithelial cells [126].
Microorganisms may derive colorectal cancer [126]
6.9 Gut Microbiota and Malabsorption Syndrome
Malabsorption syndrome is not exceptional, and it refers to the number of intestinal disorders which mimic the functional GI tract disorders. It is mainly due to the poor absorption of dietary carbohydrates, like fructose, lactose, etc. Occurrence and degree of malabsorption due to dietary lactose are widely diverse in the world with distinct population but most common in Asia than in America and Europe [127]. Number of host factors involves in the development of malabsorption such as degree of visceral hypersensitivity, host functional issues, cognitive dysfunction, colonic transit, host gut microbiota and also on the subtypes of microorganisms; bacteria such as Methanobrevibacter smithii effects on the intestinal transit due constipation and excess production of methane, however, hydrogen sulfide (H2S) consider as a diarrhea biomarker [128].
6.10 Gut Microbiota and IBD
Irritable bowel disease related with the metabolic and compositional changes in the host intestinal microbiota. A study showed the effect on different microbial species of IBD suffering host, comprising decrease in Dialister invisus, Bifidobacterium adolescentis, Faecalibacterium prausnitzii and an increase in Ruminococcus gnavus and an unidentified member of Clostridium cluster XIVa [129]. A study revealed the wide range of data report about the host and microbial responses in 132 IBD patients, showing the host immune factors, molecular functional profile, and gut microbiome in relation of metabolome [130].
6.11 Gut Microbiota and FBD
Functional bowel disorders are known as “irritable bowel syndrome” and they are very similar to the number of GI tract diseases without any clear pathogenesis. A profound sequencing of the microbiome (150-times fold as related to the human genome and bacterial genes regulating functions) has supported that the irritable bowel syndrome gut microbes are aberrant in count and has diverse number of bacterial families [113, 131]. This report presented that the Firmicutes and Bacteroides ratio might act as an indicator of microbial imbalance in irritable bowel syndrome [132].
6.12 Gut Microbiota and CDI
Clostridium difficile is a potential pathogen associated mostly with diarrhea caused by the frequent intake of antibiotics. The infections caused by C. difficile possess major health issues and are known as Clostridium difficile infections (CDI). The role of gut microbes in pathogenesis of CDI grabs the attention of researchers [133]. The patients suffering from reoccurring CDI have shown alterations in gut microbial composition, also associated with frequent intake of antibiotics. A study conducted on CDI patients who have undergone fecal microbiota transplantation (FMT), reduction in Firmicutes and Bacteroidetes population, and increment in Proteobacteria was observed in pre-FMT fecal samples [134]. Another study on CDI patients showed decrease in lactate producing phylotypes and opportunistic pathogens associated with endotoxin production (Fig. 6.5). An increment in the butyrate-producing anaerobic bacteria was also reported when compared to healthy control groups [135].
Human gut microbiota and diseases [136]
6.13 Gut Microbiota and Health
The microbes of human gut can affect the physiology of host in various dimensions and their interaction built a beneficial relationship for both host and gut microbes. Mutually beneficial bacteria help in providing vital nutrients, metabolize the complex compounds, produce inhibitory compounds against pathogens, and help in the formation of intestinal architecture [137] (Table 6.3).
6.13.1 Immune Regulation
Gut microbes can stimulate the normal development of host humoral and cellular mucosal immunity. Hematopoietic and non-hematopoietic cells of innate immunity can recognized the metabolites and signals of microbes and converted into physiological functions [151]. Clinical studies reported that the GF mice have showed defects in the formation of antibodies and gut-associated lymphoid tissues as comparison to normal mice [152]. A study has showed that the tolerogenic responses produced by gut microbes affect the gut dendritic cells and ceased the anti-inflammatory pathway of Th17 helper cells [153].
6.13.2 Drug Metabolism by Gut Microbiota
Microbiome-encoded enzymes elucidate the drug-metabolizing activities of host gut microbes and different communities on the basis of their genomic structural content and significantly affect the intestinal and systemic drug metabolism of mice [154].
6.13.3 Bacterial Metabolite Enhances Athletic Performance
Veillonella strain enhance the mice treadmill run time and also increases the specific run time of marathon athletes. V. atypica improves the athlete’s performance during physical activities (running) by metabolic conversion of lactate into propionate, hence consider as a natural microbiome-encoded enzymatic process [155].
6.13.4 Alleviation of Food Allergy (FA)
In food allergic infants dysbiotic fecal microbiota developed with in time but unsuccessful in mice. Therapy with Clostridiales strains, either as a monotherapy with Subdoligranulum variable or consortium, suppressed food allergy in mice. However, immunomodulatory bacteroidales consortium bacteriotherapy induced expression by regulator T (Treg) cells of the transcription factor ROR γt in a My D88-dependent manner, which was less in food allergic mice plus infants and futilely persuaded by their microbiota [156].
6.14 Conclusions
Industrial, agricultural, and domestic use of synthetic compounds produce large amount of environmental pollutants. From past several decades’ environmental pollutants cause various health hazards and these pollutants can alter the functioning of gut microbiota. Use of probiotics will protect against the toxicity caused by these pollutants. There are number of bacterial, yeast, and fungal species which are used as probiotics. Various types of inhibitory compounds produced by probiotics shows antagonistic effect against pathogenic strains. It has been stated that probiotics produce extensive range of different bacteriocins such as nicin which constitute the major mechanism of antimicrobial act. Lactobacilli and bifidobacteria genera have been informed to produce bacteriosins, lactolin, acidophillin acidolin, and lactocidin, protection against infection with the foodborne pathogens. The identification of these species may help in understanding the interaction between probiotics and benefits with probiotics. Probiotics may increase the microbiological and nutritional balance of the gastrointestinal tract and used for the treatment of various gastrointestinal disorders like irritable bowel syndrome, Crohn’s disease, pouchitis, antibiotic-associated diarrhea. Probiotics also used for enhancing the immune system by improving gut microbiota. It is concluded that the probiotics are essential for immune regulation, improve gut microbiota and for the treatment of gastrointestinal disorders.
References
Atashgahi S et al (2018) Prospects for harnessing biocide resistance for bioremediation and detoxification. Science 360(6390):743–746
Huttenhower C et al (2012) Structure, function and diversity of the healthy human microbiome. Nature 486(7402):207–214
Ding T, Schloss PD (2014) Dynamics and associations of microbial community types across the human body. Nature 509(7500):357–360
Dietert RR, Silbergeld EK (2015) Biomarkers for the 21st century: listening to the microbiome. Toxicol Sci 144(2):208–216
Sousa T et al (2008) The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm 363(1–2):1–25
Koppel N, Rekdal VM, Balskus EP (2017) Chemical transformation of xenobiotics by the human gut microbiota. Science 356(6344):1246–1257
Metchnikoff E (1907) The prolongation of life: optimistic studies, trans. P. Chalmers Mitchell. New York: GP Putnam’s Sons. Harvard
Thomas CM, Versalovic JJGM (2010) Probiotics-host communication: modulation of signaling pathways in the intestine. 1(3):148–163
He MQ, Shi BY (2017) Gut microbiota as a potential target of metabolic syndrome: the role of probiotics and prebiotics. Cell Biosci 7:54
Jandhyala SM et al (2015) Role of the normal gut microbiota. World J Gastroenterol 21(29):8787–8803
Jakobsson HE et al (2015) The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 16(2):164–177
Spanogiannopoulos P et al (2016) The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol 14(5):273–287
Charbonneau MR et al (2016) Sialylated Milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 164(5):859–871
Lange K et al (2016) Effects of antibiotics on gut microbiota. 34(3):260–268
Fei N, Zhao LP (2013) An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J 7(4):880–884
Pizzorno JE, Murray MT, Joiner-Bey H (2016) The clinician’s handbook of natural medicine e-book. Elsevier Health Sciences
Rao V, Rao L (2016) Probiotics and prebiotics in human nutrition and health. BoD–Books on Demand
Makarova K et al (2006) Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci U S A 103(42):15611–15616
Walker WA (2013) Initial intestinal colonization in the human infant and immune homeostasis. Ann Nutr Metab 63(Suppl. 2):8–15
Chen JJ, Cai W, Feng Y (2007) Development of intestinal bifidobacteria and lactobacilli in breast-fed neonates. Clin Nutr 26(5):559–566
Ruiz L, Margolles A, Sanchez B (2013) Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol 4:396
Fijan S (2014) Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health 11(5):4745–4767
Elshaghabee FMF et al (2017) Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol 8:1490
Konuray G, Erginkaya ZJF (2018) Potential use of Bacillus coagulans in the food industry. Foods 7(6):92
Chmielewska A, Szajewska H (2010) Systematic review of randomised controlled trials: probiotics for functional constipation. World J Gastroenterol 16(1):69–75
Xia P, Zhu J, Zhu G (2013) Escherichia coli Nissle 1917 as safe vehicles for intestinal immune targeted therapy—a review. 53(6):538–544
Behnsen J et al (2013) Probiotics: properties, examples, and specific applications. Cold Spring Harb Perspect Med 3(3):a010074
Hutkins RW, Goh Y (2014) Streptococcus: Streptococcus thermophilus. In: Encyclopedia of food microbiology: second edition. Elsevier Inc., pp 554–559
Hadji-Sfaxi I et al (2011) Antimicrobial activity and safety of use of Enterococcus faecium PC4.1 isolated from Mongol yogurt. Food Control 22(12):2020–2027
Garcia EF et al (2016) Identification of lactic acid bacteria in fruit pulp processing byproducts and potential probiotic properties of selected lactobacillus strains. Front Microbiol 7:1371
Pieniz S et al (2013) Production of selenium-enriched biomass by enterococcus durans. Biol Trace Elem Res 155(3):447–454
Hempel S et al (2012) Probiotics for the prevention and treatment of antibiotic-associated diarrhea a systematic review and meta-analysis. JAMA 307(18):1959–1969
DiRienzo DB (2014) Effect of probiotics on biomarkers of cardiovascular disease: implications for heart-healthy diets. Nutr Rev 72(1):18–29
Bednorz C et al (2013) Feeding the probiotic Enterococcus faecium strain NCIMB 10415 to piglets specifically reduces the number of Escherichia coli Pathotypes that adhere to the gut mucosa. Appl Environ Microbiol 79(24):7896–7904
Yang X, Wang Y, Huo GJGA (2013) Complete genome sequence of Lactococcus lactis subsp. lactis KLDS4. 0325. Genome Announc 1(6):e00962-13
Gao Y et al (2011) Complete genome sequence of Lactococcus lactis subsp. lactis CV56, a probiotic strain isolated from the vaginas of healthy women. J Bacteriol 193(11):2886–2887
Johnston BC et al (2011) Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev (11):CD004827
McGuckin MA et al (2009) Intestinal barrier dysfunction in inflammatory bowel diseases. 15(1):100–113
Blaut M, Klaus S (2012) Intestinal microbiota and obesity. In: Appetite control. Springer, pp 251–273
Lebeer S et al (2012) Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl Environ Microbiol 78(1):185–193
Yan F, Polk DB (2002) Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 277(52):50959–50965
van Baarlen P et al (2011) Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc Natl Acad Sci U S A 108:4562–4569
Wang H et al (2014) Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS One 9(3):e90153
Liao SFF, Nyachoti M (2017) Using probiotics to improve swine gut health and nutrient utilization. Anim Nutr 3(4):331–343
Razdan K, Parihar J, Bajaj B (2012) Isolation and characterization of a lipolytic and phytase producing probiotic for potential application in poultry feed. 2:369–377
Nielsen DS et al (2010) The effect of bacteriocin-producing Lactobacillus plantarum strains on the intracellular pH of sessile and planktonic Listeria monocytogenes single cells. 141:S53–S59
Hassan M et al (2012) Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol 113(4):723–736
Vilà i Miquel B, Esteve-Garcia E, Brufau de Barberà J (2010) Probiotic microorganisms: 100 years of innovation and efficacy. Modes of action
Corr SC et al (2007) Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. 104(18):7617–7621
Bermudez-Brito M et al (2012) Probiotic mechanisms of action. 61(2):160–174
Bujalance C et al (2014) Lack of correlation between in vitro antibiosis and in vivo protection against enteropathogenic bacteria by probiotic lactobacilli. Res Microbiol 165(1):14–20
Reis JA et al (2012) Lactic acid bacteria antimicrobial compounds: characteristics and applications. Food Eng Rev 4(2):124–140
Shin JM et al (2016) Biomedical applications of nisin. J Appl Microbiol 120(6):1449–1465
Karpiński TM, Szkaradkiewicz AKJPJM (2013) Characteristic of bacteriocines and their application. Pol J Microbiol 62(3):223–235
Burgos MJG et al (2014) The cyclic antibacterial peptide Enterocin AS-48: isolation, mode of action, and possible food applications. Int J Mol Sci 15(12):22706–22727
Karpinski TM, Szkaradkiewicz AK (2013) Characteristic of bacteriocines and their application. Pol J Microbiol 62(3):223–235
Vera ECS et al (2018) Optimization of biosurfactant and bacteriocin-like inhibitory substance (BLIS) production by Lactococcus lactis CECT-4434 from agroindustrial waste. Biochem Eng J 133:168–178
Wong FWF et al (2017) Recovery of a bacteriocin-like inhibitory substance from Pediococcus acidilactici Kp10 using surfactant precipitation. Food Chem 232:245–252
Arakawa K et al (2016) Production of a bacteriocin-like inhibitory substance by Leuconostoc mesenteroides subsp. dextranicum 213M0 isolated from Mongolian fermented mare milk, airag. 87(3):449–456
Stevens M et al (2011) The potential of reuterin produced by Lactobacillus reuteri as a broad spectrum preservative in food. In: Protective cultures, antimicrobial metabolites and bacteriophages for food and beverage biopreservation. Elsevier, pp 129–160
Rattanachaikunsopon P, Phumkhachorn P (2010) Lactic acid bacteria: their antimicrobial compounds and their uses in food production 1(4):218–228
Singh VP (2018) Recent approaches in food bio-preservation-a review. 8(1):104–111
Whittenbury RJM (1964) Hydrogen peroxide formation and catalase activity in the lactic acid bacteria. 35(1):13–26
Elshaghabee FMF et al (2016) Ethanol production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Front Microbiol 7:47
Van Tassell ML, Miller MJ (2011) Lactobacillus adhesion to mucus. 3(5):613–636
Buck BL et al (2005) Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl Environ Microbiol 71(12):8344–8351
Ohland CL, MacNaughton WK (2010) Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 298(6):G807–G819
Sun J et al (2007) Factors involved in binding of Lactobacillus plantarum Lp6 to rat small intestinal mucus. Lett Appl Microbiol 44(1):79–85
Collado MC et al (2006) Adhesion properties and competitive pathogen exclusion ability of Bifidobacteria with acquired acid resistance. J Food Prot 69(7):1675–1679
Caballero-Franco C et al (2007) The VSL# 3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. 292(1):G315–G322
Prince T, McBain AJ, O’Neill CA (2012) Lactobacillus reuteri protects epidermal keratinocytes from Staphylococcus aureus-induced cell death by competitive exclusion. Appl Environ Microbiol 78(15):5119–5126
Hojo K et al (2007) Reduction of vitamin K concentration by salivary Bifidobacterium strains and their possible nutritional competition with Porphyromonas gingivalis. J Appl Microbiol 103(5):1969–1974
Martin FPJ et al (2008) Probiotic modulation of symbiotic gut microbial–host metabolic interactions in a humanized microbiome mouse model. 4(1):157
Elli M et al (2000) Iron requirement of Lactobacillus spp. in completely chemically defined growth media. J Appl Microbiol 88(4):695–703
Woo J, Ahn J (2013) Probiotic-mediated competition, exclusion and displacement in biofilm formation by food-borne pathogens. 56(4):307–313
van Hemert S, Verwer J, Schütz B (2013) Clinical studies evaluating effects of probiotics on parameters of intestinal barrier function. 3:212–221
Hyland NP, Quigley EM, Brint E (2014) Microbiota-host interactions in irritable bowel syndrome: epithelial barrier, immune regulation and brain-gut interactions. World J Gastroenterol 20(27):8859–8866
Tsai YT, Cheng PC, Pan TM (2012) The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl Microbiol Biotechnol 96(4):853–862
Rajput IR et al (2014) Saccharomyces boulardii and Bacillus subtilis B10 modulate TLRs mediated signaling to induce immunity by chicken BMDCs. J Cell Biochem 115(1):189–198
Ng SC et al (2009) Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15(2):300–310
Sharma R et al (2014) Improvement in Th1/Th2 immune homeostasis, antioxidative status and resistance to pathogenic E. coli on consumption of probiotic Lactobacillus rhamnosus fermented milk in aging mice. Age 36(4):9686
Kondepudi KK et al (2014) A novel multi-strain probiotic and synbiotic supplement for prevention of Clostridium difficile infection in a murine model. 58(10):552–558
O’Shea EF et al (2012) Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: Bacteriocins and conjugated linoleic acid. Int J Food Microbiol 152(3):189–205
Van Guelpen B et al (2006) Low folate levels may protect against colorectal cancer. Gut 55(10):1461–1466
Tooley KL et al (2006) Oral ingestion of Streptococcus thermophilus diminishes severity of small intestinal mucositis in methotrexate treated rats. Cancer Biol Ther 5(6):593–600
Lin C-S et al (2014) Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. 37(5):259–268
Ceapa C et al (2013) Influence of fermented milk products, prebiotics and probiotics on microbiota composition and health. Best Pract Res Clin Gastroenterol 27(1):139–155
Islam SUJM (2016) Clinical uses of probiotics. 95(5):e2658
Sullivan Å, Nord C (2005) Probiotics and gastrointestinal diseases. 257(1):78–92
Jafarnejad S et al (2016) Probiotics reduce the risk of antibiotic-associated diarrhea in adults (18–64 years) but not the elderly (> 65 years) a meta-analysis. 31(4):502–513
Szajewska H, Kołodziej M (2015) Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. 42(10):1149–1157
Szajewska H, Kołodziej M (2015) Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. 42(7):793–801
Patro-Golab B, Shamir R, Szajewska H (2015) Yogurt for treating antibiotic-associated diarrhea: systematic review and meta-analysis. Nutrition 31(6):796–800
Ringel Y, Carroll IM (2009) Alterations in the intestinal microbiota and functional bowel symptoms. 19(1):141–150
Salonen A, de Vos WM, Palva A (2010) Gastrointestinal microbiota in irritable bowel syndrome: present state and perspectives. Microbiology 156:3205–3215
Moayyedi P et al (2010) The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 59(3):325–332
Whorwell PJ et al (2006) Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 101(7):1581–1590
O’Mahony L et al (2005) Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 128(3):541–551
Nobaek S et al (2000) Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol 95(5):1231–1238
McFarland LV, Dublin S (2008) Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol 14(17):2650–2661
Carroll IM et al (2011) Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 301(5):G799–G807
Zocco MA et al (2006) Efficacy of lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 23(11):1567–1574
Kruis W et al (2004) Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 53(11):1617–1623
Prantera C et al (2002) Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with lactobacillus GG. Gut 51(3):405–409
Guslandi M (2015) Role of probiotics in Crohn’s disease and in Pouchitis. J Clin Gastroenterol 49:S46–S49
Guslandi M et al (2000) Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig Dis Sci 45(7):1462–1464
Kollman KA, Goulet O, Vanderhoof JA (2001) Saccharomyces boulardii does not stimulate mucosal hyperplasia after intestinal resection in the rat. J Pediatr Gastroenterol Nutr 32(4):454–457
McLaughlin S et al (2008) Restorative proctocolectomy, indications, management of complications and follow-up–a guide for gastroenterologists. 27(10):895–909
Shen J, Zuo ZX, Mao AP (2014) Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and Pouchitis: meta-analysis of randomized controlled trials. Inflamm Bowel Dis 20(1):21–35
Persborn M et al (2013) The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment Pharmacol Ther 38(7):772–783
Holubar SD et al (2010) Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. (6):CD001176
Marotta A et al (2019) Effects of probiotics on cognitive reactivity, mood, and sleep quality. Front Psych 10:164
Qin JJ et al (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285):59–65
O’Hara AM, Shanahan F (2006) The gut flora as a forgotten organ. EMBO Rep 7(7):688–693
Briliute J et al (2019) Complex N-glycan breakdown by gut Bacteroides involves an extensive enzymatic apparatus encoded by multiple co-regulated genetic loci. Nat Microbiol 4(9):1571–1581
Rowland I et al (2018) Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr 57(1):1–24
Org E, Mehrabian M, Lusis AJ (2015) Unraveling the environmental and genetic interactions in atherosclerosis: central role of the gut microbiota. 241(2):387–399
Mazmanian SK et al (2005) An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122(1):107–118
Rakoff-Nahoum S et al (2004) Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118(2):229–241
Arpaia N, Barton GM (2013) The impact of toll-like receptors on bacterial virulence strategies. Curr Opin Microbiol 16(1):17–22
Smith PM et al (2013) The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. 341(6145):569–573
Mathipa MG, Thantsha MS (2017) Probiotic engineering: towards development of robust probiotic strains with enhanced functional properties and for targeted control of enteric pathogens. Gut Pathog 9:28
Stecher B et al (2007) Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5(10):2177–2189
Almeida A et al (2019) A new genomic blueprint of the human gut microbiota. Nature 568(7753):499–504
Kinross JM, Darzi AW, Nicholson JK (2011) Gut microbiome-host interactions in health and disease. Genome Med 3:14
Garrett WS (2019) The gut microbiota and colon cancer. Science 364(6446):1133–1135
Ghoshal UC, Ghoshal U (2020) Investigations for dietary carbohydrate malabsorption and gut microbiota, pp 359–370
Sharma A et al (2014) Fructose malabsorption is not uncommon among patients with irritable bowel syndrome in India: a case–control study. Indian J Gastroenterol 33(5):466–470
Joossens M et al (2011) Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 60(5):631–637
Lloyd-Price J et al (2019) Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569(7758):655–662
Rajilic-Stojanovic M et al (2011) Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 141(5):1792–1801
Jeffery IB et al (2012) An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 61(7):997–1006
Lessa FC et al (2015) Burden of Clostridium difficile infection in the United States. N Engl J Med 372(24):2369–2370
Weingarden AR et al (2014) Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol 306(4):G310–G319
Antharam VC et al (2013) Intestinal Dysbiosis and depletion of Butyrogenic Bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol 51(9):2884–2892
Britton RA, Young VB (2012) Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol 20(7):313–319
Round JL, Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease (vol 9, pg 313, 2009). Nat Rev Immunol 9(8):600
Nicholson JK et al (2012) Host-gut microbiota metabolic interactions. Science 336(6086):1262–1267
LeBlanc JG et al (2013) Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol 24(2):160–168
Martins SV et al (2015) Adipocyte membrane glycerol permeability is involved in the anti-adipogenic effect of conjugated linoleic acid. Biochem Biophys Res Commun 458(2):356–361
Daliri EB-M, Lee BH (2015) New perspectives on probiotics in health and disease. 4(2):56–65
de Diego-Cabero N et al (2015) Bile acid mediated effects on gut integrity and performance of early-weaned piglets. BMC Vet Res 11:111
Kelly CJ et al (2015) Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17(5):662–671
den Besten G et al (2015) Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. 64(7):2398–2408
Chao M-R et al (2015) Simultaneous detection of 3-nitrotyrosine and 3-nitro-4-hydroxyphenylacetic acid in human urine by online SPE LC-MS/MS and their association with oxidative and methylated DNA lesions. 28(5):997–1006
Craciun S, Balskus EP (2012) Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A 109(52):21307–21312
Shimada Y et al (2013) Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One 8(11):e80604
Li L et al (2014) Immunoregulatory effects on Caco-2 cells and mice of exopolysaccharides isolated from Lactobacillus acidophilus NCFM. Food Funct 5(12):3261–3268
Graham SF et al (2015) Untargeted metabolomic analysis of human plasma indicates differentially affected polyamine and L-Arginine metabolism in mild cognitive impairment subjects converting to Alzheimer’s disease. PLoS One 10(3):e0119452
Pandeya DR et al (2012) Host-microbial interaction in the mammalian intestine and their metabolic role inside. Biomed Res 23(1):9–21
Thaiss CA et al (2016) The microbiome and innate immunity. Nature 535(7610):65–74
Madsen KL et al (1999) Lactobacillus species prevents colitis in interleukin 10 gene–deficient mice. 116(5):1107–1114
Magrone T, Jirillo E (2013) The interplay between the gut immune system and microbiota in health and disease: Nutraceutical intervention for restoring intestinal homeostasis. Curr Pharm Des 19(7):1329–1342
Zimmermann M et al (2019) Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 570(7762):462–467
Scheiman J et al (2019) Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med 25(7):1104–1109
Abdel-Gadir A et al (2019) Microbiota therapy acts via a regulatory T cell MyD88/ROR gamma t pathway to suppress food allergy. Nat Med 25(7):1164–1174
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
El-Dalatony, M.M., El-Sheekh, M., Li, X. (2020). Environmental Pollutants that Can Be Metabolized by the Host, but Would Be Harmful to Humans (e.g., Causing Cancers, etc.). In: Li, X., Liu, P. (eds) Gut Remediation of Environmental Pollutants. Springer, Singapore. https://doi.org/10.1007/978-981-15-4759-1_6
Download citation
DOI: https://doi.org/10.1007/978-981-15-4759-1_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4758-4
Online ISBN: 978-981-15-4759-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)